Abstract
Parkinson's disease (PD) is a movement neurodegenerative disorder, characterized by bradykinesia, rigidity and tremor, constituting difficulties in walking and abnormal gait. Previous research shows that Drosophila expressing human α-synuclein A30P (A30P) develop deficits in geotaxis climbing; however, geotaxis climbing is a different movement modality from walking. Whether A30P flies would exhibit abnormal walking in a horizontal plane, a measure more relevant to PD, is not known. In this study, we characterized A30P fly walking using a high-speed camera and an automatic behavior tracking system. We found that old but not young A30P flies exhibited walking abnormalities, specifically decreased total moving distance, distance per movement, velocity, angular velocity and others, compared with old control flies. Those features match the definition of bradykinesia. Multivariate analysis further suggested a synergistic effect of aging and A30P, resulting in a distinct pattern of walking deficits, as seen in aged A30P flies. Psychiatric problems are common in PD patients with anxiety affecting 40–69% of patients. Central avoidance is one assessment of anxiety in various animal models. We found old but not young A30P flies exhibited increased centrophobism, suggesting possible elevated anxiety. Here, we report the first quantitative measures of walking qualities in a PD fly model and propose an alternative behavior paradigm for evaluating motor functions apart from climbing assay.
Keywords: A30P, bradykinesia, centrophobism, Drosophila, multivariate analysis, neurodegenerative diseases, open field, Parkinson's disease, walking deficits, α-synuclein
Parkinson's disease is motor neurodegenerative disease, characterized by degeneration of dopamine neurons in the substantia nigra of midbrain. Bradykinesia is one of three cardinal motor symptoms with rigidity and tremor as the others, referring to slowness in the execution of movements or a decrease of amplitude or the range of movements. One major histopathological hallmark of PD is Lewy bodies. They are intracellular, cytoplasmic abnormal proteinaceous fibrillar aggregates, heavily stained with α-synuclein (αSyn). The duplication, triplication or point mutations of αSyn were identified in rare cases of familial early-onset PD, suggesting a direct contribution of αSyn overexpression to PD pathology (Kruger et al. 1998; Polymeropoulos et al. 1997; Singleton et al. 2003; Van Der Geest et al. 1998; Zarranz et al. 2004).
Expression of human αSyn or its mutant forms (A30P, A53T or E46K) in Drosophila using pan-neurons Elav driver (Feany & Bender 2000) captured three major hallmarks of PD: (1) filamentous intraneuronal inclusions containing αSyn, resembling Lewy bodies, as seen in human PD postmortem brain, (2) a selective loss of subpopulation of dopaminergic neurons and (3) a l-dopa treatment reversible climbing deficit (Feany & Bender 2000; Pendleton et al. 2002; Trinh et al. 2008), which directly indicating a specific involvement of dopamine system.
While PD fly models were widely used, the motor function of most fly PD models is mostly evaluated through fly climbing, taking advantage of the negative geotaxis nature of fruit fly. Negative geotaxis is an innate escape response, during which while flies were in a confined environment, and tapped to the bottom, flies will ascend the wall of the container in hope to escape. This assay is sensitive to deficits of motor coordination and of muscle tone regulation. Climbing assay, however, is a different assessment from fly walking, which is more relevant to the motor assessment used in rat and mouse PD models or in PD patients. Here, we asked whether A30P expression would affect fly walking. If so, we asked what properties of walking were affected, whether any observed deficits resembled features of bradykinesia and how they were compared with the locomotive deficits of mice expressing αSyn or its mutants. We were also curious whether aging and A30P affect A30P flies' walking properties independently or synergistically.
Changes in locomotive activity in an open flied positively correlated with emotional reactivity and exploratory behavior in rodents (Denenberg 1969) and in flies. Animals that spend more time in the center of an arena are considered less fearful and less anxious. In PD patients, generalized anxiety, panic attacks and social phobias are common (Lauterbach 2005). About 40% of the patients suffer from anxiety (Cummings et al. 1996; Leentjens et al. 2008) or a more prevalent 69% in a very recent report (Kulisevsky et al. 2008). Here, we found that old but not young A30P flies exhibit an increased center-avoidance, consistent with what reported in synuclein transgenic mice (George et al. 2008), providing first evidence of centrophobism in A30P flies. Similar phenotypes caused by A30P in both fly and mouse may suggest a conserved neural circuit that is particularly vulnerable to A30P toxicity.
Materials and methods
Fly stock
Fly stocks used were wild-type 2U, Elav-GAL4, MJ85b-GAL4, nSyb-GAL4 (Bloomington Drosophila Stock Center) and UAS-A30P (from Mel B. Feany's Lab). All the flies were outcrossed with 2U wild-type flies for five or more generations to equilibrate genetic background within 3 months before experiments. All flies were raised under a 12:12 light:dark cycle at 27°C and 70% humidity. l-Dopa containing food at the final concentration of 1 mm was made by mixing with ascorbic acid (25 mg/100 ml) and then added into 55°C freshly made food to flasks. Ascorbic acid was used to prevent drug oxidation. Food was changed every 3 days.
Climbing assay
Startle-induced negative geotactic climbing was measured in a countercurrent device using samples of ∼30 flies over five consecutive 10-second intervals (Benzer 1973). A PI was calculated as the percentage of transitions by flies of each group in which they climbed a height of 8 cm (see details: (Chen et al. 2014). Experiments were conducted 2 h before dark cycle, in an environmental chamber.
Immunohistochemistry
Whole-mount immunolabeling of 30-day-old adult brains was performed as described by Xia et al. (2005). Briefly, fly brains were dissected in cold phosphate-buffered saline (PBS), fixed in cold PBS with 4% paraformaldehyde in for 2 h, transferred to PBS with 4% paraformaldehyde in and 2% Triton X-100 in vacuum for 1 h at room temperature, and blocked for 2 days at 4°C in PBS with 2% Triton X-100 and 10% normal goat serum (NGS). Brains were incubated with primary anti-human α-synuclein (1: 2000; LB509 of Zymed, Carlsbad, CA, USA) for 2 days at 4°C in PBS with 1% Triton X-100 and 0.25% NGS, washed in PBS and immunoprobed with secondary anti-mouse Alexa-486 (Life Technologies, Carlsbad, CA, USA). Tissue was cleared and mounted in FocusClear (CelExplorer Lab, Hsinchu, Taiwan). Brains were imaged with a Zeiss LSM 510 confocal microscope. Three-dimensional images were stacked for dopamine neuron cluster identification and quantification, blind to the genotypes. Only well-identified clusters were included for analysis.
Setup and locomotion tracking
The experimental setup was shown as in Fig. 2. Four individual flies (one per arena) were simultaneously monitored and recorded for 2 min (Figs. 6) or 5 min (Fig. 4) per trial. The walking patterns from each fly was tracked at 17 frames per second and quantified with EthoVision XT. Each arena was 6 cm in diameter, covered with a thin transparent plastic ceiling of 2 mm in height. The shallow depth prevents non-walking and ensures monolayer movements. The arena was tapped three times (with 1–1.5 kg force) before each trial to activate the locomotion. Experiments were carried out between 4:00 and 7:00 pm with constant visual cues before the dark cycle, 8 am to 8 pm daily.
Figure 2.
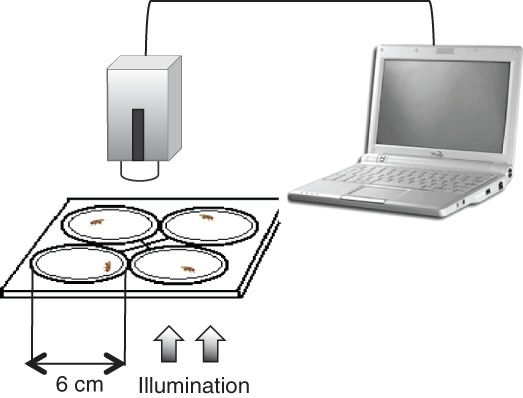
Open-field assay for flies. Transparent acrylic plates were illuminated by 300 lux of white light from beneath to elevate basal locomotive activity and to provoke center-avoidance behavior. A panel of four arenas, 6 cm in diameter, was used, allowing a simultaneous recording of four flies.
Figure 6.
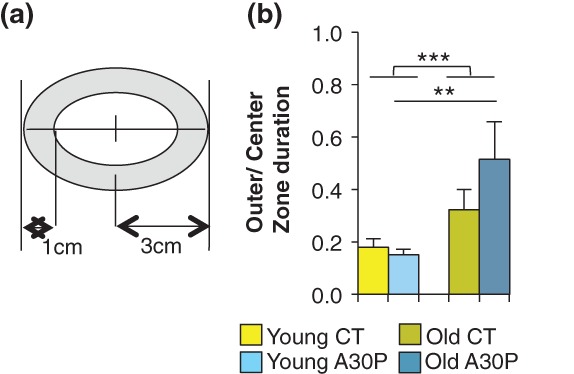
Age-dependent anxiety-like phenotype in A30P flies. (a) The outer zone is 1-cm wide of ring area in the area. (b) Old A30P flies showed increased movement duration in the outer zone, shown as an increase in the ratio of the outer to center zone duration. Each trial is 2 min. Data present mean ± 95% confidence interval of the mean (1.96 SEM). nYoung = 23; nold = 16. Student's t-test. ***P < 0.001. **P < 0.01.
Figure 4.
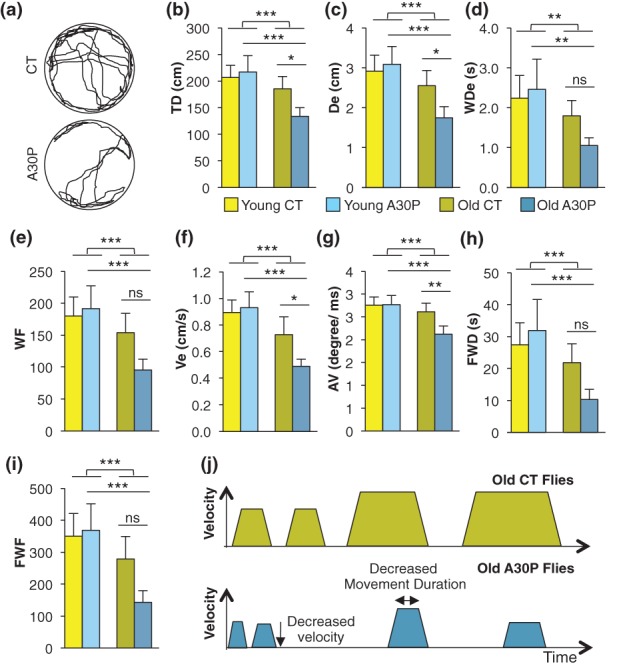
Age-dependent walking deficits in A30P flies. The trial is 5 min. (a) Sample tracings of old CT and A30P flies' walking pattern. (b) Total distance moved (TD; cm). (c) Distance per episode (De; cm). (d) Walking Distance per episode (WDe; second). (e) Walking frequency (WF). (f) Velocity (Ve; cm/s). (g) Angular velocity (degree/millisecond). (h) Fast walking duration (FWD; second). (i) Fast walking frequency (FWF). Fast walking was defined as walking at Ve above 1 cm/second. (j) A summary of multiple walking deficits identified in old A30P files. Data present mean ± 95% confidence interval of the mean (1.96 SEM). nYoung = 23; nold = 20. Student's t-test was used for comparisons between mixed young vs. old. Two-way anova with Tukey post hoc was used for comparisons between four groups. *P < 0.05; **P < 0.01; ***P < 0.001.
Western blotting
Thirty fly heads were homogenized in 150 µl 2× Laemmli sample buffer (Biorad, Hercules, CA, USA) and centrifuged for 20 min at 13 000 g at 4°C. Protein extract from about five heads was used per lane on SDS–PAGE (sodium dodecyl sulphate–polyacrylamide gel electrophoresis) gels to visualize human αSyn A30P protein, probed with a specific anti-human αSyn antibody (Cell Signaling Technology, Danvers, MA, USA). An antibody recognizing pan-actin (Sigma, St. Louis, MO, USA) was used as an internal control of the total protein.
Statistics
All data that were subjected to one- or two-way analysis of variance (anova) with Tukey post hoc pairwise comparisons or unpaired or paired Student's t-test were analyzed using Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Not normally distributed data in Fig. 4 were subjected to a Box-Cox transformation (JMP 9, SAS Institute Inc., Cary, NC, USA) to render error variances homogenous across groups to achieve normal distribution (Olson et al. 2005) before comparisons, using Prism 6. For Fig. 5, transformed data were scaled separately between 0 and 1 before multivariate analysis, using R (R 2.14.0).
Figure 5.
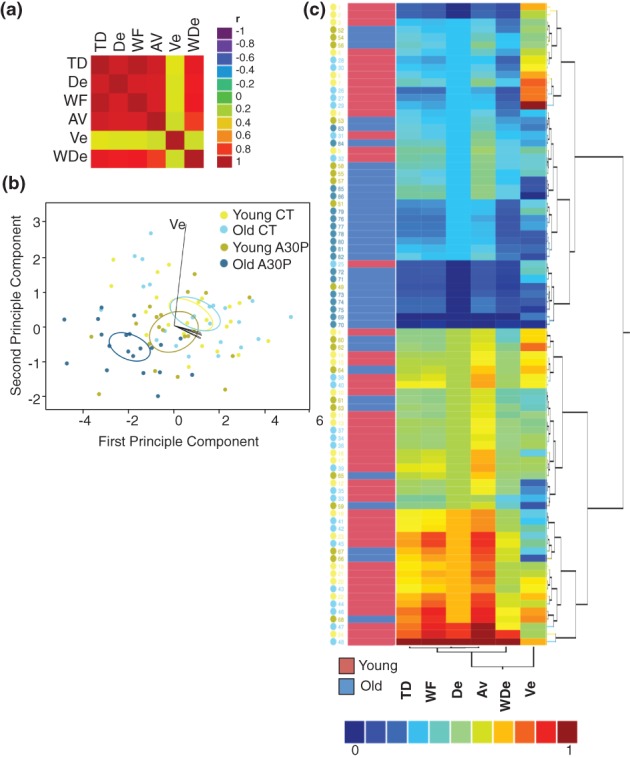
Distinct walking pattern of aged A30P flies. (a) Correlation heat map. Five walking qualities (TD, De, WF, AV and WDe) are highly correlated with each other [the correlation coefficients (Rs) range from 0.79 to 0.99], but not with Ve (Rs range from 0.267 to 0.298). The inputs are data of all flies from Fig. 4. (b) Biplot of a discriminative analysis. Each point is data of one fly. Each linear trajectory represents one variable. Each ellipse corresponds to a 95% confidence limit for a mean. Discriminative analysis was constructed using ‘quadratic method’. A summary of discrimination power was tabulated in Table2. (c) Clusters of fly walking, based on the measurements of TD, MF, De, AV, Ade and Ve, and ages, young or old. For multivariate analysis, all data were scaled linearly between 0 and 1.
Results
Characterization of nSyb/A30P transgenic flies
To ensure a high and robust expression of human A30P αSyn (A30P) in flies, we compared A30P expression levels driven by three different pan-neural promoters: Elav-GAL4, MJ85b-GAL4 and nSyb-GAL4 (Liu et al. 2012; Mehren & Griffith 2004; Yu et al. 2010), in flies rearing at 27°C. A30P expression in heads of 1- to 5-day-old flies were quantified by immunoblotting, using antibody specific to human α-synuclein (hαSyn). We found that A30P was expressed at the highest level in nSyb/A30P flies, showing an additional 60% mean increase, comparing to Elav/A30P (n = 4; Fig. 1a). nSyb is a neural synaptobrevin promoter. Hereafter, we expressed A30P transgene using nSyb-GAL4 in all experiments.
Figure 1.
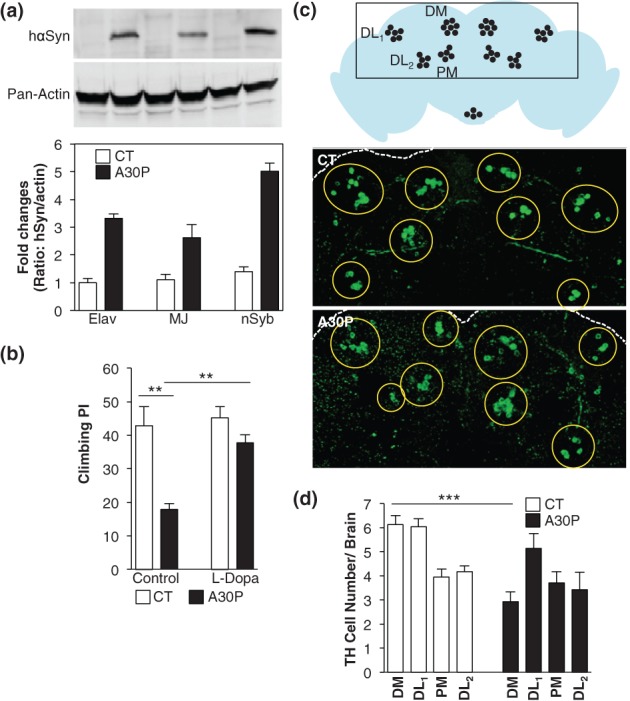
Properties of A30P transgenic flies. (a) Promoter strength of pan-neural GAL4 drivers. A30P expression was driven by Elav, MJ-85b (MJ) or nSyb GAL4 drivers. Protein extracts from adult heads, age 1–5 days, were probed with specific anti-human αSyn antibody (upper panel). The A30P-specific signal was normalized to its actin signal and then further normalized as ratios to Elav/CT controls for four independent blots (lower panel). Elav/CT is Elav/+; +/+. Elav/A30P is Elav/+; A30P/+. MJ/CT is MJ-85b/+; +/+. MJ/A30P is MJ-85b/+; A30P/+. nSyb/CT is nSyb/+; +/+. nSyb/A30P is nSyb/+; A30P/+. n = 4. (b) Climbing PI showed l-Dopa reversible motor deficit in A30P flies. Flies were 15 days old, treated with (l-Dopa) or without l-Dopa (control). See Materials and methods for PI calculation. The CT is nSyb/+; +/+; A30P is nSyb/+; A30P/+. Both transgenic flies were used hereafter in all figures. n = 4 per group. Comparisons between CT and A30P of the control group and between l-Dopa-treated and not treated A30P were made using unpaired and paired Student's t-test, respectively. (c) Diagram showing four major clusters of dopamine neuron in an adult fly brain. DM, dorsal medial cluster; DL1, dorsal lateral 1; DL2, dorsal lateral 2; PM, posterior medial (upper panel). Anti-TH was used to identify dopamine neurons in 30-day-old CT and A30P fly brains using immunostaining (lower panel). (d) Quantification of TH-stained cells in (c). 8 < n < 25. Comparisons between TH-stained cell number from the same cluster of CT and of A30P were made using unpaired Student's t-test. All data represented means ± SEM; **P < 0.01; ***P < 0.01.
To determine whether nSyb/A30P flies (called A30P flies hereafter; nSyb/+; A30P/+) in this study showed the previously reported geotaxis climbing deficit (Botella et al. 2008; Feany & Bender 2000; Hernandez-Vargas et al. 2011) and whether such motor deficit could be corrected by l-Dopa, a dopamine precursor and a common medication for PD, we measured the climbing performance indexes (PIs) of A30P flies and of the wild-type control (CT) that were fed a diet with and without l-Dopa (Fig. 1b). We found that 15-day-old A30P showed significant decline in climbing [CT vs. A30P: t(6) = 4.241, P < 0.01], and such decline was correctable by l-Dopa treatment [A30P vs. A30P + l-Dopa: t(3) = 5.526; P < 0.05; Fig. 2b].
To determine whether aged A30P flies would develop dopamine neuron loss, we immunostained dopamine neurons in the brains of 30-day-old CT or A30P flies, using tyrosine hydroxylase (TH) as a marker. Tyrosine hydroxylase neurons reside in clusters in brain (Fig. 1c). One prominent cluster is dorsomedial (DM) dopamine neurons and was found significantly reduced in aged A30P flies [CTDM vs. A30PDM: t(38) = 5.325, P < 0.001; Fig. 1d].
Starvation-dependent hyperactivity without affecting velocity
Locomotion may be spontaneous or elicited. Flies, similar to rodents, show an initially elevated level of activity followed by habituation when placed in an open-field arena. As mice expressing mutant or wild-type αSyn tend to be hypoactive along aging (Maries et al. 2003; Oksman et al. 2009; Yavich et al. 2005), we set forward to optimize a condition evoking a higher basal activity in flies to avoid ‘floor effect’.
To elevate basal spontaneous walking, we applied 300 lux of white light to evenly illuminate the arenas from beneath using an X-ray box with constant visual cues. To ensure testing groups were well balanced and directly comparable, four animals, one animal from each four testing groups (young, 15 days old, and old, 30 days old, of male control (CT) and A30P flies) were evaluated in parallel using a multiplex arena of 6 cm in diameter for imaging and tracking (Fig. 2). To ensure a monolayer movement during tracking, each arena is made with 0.2 mm high of ceiling. As starvation was suggested to elevate basal locomotive activity, we evaluated the effect of starvation on locomotion, using 2U wild-type flies. Two- to three-day-old young wild-type males (M) or females (F) were food starved for 0, 4, 8 h or 8 h, respectively, before experiments (Fig. 3). During starvation, flies were individually housed in empty plastic tubes with water-wetted filter paper as water sources. We found elevated walking activity in male flies with 8-h food starvation, displaying increased total distance (TD), movement duration and moving frequency (MF) (0 vs. 8 h, males: Tukey post hoc test: **P < 0.01). The trial is 2-min long.
Figure 3.
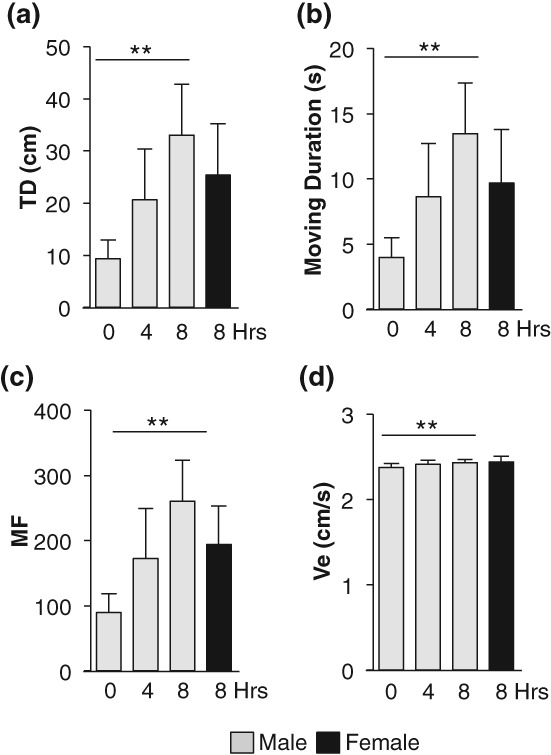
Starvation induced hyperactivity without affecting Ve. A 2-min walking was measured in 2- to 3-day-old wild-type male flies after 0, 4 or 8 h of food depletion and in the same-aged female flies after 8 h of food depletion. Starvation consistently increased the locomotive activity of male. (a) Total distance moved (TD; cm). (b) Moving duration (seconds). (c) Mean MF. (d) Moving velocity (cm/second). Data present mean ± 95% confidence interval of the mean (1.96 SEM). 10 < n < 13. One-way anova with Tukey post hoc was used for within-male comparisons. Student's t-test was used to compare 8-h-starved male to 8-h-starved female. *P < 0.05; **P < 0.01.
Between 8-h-starved male and female flies, males constantly show higher, although not statistically significant, walking activity (Fig. 3a–c; n = 10, 12, 13, 12 for 0, 4 and 8-h-starved males and 8-h-starved females, respectively). No difference in moving velocity was found between flies with or without food starvation and between males and females (Fig. 3d). To detect a likely hypoactivity in A30P flies, we hereafter measured fly walking using 8-h food-depleted male flies of the control (CT: nSyb/+; +/+) and A30P (A30P: nSyb/+; A30P/+).
Age-dependent walking deficits in A30P flies
To reveal walking qualities in A30P flies, we measured walking parameters, including TD walked, Distance per episode (De), Walking Duration per episode (WDe), Velocity (Ve), Angular Velocity (AV) and Walking Frequency (WF) of the A30P and CT flies of both young (15 days old) and old (30 days old) flies. While Ve was above 1 cm/second, two additional measurements, the sum of fast walking duration (FWD) and fast walking frequency (FWF), were calculated separately. Sample tracings of old CT and A30P flies' walking pattern are shown in Fig. 4a. We found that aging leads to significant decreases in walking activity [TD: t(86) = 4.067, P < 0.001; De: t(86) = 4.039, P < 0.001; WDe: t(86) = 3.246, P < 0.01; WF: t(86) = 3.847, P < 0.001; Ve: t(86) = 5.446, P < 0.001; AV: t(86) = 3.914, P < 0.001; FWD: t(86) = 3.602, P < 0.001; FWF: t(86) = 4.028, P < 0.001; Fig. 4b–i: Student's t-test between the pooled young and old flies]. The aging effect is mostly contributed by old A30P flies, as the comparisons between young CT and old CT are not significant (Table1). Next, A30P expression in aged flies (old A30P) showed further decreases in TD, De, Ve (old CT vs. old A30P: P < 0.05) and in AV (old CT vs. old A30P: P < 0.01), but has no effect on WDe, WF and FWD, FWF (old CT vs. old A30P, P = ns; all pairwise comparisons are done with Turkey's post hoc test; Fig. 4b–i).
Table 1.
Summary statistics of walking properties of A30P flies
| Two-way anova | ||||||||
|---|---|---|---|---|---|---|---|---|
| TD | De | WDe | Ve | AV | FWD | FWF | WF | |
| Interaction P value | * | * | ns | * | * | * | * | * |
| Tukey's multiple comparisons tests | ||||||||
| Young CT vs. old CT | ns | ns | ns | ns | ns | ns | ns | ns |
| Young A30P vs. old A30P | **** | **** | ** | **** | **** | *** | *** | *** |
| Young CT vs. young A30P | ns | ns | ns | ns | ns | ns | ns | ns |
| Old CT vs. old A30P | * | * | ns | * | ** | ns | ns | ns |
Data analysis was performed using normally distributed data generated by Box-Cox transformation. FTD,1,84 = 6.165, PTD = 0.015; FDe,1,84 = 5.606, PDe = 0.020; FWDe,1,84 = 2.878, PWDe = 0.094; FVe,1,84 = 6.499, PVe = 0.013; FAV,1,84 = 6.827, PAV = 0.011; FFWD,1,84 = 4.543, PFWD = 0.036; FFWF,1,84 = 4.526, PFWF = 0.036; FWF,1,84 = 5.168, PWF = 0.026. P values are <0.05 (*), < 0.01 (**), < 0.001 (***) or < 0.0001 (****). ns, not significant. Analysis was performed with Prism 6 (GraphPad Software, Inc.).
The decreased De and WDe in aged A30P but not in aged CT may suggest that old A30P flies are less effective in maintaining movement. The decreased WF may suggest that old A30P flies are less effective in initiating movement (young A30P vs. old A30P: P < 0.001). The decreased AV in old A30P flies may reflect poor movement coordination because faster AV requires smooth stepping and motion coordination. Two-way anova supports that aging and A30P expression are not independent factors but factors that work synergistically, enhancing walking deficits in most measurements other than walking distance per episode (WDe; Table1). Collectively, walking patterns of old A30P flies indicate hypoactivity and the definition of bradykinesia in Parkinson's disease (PD). Figure 4j summarizes walking patterns of old CT and A30P flies.
Distinct walking in old A30P flies
To determine the relationship between walking qualities, and how those qualities may be affected by aging and by A30P, data from all flies (young and old CT and A30P flies) were subjected to multivariate analysis. All measurements were either normally distributed or were transformed into representative data that is normally distributed using Box-Cox, and then scaled from 0 to 1 using linear relationship, prior to further analysis. As FWD is the sum of WDe at a higher speed and FWF is a subset measurement of WF, both FWD and FWF were excluded in further analysis.
Interestingly, in the correlation analysis, five out of six properties (TD, De, WF, WDe and AV) between all animals were highly correlated with one another (Rs range from 0.79 to 0.99, Fig. 5a) but were poorly correlated with Ve (R < 0.3, ranging from 0.267 to 0.298). The ‘R’ is correlation coefficient. The weak correlation suggested that a different neural circuit might regulate Ve separately. Next, we set out to determine the relationship of walking patterns between four groups of flies. As five out of six measurements were highly correlated and the two most highly ranked principle components, determined by principle component analysis, could explain 98.31% of total variation, we performed a quadratic discriminant analysis on all flies using the top two principle components. Quadratic discriminant analysis is a statistic method used to predict group membership based on a linear combination of variables without assuming that the covariance of each variable is identical. The biplot of discriminate model displays distribution of all flies in a canonical space (Fig. 5b), and shows that only old A30P flies are well separated from the other three groups. The length and direction of each line vector indicates the contribution of each variable in separating the centroids, with Ve most separated from the other five vectors, pointing to the lower right canonical space.
A30P expression moved fly distribution to the lower left quarter (young CT vs. young A30P) and aging moved fly distribution slightly to the upper right quarter of the canonical space (young CT vs. old CT). The far disproportional separation of old A30P flies to the lower left quarter of canonical space and the directionality of the old A30P flies distribution, suggesting that old A30P flies exhibited enhanced walking deficits (Fig. 5b). The highest discriminating power was 90% in assigning the membership of old A30P flies correctly as old A30P flies (Table2). A lower discriminating power of 66.7% in discriminating young CT, or no discriminating power ∼25%, corresponding to a random chance of assigning any member to one of four groups, was found to discriminate young A30P (33.3%) and old CT (15%) from all other groups. A high error rate of assigning young A30P flies as young CT flies (45.8%) and old CT flies as young CT flies (40%) suggested that A30P expression and aging alone did not dramatically affect the walking pattern (Table2).
Table 2.
Summary statistics of discriminant analysis
| Outputs (assigned membership) | |||||
|---|---|---|---|---|---|
| Young | Old | ||||
| Inputs | CT | A30P | CT | A30P | n |
| Young | |||||
| CT | 16 (66.7) | 6 (25.0) | 1 (4.17) | 1 (4.17) | 24 |
| A30P | 11 (45.8) | 8 (33.3) | 3 (12.5) | 2 (8.3) | 24 |
| Old | |||||
| CT | 8 (40.0) | 4 (20.0) | 3 (15.0) | 5 (25.0) | 20 |
| A30P | 0 (0) | 0 (0) | 2 (10.0) | 18 (90.0) | 20 |
Values inside parenthesis indicate percentage of discriminant power, which is the predicted output divided by n, ×100%.
To visualize individual differences on walking pattern, we ran a hierarchical cluster on six measurements and on ages, young and old (Fig. 5c), using Ward's criterion (Fionn Murtagh 2011). Ve was found most variable and appeared to be dimorphic among groups other than old A30P flies. On the contrary, a better clustering in old A30P flies again indicated that aging and A30P synergistically enhanced walking deficits. Collectively, both discrimination and clustering analysis supported that old A30P flies developed a distinct pattern of walking deficits.
A30P flies show age-dependent anxiety-like phenotype
Anxiety is common in PD and was suggested as a preclinical risk factor (Shiba et al. 2000; Weisskopf et al. 2003). It is commonly agreed that decrease in central exploratory behavior, also known as centrophobism, is negatively correlated with an increase of anxiety in many animals (Ramos 2008). Centrophobism is sexually dimorphic in flies, with females displaying more obvious avoidance than males (Martin 2004). Considering a possible increase of anxiety in aged A30P flies, we evaluate anxiety in male flies to avoid a ceiling effect. In the open-field assay, we defined center as a circular area of 4 cm in diameter and the outer zone as a ring of 1 cm in width outside of the center (Fig. 6a).
We found that young CT and A30P flies spent similar amount of time in the outer over center zone [young CT vs. young A30P: t(42) = 0.533, P = 0.597], while aged A30P spent longer time in the outer zone [young A30P vs. old A30P: t(37) = 2.879, **P = 0.0063; Fig. 6b], indicating increased centraphobism and possible elevated anxiety. Young is 15 days old and old is 30 days old, same as previous figures.
Discussion
A long-standing obstacle of studying PD is to create animal models that recapitulate essential clinical features of the disease. The Drosophila Parkinson's model was created by overexpressing human αSyn or its mutants A30P or A53T αSyn and has been shown to exhibit many PD-like pathologies (Feany & Bender 2000). These pre-characterized pathologies make PD fly models powerful in revealing pathological mechanisms and productive in identifying therapeutic targets (Auluck & Bonini 2002; Auluck et al. 2002; Bilen & Bonini 2005; Chen & Feany 2005; Whitworth et al. 2005). A key feature of fly PD model is age-dependent deterioration of motor function, which is often examined by geotaxis climbing. Geotaxis climbing assays evaluate motor function differently than measures of walking ability, which is more relevant to PD. Here, we examined several walking qualities of young and old PD flies, expressing A30P protein. We focused on Ve, AV and measurements of episodic movements. We found aging leads to particularly decreased TD traveled, De, decreased WDe, Ve, AV, FWF and WF in A30P flies but not in CT flies. Such compound effects of aging and A30P suggested that aging and A30P interactively deteriorate normal walking. Two-way anova tests for all measurements, except WDe (Table1), supported this conclusion.
Short-step gait festination is commonly reported in advance PD patients (Nutt et al. 2011; Rajesh Pahwa 2007). A similar phenotype in flies would likely lead to shortened WDe and changes in WF. Indeed, old A30P PD fly showed further decreased WDe and WF (young A30P vs. old A30P), suggesting possible difficulties in initiating and maintaining movement. While episodic walking duration was not measured in A30P mice, the reported decrease in total moving duration in old A30P mice (Yavich et al. 2005) is consistent with reduced WDe in old A30P fly in our results.
Reduced AV in old A30P flies may suggest poorly coordinated gaits. Future analysis on gait variability in old A30P flies would be beneficial. This can be achieved by using methods similar to Catwalk for fly as described by Mendes et al. (2013), which may reveal differential turning coordination in old A30P flies.
Gait festination was known to manifest by visual cue. How visual perception modulates motor control is not known and is difficult to investigate in mouse or human studies. Perception of environmental constraints has shown to slow health subjects' gait such as when subjects were approaching to a narrowed hallway or doorway. Parkinson's disease patients, suffering gait-freezing, stalled their gaits and decreased stride length to a greater degree as a doorway is approached, displaying an exaggerated response to visual cues (Almeida & Lebold 2010; Cowie et al. 2012; Giladi et al. 1992). It is not known whether a similar modulation of walking by visual perception occurred in old A30P flies. This would be interesting to investigate by evaluating walking of old A30P flies in arena equipped with constricted visual cues.
Multivariate analysis revealed how six qualities of walking changed correlatively with one another. Results supported that aging and A30P synergistically exacerbated normal walking, leading to a distinct walking pattern in old A30P flies. The high correlation between five walking properties in Fig. 5a also suggested that the presence of one walking abnormality can be a strong indicator of the other four, and fewer measurements may be used in the future to evaluate A30P-induced motor deficits or the efficacy of a therapeutic intervention in A30P flies. Low correlation between Ve and the other five parameters suggests that Ve may be regulated independently.
Velocity and other walking qualities were regulated separately. Indeed, flies with chemically ablated mushroom body (MB) showed reduced total walking duration while Ve was unchanged (Serway et al. 2009). Authors showed that instead of being regulated by MB, Ve was regulated by centreal complex (CC). Centreal complex is a prominent neuropilar structure, locating at the center of the insect brain between two protocerebral hemispheres, comprising four interconnected neuropilar regions: the fan-shaped body, the ellipsoid body, the protocerebral bridge and the paired noduli. When CC was disrupted structurally by either gene mutations or by targeted expression of tetanus toxin, walking Ve was greatly reduced (Martin et al. 1999; Strauss & Heisenberg 1993). A study later shows functional differences between two substructures of CC; fan-shaped body mediates maintaining but not initiating movement, while ellipsoid body mediates organization of temporal locomotion pattern (Martin et al. 2001). Old A30P flies showed poorly organized temporal movements (decreased WDeand WF) and walking velocity, suggesting CC as a functional target of A30P toxicity.
In addition to MB's role in controlling walking, the γ lobes of MB mediate centrophobism. The MBs are comprised of three distinct groups of fiber lobes, the α/α′, β/β′ and γ lobes (Crittenden et al. 1998). Centrophobism is greatly diminished in flies with chemical ablation of the MBs (Besson & Martin 2005). Blocking neural activity of the γ lobes, but not of the α/α′ and β/β′ lobes of MB, in particular, results in increased centrophobism (Besson & Martin 2005) through a cAMP-dependent pathway (Lebreton & Martin 2009; Wolfers et al. 2001). Dopamine neurons are particularly susceptible to the toxicity of Synuclein A30P expression. A known cluster of the protocerebral anterior medial (PAM) dopamine neurons was found projecting to the distal tip of γ lobes (Mao & Davis 2009), making PAM a prime substrate of A30P toxicity, although the involvement of other anatomical regions cannot be excluded. To determine the involvement of dopamine neurons in regulating walking, it would be interesting to evaluate whether l-dopa feeding would ameliorate the observed walking deficits and to characterize the walking properties of aged fly, when A30P was expressed exclusively in dopamine neurons or in PAM neurons.
In sum, this study is the first to characterize walking of human synuclein fly model and has demonstrated multiple walking deficits and centrophobism in old A30P flies. Dopamine neurons are widely distributed in the fly brain. This study, combined with a panoply of Drosophila genetic tools for spatial and temporal expression A30P expression, paves ways to further identify the mechanism for αSyn toxicity causing the observed walking deficits at both cellular and circuitry levels. The result of this study also presents an alternative behavior paradigm to the climbing assay for screening drug efficacy or neuroprotective genes.
Acknowledgments
This work was supported by Dart NeuroScience LLC. All authors have no conflicts of interest to declare. We thank Dr Mel Feany for their generosity of gifting UAS-A30P. We thank Dr Valente who established a prototype algorithm for locomotion tracking, which was used when optimizing walking paradigm.
References
- Almeida QJ. Lebold CA. Freezing of gait in Parkinson's disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry. 2010;81:513–518. doi: 10.1136/jnnp.2008.160580. [DOI] [PubMed] [Google Scholar]
- Auluck PK. Bonini NM. Pharmacological prevention of Parkinson disease in Drosophila. Nat Med. 2002;8:1185–1186. doi: 10.1038/nm1102-1185. [DOI] [PubMed] [Google Scholar]
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM. Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- Benzer S. Genetic dissection of behavior. Sci Am. 1973;229:24–37. doi: 10.1038/scientificamerican1273-24. [DOI] [PubMed] [Google Scholar]
- Besson M. Martin JR. Centrophobism/thigmotaxis, a new role for the mushroom bodies in Drosophila. J Neurobiol. 2005;62:386–396. doi: 10.1002/neu.20111. [DOI] [PubMed] [Google Scholar]
- Bilen J. Bonini NM. Drosophila as a model for human neurodegenerative disease. Annu Rev Genet. 2005;39:153–171. doi: 10.1146/annurev.genet.39.110304.095804. [DOI] [PubMed] [Google Scholar]
- Botella JA, Bayersdorfer F. Schneuwly S. Superoxide dismutase overexpression protects dopaminergic neurons in a Drosophila model of Parkinson's disease. Neurobiol Dis. 2008;30:65–73. doi: 10.1016/j.nbd.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Chen L. Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- Chen AY, Xia S, Wilburn P. Tully T. Olfactory deficits in an alpha-synuclein fly model of Parkinson's disease. PLoS One. 2014;9:e97758. doi: 10.1371/journal.pone.0097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie D, Limousin P, Peters A, Hariz M. Day BL. Doorway-provoked freezing of gait in Parkinson's disease. Mov Disord. 2012;27:492–499. doi: 10.1002/mds.23990. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EM, Han KA, Kalderon D. Davis RL. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem. 1998;5:38–51. [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Diaz C, Levy M, Binetti G. Litvan II. Neuropsychiatric syndromes in neurodegenerative disease: frequency and signficance. Semin Clin Neuropsychiatry. 1996;1:241–247. doi: 10.1053/SCNP00100241. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Open-field bheavior in the rat: what does it mean? Ann N Y Acad Sci. 1969;159:852–859. doi: 10.1111/j.1749-6632.1969.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Feany MB. Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- Fionn Murtagh PL. Ward's hierarchical clustering method: clustering criterion and agglomerative algorithm. Clin Orthop Relat Res. 2011 eprint arXiv:1111.6285. [Google Scholar]
- van der Geest R, van Laar T, Kruger PP, Gubbens-Stibbe JM, Bodde HE, Roos RA. Danhof M. Pharmacokinetics, enantiomer interconversion, and metabolism of R-apomorphine in patients with idiopathic Parkinson's disease. Clin Neuropharmacol. 1998;21:159–168. [PubMed] [Google Scholar]
- George S, van den Buuse M, San Mok S, Masters CL, Li QX. Culvenor JG. Alpha-synuclein transgenic mice exhibit reduced anxiety-like behaviour. Exp Neurol. 2008;210:788–792. doi: 10.1016/j.expneurol.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Giladi N, McMahon D, Przedborski S, Flaster E, Guillory S, Kostic V. Fahn S. Motor blocks in Parkinson's disease. Neurology. 1992;42:333–339. doi: 10.1212/wnl.42.2.333. [DOI] [PubMed] [Google Scholar]
- Hernandez-Vargas R, Fonseca-Ornelas L, Lopez-Gonzalez I, Riesgo-Escovar J, Zurita M. Reynaud E. Synphilin suppresses alpha-synuclein neurotoxicity in a Parkinson's disease Drosophila model. Genesis. 2011;49:392–402. doi: 10.1002/dvg.20740. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L. Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J, Pagonabarraga J, Pascual-Sedano B, Garcia-Sanchez C. Gironell A. Prevalence and correlates of neuropsychiatric symptoms in Parkinson's disease without dementia. Mov Disord. 2008;23:1889–1896. doi: 10.1002/mds.22246. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC. The neuropsychiatry of Parkinson's disease. Minerva Med. 2005;96:155–173. [PubMed] [Google Scholar]
- Lebreton S. Martin JR. Mutations affecting the cAMP transduction pathway disrupt the centrophobism behavior. J Neurogenet. 2009;23:225–234. doi: 10.1080/01677060802509160. [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE, Weintraub D, Sampaio C, Poewe W, Rascol O, Stebbins GT. Goetz CG. Anxiety rating scales in Parkinson's disease: critique and recommendations. Mov Disord. 2008;23:2015–2025. doi: 10.1002/mds.22233. [DOI] [PubMed] [Google Scholar]
- Liu Q, Liu S, Kodama L, Driscoll MR. Wu MN. Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr Biol. 2012;22:2114–2123. doi: 10.1016/j.cub.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z. Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maries E, Dass B, Collier TJ, Kordower JH. Steece-Collier K. The role of alpha-synuclein in Parkinson's disease: insights from animal models. Nat Rev Neurosci. 2003;4:727–738. doi: 10.1038/nrn1199. [DOI] [PubMed] [Google Scholar]
- Martin JR. A portrait of locomotor behaviour in Drosophila determined by a video-tracking paradigm. Behav Processes. 2004;67:207–219. doi: 10.1016/j.beproc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Martin J, Faure P. Ernst R. The power law distribution for walking-time intervals correlates with the ellipsoid-body in Drosophila. J Neurogenet. 2001;15:205–219. doi: 10.3109/01677060109167377. [DOI] [PubMed] [Google Scholar]
- Martin JR, Raabe T. Heisenberg M. Central complex substructures are required for the maintenance of locomotor activity in Drosophila melanogaster. J Comp Physiol A. 1999;185:277–288. doi: 10.1007/s003590050387. [DOI] [PubMed] [Google Scholar]
- Mehren JE. Griffith LC. Calcium-independent calcium/calmodulin-dependent protein kinase II in the adult Drosophila CNS enhances the training of pheromonal cues. J Neurosci. 2004;24:10584–10593. doi: 10.1523/JNEUROSCI.3560-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CS, Bartos I, Akay T, Marka S. Mann RS. Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila melanogaster. ELife. 2013;2:e00231. doi: 10.7554/eLife.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB. Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–744. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksman M, Tanila H. Yavich L. Behavioural and neurochemical response of alpha-synuclein A30P transgenic mice to the effects of L-DOPA. Neuropharmacology. 2009;56:647–652. doi: 10.1016/j.neuropharm.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Olson AJ, Tully T. Sachidanandam R. GeneSeer: a sage for gene names and genomic resources. BMC Genomics. 2005;6:134. doi: 10.1186/1471-2164-6-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton RG, Parvez F, Sayed M. Hillman R. Effects of pharmacological agents upon a transgenic model of Parkinson's disease in Drosophila melanogaster. J Pharmacol Exp Ther. 2002;300:91–96. doi: 10.1124/jpet.300.1.91. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI. Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Rajesh Pahwa KEL. Handbook of Parkinson's Disease. Boca Raton, FL: CRC Press; 2007. 4 Chap. 4. edn. [Google Scholar]
- Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Serway CN, Kaufman RR, Strauss R. de Belle JS. Mushroom bodies enhance initial motor activity in Drosophila. J Neurogenet. 2009;23:173–184. doi: 10.1080/01677060802572895. [DOI] [PubMed] [Google Scholar]
- Shiba M, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ. Rocca WA. Anxiety disorders and depressive disorders preceding Parkinson's disease: a case-control study. Mov Disord. 2000;15:669–677. doi: 10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Strauss R. Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh K, Moore K, Wes PD, Muchowski PJ, Dey J, Andrews L. Pallanck LJ. Induction of the phase II detoxification pathway suppresses neuron loss in Drosophila models of Parkinson's disease. J Neurosci. 2008;28:465–472. doi: 10.1523/JNEUROSCI.4778-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Chen H, Schwarzschild MA, Kawachi I. Ascherio A. Prospective study of phobic anxiety and risk of Parkinson's disease. Mov Disord. 2003;18:646–651. doi: 10.1002/mds.10425. [DOI] [PubMed] [Google Scholar]
- Whitworth AJ, Theodore DA, Greene JC, Benes H, Wes PD. Pallanck LJ. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. Proc Natl Acad Sci USA. 2005;102:8024–8029. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S. Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- Xia S, Miyashita T, Fu TF, Lin WY, Wu CL, Pyzocha L, Lin IR, Saitoe M, Tully T. Chiang AS. NMDA receptors mediate olfactory learning and memory in Drosophila. Curr Biol. 2005;15:603–615. doi: 10.1016/j.cub.2005.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L, Oksman M, Tanila H, Kerokoski P, Hiltunen M, van Groen T, Puolivali J, Mannisto PT, Garcia-Horsman A, MacDonald E, Beyreuther K, Hartmann T. Jakala P. Locomotor activity and evoked dopamine release are reduced in mice overexpressing A30P-mutated human alpha-synuclein. Neurobiol Dis. 2005;20:303–313. doi: 10.1016/j.nbd.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Yu JY, Kanai MI, Demir E, Jefferis GS. Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG. de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]


