Abstract
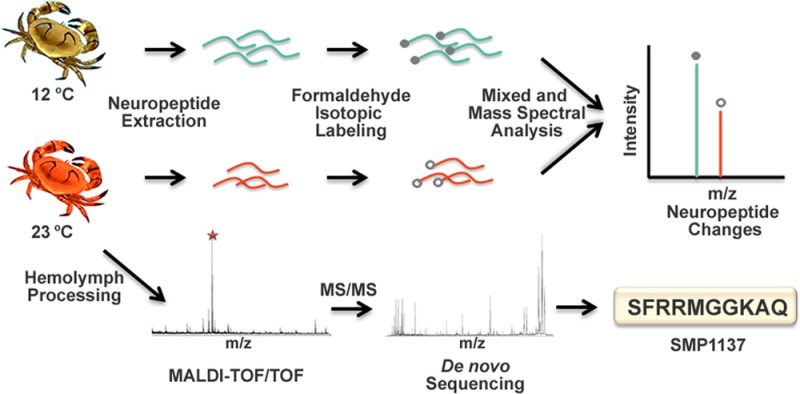
Temperature changes influence the reaction rates of all biological processes, which can pose dramatic challenges to cold-blooded organisms, and the capability to adapt to temperature fluctuations is crucial for the survival of these animals. In order to understand the roles that neuropeptides play in the temperature stress response, we employed a mass spectrometry-based approach to investigate the neuropeptide changes associated with acute temperature elevation in three neural tissues from the Jonah crab Cancer borealis. At high temperature, members from two neuropeptide families, including RFamide and RYamide, were observed to be significantly reduced in one of the neuroendocrine structures, the pericardial organ, while several orcokinin peptides were detected to be decreased in another major neuroendocrine organ, the sinus gland. These results implicate that the observed neuropeptides may be involved with temperature perturbation response via hormonal regulation. Furthermore, a temperature stress marker peptide with the primary sequence of SFRRMGGKAQ (m/z 1137.7) was detected and de novo sequenced in the circulating fluid (hemolymph) from animals under thermal perturbation.
Keywords: Cancer borealis, neuropeptide, temperature change, dimethyl labeling, quantitative peptidomics, mass spectrometry, pericardial organ, sinus gland, MALDI-TOF/TOF, MALDI-FTMS
Introduction
Environmental temperature fluctuations can pose dramatic challenges to cold-blooded organisms, such as insects, fish, and crustaceans, since temperature changes can cause global perturbation that affects the reaction rates of all the biological processes. When the homeostasis of life is challenged, complex responses, including physiological, neurological, and behavioral, may co-mediate the response and rebuild the balance to prolong the animals’ survival.1−3 Using four congeneric species of crabs as study models, Stillman has suggested that the temperature acclimation capacity of these marine invertebrates is crucial for their survival in the face of global warming.4 By unveiling the underlying mechanism of how organisms respond to increasing habitat temperature, we can obtain a better understanding of the direct impacts that climate changes have on life.
It has been a lasting effort to probe the mechanism of the thermal perturbation response in invertebrates over the past decade. In the lobster Homarus americanus, a large spectrum of physiological parameters in the circulating hemolymph has been observed to be influenced negatively by high temperature, including glucose, total protein concentration, cholesterol, chloride and calcium concentration, etc.5 Temperature also has a considerable impact on the outputs of neuronal circuits.6,7 For example, the pyloric rhythm of the stomatogastric ganglion (STG) from Cancer borealis was shown to be robust to temperature changes from 7 to 23 °C, but the system “crashed” at high acute temperatures (>23 °C).8−10 A recent study in Cancer borealis and Cancer pagurus showed that the pyloric phases were maintained in intact animals at high temperature (26 °C), while pyloric frequency increased significantly. However, the frequency range was more restricted than it was in vitro, which might be caused by sensory feedback and neuromodulatory input.11 Temperature changes are also correlated with cardiac performance in multiple organisms.4,12−14 In H. americanus, the amplitude and frequency of heartbeats are both strongly affected by increasing temperature.13 Interestingly, although the Q10 values are similar in vivo and in vitro, the heartbeat rates are faster in intact animals, and less heart failures are observed at higher temperatures (above 20 °C) compared to the in vitro preparations. This is intriguing because it may suggest that endogenous neural and/or hormonal signals might protect the cardiac performance integrity in intact animals from temperature ramp. However, to date, there has been no direct evidence regarding the involvement of neuromodulators in the regulation of the temperature perturbation response.
To address this knowledge gap, we employed a mass spectrometry-based strategy to examine the quantitative changes of neuropeptides caused by temperature elevation. The Jonah crab C. borealis was selected as an experimental model, which is an ideal system for investigating neuromodulation due to its simple and well-defined neural network.15 The neural network of C. borealis includes two features: the stomatogastric nervous system (STNS), which generates rhythmic motor patterns that control the movement of the stomach, and the central nervous system (CNS), which consists of the brain, thoracic ganglion, etc. Other major neuroendocrine components are the pericardial organ (PO), which surround the heart, and the paired sinus gland (SG) located in the eyestalks. Neuropeptides from neurohemal organs, such as PO, are released into the hemolymph and pumped through the stomatogastric ganglion (STG) to the brain, which can influence the output of the neural networks. A large number of neuropeptides from C. borealis have already been identified in previous studies using mass spectrometry, facilitating us to further assess their functions.16−19
In this study, we measured the neuropeptide changes in three different neural organs in the nervous system of C. borealis in response to acute temperature elevation using dimethyl labeling. A number of neuropeptides were found to be decreased in two neuroendocrine organs, PO and SG, indicating that these two organs were actively involved in the temperature perturbation response. In addition, the neuropeptidome changes of the brain and circulating fluid hemolymph were also examined. A temperature stress marker peptide was discovered in the hemolymph and de novo sequenced.
Methods
Chemicals and Materials
Methanol, acetonitrile, formic acid, acetic acid, and EDTA disodium salt were purchased from Fisher Scientific (Pittsburgh, PA). Borane pyridine, formaldehyde, and deuterium formaldehyde were from Sigma-Aldrich (St. Louis, MO). 2,5-Dihydroxybenzoic acid (DHB) used as MALDI matrix was obtained from MP Biomedicals, Inc. (Solon, OH). Acidified methanol was prepared using 90% methanol, 9% acetic acid, and 1% water.
Animals and Temperature Elevation Experiments
Jonah crabs Cancer borealis were purchased from The Fresh Lobster Company (Gloucester, MA) and maintained without food in artificial seawater at 12–13 °C for at least 1 week before experiments. To increase the ambient temperature, crabs were placed in a bucket filled with artificial seawater at 12–13 °C, and the bucket was then placed in a water bath preheated to around 50 °C. The temperature within the bucket was increased to around 23 °C in 15 min. The crabs were then subjected to dissection, which usually took 20–30 min. The neural tissues, including PO, SG, and brain, were dissected in chilled (approximately 10 °C) physiological saline (composition: 440 mM NaCl; 11 mM KCl; 13 mM CaCl2; 26 mM MgCl2; 10 mM HEPES acid; pH 7.4). Details of the dissection procedure were described previously.20 Hemolymph samples were collected by inserting a 22-gauge needle attached to a 3 mL plastic syringe through the junction of the thorax and abdomen into the pericardial chamber. All the crabs used in this study were male, and animals with similar shell color, size, and weight were paired for each comparison group.
Hemolymph Sample Preparation
The peptides in the hemolymph were extracted as described before.21 Briefly, a mixture of 0.45 mL of acidified methanol and 0.3 mL of EDTA solution (20 mM aqueous solution) was spiked into 0.75 mL of freshly obtained hemolymph to extract peptides and precipitate large proteins. EDTA was added to prevent cation-triggered hemolymph clotting during the sample preparation process. The samples were centrifuged at 16 000g for 10 min, after which the supernatant was collected followed by ultrafiltration through a 10 kDa MWCO tube by centrifugation at 15 000g. The low mass filtrate was concentrated to dryness using a Savant SC 110 SpeedVac concentrator (Thermo Electron Corporation, West Palm Beach, FL) and was resuspended in 80 μL of 0.1% FA in water. After sonication for 10 min, the sample was desalted by C18 micro spin column (Argos, Elgin, IL) according to the product manual and eluted in 6 μL of 0.1% FA in 50% acetonitrile (v/v). The sample was then directly spotted for MALDI-TOF/TOF analysis.
Tissue Extraction and Dimethyl Labeling
Tissue extractions were obtained by homogenizing the neural tissues in 200 μL of cooled acidified methanol. The undissolved tissue pellets were removed by centrifugation at 16 000 rcf for 10 min. The supernatants were dried in a Savant SC 110 SpeedVac concentrator (Thermo Fisher Scientific, Waltham, MA) and resuspended in 20 μL of 0.1% formic acid, after which the samples were desalted by a C18 Ziptip (Millipore, Bedford, MA) according to the product instruction. The peptides were eluted with 10 μL of 50% acetonitrile. A 3 μL aliquot of tissue extract from neural tissues was first mixed with 0.7 μL of borane pyridine (C5H8BN, 120 mM in 10% methanol), after which 0.5 μL of formaldehyde (FH2, 15% in H2O) for control samples or 0.5 μL of deuterium formaldehyde (FD2, 15% in H2O) for temperature stress samples was added. The mixtures were then placed in a 37 °C water bath for 20 min for the labeling reaction to complete. Samples from control and stressed animals were mixed with a 1:1 ratio before mass spectrometry analysis.
Mass Spectrometry and Data Analysis
A model 4800 MALDI-TOF/TOF (Applied Biosystems, Framingham, MA) equipped with a 200 Hz, 355 nm Nd:YAG laser was used for neuropeptide quantification analysis. Acquisitions were performed in positive ion reflectron mode, and instrument parameters were set using the 4000 Series Explorer software (Applied Biosystems, Framingham, MA). Mass spectra were obtained by averaging 900 laser shots covering mass range m/z 500–4000, and MS/MS were acquired by 1 kV collisionally induced dissociation (CID) using air as collision gas.
To elucidate the primary sequence of the derivatized stress marker peptide, sustained off-resonance irradiation collision-induced dissociation (SORI-CID) was performed on a MALDI-FTMS (Varian, Lake Forest, CA) equipped with a 7.0 T actively shielded superconducting magnet. The FTMS instrument consisted of an external high-pressure MALDI source with a 355 nm Nd:YAG laser (Laser Science, Inc., Franklin, MA) to create ions that were accumulated in the external hexapole storage trap before being transferred through a quadrupole ion guide to the ICR cell. All mass spectra were collected in positive ion mode, and detection was performed in broadband mode from m/z 108.00 to 2500.00. For SORI-CID, an arbitrary waveform with a ±10 Da isolation window was introduced to isolate the ion of interest, and ions were excited with SORI Burst excitation (2.648 V, 2500–3000 ms). A pulse of nitrogen gas was introduced through a pulse valve from 2500 to 2750 ms to induce collision activation.
The neuropeptide identification was based on mass matching with previously characterized neuropeptides from C. borealis.16−19 Fragmentation was performed on selected peptides to further confirm their amino acid sequences. De novo sequencing of the stress marker peptide was done manually without the assistance of any software. For relative quantification of neuropeptides, each labeled mixture sample was spotted on the MALDI plate twice, and two replicate spectra were acquired for each spot, resulting in four replicate spectra for each sample. Peak lists were extracted from the crude spectra without any postacquisition processing using the Data Explorer software (Applied Biosystems, Framingham, MA). The peak pairs generated from the known neuropeptides were selected for quantitative analysis. The relative abundance ratio for each neuropeptide between the temperature-stressed sample and the control sample was determined by dividing the heavy labeled peak intensity with the light labeled peak intensity. Four replicate spectra were used to calculate the average relative abundance ratios. Student’s t test was performed to evaluate the differences of each peptide, and a P value <0.005 was considered to be statistically significant.
Results and Discussion
It is rather difficult to study the expression of each individual neuropeptide in different physiological conditions using traditional biological assays, such as Western blotting, because neuropeptide families usually contain a large number of isoforms sharing similar amino acid sequences that could cause antibody cross-reactivity. To solve such a problem, a number of mass spectrometry-based methods have been developed, leading to the discovery and mapping of numerous neuropeptides in different animals.19,22−27 It is our ultimate goal to utilize such tools to elucidate the functions of neuropeptides in the regulation of neural circuits. Mass spectrometry-based quantitation methods have also been used to accurately measure the quantitative changes of neuropeptide levels in animals under different conditions. For example, label-free peptidomics was employed to study the neuropeptide alterations in the tree shrew hypothalamus during volatile anesthesia.28 Another method, dimethyl labeling, has been reported as a fast and simple reaction that can be applied for differential proteomic and peptidomic analyses.29 In our previous work, dimethyl labeling was used to study the involvement of neuropeptides in feeding.30 In this current study, we used a similar strategy to examine the quantitative changes of neuropeptides in three different neural organs from C. borealis induced by acute temperature elevation.
RFamides and RYamides Were Reduced in the PO in Response to Temperature Elevation
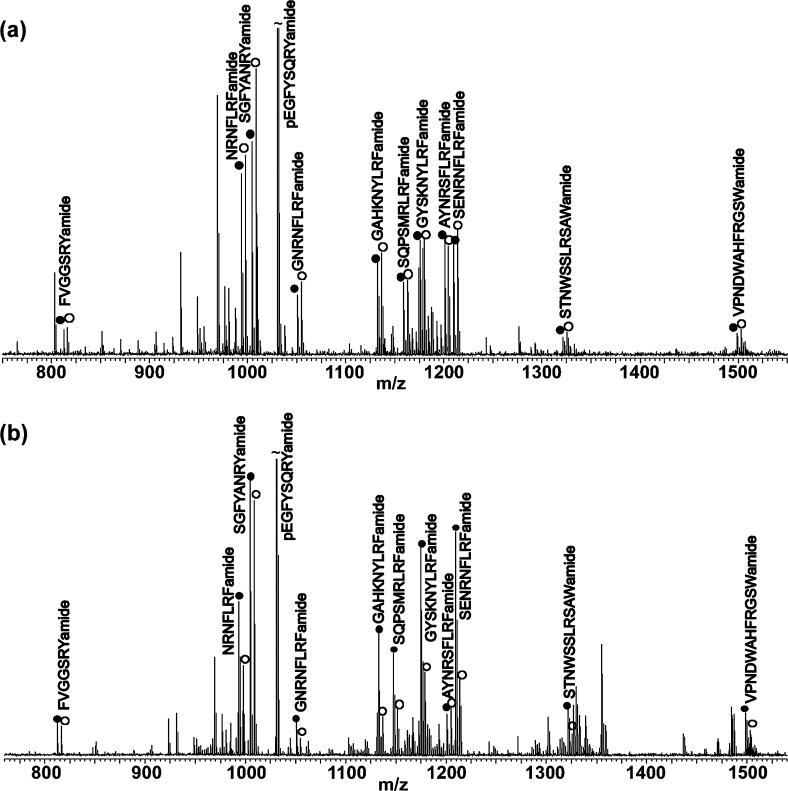
The PO is a major neurohemal organ surrounding the heart, which can release hormones into the circulating hemolymph and regulate the functions of heart and other distant organs.31 To determine the variability of neuropeptide expression between different animals, we initially compared two groups of control crabs. A representative MALDI-TOF/TOF spectrum of a dimethyl-labeled mixture of two control extracts from PO is shown in Figure 1a, and the peak pairs of labeled neuropeptides are indicated with their amino acid sequences. No significant changes were observed between these two control samples. However, as shown in Figure 1b, the intensities of many detected neuropeptides were significantly reduced in the temperature stress sample.
Figure 1.
MALDI-TOF/TOF mass spectra of dimethyl-labeled mixtures of (a) two control pericardial organ extracts and (b) pericardial organ extracts from control (12 °C) versus temperature-stressed (23 °C) animals. The H2-labeled dimethylated peaks (control) are indicated with closed circles, and the D2-labeled dimethylated peaks (stressed) are indicated with open circles. The labeling reaction resulted in 4 Da mass differences per label between each peak pair. Peaks are annotated with their corresponding neuropeptide sequences.
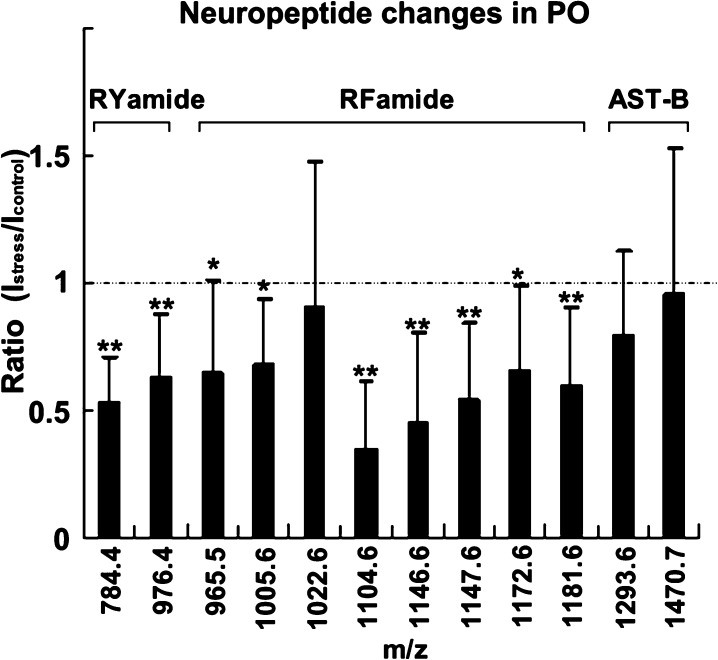
To further verify the observation, 15 pairs of control and stressed crabs were compared, where each single crustacean contributed a set of paired PO. In the quantification experiments, three families of neuropeptides were detected in the PO, including RFamide-related peptide (FaRP), RYamide, and B-type allatostatin (AST-B) (Table 1). Table 1 and Figure 2 show the average abundance ratio between control and stressed animals for each individual neuropeptide. Peptides from both the RFamide and RYamide families were significantly reduced in the PO after acute temperature shock (P < 0.005). The ratios between the temperature stress and control samples ranged from 0.34 to 0.68, although different isoforms in the same family were observed to have varying degrees of change. GAHKNYLRFa (m/z 1104.61) showed the largest level of decrease upon temperature change (0.34, P < 0.001), while GNRNFLRFa (m/z 1022.56) was the only RFamide peptide with no significant change upon temperature elevation. Both of the two observed RYamides, FVGGSRYa (m/z 784.41) and SGFYANRYa (m/z 976.46), were reduced in the PO at high temperature. Two isoforms of AST-B were detected in the PO extract in this study, although neither of them showed significant changes. It is interesting that these neuropeptide families respond differently to the thermal challenge, indicating that they may play distinct roles in response to temperature changes.
Table 1. Quantitative Changes of Neuropeptides in the Pericardial Organ in Response to Temperature Elevation (n = 15)a.
| neuropeptide family | sequences | m/z | ratios/c | std | P value |
|---|---|---|---|---|---|
| RYamide | FVGGSRYa** | 784.41 | 0.53 | 0.18 | <0.001 |
| SGFYANRYa** | 976.46 | 0.63 | 0.24 | <0.001 | |
| FaRP | NRNFLRFa* | 965.54 | 0.65 | 0.36 | 0.004 |
| GPRNFLRFa* | 1005.57 | 0.68 | 0.26 | 0.005 | |
| GNRNFLRFa | 1022.56 | 0.91 | 0.57 | 0.12 | |
| GAHKNYLRFa** | 1104.61 | 0.34 | 0.27 | <0.001 | |
| GYSKNYLRFa** | 1146.61 | 0.45 | 0.35 | <0.001 | |
| APQRNFLRFa** | 1147.64 | 0.54 | 0.30 | <0.001 | |
| AYNRSFLRFa* | 1172.63 | 0.66 | 0.33 | 0.004 | |
| SENRNFLRFa** | 1181.62 | 0.59 | 0.32 | <0.001 | |
| AST-B | STNWSSLRSAWa | 1293.63 | 0.79 | 0.34 | 0.03 |
| VPNDWAHFRGSWa | 1470.68 | 0.96 | 0.57 | 0.26 |
Neuropeptides with significant changes are indicated with an asterisk (*P ≤ 0.005, **P < 0.001). FaRP, FMRFamide-related peptide; AST-B, B-type allatostatin. Ratios/c: ratio of neuropeptide signal intensities between stressed (23 °C) and control (12 °C) crabs. The observed neuropeptides listed in the table were identified in previous studies.16−19
Figure 2.
Neuropeptide changes in the pericardial organ upon acute temperature elevation. Ratios of peptide levels between stressed (23 °C) and control (12 °C) were determined using dimethyl labeling (columns, average of ratios calculated from 15 groups of comparison; bars, standard deviation; *P < 0.005; **P < 0.001). AST-B, B-type allatostatins.
The peptide hormones released by the PO have rapid and direct access to the heart, which suggests that heart is a primary target of the pericardial hormones. RFamides are present in many different organisms with a large diversity of amino acid sequences.32−34 In crustaceans, FaRPs are shown to act predominantly on excitable tissues.31 In an electrophysiological study, it was observed that the application of FLRFamide peptides can increase the output activity of the cardiac ganglion that drives the contractions of the heart.35−37 It was also reported that the heart beat rate in lobster H. americanus increased with environmental temperature in vivo and in vitro, while its contraction amplitude was reduced.13 Therefore, it is quite likely that FaRPs play a key role in this heart functional change in response to temperature stress. FaRPs are also reported to alter the arterial hemolymph flow and regulate the circulatory system.38 These processes may be important for the animal to manage oxygen regulation under a stressful environment. On the other hand, the RYamide peptide family was first discovered in the pericardial organ of C. borealis, and its physiological effects are largely unknown.17 Recently, two peptides with a similar RYamide motif were identified in Drosophila and believed to be involved with feeding;39,40 however, it is unclear whether they also have cardiac effects. Our findings suggest that the RYamides may be involved with temperature stress response. Still, further investigation is required to better understand their physiological roles.
Quantitative Changes of Neuropeptides in the SG in Response to Temperature Elevation
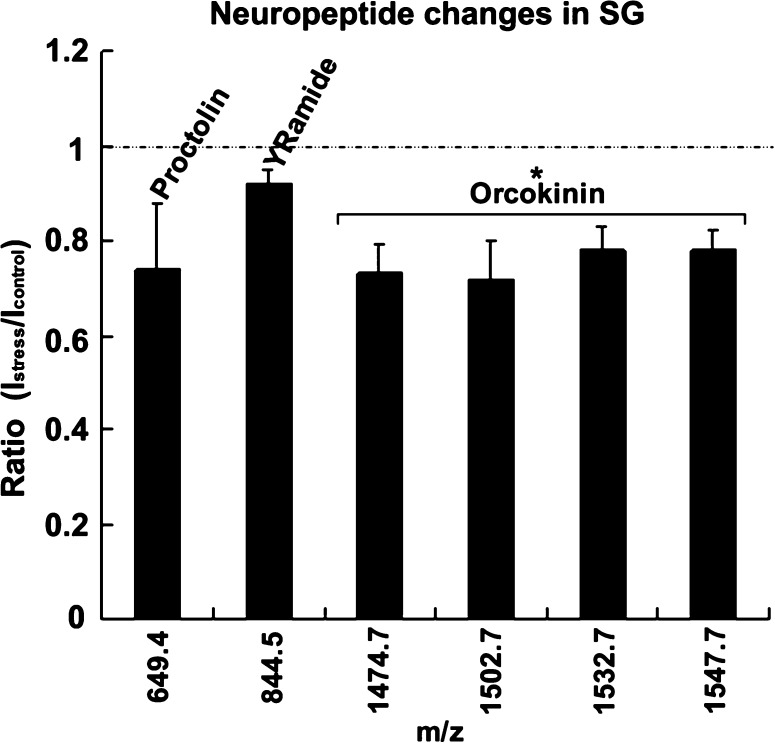
Another major neuroendocrine organ in crustaceans is the SG, which is a paired organ located in the two eyestalks, and the quantitative changes between temperature stress and control preparations were also determined. Since the total amount of neuropeptides was lower in the SG compared to PO, four SG tissues were used for each extract. Overall, five groups of samples were analyzed, from which six neuropeptides were detected (Figure 3 and Table 2). As shown in Figure 3, four orcokinin peptides were detected to be reduced at high temperature (P < 0.005), specifically NFDEIDRSGFGFA (m/z 1474.7), NFDEIDRSGFGFV (m/z 1502.7), NFDEIDRSSFGFV (m/z 1532.7), and NFDEIDRSSFGFN (m/z 1547.7). The ratios of these four peptides between the stress and control samples were similar, between 0.7 and 0.8. The other two observed neuropeptides, proctolin and YRamide, did not show significant changes.
Figure 3.
Neuropeptide changes in the sinus gland in response to acute temperature elevation. Ratios of peptide levels between stressed (23 °C) and control (12 °C) were determined using dimethyl labeling (columns, average of ratios calculated from five groups of stress experiments for each neuropeptide; bars, standard deviation; *, student’s t test, P < 0.005).
Table 2. Quantitative Changes of Neuropeptides in the Sinus Gland in Response to Temperature Elevation (n = 5)a.
| neuropeptide family | sequences | m/z | ratios/c | std | P value |
|---|---|---|---|---|---|
| proctolin | RYLPT | 649.4 | 0.74 | 0.14 | 0.02 |
| YRamide | HIGSLYRa | 844.5 | 0.92 | 0.03 | 0.006 |
| orcokinin | NFDEIDRSGFGFA* | 1474.7 | 0.73 | 0.06 | 0.001 |
| NFDEIDRSGFGFV* | 1502.7 | 0.72 | 0.08 | 0.003 | |
| NFDEIDRSSFGFV** | 1532.7 | 0.78 | 0.05 | <0.001 | |
| NFDEIDRSSFGFN** | 1547.7 | 0.78 | 0.04 | <0.001 |
The SG is known to be involved in various forms of environmental stresses, such as temperature changes, salinity changes, pollution, and hypoxia.41−44 It was found that crustacean hyperglycemic hormones (CHHs) were released from the SG system during stress, resulting in subsequent elevation of the blood glucose level.44−47 Although we were unable to detect and quantify the presence of CHHs in this work, we found that neuropeptide orcokinins were decreased in the SG under temperature elevation. Generally, orcokinins are myotropic peptides that have excitatory effects on different tissues. Bungart et al. have reported that orcokinins are measurable in the hemolymph with an approximate concentration of 10–11 M,48 indicating that orcokinins may be released into hemolymph and function as a hormone in addition to its role as a locally acting neurotransmitter. In this study, we demonstrated that the orcokinin levels were reduced in the SG upon temperature stress, implicating that orcokinins may be involved in the stress response via a hormonal route.
Neuropeptide Levels in the Brain Were Not Affected by Temperature Elevation
To evaluate the neuropeptide expression changes in the CNS, C. borealis brain extracts were also investigated. Two brains were used for each extract sample, and 10 comparison groups were conducted. Compared to the PO and the SG, a larger number of neuropeptides were detected that reside from eight different families, including FaRP, RYamide, YRamide, Cancer borealis tachykinin-related peptide (CabTRP), crustacean cardioactive peptide (CCAP), proctolin, SIFamide, and orcokinins. As shown in Table 3, no significant changes were observed for any of these neuropeptide families in the brain.
Table 3. Quantitative Analysis of Neuropeptides in the Brain in Response to Temperature Elevation (n = 10)a.
| neuropeptide family | sequences | m/z | ratios/c | std | P value |
|---|---|---|---|---|---|
| proctolin | RYLPT | 649.4 | 0.92 | 0.30 | 0.18 |
| YRamide | HIGSLYRa | 844.5 | 1.03 | 0.87 | 0.35 |
| CabTRP | APSGFLGMRa | 934.5 | 0.90 | 0.45 | 0.20 |
| TPSGFLGMRa | 964.5 | 1.26 | 0.26 | 0.009 | |
| CCAP | PFCNAFTGCa | 956.4 | 1.16 | 0.23 | 0.06 |
| RYamide | SGFYANRYa | 976.4 | 1.16 | 0.52 | 0.79 |
| RaRP | NRNFLRFa | 965.4 | 0.88 | 0.38 | 0.17 |
| GNRNFLRFa | 1022.5 | 0.89 | 0.37 | 0.19 | |
| APNKNFLRFa | 1105.5 | 0.87 | 0.21 | 0.07 | |
| GAHKNYLRFa | 1104.7 | 0.90 | 0.41 | 0.19 | |
| APQRNFLRFa | 1147.5 | 0.87 | 0.33 | 0.12 | |
| AYNRSFLRFa | 1172.7 | 1.14 | 0.46 | 0.56 | |
| SENRNFLRFa | 1181.6 | 0.90 | 0.33 | 0.21 | |
| DVRTPALRLRFa | 1342.8 | 0.92 | 0.43 | 0.20 | |
| SIFamide | GYRKPPFNGSIFa | 1381.5 | 0.84 | 0.30 | 0.08 |
| orcokinin | NFDEIDRSGFA | 1270.6 | 1.30 | 1.00 | 0.68 |
| NFDEIDRSGFGFA | 1474.7 | 0.90 | 0.19 | 0.12 | |
| NFDEIDRSGFGFV | 1502.7 | 0.84 | 0.13 | 0.005 | |
| NFDEIDRSSFGFV | 1532.7 | 1.01 | 0.27 | 0.81 | |
| NFDEIDRSSFGFN | 1547.7 | 1.00 | 0.39 | 0.60 | |
| NFDEIDRTGFGFH | 1554.8 | 0.89 | 0.33 | 0.19 |
The results from PO and SG indicate that endocrine hormonal regulation may play a very important role in response to acute temperature elevation. To the authors’ knowledge, this is the first direct demonstration that neuropeptides, including RFamides, RYamides, and orcokinins, are involved in the physiological regulation of the temperature stress response. In addition, the mass spectrometry-based methods show unique advantages for comprehensive study of multiple neuropeptides simultaneously, leading to valuable insights into their physiological functions. It should be noted that the neuropeptides in the brain are not observed to be significantly changed in this study. It is possible that the neuropeptide synthesis in the brain is not strongly affected by temperature, or it may also be due to the short time course of the temperature elevation experiment. A longer term temperature perturbation study will be conducted in the future to further explore the roles played by the central nervous system and its interaction with other neuroendocrine organs.
Discovery of a Temperature Stress Marker Peptide in the Hemolymph
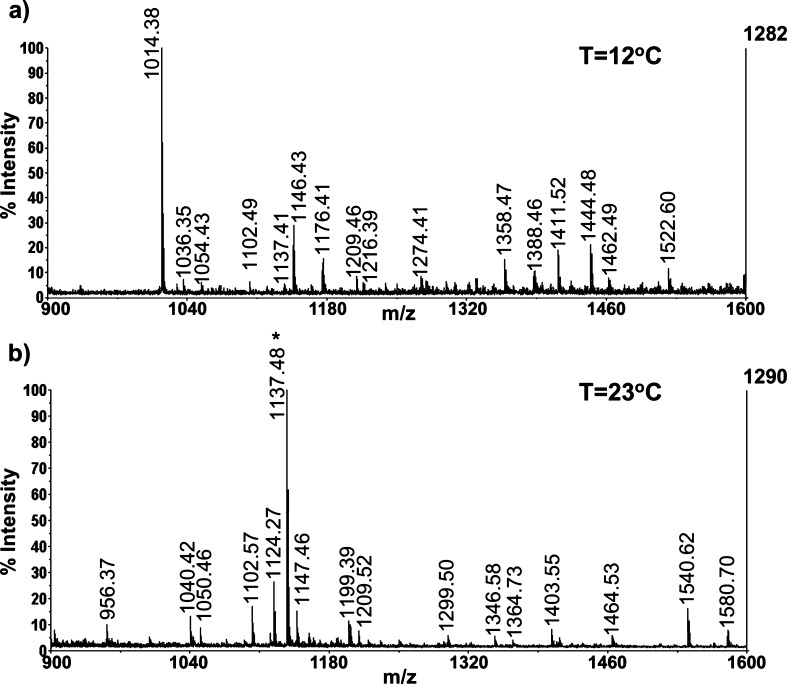
It has been reported that a number of physiological variables were changed in the hemolymph in response to temperature elevation.5 To better understand the temperature response system in the crab, we analyzed the peptidomic complements of the hemolymph from animals undergoing an acute temperature ramp. In our previous study, we developed an optimized sample preparation method for peptide extraction from the crude hemolymph samples.21 This method was utilized here to extract peptides from the C. borealis hemolymph. As shown in Figure 4, quite distinct peptide profiles were observed under different temperatures. A peptide peak with m/z 1137.6 (SMP1137) with the highest peak intensity was repeatedly observed in the temperature stress hemolymph samples (n = 5). It should be noted that this ion was also detected from the control hemolymph in some cases but at much lower peak intensities (Figure 4a).
Figure 4.
Mass spectral comparison of processed hemolymph samples from (a) control (12 °C) and (b) temperature-stressed (23 °C) crabs acquired using MALDI-TOF/TOF. The dominant peak (m/z 1137.6) observed at high temperature is indicated with an asterisk.
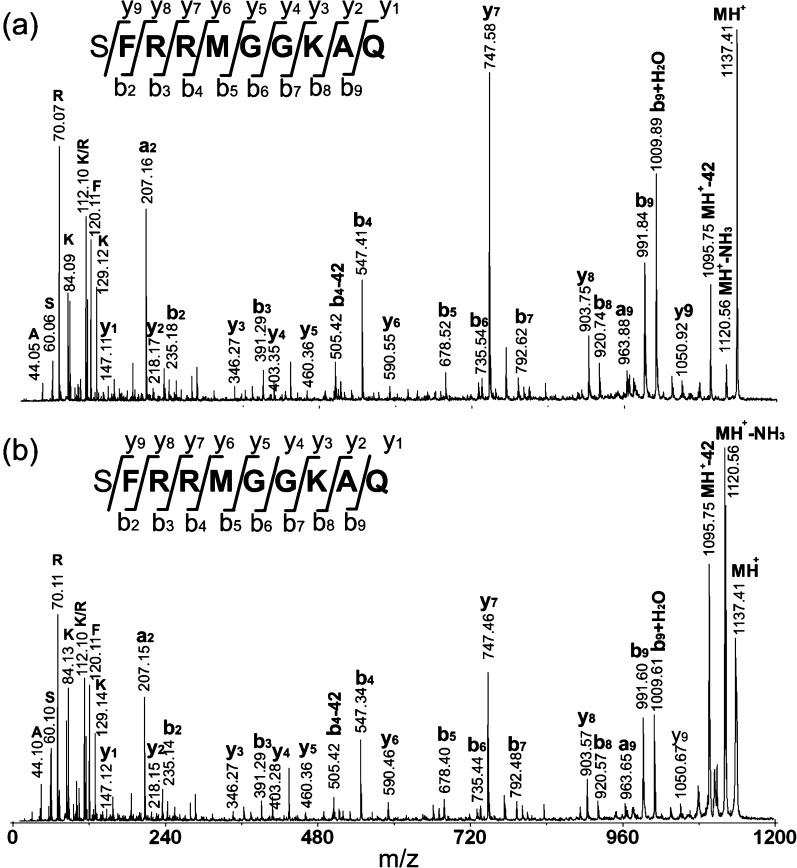
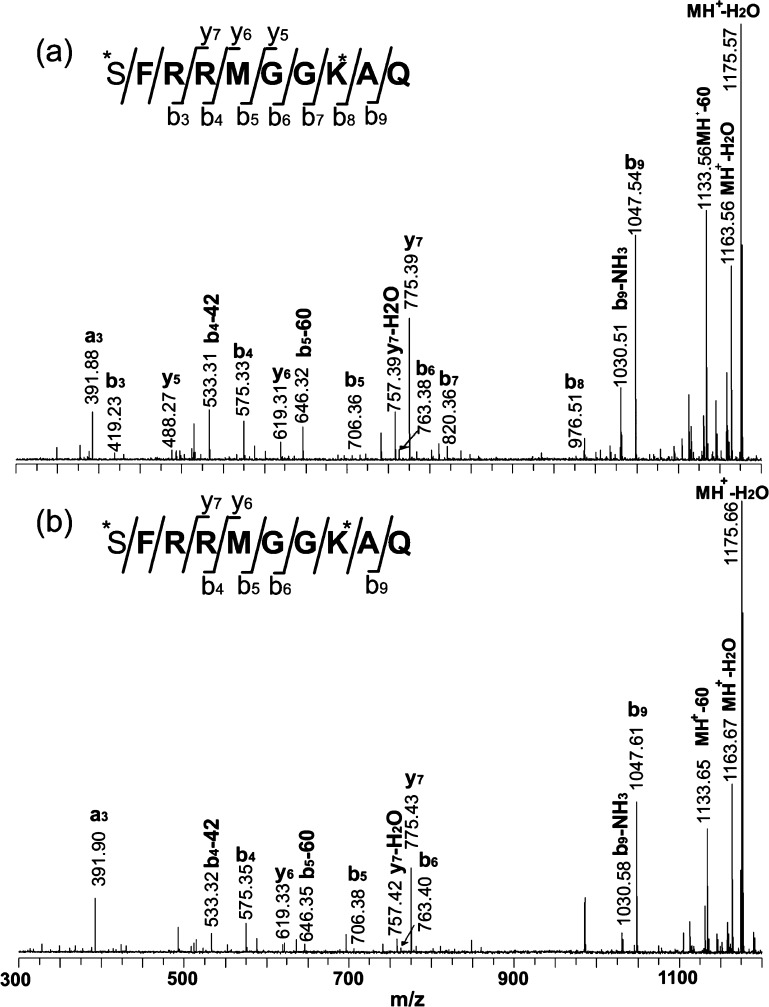
To identify this peptide, CID fragmentation was performed on a MALDI-TOF/TOF instrument to acquire peptide sequence information. As shown in Figure 5a, the fragmentation was quite complete, and the majority of the b- and y-ions were observed. To obtain complementary information for amino acid sequence assignment, dimethylation derivatization was carried out. Formaldehyde could react with all the primary amine groups in the peptide, leading to differentiation of the lysine residue (K) as compared to glutamine (Q) or the combination of glycine and alanine (GA or AG). The fragmentation of the dimethylated peptide is shown in Figure 6a. A Δ56 Da mass shift was observed after the dimethylation derivatization, suggesting the presence of a lysine residue in the sequence. The fragmentation spectrum further confirmed the position of the lysine (K) residue in the primary sequence. To further validate the deduced sequence, a peptide standard was synthesized and analyzed using the same activation methods (Figure 5b and Figure 6b), which showed a fragmentation pattern similar to that of the putative SMP1137 peptide from the hemolymph.
Figure 5.
MS/MS spectra acquired by MALDI-TOF/TOF using collision-induced dissociation for (a) putative SMP1137 from the hemolymph sample and (b) synthetic peptide standard with the proposed sequence at a concentration of 1 μM. The presence of b- and y-ions is indicated by horizontal lines below (b-ions) or above (y-ions) the corresponding amino acid residues in the peptide sequence.
Figure 6.
MS/MS spectra acquired by MALDI-FTICR for (a) dimethyl-labeled putative SMP1137 from the hemolymph sample and (b) dimethyl-labeled synthetic standard with the predicted sequence at a concentration of 1 μM. The asterisk indicates the two dimethylated sites, including the N terminus and the lysine (K) residue. The presence of b- and y-ions is indicated by horizontal lines below (b-ions) or above (y-ions) the corresponding amino acid residues in the peptide sequence.
To the best of the authors’ knowledge, this peptide does not belong to any known neuropeptide families, and it is possibly a fragment from a certain protein in the hemolymph. Notably, Lorenzon et al. reported that the protein concentration in the lobster hemolymph increased significantly with the body temperature.5 It has also been reported that heat shock proteins, whose primary function was to promote initial folding of other proteins at the ribosome and the refolding of unfolded proteins when they were partially denatured, were elevated in crustacean hemolymph when the animal undergoes stressful conditions.45 However, we performed a BLAST search using the sequence of SMP1137 against the protein database, with no match of any relevant proteins found, suggesting that the SMP1137 could be a fragment cleaved from an unknown protein that is related to temperature stress response. Future experiments will be performed to identify the origin of SMP1137 and to determine whether this peptide has any physiological activities. In addition, it is also of great interest to investigate whether SMP1137 is related to other types of environmental stresses, such as salinity changes, pollution, and hypoxia, or it is a unique signature for temperature change.
Conclusions
In this study, quantitative peptidomics was employed to study the neuropeptide changes in the Jonah crab Cancer borealis associated with acute temperature elevation. The results showed that neuroendocrine organs, including the pericardial organ and the sinus gland, were actively involved with the temperature perturbation response. The neuropeptides released by these two organs may be involved with temperature change response via hormonal regulation. This study provided direct evidence that neuropeptides may play an important role in the regulation of biological changes in crustaceans in response to environmental perturbation.
Acknowledgments
The authors thank Dr. Eve Marder from Brandeis University for helpful discussions. We also would like to thank Dr. Amy Harms and Dr. Mike Sussman at the University of Wisconsin Biotechnology Center Mass Spectrometry Facility for the access to the MALDI-TOF/TOF instrument. This study was supported by the National Science Foundation Grant CHE-1413596 to L.L., and National Institutes of Health Grants 1R01DK071801 and 1R56DK071801 to L.L. Financial support for this study was also provided by grants from National Natural Science Foundation of China (21205088 to R.C.) and Doctoral Research Fund from the Ministry of Education of China (20121202120001 to R.C.). L.L. acknowledges an H.I. Romnes Faculty Research Fellowship.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Dillon M. E.; Wang G.; Garrity P. A.; Huey R. B. Review: thermal preference in Drosophila. J. Therm. Biol. 2009, 343109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz P. H.; Hower A. E.; Hartline D. K. Temperature compensation in the escape response of a marine copepod, Calanus finmarchicus (Crustacea). Biol. Bull. 2005, 209175–85. [DOI] [PubMed] [Google Scholar]
- Garrity P. A.; Goodman M. B.; Samuel A. D.; Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 2010, 24212365–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman J. H. Acclimation capacity underlies susceptibility to climate change. Science 2003, 301562965. [DOI] [PubMed] [Google Scholar]
- Lorenzon S.; Giulianini P. G.; Martinis M.; Ferrero E. A. Stress effect of different temperatures and air exposure during transport on physiological profiles in the American lobster Homarus americanus. Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol. 2007, 147194–102. [DOI] [PubMed] [Google Scholar]
- Szabo T. M.; Brookings T.; Preuss T.; Faber D. S. Effects of temperature acclimation on a central neural circuit and its behavioral output. J. Neurophysiol. 2008, 10062997–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. M.; Money T. G. Temperature and neuronal circuit function: compensation, tuning and tolerance. Curr. Opin. Neurobiol. 2012, 224724–734. [DOI] [PubMed] [Google Scholar]
- Tang L. S.; Taylor A. L.; Rinberg A.; Marder E. Robustness of a rhythmic circuit to short- and long-term temperature changes. J. Neurosci. 2012, 322910075–10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L. S.; Goeritz M. L.; Caplan J. S.; Taylor A. L.; Fisek M.; Marder E. Precise temperature compensation of phase in a rhythmic motor pattern. PLoS Biol. 2010, 88e1000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg A.; Taylor A. L.; Marder E. The effects of temperature on the stability of a neuronal oscillator. PLoS Comput. Biol. 2013, 91e1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soofi W.; Goeritz M. L.; Kispersky T. J.; Prinz A. A.; Marder E.; Stein W. Phase maintenance in a rhythmic motor pattern during temperature changes in vivo. J. Neurophysiol. 2014, 111122603–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman R. C.; Norris B. J.; Calabrese R. L. Animal-to-animal variability of connection strength in the leech heartbeat central pattern generator. J. Neurophysiol. 2012, 10761681–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden M. K.; Clark C. M.; Conaway M.; Qadri S. A. Temperature dependence of cardiac performance in the lobster Homarus americanus. J. Exp. Biol. 2006, 20961024–1034. [DOI] [PubMed] [Google Scholar]
- Camacho J.; Qadri S. A.; Wang H.; Worden M. K. Temperature acclimation alters cardiac performance in the lobster Homarus americanus. J. Comp. Physiol., A 2006, 192121327–1334. [DOI] [PubMed] [Google Scholar]
- Marder E.; Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu. Rev. Physiol. 2007, 691291–316. [DOI] [PubMed] [Google Scholar]
- Huybrechts J.; Nusbaum M. P.; Bosch L. V.; Baggerman G.; De Loof A.; Schoofs L. Neuropeptidomic analysis of the brain and thoracic ganglion from the Jonah crab Cancer borealis. Biochem. Biophys. Res. Commun. 2003, 3083535–544. [DOI] [PubMed] [Google Scholar]
- Li L.; Kelley W. P.; Billimoria C. P.; Christie A. E.; Pulver S. R.; Sweedler J. V.; Marder E. Mass spectrometric investigation of the neuropeptide complement and release in the pericardial organs of the crab Cancer borealis. J. Neurochem. 2003, 873642–656. [DOI] [PubMed] [Google Scholar]
- Fu Q.; Goy M. F.; Li L. Identification of neuropeptides from the decapod crustacean sinus glands using nanoscale liquid chromatography tandem mass spectrometry. Biochem. Biophys. Res. Commun. 2005, 3373765–778. [DOI] [PubMed] [Google Scholar]
- Ma M.; Wang J.; Chen R.; Li L. Expanding the crustacean neuropeptidome using a multifaceted mass spectrometric approach. J. Proteome Res. 2009, 852426–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz K. K.; Schmidt J. J.; Li L. In situ tissue analysis of neuropeptides by MALDI FTMS in-cell accumulation. Anal. Chem. 2004, 76195630–5640. [DOI] [PubMed] [Google Scholar]
- Chen R.; Ma M.; Hui L.; Zhang J.; Li L. Measurement of neuropeptides in crustacean hemolymph via MALDI mass spectrometry. J. Am. Soc. Mass Spectrom. 2009, 204708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.; Jiang X.; Conaway M. C.; Mohtashemi I.; Hui L.; Viner R.; Li L. Mass spectral analysis of neuropeptide expression and distribution in the nervous system of the lobster Homarus americanus. J. Proteome Res. 2010, 92818–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L.; D’Andrea B. T.; Jia C.; Liang Z.; Christie A. E.; Li L. Mass spectrometric characterization of the neuropeptidome of the ghost crab Ocypode ceratophthalma (Brachyura, Ocypodidae). Gen. Comp. Endrocrinol. 2013, 184122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L.; Cunningham R.; Zhang Z.; Cao W.; Jia C.; Li L. Discovery and characterization of the crustacean hyperglycemic hormone precursor related peptides (CPRP) and orcokinin neuropeptides in the sinus glands of the blue crab Callinectes sapidus using multiple tandem mass spectrometry techniques. J. Proteome Res. 2011, 1094219–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q.; Kutz K. K.; Schmidt J. J.; Hsu Y. W.; Messinger D. I.; Cain S. D.; de la Iglesia H. O.; Christie A. E.; Li L. Hormone complement of the Cancer productus sinus gland and pericardial organ: an anatomical and mass spectrometric investigation. J. Comp. Neurol. 2005, 4934607–626. [DOI] [PubMed] [Google Scholar]
- Verhaert P. D.; Pinkse M. W.; Strupat K.; Conaway M. C. Imaging of similar mass neuropeptides in neuronal tissue by enhanced resolution MALDI MS with an ion trap—orbitrap hybrid instrument. Methods Mol. Biol. 2010, 656, 433–449. [DOI] [PubMed] [Google Scholar]
- Chen R.; Li L. Mass spectral imaging and profiling of neuropeptides at the organ and cellular domains. Anal. Bioanal. Chem. 2010, 39783185–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouillen L.; Petruzziello F.; Veit J.; Bhattacharyya A.; Kretz R.; Rainer G.; Zhang X. Neuropeptide alterations in the tree shrew hypothalamus during volatile anesthesia. J. Proteomics 2013, 80, 311–319. [DOI] [PubMed] [Google Scholar]
- Hsu J. L.; Huang S. Y.; Chow N. H.; Chen S. H. Stable-isotope dimethyl labeling for quantitative proteomics. Anal. Chem. 2003, 75246843–6852. [DOI] [PubMed] [Google Scholar]
- Chen R.; Hui L.; Cape S. S.; Wang J.; Li L. Comparative neuropeptidomic analysis of food intake via a multi-faceted mass spectrometric approach. ACS Chem. Neurosci. 2010, 13204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard D. M. Thoracic neurosecretory structures in brachyura. II. Secretory neurons. Gen. Comp. Endrocrinol. 1961, 1, 237–263. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld L. J. Driving reproduction: RFamide peptides behind the wheel. Horm. Behav. 2006, 505655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVeigh P.; Leech S.; Mair G. R.; Marks N. J.; Geary T. G.; Maule A. G. Analysis of FMRFamide-like peptide (FLP) diversity in phylum Nematoda. Int. J. Parasitol. 2005, 35101043–1060. [DOI] [PubMed] [Google Scholar]
- Predel R.; Neupert S.; Wicher D.; Gundel M.; Roth S.; Derst C. Unique accumulation of neuropeptides in an insect: FMRFamide-related peptides in the cockroach Periplaneta americana. Eur. J. Neurosci. 2004, 2061499–1513. [DOI] [PubMed] [Google Scholar]
- Fort T. J.; Brezina V.; Miller M. W. Regulation of the crab heartbeat by FMRFamide-like peptides: multiple interacting effects on center and periphery. J. Neurophysiol. 2007, 9852887–2902. [DOI] [PubMed] [Google Scholar]
- Cruz-Bermudez N. D.; Marder E. Multiple modulators act on the cardiac ganglion of the crab Cancer borealis. J. Exp. Biol. 2007, 210162873–2884. [DOI] [PubMed] [Google Scholar]
- Krajniak K. G. The identification and structure–activity relations of a cardioactive FMRFamide-related peptide from the blue crab Callinectes sapidus. Peptides 1991, 1261295–1302. [DOI] [PubMed] [Google Scholar]
- Mercier A. J.; Friedrich R.; Boldt M. Physiological functions of FMRFamide-like peptides (FLPs) in crustaceans. Microsc. Res. Tech. 2003, 603313–324. [DOI] [PubMed] [Google Scholar]
- Ida T.; Takahashi T.; Tominaga H.; Sato T.; Kume K.; Ozaki M.; Hiraguchi T.; Maeda T.; Shiotani H.; Terajima S.; Sano H.; Mori K.; Yoshida M.; Miyazato M.; Kato J.; Murakami N.; Kangawa K.; Kojima M. Identification of the novel bioactive peptides dRYamide-1 and dRYamide-2, ligands for a neuropeptide Y-like receptor in Drosophila. Biochem. Biophys. Res. Commun. 2011, 4104872–877. [DOI] [PubMed] [Google Scholar]
- Collin C.; Hauser F.; Krogh-Meyer P.; Hansen K. K.; Gonzalez de Valdivia E.; Williamson M.; Grimmelikhuijzen C. J. Identification of the Drosophila and Tribolium receptors for the recently discovered insect RYamide neuropeptides. Biochem. Biophys. Res. Commun. 2011, 4124578–583. [DOI] [PubMed] [Google Scholar]
- Chang E. S.; Keller R.; Chang S. A. Quantification of crustacean hyperglycemic hormone by ELISA in hemolymph of the lobster, Homarus americanus, following various stresses. Gen. Comp. Endrocrinol. 1998, 1113359–366. [DOI] [PubMed] [Google Scholar]
- Webster S. Measurement of crustacean hyperglycaemic hormone levels in the edible crab Cancer pagurus during emersion stress. J. Exp. Biol. 1996, 19971579–1585. [DOI] [PubMed] [Google Scholar]
- Lorenzon S.; Francese M.; Ferrero E. A. Heavy metal toxicity and differential effects on the hyperglycemic stress response in the shrimp Palaemon elegans. Arch. Environ. Contam. Toxicol. 2000, 392167–176. [DOI] [PubMed] [Google Scholar]
- Lorenzon S.; Edomi P.; Giulianini P. G.; Mettulio R.; Ferrero E. A. Variation of crustacean hyperglycemic hormone (cHH) level in the eyestalk and haemolymph of the shrimp Palaemon elegans following stress. J. Exp. Biol. 2004, 207244205–4213. [DOI] [PubMed] [Google Scholar]
- Chang E. S. Stress-out lobsters: crustacean hyperglycemic hormone and stress proteins. Integr. Comp. Biol. 2005, 45143–50. [DOI] [PubMed] [Google Scholar]
- Chung J. S.; Zmora N. Functional studies of crustacean hyperglycemic hormones (CHHs) of the blue crab, Callinectes sapidus—the expression and release of CHH in eyestalk and pericardial organ in response to environmental stress. FEBS J. 2008, 2754693–704. [DOI] [PubMed] [Google Scholar]
- Lorenzon S.; Edomi P.; Giulianini P. G.; Mettulio R.; Ferrero E. A. Role of biogenic amines and cHH in the crustacean hyperglycemic stress response. J. Exp. Biol. 2005, 208173341–3347. [DOI] [PubMed] [Google Scholar]
- Bungart D.; Dircksen H.; Keller R. Quantitative determination and distribution of the myotropic neuropeptide orcokinin in the nervous system of astacidean crustaceans. Peptides 1994, 153393–400. [DOI] [PubMed] [Google Scholar]