Abstract
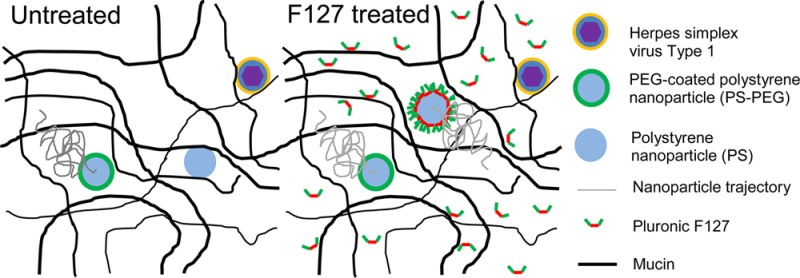
Mucosal drug delivery nanotechnologies are limited by the mucus barrier that protects nearly all epithelial surfaces not covered with skin. Most polymeric nanoparticles, including polystyrene nanoparticles (PS), strongly adhere to mucus, thereby limiting penetration and facilitating rapid clearance from the body. Here, we demonstrate that PS rapidly penetrate human cervicovaginal mucus (CVM), if the CVM has been pretreated with sufficient concentrations of Pluronic F127. Importantly, the diffusion rate of large polyethylene glycol (PEG)-coated, nonmucoadhesive nanoparticles (PS–PEG) did not change in F127-pretreated CVM, implying that F127 did not significantly alter the native pore structure of CVM. Additionally, herpes simplex virus type 1 (HSV-1) remains adherent in F127-pretreated CVM, indicating that the presence of F127 did not reduce adhesive interactions between CVM and the virions. In contrast to treatment with a surfactant that has been approved for vaginal use as a spermicide (nonoxynol-9 or N9), there was no increase in inflammatory cytokine release in the vaginal tract of mice after daily application of 1% F127 for 1 week. Pluronic F127 pretreatment holds potential as a method to safely improve the distribution, retention, and efficacy of nanoparticle formulations without compromising CVM barrier properties to pathogens.
Introduction
Mucus is a viscoelastic, adhesive gel that coats and protects most epithelial surfaces. Mucus barriers efficiently trap most foreign particulates and pathogens through adhesive and steric interactions, followed by rapid clearance.1 Similarly, conventional polymeric nanoparticles adhere avidly to mucus.2−5 In contrast, we demonstrated that nanoparticles coated with a high density of low-molecular weight polyethylene glycol (PEG) rapidly penetrate human cervicovaginal mucus (CVM), as well as other types of human and animal mucus.2,3,6,7 So-called mucus-penetrating particles provided improved vaginal distribution, retention, protection against vaginal herpes infection, and safety in mice in vivo.2 Similarly, mucus-penetrating gene carriers provided improved airway distribution, retention, and gene expression in mice in vivo without eliciting acute inflammatory responses.8 Biodegradable mucus-penetrating nanoparticles composed of poly(lactic-co-glycolic acid) (PLGA) and a Pluronic F127 coating provided improved cervical tumor suppression compared to mucoadhesive PLGA nanoparticles when administered locally to the vaginal mucosa.9
A muco-inert PEG coating has been achieved by covalent attachment of PEG on the particle surface3,10,11 or by physically adsorbing specific triblock copolymers of poly(propylene oxide) (PPO) flanked by two PEG groups (PEG–PPO–PEG, known as Pluronics) to the particle surface.2,9,12 In the latter case, it was found that the relatively hydrophobic PPO segment (if above a minimum molecular weight) associated with the hydrophobic nanoparticle surface, while the PEG chains extended outward to form a mucoinert surface coating.12 Thus, we hypothesized that pretreating mucus with Pluronic solution prior to nanoparticle addition may be another method for imparting mucoinert surface properties to hydrophobic nanoparticles. In this case, unmodified mucoadhesive nanoparticles could potentially be delivered more efficiently to mucosal surfaces.
Here, we investigated the transport behavior of mucoadhesive polystyrene nanoparticles (PS) in fresh, undiluted human CVM pretreated with various concentrations of Pluronic F127 solution. We used large muco-inert, PEG-coated PS nanoparticles (PS–PEG) to examine the potential effects of F127 pretreatment on the native pore structure of CVM. We then observed the transport behavior of herpes simplex virus (HSV-1) in F127-pretreated CVM to ensure that the barrier properties were not compromised. Finally, we characterized vaginal cytokine release in mice to ensure that F127-pretreatment was not toxic to the vaginal mucosal epithelium.
Materials and Methods
Nanoparticle and Virus Preparation and Characterization
Fluorescent, carboxylate (COOH)-modified polystyrene (PS-COOH) nanoparticles, sized 200 and 500 nm, were purchased from Molecular Probes (Eugene, OR). These particles have negative surface charge at neutral pH (Table S1). In order to produce nonmucoadhesive nanoparticles (PS–PEG) for use in characterizing mucus pore structure, PS-COOH particles were covalently modified with 2 kDa or 5 kDa amine-modified PEG (Creative PEGworks, Winston Salem, NC) as previously described.13 Particles were added to CVM at a concentration of 0.2–0.4 mg/mL. Poly(lactic-co-glycolic acid) (PLGA) nanoparticles were made as previously described.12 PLGA 2A (50:50) was purchased from Lakeshore Biomaterials (Birmingham, AL), and Pluronic F127 was purchased from BASF (Florham Park, NJ). Briefly, 10 mg/mL of PLGA was dissolved in acetone and added dropwise to 40 mL of aqueous 1% F127 solution. After stirring for 2 h, particles were filtered through a 5 μm syringe filter and collected by centrifugation (Sorvall RC-6+, ThermoScientific, Waltham, MA), and washed twice with 0.1% F127 solution. PLGA nanoparticles were suspended in 1% F127 solution at a particle concentration of 0.2 mg/mL.
Particle size and ζ-potential were determined by dynamic light scattering and laser Doppler anemometry, respectively, using a Zetasizer Nano ZS90 (Malvern Instruments, Southborough, MA). Size measurements were performed at 25 °C at a scattering angle of 90°. Samples were diluted in 10 mM NaCl solution (pH 7) and measurements performed according to instrument instructions. A near-neutral ζ-potential was used to confirm PEG surface coating as previously described (Table S1).10
HSV-1 virus was a generous gift from Prashant Desai (Johns Hopkins University). The recombinant virus expresses red fluorescent protein (RFP) internally,14 so the fluorescent label should not affect interactions between the viral surface and mucus. The viral titer was 1.44E9 PFU/mL, and the virus was used at this concentration.
Collection of Human CVM
CVM samples were collected as previously described.15 Briefly, undiluted cervicovaginal secretions from women with normal vaginal microbiota were obtained using a self-sampling method following a protocol approved by the Institutional Review Board of Johns Hopkins Medicine. The device (Instead Softcup, Evofem, Inc., San Diego, CA) was inserted into the vagina for ∼30 s, removed, and placed into a 50 mL conical tube. Samples were centrifuged at 200g for 2 min to collect the mucus secretions. None of the samples used in this study were ovulatory by visual inspection for spinnbarkeit. The CVM samples used in this study were from women with healthy microbiota, indicated by sample pH < 4.5. Mucus samples were stored at 4 °C prior to use (always within a few hours of collection).
CVM Sample Preparation
F127 solution was added at specified concentrations to ∼30 μL of CVM at a 3% (v/v) ratio in custom-made chamber slides. Phosphate buffered saline (PBS) was added to control CVM slides to account for possible dilution effects. The slides were incubated at 37 °C in saturated humidity for 15 min prior to nanoparticle addition (3% vol/vol). We found that 15 min was sufficient incubation time; we observed no difference in nanoparticle transport if the pretreated samples were incubated for up to 2 h prior to nanoparticle addition (data not shown). After particle addition, the slides were immediately sealed with super glue and imaged. CVM samples were neutralized with minimal amounts (1–2 μL per 100 μL of mucus) of 5 M sodium hydroxide for use in HSV-1 experiments. The initial and final pH of all samples was confirmed by blotting a small mucus sample onto pH paper. Mucus samples were gently mixed to minimize any perturbations to the mucus structure.
Multiple Particle Tracking in CVM
The trajectories of fluorescent nanoparticles were recorded using a silicon-intensified target camera (VE-1000, Dage-MTI, Michigan City, IN) mounted on an inverted epifluorescence microscope equipped with 100× oil-immersion objective (numerical aperture 1.3). Movies were captured with Metamorph software (Molecular Devices, Sunnyvale, CA) at a temporal resolution of 66.7 ms for 20 s. Trajectories of n > 100 particles were analyzed for each experiment, and at least three independent experiments were performed using mucus samples from different women. The coordinates of particle centroids were transformed into time-averaged mean square displacements (MSD), calculated as <Δr2(τ) >= [x(t + τ) – x(t)]2 + [y(t + τ) – y(t)]2, where τ is time scale (or time lag), x and y are the corresponding particle coordinates at time t. This equation was used to calculate particle MSDs as previously demonstrated.3
Mucus samples were assumed to be locally isotropic (but not homogeneous), such that 2D diffusion can be extrapolated to 3D diffusion.16 We have previously estimated that the static error for our system is much smaller than the overall particle displacements for PS–PEG, thus the static error is not expected to have a significant impact on the quantification of particle MSD.17 Although we are unable to determine the dynamic error for a complex fluid such as mucus, we minimize contributions of dynamic error by comparing data at a long time scale (1 s) relative to the time interval between frames.
In Vivo Cytokine Release
Female 6–8 week-old CF-1 mice were purchased from Harlan (Indianapolis, IN). Depo-Provera (150 mg/mL) was purchased from Pharmacia and Upjohn Company (New York, NY). Mice were given a subcutaneous flank injection of 2.5 mg Depo-Provera in 100 μL of phosphate buffered saline (PBS) 7 days prior to experiments. This treatment is commonly used to arrest and synchronize the mouse in the diestrus phase of the estrous cycle.18,19 A total of 20 μL of each test agent was administered intravaginally once a day for 7 days. HEC gel and nonoxynol-9 (N9) were provided by Thomas Moench (ReProtect). On the eighth day, each mouse was lavaged twice with 50 μL of PBS. Cervicovaginal lavage fluid was diluted with an additional 200 μL of PBS and centrifuged to remove the mucus plug. The supernatant was removed and split into 50 μL aliquots for each of the two (IL-1α, IL-1β) Quantikine ELISA (enzyme-linked immunosorbent assay) kits (R&D Systems, Minneapolis, MN). ELISAs were conducted per manufacturer instructions. All experimental protocols were approved by the Johns Hopkins Animal Care and Use Committee.
Statistics
The Wilcoxon rank sum test was used to compare individual data sets, and the Kruskal–Wallis test was used to compare multiple samples. These tests are the nonparametric version of the t test and ANOVA test, respectively, which is more appropriate in situations where data sets are not assumed to fit a Gaussian distribution.20
Results and Discussion
Transport of Polystyrene Nanoparticles in F127-Pretreated CVM
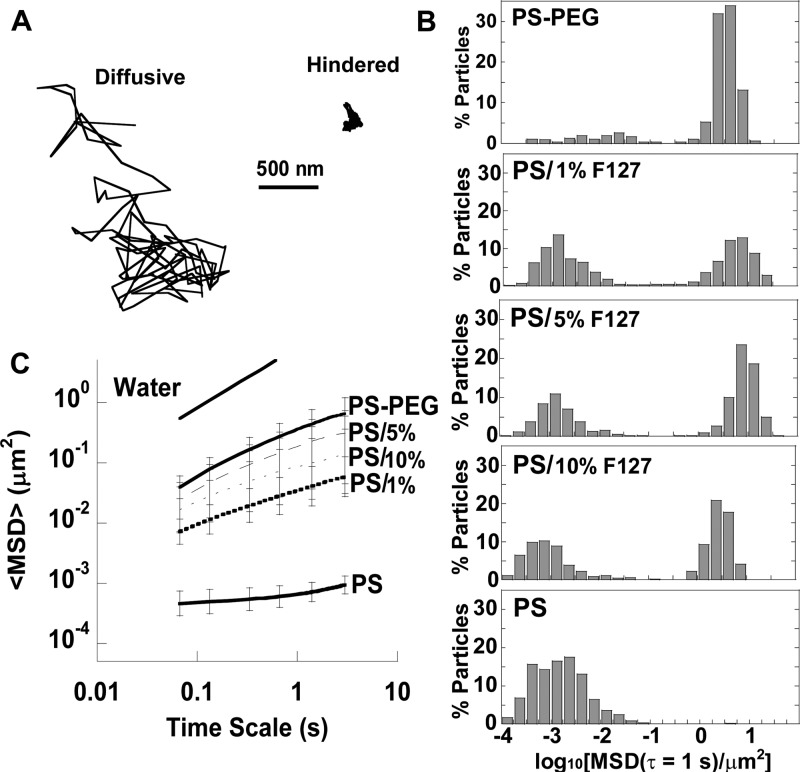
We chose to test conventional mucoadhesive polystyrene nanoparticles (PS) 200 nm in size; 200 nm PS particles are highly adhesive to healthy CVM, but diffuse rapidly in CVM when the hydrophobic core is densely coated with low-molecular weight PEG (PS–PEG).3 Currently, Pluronic concentrations in FDA-approved products for topical use range from 0.01–15.5%, so we tested pretreatment solutions with concentrations in this range. When CVM was pretreated with 0.01% (w/v) F127, PS remained immobilized, similar to PS in untreated CVM (Figure 1A). However, when CVM was pretreated with 1% F127 solution, PS diffused at similar rates to PS–PEG (Figure 1A). The ensemble-averaged mean square displacement (⟨MSD⟩) of PS in CVM pretreated with 1% F127 was significantly higher than the ⟨MSD⟩ of PS particles in untreated CVM and 0.01% F127-pretreated CVM, and not statistically different than the ⟨MSD⟩ of PS–PEG in CVM (Figure 1B). However, analysis of individual particle MSD indicated that only ∼50% of PS in 1% F127-pretreated CVM were highly diffusive, compared to nearly all PS–PEG (data not shown). Since PS were not uniformly diffusive in 1% F127-pretreated CVM, we investigated whether pretreating CVM with 5% and 10% (w/v) F127 solutions would increase the percentage of diffusive PS. However, it was evident that pretreatment of CVM with higher concentrations of F127 did not lead to an increase in the fraction of rapidly diffusive PS particles. Typical trajectories of diffusive PS and hindered PS in the same CVM sample pretreated with 1% F127 are shown in Figure 2A. The distribution of individual particle MSDs in Figure 2B highlights the two distinct particle populations. Based on these results, it is unclear whether the F127 was interacting directly with the hydrophobic particle surface, hydrophobic portions of CVM, or both. It is possible that some PS were able to adhere to mucins or cell debris before the hydrophobic polymer core or hydrophobic portions of the mucin proteins are completely shielded by F127. In contrast, PS–PEG were nearly uniformly diffusive in CVM (Figure 2B), and mucoadhesive nanoparticles preincubated in F127 solution were nearly uniformly diffusive in CVM.12 Thus, it appears that direct modification of the nanoparticle surface with F127 resulted in more uniform mucus-penetrating character. Of note, some of the fastest PS in F127-pretreated CVM had slightly higher MSD values than the fastest PS–PEG (Figure 2B), which could be due to the minor increase in hydrodynamic diameter of PS–PEG after PEGylation (Table S1). As shown in Figure 2C, pretreatment of CVM with 5% and 10% F127 resulted in a modest increase in the ⟨MSD⟩ of PS compared to pretreatment of CVM with 1% F127, but all three treatment groups and PS–PEG were statistically indistinguishable.
Figure 1.
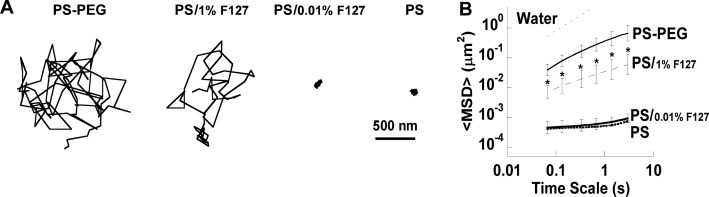
Transport of 200 nm particles in CVM pretreated with low concentrations of F127 (indicated by /% F127). (A) Trajectories of PS–PEG and PS in CVM after pretreatment with varying concentrations of F127. (B) Ensemble-averaged geometric mean square displacement (⟨MSD⟩) as a function of time scale for PS–PEG and PS, including the theoretical MSD in water (W). Data are means ± SEM (≥3 independent experiments, with n ≥ 100 particles per experiment). *P < 0.05 compared to PS in untreated mucus (PS), Wilcoxon rank sum test.
Figure 2.
Transport of 200 nm particles in CVM pretreated with high concentrations of F127 (indicated by /% F127). (A) Representative trajectories of diffusive (left) and hindered (right) PS in CVM pretreated with 1% F127. (B) Distributions of the logarithms of individual particle mean square displacement (MSD) at a time scale of 1 s. (C) Ensemble-averaged geometric MSD (⟨MSD⟩) as a function of time scale for PS–PEG and PS, including the theoretical MSD in water (W). Data are means ± SEM (≥3 independent experiments, with n ≥ 100 particles).
Effect of F127 Pretreatment on CVM Pore Structure
We next sought to determine whether pretreating CVM with F127 would affect the structural properties of the mucus mesh. It was previously demonstrated that the addition of polymers such as polyvinylpyridine and PEG to ovulatory cervical mucus affected the mucus structure, which altered cell migration rates through the mucus.21,22 Similarly, we described the use of nonadhesive PS–PEG as probes for characterizing the microscopic structural and viscoelastic properties of mucus.23,24 We demonstrated that pretreatment of CVM with a nonionic detergent, N9, led to rearrangement of mucin bundles and an overall decrease in pore size in CVM, without changing the macroscopic viscoelasticity.23 Such a decrease in mesh spacing would reduce the size of nanoparticle that could efficiently penetrate without becoming sterically trapped.
To determine whether the surfactant properties of F127 would also affect the pore structure of CVM, we used PS–PEG in the size range of the average pore size of CVM.24 The transport behavior of 500 nm PS–PEG is sensitive to small reductions in average mucus mesh pore size.23 As shown in Figure 3A, 500 nm PS–PEG displayed similarly diffusive trajectories in CVM, regardless of the concentration of F127 used to pretreat the mucus (1–10%). The ⟨MSD⟩ of 500 nm PS–PEG in untreated and F127-pretreated CVM were indistinguishable, indicating that CVM pore structure was not affected by F127 pretreatment (Figure 3B). F127 is a triblock copolymer, so it does not have the typical structure of a surfactant with a polar head and a hydrophobic tail. In contrast, F127 structure alternates between hydrophilic, hydrophobic, and hydrophilic regions. Thus, it would be less energetically favorable for F127 to incorporate into mucin protein bundles and affect the overall pore structure. Also, F127 is uncharged, so it would not interact electrostatically with the highly negatively charged portions of mucins.
Figure 3.
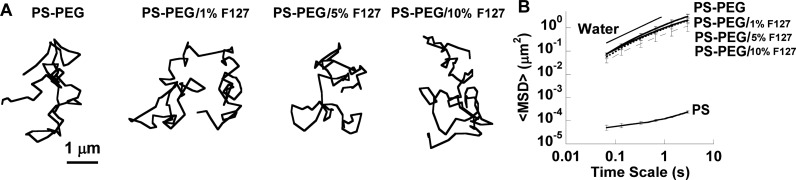
Transport of 500 nm PS–PEG in CVM pretreated with high concentrations of F127 (indicated by /% F127). (A) Trajectories of PS–PEG in CVM after pretreatment with varying concentrations of F127. (B) Ensemble-averaged geometric mean square displacement (⟨MSD⟩) as a function of time scale for PS–PEG, including the theoretical MSD in water (W). Data are means ± SEM (three independent experiments, with n ≥ 100 particles per experiment).
Effect of F127 Pretreatment on CVM as a Barrier to Herpes Simplex Virus Type 1
The healthy human vagina is acidified to pH 3.5–4 by the presence of lactic acid secreting lactobacilli,25,26 and we previously demonstrated that HSV-1 (diameter ∼ 180 nm) is adhesively, not sterically, trapped in healthy CVM.24 However, vaginal pH is transiently neutralized by the presence of semen.27 Additionally, bacterial vaginosis, a condition characterized by a lack of lactic acid secreting bacteria and more neutral vaginal pH, is known to be a risk factor for acquiring sexually transmitted infections.28 Interestingly, recent work demonstrated that HSV-1 diffuses rapidly through neutralized CVM.29 Thus, the effect of pH on viral adhesion could potentially explain the higher incidence of HSV infections in women with bacterial vaginosis as compared to women with healthy vaginal microbiota. Since the presence of F127 reduced adhesive interactions between CVM and PS, we wanted to ensure that the presence of F127 in CVM would not compromise the barrier properties of CVM to pathogens, such as HSV-1.
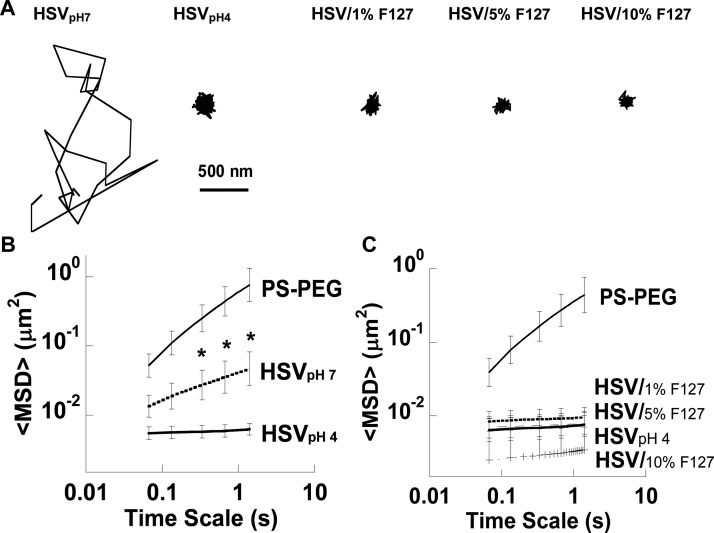
Here, we compared the diffusion of HSV-1 in neutralized (pH ∼ 7) CVM (Figure 4A) and F127-pretreated CVM. In neutralized CVM, two populations of HSV-1 were observed and tracked: a fraction that was immobilized and a fraction that diffused very rapidly (some virions were not in focus long enough to track). Overall, the ⟨MSD⟩ of all virions in the neutralized CVM sample was only ∼15-fold reduced compared to similarly sized PS–PEG at a time scale of 1 s (Figure 4B). In contrast, HSV-1 remained adhesively immobilized in non-neutralized, healthy CVM pretreated with 1–10% F127 solution, as indicated by the lower, constant ⟨MSD⟩ over time (statistically indistinguishable compared to HSV-1 in pH 4 CVM; Figure 4C).
Figure 4.
Transport of HSV in neutralized CVM or CVM pretreated with high concentrations of F127 (indicated by /% F127). CVM samples are at pH ∼ 4 unless otherwise noted. (A) Trajectories of HSV in untreated CVM (HSVpH4), CVM after neutralization (HSVpH7), and CVM after pretreatment with varying concentrations of F127. Ensemble-averaged geometric mean square displacement (⟨MSD⟩) as a function of time scale for PS–PEG compared to (B) HSV in neutralized (pH 7) and untreated (pH 4) CVM and (C) HSV in untreated CVM (pH 4) or after pretreatment with varying concentrations of F127. Data are means ± SEM (three independent experiments, with n ≥ 100 particles per experiment). *P < 0.05 compared to HSVpH4, Wilcoxon rank sum test.
The amount of visible virus under acidic conditions was reduced compared to the amount of visible virus under neutralized conditions (visual observation). In addition to the pH-dependent viral adhesion to CVM, it has also been demonstrated that acidity reduces the infectivity of HSV-1 by irreversibly disrupting the virus; the fraction of infectious HSV-1 decreased as pH was reduced from 4.5 to 3.5.30 However, all remaining visible HSV-1 was adhesively immobilized in acidic CVM, regardless of F127 pretreatment. Thus, CVM acts as both an adhesive and inactivating barrier to infection in the healthy vagina, and the adhesive interactions with HSV-1 were not affected by the presence of F127.
Of note, we previously found that the microstructure and bulk rheology of CVM was remarkably resistant to pH changes in the range of pH 1–2 to pH 8–9.31 PS–PEG 210 nm in size rapidly diffused in both pH 4 and pH 6–7 CVM.31 Thus, trapping of HSV-1 (∼180 nm) in acidic CVM must be due to adhesive interactions that are reduced at neutral pH. It is possible that HSV-1 envelope proteins play a role in its adhesion to CVM, and that pH changes induce charge and conformational changes in surface proteins. Similarly, we previously found that HIV was adhesively trapped in healthy CVM, but diffused rapidly in neutralized CVM.32 When HIV was incubated in lactic acid at pH 4, mimicking healthy CVM, the surface charge was near 0. In contrast, at pH 7, the virion surface charge was highly negative.32 If the interactions of HSV-1 with CVM are similarly based on surface proteins and surface charge, then it is unlikely that the shielding of hydrophobic interactions by F127 would have an impact on HSV-2 adhesion.
Safety of Vaginal F127 Administration In Vivo
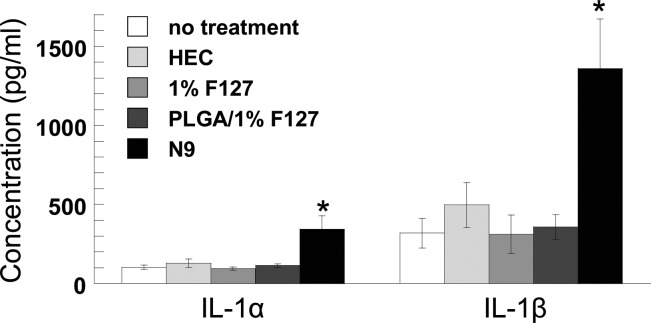
When proposing to use a surfactant solution as a pretreatment for a mucosal surface, it is important to ensure that the solution does not cause local toxicity or inflammation. The epithelium itself serves as a barrier to pathogens at mucosal surfaces; damage to the epithelium and local inflammation can increase risk of viral transmission, potentially diminishing the therapeutic effects of a delivered drug.33,34 The surfactant N9 was shown to cause local toxicity in the vagina that led to an increase in HIV transmission.35 In this case, N9 caused local epithelial irritation and toxicity, diminishing the barrier properties of the epithelium. We have previously demonstrated that a single dose of 2% N9 significantly increases susceptibility to HSV-2 in a mouse model.36 Furthermore, repeated vaginal administration of N9 in mice caused an increase in inflammatory cytokines IL-1α and IL-1β (but not TNF-α or IL-6), whereas repeated vaginal administration of hydroxyethylcellulose (HEC) placebo gel was indistinguishable from no treatment.2 IL-1α/β are secreted by the epithelium in response to injury.37 Here, we show that daily vaginal administration of 1% F127 solution for one week did not elevate IL-1α/β compared to controls (Figure 5). Additionally, cytokine levels were not elevated in response to daily administration of PLGA nanoparticles in a 1% F127 solution (PLGA/1% F127). In contrast, treatment with 5% N9 solution caused a significant increase in both IL-1α and IL-1β levels, indicating epithelial injury that leads to diminished barrier properties.
Figure 5.
IL-1α and IL-1β concentrations in mouse vaginal lavage fluid after daily vaginal treatments for 1 week. Data are means ± SEM; *P < 0.05 compared to no treatment, Wilcoxon rank sum test. N9 and HEC data reprinted with permission from ref (5). Copyright 2012 AAAS.
F127 has a long history of safety, and many products considered safe for ingestion and for application to the eye and buccal mucosa contain Pluronics,38,39 including drug delivery devices.40,41 Indeed, previous studies with particles formulated for oral delivery have employed 1% F127 as a suspending solution,42,43 which may have reduced mucoadhesion and increased overall effectiveness of the particles. In contrast to vaginal F127 treatment, vaginal N9 treatment resulted in a significant increase in both IL-1α and IL-1β2. Similar to treatment with F127 alone, there was no increase in IL-1α and IL-1β release for F127-coated PLGA nanoparticles.
F127-coated PLGA nanoparticles have already been demonstrated to rapidly penetrate CVM,12 distribute over the entire vaginal and ectocervical surfaces,2 and provide enhanced cervical tumor suppression.9 Similar mucus-penetrating characteristics were already demonstrated for F127-coated polycaprolactone (PCL).12 In contrast, PLGA particles first coated in a relatively hydrophilic surfactant, poly(vinyl alcohol) (PVA), were found to be mucoadhesive, and could not be further coated by Pluronic F127 to reduce mucoadhesion.5 Improvements in mucosal delivery would be expected for Pluronic pretreatment in combination with other hydrophobic nanoparticle formulations compared to the nanoparticles alone, which will have to be determined on a case-by-case basis. Combining a mucosal pretreatment, such as a solution in the vagina or an aerosol for inhalation into the lungs, with a mucoadhesive nanoparticle formulation may eliminate the need for any changes in manufacturing and reduce time and costs for clinical development. However, of note, more uniform diffusive character was observed for nanoparticles that were formulated either with dense PEG coatings (Figure 1) or preincubated in Pluronic F127 to form a surface coating prior to addition to mucus.12 For particles preincubated in Pluronic F127 solution and administered to mucosal surfaces, the results here suggest that excess Pluronic in solution will not negatively impact the microstructure or barrier properties of mucus to pathogens.
Conclusions
We found that pretreating human CVM with a sufficient concentration of Pluronic F127 led to a significant enhancement in the diffusion of ∼50% of conventional mucoadhesive polystyrene nanoparticles (PS). It remains to be determined whether this simple technique may be generally used with hydrophobic nanoparticle formulations to improve delivery to mucosal surfaces compared to hydrophobic nanoparticles alone. Pluronic F127 did not affect the native mucus pore structure or cause an inflammatory response after daily vaginal administration for one week. Although HSV-1 rapidly diffuses through neutralized CVM, F127-pretreatment did not reduce adhesion between HSV-1 and healthy, acidic CVM. Therefore, Pluronic pretreatment of mucosal tissues may improve the effectiveness of conventional drug delivery nanotechnologies in a safe manner.
Acknowledgments
We thank S. Chen and J. Teng (multiple particle tracking), H. Patel (ELISA analysis), and the animal husbandry staff at Johns Hopkins. This work was supported by the NIH (Grants R01HD062844, R33AI079740, R01CA140746; J.H. and R.C.) and the W.W. Smith Charitable Trust (L.M.E.).
Supporting Information Available
Supplemental text contains a table of nanoparticle physiochemical characteristics. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
△ Division of Molecular Pharmaceutics, Eshelman School of Pharmacy, University of North Carolina-Chapel Hill, Chapel Hill, North Carolina, United States (S.K.L.).
The authors declare the following competing financial interest(s): The mucus penetrating particle technology is being developed by Kala Pharmaceuticals. J.H. is a co-founder of Kala. J.H. and R.C. own company stock, which is subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Cone R. A. Barrier properties of mucus. Adv. Drug Delivery Rev. 2009, 61, 75–85. [DOI] [PubMed] [Google Scholar]
- Ensign L. M.; Tang B. C.; Wang Y. Y.; Tse T. A.; Hoen T.; Cone R.; Hanes J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci. Transl. Med. 2012, 4, 138ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. K.; O’Hanlon D. E.; Harrold S.; Man S. T.; Wang Y. Y.; Cone R.; Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 1482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. K.; Wang Y. Y.; Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Delivery Rev. 2009, 61, 158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.; Lai S. K.; Yu T.; Wang Y. Y.; Happe C.; Zhong W.; Zhang M.; Anonuevo A.; Fridley C.; Hung A.; Fu J.; Hanes J. Nanoparticle penetration of human cervicovaginal mucus: The effect of polyvinyl alcohol. J. Controlled Release 2014, 192, 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster B. S.; Suk J. S.; Woodworth G. F.; Hanes J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials 2013, 34, 3439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk J. S.; Lai S. K.; Wang Y. Y.; Ensign L. M.; Zeitlin P. L.; Boyle M. P.; Hanes J. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials 2009, 30, 2591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk J. S.; Kim A. J.; Trehan K.; Schneider C. S.; Cebotaru L.; Woodward O. M.; Boylan N. J.; Boyle M. P.; Lai S. K.; Guggino W. B.; Hanes J. Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J. Controlled Release 2014, 178, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.; Yu T.; Wang Y. Y.; Lai S. K.; Zeng Q.; Miao B.; Tang B. C.; Simons B. W.; Ensign L. M.; Liu G.; Chan K. W.; Juang C. Y.; Mert O.; Wood J.; Fu J.; McMahon M. T.; Wu T. C.; Hung C. F.; Hanes J. Vaginal delivery of paclitaxel via nanoparticles with non-mucoadhesive surfaces suppresses cervical tumor growth. Adv. Healthcare Mater. 2013, 3, 1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Y.; Lai S. K.; Suk J. S.; Pace A.; Cone R.; Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew. Chem., Int. Ed. 2008, 47, 9726–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q.; Boylan N. J.; Cai S.; Miao B.; Patel H.; Hanes J. Scalable method to produce biodegradable nanoparticles that rapidly penetrate human mucus. J. Controlled Release 2013, 170, 279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.; Lai S. K.; Wang Y. Y.; Zhong W.; Happe C.; Zhang M.; Fu J.; Hanes J. Biodegradable nanoparticles composed entirely of safe materials that rapidly penetrate human mucus. Angew. Chem., Int. Ed. 2011, 50, 2597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance E. A.; Woodworth G. F.; Sailor K. A.; Shih T. Y.; Xu Q.; Swaminathan G.; Xiang D.; Eberhart C.; Hanes J. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl. Med. 2012, 4, 149ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P.; Sexton G. L.; Huang E.; Person S. Localization of herpes simplex virus type 1 UL37 in the Golgi complex requires UL36 but not capsid structures. J. Virol 2008, 82, 11354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey E. R.; Moench T. R.; Hees P. S.; Cone R. A. A self-sampling method to obtain large volumes of undiluted cervicovaginal secretions. Sex. Transm. Dis. 2003, 30, 107–9. [DOI] [PubMed] [Google Scholar]
- Suh J.; Dawson M.; Hanes J. Real-time multiple-particle tracking: applications to drug and gene delivery. Adv. Drug Delivery Rev. 2005, 57, 63–78. [DOI] [PubMed] [Google Scholar]
- Kim A. J.; Boylan N. J.; Suk J. S.; Hwangbo M.; Yu T.; Schuster B. S.; Cebotaru L.; Lesniak W. G.; Oh J. S.; Adstamongkonkul P.; Choi A. Y.; Kannan R. M.; Hanes J. Use of single-site-functionalized PEG dendrons to prepare gene vectors that penetrate human mucus barriers. Angew. Chem., Int. Ed. 2013, 52, 3985–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achilles S. L.; Shete P. B.; Whaley K. J.; Moench T. R.; Cone R. A. Microbicide efficacy and toxicity tests in a mouse model for vaginal transmission of Chlamydia trachomatis. Sex. Transm. Dis. 2002, 29, 655–64. [DOI] [PubMed] [Google Scholar]
- Zeitlin L.; Whaley K. J.; Hegarty T. A.; Moench T. R.; Cone R. A. Tests of vaginal microbicides in the mouse genital herpes model. Contraception 1997, 56, 329–35. [DOI] [PubMed] [Google Scholar]
- Kitchen C. M. Nonparametric vs parametric tests of location in biomedical research. Am. J. Ophthalmol. 2009, 147, 571–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willits R. K.; Saltzman W. M. Synthetic polymers alter the structure of cervical mucus. Biomaterials 2001, 22, 445–52. [DOI] [PubMed] [Google Scholar]
- Willits R. K.; Saltzman W. M. The effect of synthetic polymers on the migration of monocytes through human cervical mucus. Biomaterials 2004, 25, 4563–71. [DOI] [PubMed] [Google Scholar]
- Lai S. K.; Wang Y. Y.; Cone R.; Wirtz D.; Hanes J. Altering mucus rheology to “solidify” human mucus at the nanoscale. PLoS One 2009, 4, e4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. K.; Wang Y. Y.; Hida K.; Cone R.; Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hanlon D. E.; Moench T. R.; Cone R. A. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One 2013, 8, e80074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey E. R.; Telsch K. M.; Whaley K. J.; Moench T. R.; Cone R. A. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect. Immun. 1999, 67, 5170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. A.; Meldrum S. J.; Watson B. W. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J. Reprod. Fertil. 1973, 33, 69–75. [DOI] [PubMed] [Google Scholar]
- Allsworth J. E.; Lewis V. A.; Peipert J. F. Viral sexually transmitted infections and bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Sex. Transm. Dis. 2008, 35, 791–6. [DOI] [PubMed] [Google Scholar]
- Wang Y. Y.; Kannan A.; Nunn K. L.; Murphy M. A.; Subramani D. B.; Moench T.; Cone R.; Lai S. K. IgG in cervicovaginal mucus traps HSV and prevents vaginal Herpes infections. Mucosal Immunol. 2014, 7, 1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyama A. C.; Cheshenko N.; Carlucci M. J.; Li J. H.; Goldberg C. L.; Waller D. P.; Anderson R. A.; Profy A. T.; Klotman M. E.; Keller M. J.; Herold B. C. ACIDFORM inactivates herpes simplex virus and prevents genital herpes in a mouse model: optimal candidate for microbicide combinations. J. Infect. Dis. 2006, 194, 795–803. [DOI] [PubMed] [Google Scholar]
- Wang Y. Y.; Lai S. K.; Ensign L. M.; Zhong W.; Cone R.; Hanes J. The microstructure and bulk rheology of human cervicovaginal mucus are remarkably resistant to changes in pH. Biomacromolecules 2013, 14, 4429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. K.; Hida K.; Shukair S.; Wang Y. Y.; Figueiredo A.; Cone R.; Hope T. J.; Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J. Virol. 2009, 83, 11196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench T. R.; Mumper R. J.; Hoen T. E.; Sun M.; Cone R. A. Microbicide excipients can greatly increase susceptibility to genital herpes transmission in the mouse. BMC Infect. Dis. 2010, 10, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. S.; Cheshenko N.; Fakioglu E.; Mesquita P. M.; Keller M. J.; Herold B. C. Susceptibility to genital herpes as a biomarker predictive of increased HIV risk: expansion of a murine model of microbicide safety. Antiviral Ther. 2009, 14, 1113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme L.; Ramjee G.; Alary M.; Vuylsteke B.; Chandeying V.; Rees H.; Sirivongrangson P.; Mukenge-Tshibaka L.; Ettiegne-Traore V.; Uaheowitchai C.; Karim S. S.; Masse B.; Perriens J.; Laga M. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 2002, 360, 971–7. [DOI] [PubMed] [Google Scholar]
- Cone R. A.; Hoen T.; Wong X.; Abusuwwa R.; Anderson D. J.; Moench T. R. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect. Dis. 2006, 6, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins J. E. Jr.; Doncel G. F. Biomarkers of cervicovaginal inflammation for the assessment of microbicide safety. Sex. Transm. Dis. 2009, 36, S84–91. [DOI] [PubMed] [Google Scholar]
- Mao Y.; Thompson M. J.; Wang Q.; Tsai E. W. Quantitation of poloxamers in pharmaceutical formulations using size exclusion chromatography and colorimetric methods. J. Pharm. Biomed. Anal. 2004, 35, 1127–42. [DOI] [PubMed] [Google Scholar]
- Urban-Morlan Z.; Castro-Rios R.; Chavez-Montes A.; Melgoza-Contreras L. M.; Pinon-Segundo E.; Ganem-Quintanar A.; Quintanar-Guerrero D. Determination of poloxamer 188 and poloxamer 407 using high-performance thin-layer chromatography in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2008, 46, 799–803. [DOI] [PubMed] [Google Scholar]
- Batrakova E. V.; Kabanov A. V. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Controlled Release 2008, 130, 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanov A. V.; Batrakova E. V.; Alakhov V. Y. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J. Controlled Release 2002, 82, 189–212. [DOI] [PubMed] [Google Scholar]
- Furtado S.; Abramson D.; Burrill R.; Olivier G.; Gourd C.; Bubbers E.; Mathiowitz E. Oral delivery of insulin loaded poly(fumaric-co-sebacic) anhydride microspheres. Int. J. Pharm. 2008, 347, 149–155. [DOI] [PubMed] [Google Scholar]
- Thanos C. G.; Yip K. P.; Mathiowitz E. Intestinal uptake of polymer microspheres in the rabbit studied with confocal microscopy. J. Bioact. Compat. Polym. 2004, 19, 247–266. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.