Abstract
Over the last century, terrestrial yeasts have been widely used in various industries, such as baking, brewing, wine, bioethanol and pharmaceutical protein production. However, only little attention has been given to marine yeasts. Recent research showed that marine yeasts have several unique and promising features over the terrestrial yeasts, for example higher osmosis tolerance, higher special chemical productivity and production of industrial enzymes. These indicate that marine yeasts have great potential to be applied in various industries. This review gathers the most recent techniques used for marine yeast isolation as well as the latest applications of marine yeast in bioethanol, pharmaceutical and enzyme production fields.
Keywords: marine yeast, isolation, biofuel, enzyme, pharmaceuticals, seawater
Introduction
Marine microorganisms have potential applications in metal detoxification, nutrient cycling, greenhouse gas reduction and form the basis of food webs. Marine microorganisms, including marine yeasts, live in extreme environments, and this provides a unique potential for the synthesis of functional biomolecules (Connell et al., 2008). Marine yeasts, defined as the yeasts that are isolated from marine environments, are able to grow better on a medium prepared using seawater rather than freshwater (Chi et al., 2010). The first marine yeasts were isolated by Bernhard Fischer in 1894 from the Atlantic Ocean, and those were identified as Torula sp. and Mycoderma sp. (Kutty & Philip, 2008). Following this discovery, various other marine yeasts have been isolated from around the world from different sources, including seawater, seaweeds, marine fish and mammals as well as seabirds. Among these isolates, some marine yeasts originated from terrestrial habitats (grouped as facultative marine yeast), which were brought to and survived in marine environments. The other marine yeasts were grouped as obligate or indigenous marine yeasts, which confine to marine habitats (Kohlmeyer & Kohlmeyer, 1979; Kutty & Philip, 2008). However, no sufficient evidence has been found to explain the indispensability of seawater for obligate marine yeasts. It has been reported that marine yeasts are able to produce many bioactive substances, such as amino acids, glucans, glutathione, toxins, enzymes, phytase and vitamins with potential application in the food, pharmaceutical, cosmetic and chemical industries as well as for marine culture and environmental protection (Chi et al., 2009; Sarkar et al., 2010).
Marine yeast isolation methods
Over the years, microbiologists have developed several methods for marine yeast isolation. These methods differ in their sampling, sample preparation, medium composition and strain maintenance. This variation is required to cope with the diverse marine habitats, the target properties required in the isolates (e.g. the ability of utilizing xylose) and the likely cell density of the sample.
Surface seawater samples can be collected using simple plastic or glass bottles (1–5 L). Bottles should have screw caps for easy handling as well as for preventing contamination and leaks. For aseptic reason, bottles should be opened under water and washed thoroughly using the seawater 3–5 times before filling with sample. Sterilized plastic bags, jars and vials can also be employed in collecting surface samples. Surface seawater samples are suitable for isolating aerobic and facultative anaerobic yeasts. A ‘near shore’ location is more suitable for sampling yeasts that are capable of carbohydrate fermentation (Fell, 2001). Samples of 250 mL are generally enough when they are taken near shore, whereas samples from the open ocean should be at least 1 L as a lower microorganism density is expected. Fifty millilitres of sediment samples is generally considered adequate. Experiment design and replication should be taken into account for the required sample volume (Fell, 2001).
More advanced devices have been designed and used to collect deep sea samples (water and sediments). The first water sampler that was able to maintain in situ hydrostatic pressure was reported by Jannasch et al. (1973). Generally, Niskin, Van Dorn and Kemmerer samplers are the most common apparatuses that have been used for deep sea sampling, as shown in Fig.1. Niskin samplers can be used singly or in series or in a rosette of up to 12 samplers per rack. Van Dorn is a horizontal sampler, while Kemmerer is a vertical sampler so that it could fit narrow areas. These devices can collect samples from as deep as 6000 m. However, those devices do not maintain in situ hydrostatic pressure. These samplers usually consist of cylindrical tube(s) with a stopper at each end (between 1 and 121 tubes per frame). These stoppers could be controlled remotely from the surface (Dorschel, 2007; Singh, 2011). Research submarines can also be used to collect deep sea samples. This device is larger in size, very complicated and massively expensive. On the other hand, research submarines allow the collection of large amounts of samples, good observation of the sample environment and instant work on the samples as it can also carry all the laboratory equipment needed (Singh, 2011).
Figure 1.
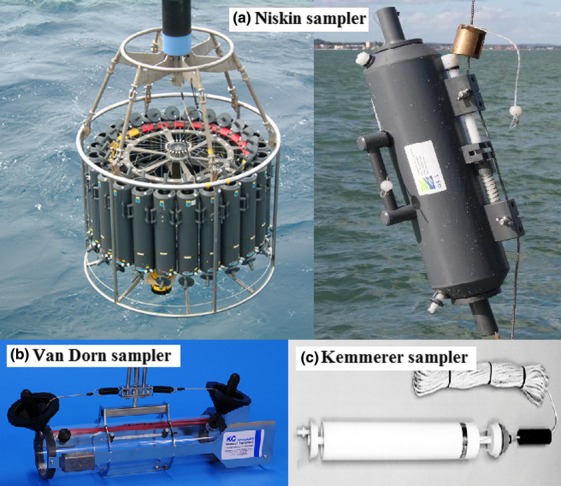
The commonly used samplers for deep seawater sampling. (a) Niskin sampler (http://www.godac.jamstec.go.jp/darwin/instrument/mirai/e), (b) Van Dorn sampler (http://www.kc-denmark.dk/products/water-sampler/van-dorn-water-sampler.aspx) and (c) Kemmerer sampler (http://www.rickly.com/as/kemmerer.htm).
Sample preparation for marine yeast isolation is depended on two main factors (1) the desired characteristics of the isolates and (2) the expected number of yeast cells per millilitre. Samples collected from the open sea usually contained around 10 or fewer cells per millilitre (Fell, 2001; Kutty & Philip, 2008). Therefore, filtration of 5 L seawater is required followed by the resuspension of the cells remaining on the filter in 15 mL of the same seawater filtrate. In contrast, samples collected from the high organic matter containing surface near shore can contain thousands of yeast cells per millilitre (Kutty & Philip, 2008). So, filtration of 100 mL is usually enough. Alternatively, samples could be subject to an enrichment step for a couple of days before isolation to select desirable strains with specific characteristics. For extraction from solid samples, such as seaweed, sea sand, dead marine plant and animal material, a known weight of solid particles can be transferred into a broth medium for enrichment, or be placed directly on an agar plate. Serial dilution is required prior to isolation if more than 300 isolates are expected per millilitre (Fell, 2001).
Several different medium recipes have been used for the isolation of marine yeasts. Although both natural and artificial seawater have been used for preparing medium, natural seawater is preferable as it is closer to the natural environment that the yeasts inhabit. A mixture of broad-spectrum antibiotics have been used in isolation media, which have been shown to be more effective than single antibiotic in inhibiting the growth of bacteria and were less harmful to yeast cells (Beuchat, 1979; Thomson, 1984). Different inhibitors, including rose Bengal (Jarvis, 1973; King et al., 1979), dichloran (Jarvis, 1973) and propionate (Bowen & Beech, 1967) have been added to the media to inhibit the growth of moulds (Kutty, 2009). Usually, the same medium as used for isolation is also used for maintenance but without added antibiotics. Plates should be incubated at a temperature similar to the environment where the samples were collected. The optimum temperature for marine yeasts varies (Watson, 1987). For taxonomic tests, yeasts are usually incubated at 25 °C (Buhagiar & Barnett, 1971). The following list gives some media and incubation conditions suggested by researchers for the isolation of marine yeasts:
Wickerham's yeast malt medium (Wickerham, 1951): This medium is widely used for marine yeast isolation. It contains (w/v) 1% glucose, 0.3% yeast extract, 0.3% malt extract, 0.5% peptone and 2% agar. All the chemicals are prepared in seawater at a salinity equivalent to the sample site. About 200 mg L−1 of chloramphenicol was added into the medium prior to autoclaving and the final pH was adjusted to 7.0. Alternatively, an antibiotic mixture of penicillin G and streptomycin sulphate (each at 150–500 mg L−1) can be added to the autoclaved, cooled (below 45 °C) medium.
Chi et al. (2007) modified a liquid YPD Medium (2.0% glucose, 2.0% polypeptone and 1.0% yeast extract, w/v) by preparing the medium with natural seawater instead of fresh water. 0.05% (w/v) chloramphenicol was also added. This medium should be prepared immediately after sampling and cultivated at the natural temperature for 5 days.
Wang et al. (2007) prepared a seawater nutrient agar medium consisting of (w/v) 2.0% glucose, 2.0% peptone, 1.0% yeast extract, 2.0% agar. Components were dissolved in half-strength artificial seawater, and the pH of the medium was then adjusted to 4.5. The agar plates were incubated for 5 days at 20 °C. The composition of the artificial seawater was (per litre) as follows: NaCl, 20 g; KCl, 0.35 g; MgCl2·6H2O, 5.4 g; MgSO4·7H2O, 2.7 g; CaCl2·2H2O, 0.5 g.
Masuda et al. (2008) used an YPD agar, containing (w/v) 2.0% glucose, 2.0% peptone, 1.0% yeast extract and 2.0% agar, supplemented with 3.0% NaCl and 100 ng μL−1 of chloramphenicol at pH 6.0. The plates were incubated at 25 °C for 5–7 days.
Hernandez-Saavedra et al. (1995) used an isolation medium consists of (w/v) 2.0% glucose, 1.0% peptone, 5.0% yeast extract and 2.0% agar prepared in filtered seawater. The pH was adjusted to 4.5 with 0.1 N HCl.
Loureiro et al. (2005) used a modified Sabouraud's dextrose agar medium (0.5% peptic digest of animal tissue, 0.5% pancreatic digest of casein, 4.0% dextrose and 1.5% agar, w/v). They added yeast extract and chloramphenicol to the medium and incubated the plates at 28 ± 1 °C. The concentrations of yeast extract and chloramphenicol were not reported.
Dinesh et al. (2011) used Sabauroud's dextrose agar medium prepared in 50% seawater. The plates were incubated at 35 °C for 48 h.
Nagahama et al. (1999) prepared an YM agar medium from Difco, which was dissolved in artificial seawater (3% NaCl, 0.07% KCl, 1.08% MgCl2, 0.54% MgSO4, 0.1% CaCl2, w/v). This was then used for isolating yeast from a cold marine habitat. Medium was then supplemented with 0.01% (w/v) chloramphenicol and 0.002% (w/v) streptomycin. The plates were incubated at a low temperature (5–10 °C) for 2 weeks and then at 20 °C for 1 month.
Sarlin and Philip (2011) suggested a malt extract agar medium containing (w/v) 2.0% malt extract, 0.5% mycological peptone and 2.0% agar. It was suspended in around 50% diluted seawater at pH 6.
Kodama (1999) described a medium consisting of (w/v): sucrose 20%, polypeptone 3%, yeast extract 0.3%, chloramphenicol 100 mg L−1, agar 1.5%. The medium was prepared using filtered seawater, and the pH was adjusted to 5.6.
Khambhaty et al. (2013) suggested a method combining filtration followed by enrichment before isolation. The enrichment medium was GYP broth consisting of (w/v): glucose 1%, peptone 0.5%, yeast extract 0.5% and sodium chloride 2.5%. Enrichment was carried out for 24 h at 30 °C in a shaking incubator. A loopful of the suspension was spread on GYP plates consisting of (w/v): glucose 1%, peptone 0.5%, yeast extract 0.5%, sodium chloride 2.5% and agar 2.5%. The plates were incubated at 30 °C for 2–3 days.
We have recently developed a three-step method for marine yeast isolation. In the first step, samples were subjected to three cycles of enrichment using a nutrient rich media supplemented with a mixture of antibiotics (penicillin G 500 mg L−1 and streptomycin sulphate 500 mg L−1). The medium in the first and second cycles consist of 3.0% glucose, 3.0% xylose, 0.3% malt extract, 0.3% yeast extract, 0.5% peptone, 0.1% (NH4)2SO4, 0.05% KH2PO4. The medium composition for the last cycle was 6.0% of the desired carbon source (e.g. glucose or xylose or galactose), 0.3% yeast extract, 0.5% peptone, 0.1% (NH4)2SO4, 0.05% KH2PO4. All media were prepared using natural seawater and adjusted to pH 5. Each cycle of enrichment was carried out in a shaking incubator at 150 r.p.m., 30 °C for 48 h. In the second step, the culture from the final cycle of enrichment was incubated at 30 °C for 24–48 h on agar plates. In the third step, single colonies from step 2 were transfer to new agar plates and were checked for their morphology under the microscope.
The above-mentioned incubation conditions, including temperature, were suggested by the researchers and could be changed according to the experiment requirement. The growth on plates should be observed daily. Parts of any suspected yeast colonies should be picked up and transferred onto a microscope slide for inspection. Streak plate technique should be applied on confirmed yeast colonies using YPD seawater agar medium without antibiotics. Streak plate should be repeated to ensure the purity of the isolate. Colonies of interest can be transferred into a slant culture tube for further study.
Use of marine yeast for bioethanol production
Bioethanol and biodiesel are two important liquid biofuels. Bioethanol production has been increased worldwide gradually in the last decade to around 85 billion litres in 2011 (Pensupa et al., 2013). Due to the increasing demand on energy with an ever growing in world population and the limited supply of fossil fuels, the contribution of bioethanol is expected to increase further over the next decades. Currently, bioethanol production is carried out using media containing freshwater and terrestrial yeast strains. In the fermentation step, bioethanol production consumes 2.7–5.8 gallons of freshwater per gallon of bioethanol produced when corn is used as the carbohydrate substrate (Wu & Chiu, 2011). In a cellulosic to bioethanol process, it has been estimated that between 4.5 and 5.3 gallons of freshwater would be consumed to produce one gallon of bioethanol (Wu & Chiu, 2011). Seawater, which accounts for 97% of the world's water, can be a promising alternative water resource for bioethanol production in coastal cities, especially in the Middle East and other arid zones where fresh water is increasingly precious. Additionally, seawater contains a spectrum of minerals and as such may avoid the addition of essential nutrients currently required for commercial fermentation medium (Lin et al., 2011). Thus, using seawater in fermentations could potentially improve the overall economics of the process by both reducing the freshwater intake and producing freshwater through distillations in the biorefinery. Therefore, the development of seawater-based bioethanol strategies can certainly make a strong impact on overcoming both the fresh water and energy crises.
Over the last few decades, halo-tolerant yeasts have been investigated as promising alternative candidates for bioethanol production. Urano et al. (2001) isolated several marine yeasts from various aquatic environments. Most of these isolates belonged to two genera, namely Candida and Debaryomyces. These isolates were preliminary tested for their fermentation capabilities by observing gas production in media containing sodium chloride. But the production of ethanol was not reported. Limtong et al. (1998) hybridized Saccharomyces cerevisiae M30, a high ethanol producing strain, with Zygosaccharomyces rouxii TISTR1750, a halo-tolerant strain, using polyethylene glycol–induced protoplast fusion. Compared with the parental strains, one of the derived strains (Fusant RM11) exhibited higher ethanol producing capacity in terms of both ethanol concentration and ethanol yield, in salted glucose broth media containing 1.5%, 3%, 5% or 7% sodium chloride. Using the medium containing 18% glucose and 3% sodium chloride, the Fusant RM11 showed maximal ethanol production of 68.5 g L−1, while the parental strains, S. cerevisiae M30 and Z. rouxii TISTR1750, produced 65.0 and 63.6 g L−1 bioethanol, respectively. The fermentations were carried out at 30 °C for 60 h.
Kathiresan et al. (2011) isolated 10 marine yeast strains from mangrove sediments on the south-east coast of India. These isolated strains were Candida albicans, Candida tropicals, Debaryomyces hansenii, Geotrichum sp., Pichia capsulata, Pichia fermentans, Pichia salicaria, Rhodotorula minuta, Cryptococcus dimennae and Yarrowia lipolylica. They reported that Pichia salicaria was the best strain for ethanol production with 12.3 ± 0.8 g L−1 bioethanol from sawdust filtrates at 2% concentration after 120 h of incubation. When 2% sawdust hydrolysis (hydrolysed by dilute phosphoric acid) as the carbohydrate source, 26.2 ± 8.9 g L−1 bioethanol was produced by P. salicaria. Later, Senthilraja et al. (2011) reported that in fermentations using free cells, P. salicaria produced the highest ethanol concentration of 28.5 ± 4.32 g L−1 among these 10 isolates. When these yeast cells were immobilized in sodium alginate, improved ethanol production was observed in fermentations using all strains. Candida albicans exhibited the highest ethanol production of 47.3 ± 3.1 g L−1.
Obara et al. (2012) studied the bioethanol production from the hydrolysate of paper shredder scrap using a marine yeast isolated from Tokyo Bay. It was found that the marine yeast – S. cerevisiae – (strain C-19) showed high osmotic tolerance and high ethanol production. It produced 122.5 g L−1 of ethanol from a medium containing 297 g L−1 of glucose. The maximum bioethanol concentrations for the control strains, S. cerevisiae NBRC 10217 and S. cerevisiae K-7, were 37.5 and 98.5 g L−1, respectively. Moreover, the fermentation using the marine yeast C-19 reached peak ethanol production at day 3, while both control strains required 7 days to achieve their maximum bioethanol production. The high osmotic tolerance of the marine yeast strain was considered to contribute to its promising performance. As this strain belongs to S. cerevisiae species, it could be amenable to the existing genetic modification tools that developed on the basis of Saccharomyces sp. for further improvement.
Saravanakumar et al. (2013) compared bioethanol production using a marine S. cerevisiae strain and a terrestrial S. cerevisiae strain. In fermentations using the hydrolysate of sawdust as the substrate, the marine strains showed maximum ethanol production of 25.1 g L−1, while the terrestrial strain produced only 13.8 g L−1 ethanol.
Khambhaty et al. (2013) isolated a marine yeast strain (Candida sp.) from Veraval, on the west coast of India. This strain was able to convert galactose, sugar cane bagasse hydrolysate as well as the hydrolysate of a red seaweed Kappaphycus alvarezii into bioethanol under a wide range of pH (2.0–11.0) conditions and in the presence of high concentration of salts (2.5–15% w/v). Sugar cane bagasse hydrolysates were prepared using H2SO4 and HCl, resulting in 7.17% and 7.57% reducing sugar, respectively. Around 22.8 and 18.9 g L−1 ethanol were obtained, equating to conversion efficiencies of 66% and 55%, respectively. In a seaweed hydrolysate containing 5.5% reducing sugar with 11.25% salt concentration, around 12.3 g L−1 ethanol was produced after 72 h of incubation, representing 50% conversion efficiency. When the seaweed hydrolysate was diluted by freshwater with a ratio of 3 : 1 or 1 : 1, 100% carbohydrate conversions were observed within 48 h. Moreover, c. 21–24 g L−1 bioethanol was produced in fermentation using a GYE broth media containing 5% galactose in the presence of 0–10% of KCl, CaCl2 and NaCl.
Khambhaty et al. (2013) concluded that the presence of 2–13% salt benefited the growth of their isolate. Although fermentation efficiency was relatively low in a medium containing 11.25% salt, 100% fermentation efficiency could be achieved in fermentations using media containing 6.25–9% salt. Their isolate could also tolerate a wide range of pH from 4 up to 10 with very little growth difference. They claim that the pH and salt tolerance of the marine yeast made it a promising candidate for fermentations under different environmental conditions. Khambhaty's findings were in line with a study conducted by Gupta (1996) who reported that various species of yeasts such as Debaryomyces, Rhodotorula, Candida and Saccharomyces could tolerate high concentration of NaCl up to 16%. In addition, yeasts that could tolerate NaCl up to 3.5 M (20.5%) have also been reported (Kutty & Philip, 2008).
Various biological materials have been investigated for the generation of bioethanol, such as wheat straw (Pensupa et al., 2013), sugarcane bagasse (Chandel et al., 2013) and corn stover (Bondesson et al., 2013). Recently, various marine biomass sources, for example seaweed (Khambhaty et al., 2013) and sea lettuce (Yanagisawa et al., 2011), have attracted increasing attention as a promising nonfood material for bioethanol production, as they do not compete with edible crops in terms of land and freshwater resources. The hydrolysis of marine biomass could result in a salty hydrolysate, which would require desalting (e.g. electrodialysis) before fermentation when terrestrial yeasts are used (Mody et al., 2013). However, halophilic yeasts, especially yeasts isolated from a marine environment, would be able to directly ferment the salty hydrolysate to bioethanol (Khambhaty et al., 2013). Therefore, the energy intensive step, desalting, could be avoided, making the whole fermentation process more economically competitive (Khambhaty et al., 2013). Table1 compares the bioethanol produced using various yeast strains isolated from the marine environment and their respective fermentation conditions.
Table 1.
Ethanol production by marine yeasts
| References | Yeast name | Isolation source | Substrate | Hydrolysis method | Fermentation condition (sugar conc., temp., incubation time) | Ethanol conc. g L−1 |
|---|---|---|---|---|---|---|
| Khambhaty et al. (2013) | Candida sp. | Veraval, the West coast of India | Seaweed | 2.5% H2SO4, cooked at 100 °C for 1 h | 3.77% sugar, 30 °C, 48 h | 17.6 |
| Sugarcane Bagasse | 2.28% sugar, 30 °C, 48 h | 7.7* | ||||
| Galactose | N/A | 5% galactose, 30 °C, 0–10% of KCl, 24 h | 21–24 | |||
| Saravanakumar et al. (2013) | S. cerevisiae | Mangrove soil, southeast coast of India | Sawdust | 0.8% H3PO4 | 6.84 mg L−1 sawdust, 30 °C, 89 h | 0.0024* |
| Obara et al. (2012) | S. cerevisiae | Tokyo Bay, Japan | Paper shredder scrap | 3% H2SO4 at 121 °C for 1 h then enzymatic saccharification | 29.7% of glucose from paper shredder scrap, 30 °C, 72 h | 122.5 |
| Enzymatic saccharification only (cellulase for 2 days at 50 °C and 150 r.p.m.) | ||||||
| Immobilized | 13 | |||||
| Sawdust | NaOH 4% at 121 °C for 30 min | 2% of sawdust, 28 °C, 120 r.p.m. for 72 h | 7.6 | |||
| Kathiresan et al. (2011) Senthilraja et al. (2011) | Candida albicans, C. tropicals, D. hansenii, Geotrichum sp., Pichia capsulata, Pichia fermentans, Pichia salicaria, R. minuta, C. dimennae and Y. lipolytica | Sediments, southeast coast of India | Glucose | N/A | 28 °C, 120 r.p.m. for 96 h. Nonimmobilized 28 °C, 120 r.p.m. for 96 h. Immobilized |
9.8–28.5 13–47.3 |
| Sawdust | NaOH 4% at 121 °C for 30 min | 2% of sawdust, 28 °C, 120 r.p.m. for 72 h | 1.7–12.3 |
No ethanol concentration was reported in the original papers. This value was estimated based on the conversation efficiencies reported by the references.
The recent research has shown great potential of marine yeasts in bioethanol production. But more investigation should be conducted to further demonstrate the benefits of using marine yeasts in bioethanol industry, especially in bioethanol fermentations using marine biomass based substrate. Subsequently, more marine yeasts should be isolated to explore their potential. The isolates should be selected based on their capability of utilizing and fermenting a wide range of sugars that presented in marine biomass hydrolysate, including galactose, xylose, mannitol and fucose. Also, the isolates should have high tolerance capacity to salts and inhibitors that may be generated during the hydrolysis of marine biomass.
Use of marine yeast for the production of pharmaceutical products
Several terrestrial yeasts, such as S. cerevisiae and Pichia pastoris, have been developed as hosts for the commercial production of pharmaceutical proteins, such as insulin, hepatitis B vaccines and caseinomacropeptide (Du & Webb, 2011). Similarly, several marine yeast species, such as Yarrowia sp. and Candida sp. have been reported to be able to synthesize different pharmaceutical products, as shown in Table2. In comparison with terrestrial yeasts, the investigation on marine yeasts for the pharmaceutical production is still at its early stage. This review will discuss three examples; astaxanthin, siderophore and riboflavin, as they are the most widely explored pharmaceutical products that could be produced using marine yeasts.
Table 2.
Products of industrial marine yeast and their applications
| Products | Marine yeasts species | Applications | Source |
|---|---|---|---|
| Fuels | |||
| Bio-ethanol | Candida albicans, Candida tropicals, Debaryomyces hansenii, Geotrichum sp., Pichia capsulata, Pichia fermentans, Pichia salicaria, Rhodotorula minuta, Cryptococcus dimennae and Yarrowia lipolytica | Biofuel industries | Kathiresan et al. (2011) |
| Saccharomyces cerevisiae C-19 | Obara et al. (2012) | ||
| Candida sp. | Khambhaty et al. (2013) | ||
| Microbial oil | Rhodotorula mucilaginosa | Biodiesel industries | Li et al. (2010a, b) |
| Yarrowia lipolytica | Katre et al. (2012) | ||
| Pharmaceuticals | |||
| Alginate lyase | Yarrowia lipolytica | Pharmaceutical industries | Liu et al. (2009) |
| Exo-β-1,3 glucanase | Williopsis saturnus WC91-2 | Pharmaceutical industries | Peng et al. (2009, 2011) |
| Riboflavin | C. membranifaciens subsp. flavinogenie | Food and pharmaceutical industries | Wang et al. (2008) |
| Candida tropicalis | Amornrattanapan (2013) | ||
| Silver nanoparticles | Yarrowia lipolytica NCYC 789 | Antimicrobial | Apte et al. (2013) |
| Copper-zinc superoxide dismutase | Debaryomyces hansenii | Anticancer | Hernandez-Saavedra & Ochoa (1999) |
| Industrial enzymes | |||
| Lipase | Aureobasidium pullulans | Chemical industries | Zhang & Chi (2007) |
| Leucosporidium scottii Cryptococcus adeliensis | Duarte et al. (2013) | ||
| Cellulase | Aureobasidium pullulans | Chi et al. (2009) | |
| Pichia salicaria | Kathiresan et al. (2011) | ||
| Inulinase | Pichia guilliermondii | Food and fuel industries | Chi et al. (2009) |
| Acid protease | Metschnikowia reukaufii W6b | Food and pharmaceutical industries | Li et al. (2010a, b) |
| Protease | Rhodotorula mucilaginosa | Feed industries | Duarte et al. (2013) |
| α Glucosidases | Leucosporidium antaracticum | Pharmaceutical industries | Turkiewicz et al. (2005) |
| Endoxylanase | Candida davisiana, Cryptococcus adeliensis, Guehomyces pullulans | Chemical industries | Duarte et al. (2013) |
| Phytase | Kodamea ohmeri | Feed industries | Li et al. (2008) |
| Other products | |||
| Silver nanoparticles | Candida albicans, C. tropicals, Debaryomyces hansenii, Geotrichum sp., Pichia capsulata, Pichia fermentans, Pichia salicaria, Rhodotorula minuta, Cryptococcus dimennae and Yarrowia lipolylica | Biomaterial industry | Subramanian et al. (2010) |
| Nanoparticles | Yarrowia lipolytica | Agnihotri et al. (2009) | |
| Degrader of pollutants | Yarrowia lipolytica | Bioremediation | Bankar et al. (2009) |
| Degrader of pollutants | Yarrowia lipolytica | Hydrocarbon degradation | Oswal et al. (2002) |
| Viable cells | Yarrowia lipolytica | Bioremediation of TNT-polluted marine environments | Jain et al. (2004) |
| Single-cell protein | Cryptococcus aureus, Yarrowia lipolytica | Food and feed industries | Zhang et al. (2009a, b) |
| Cryptococcus aureus G7a | Gao et al. (2007a, b) | ||
| Carotene | Rhodotorula sp. | Food colouring | Cong et al. (2007) |
| Rhodotorula mucilaginosa, Arxula adeninivorans | Libkind et al. (2004) | ||
Astaxanthin is a carotenoid compound, responsible for the orange-red colour of some living organisms. It is the main carotenoid used in the aquaculture industry worldwide (Higuera-Ciapara et al., 2006). It has been reported that astaxanthin has a wide range of pharmacological properties including antioxidant and antimicrobial activity and reducing risk of certain cancers and cardiovascular diseases (Neuman et al., 1999; Ushakumari & Ramanujan, 2013). In addition, astaxanthin can enhance the immune response to viral, bacterial, fungal and parasitic infections as well as reducing the risk of cataracts, atherosclerosis and macular degeneration (Cooper et al., 1999).
Roche began the large-scale production of synthetic astaxanthin in 1990. However, an ever-growing demand for natural foods and the high cost of synthesizing the pigment have stimulated research into alternative natural sources of astaxanthin (Higuera-Ciapara et al., 2006). A marine yeast strain identified as Rhodotorula glutinis YS-185, isolated from the Pacific Ocean by He and his team, was found to be capable of producing astaxanthin (He et al., 2011). The optimum fermentation conditions for astaxanthin production were 25 °C, using a medium consisting of only glucose (8 g L−1) and peptone (8 g L−1) with an initial pH of 5.5. Temperature was reported to be a key factor for both astaxanthin production and yeast growth. An astaxanthin concentration of 2.67 μg mL−1 was achieved, which was 69.7% higher than that before the fermentation optimization. Ushakumari and Ramanujan (2013) isolated a marine yeast strain from the marine sediments collected from Kerala, India. The astaxanthin was extracted and tested for its activity against Bacillus subtilis, Salmonella typhi, Staphylococcus aureus and Pseudomonas aeruginosa. The astaxanthin solution extracted from fermentations using their marine yeast isolate exhibited better antibacterial activity than the standard chloramphenicol.
Siderophores are low-molecular-weight ligands (500–1000 Da), which have extremely high affinity as iron-chelating agents. They are synthesized by many microorganisms during extreme iron deficiency conditions to facilitate the solubilization of extracellular ferric iron (Winkelmann, 2002; Wang et al., 2009). Siderophores have wide medical, agricultural and environmental applications (Renshaw et al., 2002). For example, the ability of using siderophores could give the microorganism a competitive advantage over other microorganisms as the siderophore transport system will enable the microorganism to compete effectively for the available irons (Renshaw et al., 2002; Murugappan et al., 2012). Siderophores was also used to control the growth of some pathogenic bacteria isolated from infected marine fish (Renshaw et al., 2002).
Wang et al. (2009) isolated more than 300 yeast strains from different marine environments and screened them for their abilities to produce siderophore. Among these isolates, only one strain was found to produce high level of the siderophore. This strain was identified as Aureobasidium pullulans (black yeast). Under optimal conditions, the strain could produce 1.1 mg mL−1 of crude siderophore. The crude siderophore extract was able to inhibit the growth of Vibrio anguillarum and Vibrio parahaemolyticus that were isolated from sick marine animals.
In similar research, Murugappan et al. (2012) obtained 0.7 mg mL−1 crude siderophore after 134 h of fermentation using a marine yeast strain (A. pullulans). This strain was isolated from seaweed (Ulva fasciata) samples that were collected from the coastal region of the Gulf of Mannar, India.
Riboflavin (vitamin B2) is required by all bacteria, plants, animals and human beings. It serves as a precursor for two coenzymes: flavin mononucleotide and flavin adenine dinucleotide. Riboflavin can be synthesized only by plants and microorganisms, while human and other animals must acquire it from their diets (Chi et al., 2008). Although chemical synthesis processes still dominate the market, the biosynthesis of riboflavin offers several distinctive advantages, such as lower energy needs, less chemical waste generated and easier recovery (Stahmann et al., 2000).
So far several terrestrial yeast species have been investigated for the riboflavin biosynthesis, such as Pichia guilliermondii (Leathers & Gupta, 1997; Faiura et al., 2007) and Candida famata (Stahmann et al., 2000; Boretsky et al., 2005). Leathers and Gupta (1997) found that P. guilliermondii could produce up to 14.4 μg mL−1 of riboflavin when xylose was used as the carbon source, while no more than 5.1 μg mL−1 of riboflavin could be produced when glucose replaced the xylose. Furthermore, Boretsky et al. (2005) showed that some types of yeast (such as C. famata and P. guilliermondii) can only overproduce riboflavin in media deficient in iron because iron represses riboflavin synthesis.
Wang et al. (2008) isolated a marine yeast strain from seawater from the China Eastern Sea, which was identified as Candida membranifaciens subsp. flavinogenie. This strain produced 16.3 μg mL−1 of riboflavin using an iron-deficient medium, while it produced only 0.1 μg mL−1 of riboflavin when 0.005% FeCl3 was added. Further investigation showed that the riboflavin synthesis was enhanced by vigorous shaking during cultivation in medium containing galactose, maltose, sucrose or xylose. Around 22 μg mL−1 of riboflavin was achieved within 54 h of fermentation under these optimal conditions. A recent study carried out by Amornrattanapan (2013) isolated 47 marine yeast strains for the screening of their capacity of riboflavin production. Among those isolates, the best strain Candida tropicalis MICBUU002 produced 254.22 μg mL−1 riboflavin, which was more than 10-fold higher than that reported by Wang et al. (2008). The strain was cultured at 30 °C for 5 days using 2% glucose as the only carbon source.
Use of marine yeast for industrial enzymes production
As for terrestrial yeasts, marine yeasts (e.g. Aureobasidium sp. and Pichia sp.) have also been investigated for the production of enzymes, such as inulinase (Chi et al., 2009), amylase (Li et al., 2007a, b), superoxide dismutase (Ramirez-Orozco et al., 1998), and lipase (Chi et al., 2009). As marine yeasts live in high salinity environments, these enzymes are expected to have distinct properties, for example high salt tolerance, thermostability, barophilicity and cold adaptivity (Sarkar et al., 2010). In this mini review, inulinase and amylase were used as examples for the demonstration of marine yeast–based enzyme production. Other case studies were listed in Table2 and parts of them were also described by Chi et al. (2009) and Sarkar et al. (2010).
Inulinase catalyses the hydrolysis of inulin to fructose. Inulin is found in many types of plants, such as Jerusalem artichoke, dahlia tubers or chicory root (Baheri et al., 2001; Liu et al., 2003; Sarkar et al., 2010), it is a polymer formed of linear (β-1,2)-linked fructose. It could be used as food additive in food applications, or as feedstock in the biofuel and pharmaceutical industries (Chi et al., 2011). Inulin can be hydrolysed chemically; however, the chemical process is associated with the formation of undesired by-products, such as di-fructose anhydrides (Gill et al., 2006), while enzymatic hydrolysis of inulin yields 95% pure fructose (Gao et al., 2007a, b; Bharathi et al., 2011).
Many yeast species can produce inulinases, including Candida sp., Kluyveromyces sp., Pichia sp. and Sporotrichum sp. (Pandey et al., 1999). Gao et al. (2007a, b) screened out four marine yeast strains from 427 yeast isolates obtained from different marine habitats, including guts of marine fish, marine algae, seawater, sea sediments and salterns, for the production of inulinase. These strains were identified as P. guilliermondii, Cryptococcus aureus, Yarrowia lipolytica and D. hansenii. A maximum inulinase activity of 62.85 U mL−1 was recorded in the fermentation using the yeast strain Y. lipolytica. However, no mono- or disaccharides were detected after inulin hydrolysis using crude inulinase produced by Y. lipolytica, indicating that this crude inulinase had no exo-inulinase activity. High exo-inulinase activity was, however, detected in the crude enzyme extract obtained from the fermentation of P. guilliermondii, C. aureus and D. hansenii strains (Gao et al. 2007a, b). Later, the inulinase gene of the marine yeast P. guilliermondii was successfully cloned and expressed in P. pastoris X-33 (Zhang et al., 2009a, b). The inulinase activity achieved in fermentations using the recombinant strain reached 286.8 U mL−1. Also, high exo-inulinase activity was detected in the purified recombinant enzyme (Zhang et al., 2009a, b). The inulin hydrolysate, catalysed by the recombinant inulinase, was converted into bioethanol using Saccharomyces sp., and the ethanol concentration achieved was 140 g L−1 (Wang et al., 2013). Although inulinase production has not been commercialized, these findings indicated that the recombinant inulinase might have potential to be used in the biofuel industry in the future.
Bharathi et al. (2011) isolated a marine yeast strain (SY3) from the gut of the fish Lutjanus campechanus and identified it as Cryptococcus sp. Fermentations with the SY3 strain were carried out in shake flasks using YPD media. After partial purification of the enzyme solution by dialysis, the inulinase activity reached 62.7 U mL−1. The optimal condition for inulinase production by this strain was pH 4.0 and 37 °C using a medium consisting of 4.0% (w/v) inulin and 0.5% (w/v) yeast extract.
Amylases have been widely used in baking, food, pharmaceutical, detergent, textile and biofuel industries for the hydrolysis of starch. It has been reported that several terrestrial yeasts are able to produce extracellular amylolytic enzymes, for example Arxula adeninivorans, Candida japonica and Saccharomycopsis fibuligera (Chi et al., 2003). Similarly, Li et al. (2007a, b) have investigated crude glucoamylase production using a marine yeast strain A. pullulans N13d, which was isolated from the deep sea of the Pacific Ocean. It was found that the highest amylase yield was achieved in the late stationary phase of cell growth. Within 56 h of fermentation, 58.5 units mg protein−1 of amylase were produced under optimal condition (Li et al., 2007a, b). This enzyme was tested for its ability to hydrolyse potato starch, raw corn starch and sweet potato starch, respectively. The amylase demonstrated good hydrolytic ability on potato starch (with a yield of 68.5% within 6 h) but not on corn or sweet potato starch (Li et al., 2007a, b). In a solid-state fermentation using a marine yeast S. fibuligera A11, an amylase activity of 4296 U g−1 of dry substrate was obtained (Chen et al., 2010). The substrate contained 610.0 mL kg−1 moisture, 30.0 mL kg−1 inoculums (OD600 = 20.0), wheat bran to rice husk ratio 0.42, cassava starch 20.0 g kg−1. Then, S. fibuligera was immobilized and co-cultured with oleaginous yeast Rhodosporidium toruloides (Gen et al., 2014). The yeast strain produced amylase, which converted cassava starch to glucose, while the oleaginous yeast consumed the resulting glucose to accumulate single-cell oil. In 2-L scale fermentation, a single-cell oil yield of 64.9% (w/w) was obtained from a medium containing 60 g L−1 cassava starch.
Besides bioenergy, pharmaceutical and enzyme production, marine yeasts have also showed potential to be utilized in various other fields, such as synthesis of metal nanoparticles (Turkiewicz et al., 2005; Li et al., 2010a, b), degradation of pollutants (Agnihotri et al., 2009; Bankar et al., 2009; Subramanian et al., 2010) and use as a probiotics in marine animal culture. Several case studies are summarized in Table2.
Conclusions
Marine yeasts live in harsh environments, which provide the potential for several unique desirable properties to be used in various industries. The latest development in the methodology of marine yeast isolation and cultivation offers the opportunity of discovering novel marine yeasts. Various media have been proposed by different research groups to suit for the different requirement of marine yeasts. These media are rich in nutrients, and they are common to contain antibiotics to reduce the bacterial and mould contamination. Using marine yeasts in bioethanol production shows distinctive advantage on the osmosis tolerance, the possibility of utilization of seawater instead of fresh water and the potential of advantage in using marine biomass as a substrate. Marine yeasts have already been investigated for the production of pharmaceutical and enzymatic products, such as astaxanthin, siderophore, riboflavin, inulinase and amylases. Yet, the commercial application of marine yeasts is still limited. The current research, however, indicates the promising features of the marine yeasts for the potential industrial application and their superiority over the terrestrial ones in certain field. More direct comparison studies should be carried out to give further evidence on the advantages of marine yeasts over terrestrial yeasts.
Acknowledgments
The authors gratefully acknowledge the financial supported by the Ministry of Higher Education of Egypt and Egyptian Cultural and Educational Bureau in London for Mr. Abdelrahman S. Zaky's Scholarship (JS57/12). We also thank the Biotechnology and Biological Sciences Research Council (BBSRC) Sustainable Bioenergy Centre (BSBEC), under the programme for Lignocellulosic Conversion to Ethanol (LACE) (BB/G01616X/1) for supporting this research.
References
- Agnihotri M, Joshi S, Kumar AR, Zinjarde S. Kulkarni S. Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater Lett. 2009;63:1231–1234. [Google Scholar]
- Amornrattanapan P. Riboflavin production by Candida tropicalis isolated from seawater. Sci Res Essays. 2013;8:43–47. [Google Scholar]
- Apte M, Sambre D, Gaikawad S, Joshi S, Bankar A, Kumar AR. Zinjarde S. Psychrotrophic yeast Yarrowia lipolytica NCYC 789 mediates the synthesis of antimicrobial silver nanoparticles via cell-associated melanin. AMB Express. 2013;3:32. doi: 10.1186/2191-0855-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baheri HR, Hill GA. Roesler WJ. Modelling plasmid instability in batch and continuous fermentors. Biochem Eng J. 2001;8:45–50. doi: 10.1016/s1369-703x(00)00135-2. [DOI] [PubMed] [Google Scholar]
- Bankar A, Kumar A. Zinjarde S. Environmental and industrial applications of Yarrowia lipolytica. Appl Microbiol Biotechnol. 2009;84:847–865. doi: 10.1007/s00253-009-2156-8. [DOI] [PubMed] [Google Scholar]
- Beuchat LR. Comparison of acidified and antibiotic-supplemented potato dextrose agar from three manufacturers for its capacity to recover fungi from foods. J Food Prot. 1979;42:427–428. doi: 10.4315/0362-028X-42.5.427. [DOI] [PubMed] [Google Scholar]
- Bharathi S, Saravanan D, Radhakrishnan M. Balagurunathan R. Bioprospecting of marine yeast with special reference to inulinase production. Int J ChemTech Res. 2011;3:1514–1519. [Google Scholar]
- Bondesson PM, Galbe M. Zacchi G. Ethanol and biogas production after steam pretreatment of corn stover with or without the addition of sulphuric acid. Biotechnol Biofuels. 2013;6:11. doi: 10.1186/1754-6834-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boretsky YR, Kapustyak KY, Fayura LR, Stasyk OV, Stenchuk MM, Bobak YP, Drobot LB. Sibirny AA. Positive selection of mutants defective in transcriptional repression of riboflavin synthesis by iron in the flavinogenic yeast Pichia guilliermondii. FEMS Yeast Res. 2005;5:829–837. doi: 10.1016/j.femsyr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Bowen JF. Beech FW. Yeast flora of cider factories. J Appl Bacteriol. 1967;30:475–483. [Google Scholar]
- Buhagiar RWM. Barnett JA. The yeasts of strawberries. J Appl Bacteriol. 1971;34:727–739. doi: 10.1111/j.1365-2672.1971.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Chandel AK, Antunes FA, Silva MB. da Silva SS. Unraveling the structure of sugarcane bagasse after soaking in concentrated aqueous ammonia (SCAA) and ethanol production by ScheffersomycesPichiastipitis. Biotechnol Biofuels. 2013;6:102. doi: 10.1186/1754-6834-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chi ZM, Chi Z. Li M. Amylase production by Saccharomycopsis fibuligera A11 in solid-state fermentation for hydrolysis of Cassava starch. Appl Biochem Biotechnol. 2010;162:252–263. doi: 10.1007/s12010-009-8744-3. [DOI] [PubMed] [Google Scholar]
- Chi Z, Liu J, Ji J. Meng Z. Enhanced conversion of soluble starch to trehalose by a mutant of Saccharomycopsis fibuligera sdu. J Biotechnol. 2003;102:135–141. doi: 10.1016/s0168-1656(03)00021-x. [DOI] [PubMed] [Google Scholar]
- Chi Z, Ma C, Wang P. Li HF. Optimization of medium and cultivation conditions for alkaline protease production by the marine yeast Aureobasidium pullulans. Bioresour Technol. 2007;98:534–538. doi: 10.1016/j.biortech.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Chi Z, Wang L, Ju L. Chi Z. Optimisation of riboflavin production by the marine yeast Candida membranifaciens subsp. flavinogenie W14-3 using response surface methodology. Ann Microbiol. 2008;58:677–681. [Google Scholar]
- Chi Z, Chi Z, Zhang T, Liu G, Li J. Wang X. Production, characterization and gene cloning of the extracellular enzymes from the marine-derived yeasts and their potential applications. Biotechnol Adv. 2009;27:236–255. doi: 10.1016/j.biotechadv.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Chi ZM, Liu G, Zhao S, Li J. Peng Y. Marine yeasts as biocontrol agents and producers of bio-products. Appl Microbiol Biotechnol. 2010;86:1227–1241. doi: 10.1007/s00253-010-2483-9. [DOI] [PubMed] [Google Scholar]
- Chi Z-M, Zhang T, Cao T-S, Liu X-Y, Cui W. Zhao C-H. Biotechnological potential of inulin for bioprocesses. Bioresour Technol. 2011;102:4295–4303. doi: 10.1016/j.biortech.2010.12.086. [DOI] [PubMed] [Google Scholar]
- Cong L, Chi Z, Li J. Wang X. Enhanced carotenoid production by a mutant of the marine yeast Rhodotorula sp. hidai. J Ocean Univ China. 2007;6:66–71. [Google Scholar]
- Connell L, Redman R, Craig S, Scorzetti G, Iszard M. Rodriguez R. Diversity of soil yeasts isolated from South Victoria Land, Antarctica. Microbiol Ecol. 2008;56:448–459. doi: 10.1007/s00248-008-9363-1. [DOI] [PubMed] [Google Scholar]
- Cooper DA, Eldridge AL. Peters JC. Dietary carotenoids and certain cancers, heart disease, and age-related macular degeneration: a review of recent research. Nutr Rev. 1999;57:201–214. doi: 10.1111/j.1753-4887.1999.tb06944.x. [DOI] [PubMed] [Google Scholar]
- Dinesh KS, Karthik L, Gaurav K. Bhaskara Rao KV. Biosynthesis of silver nanoparticles from marine yeast and their antimicrobial activity against multidrug resistant pathogens. Pharmacologyonline. 2011;3:1100–1111. [Google Scholar]
- Dorschel B. Deep marine sampling: strategies, devices and techniques an overview. 2007. Available at: http://www.searchmesh.net/pdf/SWDorschel.pdf.
- Du C. Webb C. Cellular systems. In: Murray MY, editor; Comprehensive Biotechnology. Burlington, VT: Academic Press; 2011. pp. 11–23. 2nd edn Vol. 2, [Google Scholar]
- Duarte AWF, Dayo-Owoyemi I, Nobre FS, Pagnocca FC, Chaud LCS, Pessoa A, Felipe MGA. Sette LD. Taxonomic assessment and enzymes production by yeasts isolated from marine and terrestrial Antarctic samples. Extremophiles. 2013;17:1023–1035. doi: 10.1007/s00792-013-0584-y. [DOI] [PubMed] [Google Scholar]
- Faiura LR, Fedorovich DV, Prokopiv TM, Boretskii IUR. Sibirnyi AA. The pleiotropic nature of rib80, hit1, and red6 mutations affecting riboflavin biosynthesis in the yeast Pichia guilliermondii. Mikrobiologiia. 2007;76:66–71. [PubMed] [Google Scholar]
- Fell JW. Collection and identification of marine yeasts. In: John HP, editor. Methods in Microbiology. Burlington, VT: Academic Press; 2001. pp. 347–356. Vol. 30. [Google Scholar]
- Gao L, Chi Z, Sheng J, Ni X. Wang L. Single-cell protein production from Jerusalem artichoke extract by a recently isolated marine yeast Cryptococcus aureus G7a and its nutritive analysis. Appl Microbiol Biotechnol. 2007a;77:825–832. doi: 10.1007/s00253-007-1210-7. [DOI] [PubMed] [Google Scholar]
- Gao L, Chi Z, Sheng J, Wang L, Li J. Gong F. Inulinase-producing marine yeasts: evaluation of their diversity and inulin hydrolysis by their crude enzymes. Microb Ecol. 2007b;54:722–729. doi: 10.1007/s00248-007-9231-4. [DOI] [PubMed] [Google Scholar]
- Gen Q, Wang Q. Chi Z-M. Direct conversion of cassava starch into single cell oil by co-cultures of the oleaginous yeast Rhodosporidium toruloides and immobilized amylases-producing yeast Saccharomycopsis fibuligera. Renew Energy. 2014;62:522–526. [Google Scholar]
- Gill PK, Manhas RK. Singh P. Purification and properties of a heat-stable exoinulinase isoform from Aspergillus fumigatus. Bioresour Technol. 2006;97:894–902. doi: 10.1016/j.biortech.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Gupta R. Growth of marine yeast on different strength of stress solutes. In: Agadi VV, editor; Pillai VK, Abidi SAH, Ravindranathan V, Balachandran KK, editors. Proceedings of Second Workshop on Scientific Results of FORV Sagar Sampada. New Delhi, India: Department of Ocean Development; 1996. pp. 91–95. [Google Scholar]
- He L, Liu J, Qin S, Yu . Sun M. Identification of an astaxanthin-producing marine yeast strain YS-185 and optimization of its fermentation conditions. 2011. CNKI Abstract. Available at: http://en.cnki.com.cn/Article_en/CJFDTotal-HYSC201104017.htm.
- Hernandez-Saavedra NY. Ochoa JL. Copper-zinc superoxide dismutase from the marine yeast Debaryomyces hansenii. Yeast. 1999;15:657–668. doi: 10.1002/(SICI)1097-0061(19990615)15:8<657::AID-YEA410>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Hernandez-Saavedra NY, Ochoa JL. Vazquez-Dulhalt R. Osmotic adjustment in marine yeast. J Plankton Res. 1995;17:59–69. [Google Scholar]
- Higuera-Ciapara I, Felix-Valenzuela L. Goycoolea FM. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- Jain MR, Zinjarde SS, Deobagkar DD. Deobagkar DN. 2,4,6-Trinitrotoluene transformation by a tropical marine yeast, Yarrowia lipolytica NCIM 3589. Mar Pollut Bull. 2004;49:783–788. doi: 10.1016/j.marpolbul.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Jannasch HW, Wirsen CO. Winget CL. A bacteriological pressure-retaining deep-sea sampler and culture vessel. Deep Sea Res Oceanogr Abstr. 1973;20:661–662. IN663, 663-664. [Google Scholar]
- Jarvis B. Comparison of an improved rose Bengal-chlortetracycline agar with other media for the selective isolation and enumeration of moulds and yeasts in foods. J Appl Bacteriol. 1973;36:723–727. doi: 10.1111/j.1365-2672.1973.tb04157.x. [DOI] [PubMed] [Google Scholar]
- Kathiresan K, Saravanakumar K. Senthilraja P. Bio-ethanol production by marine yeasts isolated from coastal mangrove sediment. Int Multidiscip Res J. 2011;1:19–24. [Google Scholar]
- Katre G, Joshi C, Khot M, Zinjarde S. Ravikumar A. Evaluation of single cell oil (SCO) from a tropical marine yeast Yarrowia lipolytica NCIM 3589 as a potential feedstock for biodiesel. AMB Express. 2012;2:36. doi: 10.1186/2191-0855-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khambhaty Y, Upadhyay D, Kriplani Y, Joshi N, Mody K. Gandhi MR. Bioethanol from macroalgal biomass: utilization of marine yeast for production of the same. Bioenergy Res. 2013;6:188–195. [Google Scholar]
- King AD, Hocking AD. Pitt JI. Dichloran-rose Bengal medium for enumeration and isolation of molds from foods. Appl Environ Microbiol. 1979;37:959–964. doi: 10.1128/aem.37.5.959-964.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama K. Isolation of Saccharomyces cerevisiae from the marine environment and their applications. J Brew Soc Japan. 1999;94:879–883. [Google Scholar]
- Kohlmeyer J. Kohlmeyer E. Marine Micology: The Higher Fungi. New York: Academic Press; 1979. [Google Scholar]
- Kutty SN. Marine yeasts from the slope sediments of Arabian Sea and Bay of Bengal. Cochin, India: Cochin University of Science and Technology; 2009. PhD Thesis, [Google Scholar]
- Kutty SN. Philip R. Marine yeasts – a review. Yeast. 2008;25:465–483. doi: 10.1002/yea.1599. [DOI] [PubMed] [Google Scholar]
- Leathers TD. Gupta SC. Xylitol and riboflavin accumulation in xylose-grown cultures of Pichia guilliermondii. Appl Microbiol Biotechnol. 1997;47:58–61. [Google Scholar]
- Li H, Chi Z, Duan X, Wang L, Sheng J. Wu L. Glucoamylase production by the marine yeast Aureobasidium pullulans N13d and hydrolysis of potato starch granules by the enzyme. Process Biochem. 2007a;42:462–465. [Google Scholar]
- Li H, Chi Z, Wang X. Ma C. Amylase production by the marine yeast Aureobasidium pullulans N13d. J Ocean Univ China. 2007b;6:60–65. [Google Scholar]
- Li XY, Chi ZM, Liu ZQ, Yan KR. Li HJ. Phytase production by a marine yeast Kodamea ohmeri BG3. Appl Biochem Biotechnol. 2008;149:183–193. doi: 10.1007/s12010-007-8099-6. [DOI] [PubMed] [Google Scholar]
- Li J, Peng Y, Wang X. Chi Z. Optimum production and characterization of an acid protease from marine yeast Metschnikowia reukaufii W6b. J Ocean Univ China. 2010a;9:359–364. [Google Scholar]
- Li M, Liu G-L, Chi Z. Chi Z-M. Single cell oil production from hydrolysate of cassava starch by marine-derived yeast Rhodotorula mucilaginosa TJY15a. Biomass Bioenergy. 2010b;34:101–107. [Google Scholar]
- Libkind D, Brizzio S. Broock M. Rhodotorula mucilaginosa, a carotenoid producing yeast strain from a patagonian high-altitude lake. Folia Microbiol. 2004;49:19–25. doi: 10.1007/BF02931640. [DOI] [PubMed] [Google Scholar]
- Limtong S, Deejing S, Yongmanitchai W. Santisopasri W. Construction of high ethanol fermenting halotolerant hybrid by intergeneric protoplast fusion of Saccharomyces cerevisiae and Zygosaccharomyces rouxii. Kasetsart J. 1998;32:213–223. [Google Scholar]
- Lin CSK, Luque R, Clark JH, Webb C. Du C. A seawater-based biorefining strategy for fermentative production and chemical transformations of succinic acid. Energy Environ Sci. 2011;4:1471–1479. [Google Scholar]
- Liu B-L, Kao P-M, Tzeng Y-M. Feng K-C. Production of chitinase from Verticillium lecanii F091 using submerged fermentation. Enzyme Microb Technol. 2003;33:410–415. [Google Scholar]
- Liu G, Yue L, Chi Z, Yu W, Chi Z. Madzak C. The surface display of the alginate lyase on the cells of Yarrowia lipolytica for hydrolysis of alginate. Mar Biotechnol. 2009;11:619–626. doi: 10.1007/s10126-009-9178-1. [DOI] [PubMed] [Google Scholar]
- Loureiro STA, Cavalcanti MADQ, Neves RP. Passavante JZDO. Yeasts isolated from sand and sea water in beaches of Olinda, Pernambuco state, Brazil. Braz J Microbiol. 2005;36:333–337. [Google Scholar]
- Masuda K, Guo XF, Uryu N, Hagiwara T. Watabe S. Isolation of marine yeasts collected from the Pacific Ocean showing a high production of gamma-aminobutyric acid. Biosci Biotechnol Biochem. 2008;72:3265–3272. doi: 10.1271/bbb.80544. [DOI] [PubMed] [Google Scholar]
- Mody KH, Ghosh PK, Sana B, et al. Process for integrated production of ethanol and seaweed sap from kappaphycus alverezii. 2013. Patent: US20130005009 A1.
- Murugappan R, Karthikeyan M, Aravinth A. Alamelu M. Siderophore-mediated iron uptake promotes yeast-bacterial symbiosis. Appl Biochem Biotechnol. 2012;168:2170–2183. doi: 10.1007/s12010-012-9926-y. [DOI] [PubMed] [Google Scholar]
- Nagahama T, Hamamoto M, Nakase T. Horikoshil K. Kluyveromyces nonferrnentans sp. nov., a new yeast species isolated from the deep sea. Int J Syst Bacteriol. 1999;49:1899–1905. doi: 10.1099/00207713-49-4-1899. [DOI] [PubMed] [Google Scholar]
- Neuman I, Nahum H. Ben-Amotz A. Prevention of exercise-induced asthma by a natural isomer mixture of beta-carotene. Ann Allergy Asthma Immunol. 1999;82:549–553. doi: 10.1016/S1081-1206(10)63165-1. [DOI] [PubMed] [Google Scholar]
- Obara N, Ishida M, Hamada-Sato N. Urano N. Efficient bioethanol production from scrap paper shredder by a marine Saccharomyces cerevisiae derived C-19. Stud Sci Technol. 2012;1:127–132. [Google Scholar]
- Oswal N, Sarma PM, Zinjarde SS. Pant A. Palm oil mill effluent treatment by a tropical marine yeast. Bioresour Technol. 2002;85:35–37. doi: 10.1016/s0960-8524(02)00063-9. [DOI] [PubMed] [Google Scholar]
- Pandey A, Soccol CR, Selvakumar P, Soccol VT, Krieger N. Fontana JD. Recent developments in microbial inulinases. Its production, properties, and industrial applications. Appl Biochem Biotechnol. 1999;81:35–52. doi: 10.1385/abab:81:1:35. [DOI] [PubMed] [Google Scholar]
- Peng Y, Chi ZM, Wang XH. Li J. Purification and molecular characterization of exo-beta-1,3-glucanases from the marine yeast Williopsis saturnus WC91-2. Appl Microbiol Biotechnol. 2009;85:85–94. doi: 10.1007/s00253-009-2061-1. [DOI] [PubMed] [Google Scholar]
- Peng Y, Liu GL, Yu XJ, Wang XH, Jing L. Chi ZM. Cloning of exo-beta-1,3-glucanase gene from a marine yeast Williopsis saturnus and its overexpression in Yarrowia lipolytica. Mar Biotechnol (NY) 2011;13:193–204. doi: 10.1007/s10126-010-9281-3. [DOI] [PubMed] [Google Scholar]
- Pensupa N, Jin M, Kokolski M, Archer DB. Du C. A solid state fungal fermentation-based strategy for the hydrolysis of wheat straw. Bioresour Technol. 2013;149:261–267. doi: 10.1016/j.biortech.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Orozco M, Hernandez-Saavedra NY, Ascencio Valle F, Acosta Gonzalez B. Ochoa JL. Cell yield and superoxide dismutase activity of the marine yeast Debaryomyces hansenii under different culture conditions. J Mar Biotechnol. 1998;6:255–259. [PubMed] [Google Scholar]
- Renshaw JC, Robson GD, Trinci APJ, Wiebe MG, Livens FR, Collison D. Taylor RJ. Fungal siderophores: structures, functions and applications. Mycol Res. 2002;106:1123–1142. [Google Scholar]
- Saravanakumar K, Senthilraja P. Kathiresan K. Bioethanol production by mangrove-derived marine yeast Sacchromyces cerevisiae. J King Saud Univ Sci. 2013;25:121–127. [Google Scholar]
- Sarkar S, Pramanik A, Mitra A. Mukherjee J. Bioprocessing data for the production of marine enzymes. Mar Drugs. 2010;8:1323–1372. doi: 10.3390/md8041323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlin PJ. Philip R. Efficacy of marine yeasts and baker's yeast as immunostimulants in Fenneropenaeus indicus: a comparative study. Aquaculture. 2011;321:173–178. [Google Scholar]
- Senthilraja P, Kathiresan K. Saravanakumar K. Comparative analysis of bioethanol production by different strains of immobilized marine yeast. J Yeast Fungal Res. 2011;8:113–116. [Google Scholar]
- Singh P. Physiological and molecular studies of deep-sea fungi. Goa India: Goa University.; 2011. PhD Thesis,. Available at: http://drs.nio.org/drs/handle/2264/4132. [Google Scholar]
- Stahmann KP, Revuelta JL. Seulberger H. Three biotechnical processes using Ashbya gossypii Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Appl Microbiol Biotechnol. 2000;53:509–516. doi: 10.1007/s002530051649. [DOI] [PubMed] [Google Scholar]
- Subramanian M, Alikunhi NM. Kandasamy K. In vitro synthesis of silver nanoparticles by marine yeasts from coastal mangrove sediment. Adv Sci Lett. 2010;3:428–433. [Google Scholar]
- Thomson GF. Enumeration of yeasts and moulds – media trial. Food Microbiol. 1984;1:223–227. [Google Scholar]
- Turkiewicz M, Pazgier M, Kalinowska H. Bielecki S. Invertase and α-glucosidase production by the endemic Antarctic marine yeast Leucosporidium antarcticum. Pol Polar Res. 2005;26:125–136. [Google Scholar]
- Urano N, Yamazaki M. Ueno R. Distribution of halotolerant and/or fermentative yeasts in aquatic environments. J Tokyo Univ Fish. 2001;87:23–30. [Google Scholar]
- Ushakumari UN. Ramanujan R. Isolation of astaxanthin from marine yeast and study of its pharmacological activity. Int Curr Pharm J. 2013;2:67–69. [Google Scholar]
- Wang Y, Feng F. Zheng X. Biocontrol of postharvest fungal pathogens by yeasts isolated from fruits and marine source. 2007. Abstract. Available at: http://www.paper.edu.cn/en_releasepaper/content/17319.
- Wang L, Chi Z, Wang X, Ju L, Chi Z. Guo N. Isolation and characterization of Candida membranifaciens subsp. flavinogenie W14-3, a novel riboflavin-producing marine yeast. Microbiol Res. 2008;163:255–266. doi: 10.1016/j.micres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Wang WL, Chi ZM, Chi Z, Li J. Wang XH. Siderophore production by the marine-derived Aureobasidium pullulans and its antimicrobial activity. Bioresour Technol. 2009;100:2639–2641. doi: 10.1016/j.biortech.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Wang L, Du YC, Meng XF, Long XH, Liu ZP. Shao H. Direct production of bioethanol from Jerusalem artichoke inulin by gene-engineering Saccharomyces cerevisiae 6525 with exoinulinase gene. Plant Biosyst. 2013;147:1–7. [Google Scholar]
- Watson K. Temperature relations. In: Harrison JS, editor; Rose AH, editor. The Yeast. London: Academic Press; 1987. pp. 41–69. Vol. 2. [Google Scholar]
- Wickerham LJ. Taxonomy of yeasts. US Dept Agric Tech Bull. 1951;1029:1–56. [Google Scholar]
- Winkelmann G. Microbial siderophore-mediated transport. Biochem Soc Trans. 2002;30:691–696. doi: 10.1042/bst0300691. [DOI] [PubMed] [Google Scholar]
- Wu M. Chiu Y. Consumptive Water Use in the Production of Ethanol and Petroleum Gasoline. USA: Transportation Technology R&D Center; 2011. Available at: http://greet.es.anl.gov/publication-consumptive-water. [Google Scholar]
- Yanagisawa M, Ojima T. Nakasaki K. Bioethanol from sea lettuce with the use of crude enzymes derived from waste. J Mater Cycles Waste Manag. 2011;13:321–326. [Google Scholar]
- Zhang L. Chi ZM. Screening and identification of a cellulase producing marine yeast and optimization of medium and cultivation conditions for cellulase production. J Ocean Univ China. 2007;37:101–108. [Google Scholar]
- Zhang T, Chi Z. Sheng J. A highly thermosensitive and permeable mutant of the marine yeast Cryptococcus aureus g7a potentially useful for single-cell protein production and its nutritive components. Mar Biotechnol. 2009a;11:280–286. doi: 10.1007/s10126-008-9144-3. [DOI] [PubMed] [Google Scholar]
- Zhang T, Gong F, Chi Z, et al. Cloning and characterization of the inulinase gene from a marine yeast Pichia guilliermondii and its expression in Pichia pastoris. Antonie Van Leeuwenhoek. 2009b;95:13–22. doi: 10.1007/s10482-008-9281-8. [DOI] [PubMed] [Google Scholar]


