Abstract
Ferrous iron has been known to function as an electron source for iron-oxidizing microorganisms in both anoxic and oxic environments. A diversity of bacteria has been known to oxidize both soluble and solid-phase Fe(II) forms coupled to the reduction of nitrate. Here, we show for the first time Fe(II) oxidation by Sphaerotilus natans strain DSM 6575T under mixotrophic condition. Sphaerotilus natans has been known to form a sheath structure enclosing long chains of rod-shaped cells, resulting in a thick biofilm formation under oxic conditions. Here, we also demonstrate that strain DSM 6575T grows mixotrophically with pyruvate, Fe(II) as electron donors and nitrate as an electron acceptor and single cells of strain DSM 6575T are dominant under anoxic conditions. Furthermore, strain DSM 6575T forms nanoball-shaped amorphous Fe(III) oxide minerals encrusting on the cell surfaces through the mixotrophic iron oxidation reaction under anoxic conditions. We propose that cell encrustation results from the indirect Fe(II) oxidation by biogenic nitrite during nitrate reduction and that causes the bacterial morphological change to individual rod-shaped single cells from filamentous sheath structures. This study extends the group of existing microorganisms capable of mixotrophic Fe(II) oxidation by a new strain, S. natans strain DSM 6575T, and could contribute to biogeochemical cycles of Fe and N in the environment.
Keywords: iron oxidation, nitrate, Sphaerotilus natans, iron mineral, biogeochemical cycling
Introduction
Iron (Fe) exists in divalent or trivalent states within the biosphere depending on the environmental conditions (Cornell & Schwertmann, 2004). Although the abiotic redox changes between Fe(II) and Fe(III) play an important role in redox processes in the environment, microorganisms also significantly contribute to iron biogeochemical cycling in both oxic and anoxic environments on Earth (Kappler & Straub, 2005; Weber et al., 2006a,b, c), because they are able to utilize both Fe(II) and Fe(III) as electron donor and acceptor, respectively. However, the exact mechanisms of Fe mineral formation during microbial Fe(II) oxidation is barely understood (Benzerara et al., 2011).
Previous studies suggested that the anaerobic microbiological Fe(II) oxidation occurs either chemotrophically with nitrate (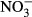 ) as the electron acceptor (Nealson & Saffarini, 1994; Hafenbradl et al., 1996; Straub et al., 1996; Lack et al., 2002) or phototrophically (Widdel et al., 1993; Ehrenreich & Widdel, 1994; Hegler et al., 2008; Poulain & Newman, 2009), which results in the formation of Fe(III) precipitates under anoxic environments. Hafenbradl et al. (1996) reported that the microbiological Fe(II) oxidation coupled with nitrate reduction was achieved using enrichment cultures and pure cultures in the absence of oxygen as a light-independent, chemotropic microbial process. The appearance of microbiological nitrate-dependent Fe(II) oxidation under anoxic natural conditions may play significant roles in coupling the redox cycling of N and Fe in sedimentary environments (Weber et al., 2001). Furthermore, the anaerobic nitrate-dependent Fe(II) oxidation has important implications for soil and sediment mineralogy and geochemistry through the formation of Fe(III) oxides, including a variety of environmentally relevant Fe(III)-bearing minerals such as ferric oxyhydroxide, goethite, haematite, green rust, and magnetite (Straub & Buchholz-Cleven, 1998; Chaudhuri et al., 2001; Lack et al., 2002; Weber et al., 2006a ,b, c; Pantke et al., 2012).
) as the electron acceptor (Nealson & Saffarini, 1994; Hafenbradl et al., 1996; Straub et al., 1996; Lack et al., 2002) or phototrophically (Widdel et al., 1993; Ehrenreich & Widdel, 1994; Hegler et al., 2008; Poulain & Newman, 2009), which results in the formation of Fe(III) precipitates under anoxic environments. Hafenbradl et al. (1996) reported that the microbiological Fe(II) oxidation coupled with nitrate reduction was achieved using enrichment cultures and pure cultures in the absence of oxygen as a light-independent, chemotropic microbial process. The appearance of microbiological nitrate-dependent Fe(II) oxidation under anoxic natural conditions may play significant roles in coupling the redox cycling of N and Fe in sedimentary environments (Weber et al., 2001). Furthermore, the anaerobic nitrate-dependent Fe(II) oxidation has important implications for soil and sediment mineralogy and geochemistry through the formation of Fe(III) oxides, including a variety of environmentally relevant Fe(III)-bearing minerals such as ferric oxyhydroxide, goethite, haematite, green rust, and magnetite (Straub & Buchholz-Cleven, 1998; Chaudhuri et al., 2001; Lack et al., 2002; Weber et al., 2006a ,b, c; Pantke et al., 2012).
In addition to the geochemical importance of the anaerobic microbiological Fe(II) oxidation, it has been widely recognized that the aerobic microbiological Fe(II) oxidation under acidic or neutral pH conditions successfully competes with the chemical iron oxidation (Blake et al., 1993; Emerson & Revsbech, 1994; Blake & Johnson, 2000; Emerson, 2000). Indeed, aerobic Fe(II)-oxidizing bacteria mostly belonging to Betaproteobacteria such as Sphaerotilus/Leptothrix, Gallionella, and Rhodocyclus spp. or to some genera in the Alpha- and Gammaproteobacteria (Emerson et al., 2010) have been found in a broad range of environments. Among Fe(II)-oxidizing bacteria, Sphaerotilus natans has been characterized by a sheath-forming bacterium enclosing long chains of rod-shaped cells (Hoeniger et al., 1973). It has been suggested that S. natans is the dominant filamentous bacterium causing bulky biofilm and pipe clogging in waste water treatments due to the formation of sheaths which allow a means of attachment to solid surfaces (Hoeniger et al., 1973). Despite the environmental issues due to this filamentous bacterium, less information is available regarding iron biomineralization by S. natans and potential importance for the scavenging of inorganic pollutants (Seder-Colomina et al., 2013). This has led many studies with S. natans to solve bulking problems caused by the filamentous growth of the cells in activated sludge (Gaudy & Wolfe, 1961; Takeda et al., 1998; Suzuki et al., 2002). In addition, it has been known that S. natans strains harbored nitrate reductase activity (Pellegrin et al., 1999). Although genetic information on nitrate reductase of S. natans was not available to date, the presence of nitrate reductase activity motivated us to study the capacity of Fe(II) oxidation by S. natans at neutral pH under nitrate-reducing conditions.
In this study, we tried to determine anaerobic Fe(II) oxidation by S. natans causing cell encrustation and to identity the formed Fe (III) oxide minerals at the cell surfaces. Interestingly, under nitrate-reducing conditions, sheath-forming filamentous S. natans displayed a morphological change to individual rod-shaped single cells encrusted by nanoball-shaped Fe(III) oxide minerals formed from the oxidation of Fe(II). Demonstration of the anaerobic nitrate-dependent Fe(II) oxidation process with formation of Fe(III) oxides encrusting single cells of S. natans could provide information about the extended bacteria group for mixotrophic iron oxidation and also essentially contributes to anaerobic Fe cycling with N.
Materials and methods
Medium and culture conditions
Iron-oxidizing bacterium S. natans strain DSM 6575T was purchased from the Deutsche Sammlung von Mikroorganismen (DSMZ, Braunschweig, Germany) and was pregrown in CGYA medium (5 g casitone, 10 g glycerol, and 1 g yeast extract L−1) (Nierman & Maglott, 1989) under aerobic conditions at 30 °C for 1 day. Pregrown culture of S. natans was inoculated to basal medium including 4 mM FeCl2, 4 mM nitrate, and 2 mM pyruvate. The basal medium for Fe(II)-oxidizing bacteria prepared as described by Enrenich and Widdel (Ehrenreich & Widdel, 1994). The composition of the basal medium was as follows: 0.14 g L−1 KH2PO4, 0.2 g L−1 NaCl, 0.5 g L−1 MgSO4·7H2O, 0.3 g L−1 NH4Cl, 0.1 g L−1 CaCl2·2H2O, 5.4 mg L−1 KH2PO·4H2O, 1 mL L−1 vitamin solution, 1 mL L−1 trace element solution, and 22 mM bicarbonate buffer, pH 6.8–7.2, and the headspace of medium was flushed with N2/CO2 (80/20%). The addition of the 5 mM FeCl2 from anoxic 1 M stock solution to the basal medium led to precipitation of a greenish-white precipitates consisting of Fe(II) phosphate and Fe(II) carbonate (Kappler & Newman, 2004). In order to exclude the presence of background of Fe(II) minerals before abiotic Fe(II) oxidation and Fe(II) mineral precipitation started, the basal medium was filtered using a 0.2-μm filter (MFS-25, Advantec MFS, Inc., Dublin, CA) in an anoxic chamber, leaving a clear solution with 3–4 mM dissolved Fe(II). The medium maintained free of Fe(II) precipitates for several weeks in the absence of Fe(II)-oxidizing bacteria, which allowed to identify biologically precipitated Fe(III)-bearing minerals by strain DSM 6575T. For abiotic Fe(II) oxidation experiment as a control, we added 4 mM FeCl2 and different concentration of nitrite (0.5, 1, 2, 4 mM) to the basal medium prepared as mentioned above under anoxic condition.
Analytical methods
All preparation processes were carried out in an anoxic glove box. For quantification of Fe(II), we used the revised ferrozine protocol for nitrite-containing samples (Klueglein & Kappler, 2013); 100 μL of culture suspension was withdrawn with a syringe and dissolved in 900 μL of 40 mM sulfamic acid for 1 h at room temperature. Sulfamic acid reacts with the nitrite present and thus preventing abiotic oxidation of Fe(II) by reactive N species formed during sample acidification. After digestion, 10 μL of the extracts were transferred to 2 mL of ferrozine (3-(2- pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4′-disulfonic acid monosodium salt, Catalog No. 82950, Fluka, Buchs, Switzerland) solution (ferrozine (1 g L−1) in HEPES buffer (50 mM) at pH 7) to make a ferrous complex, The Fe(II)-ferrozine complex was quantified at 562 nm using a UV/Vis spectrometer (Optizen POP, Mecasys, Korea). A separate calibration curve was prepared for the revised ferrozine assay using ferrous ethylene diammonium sulfate tetrahydrate (Catalog No. 44932, Fluka, Buchs, Switzerland) that was dissolved in 40 mM sulfamic acid. In order to quantify nitrate and nitrite in the aqueous medium, 1.0 mL was extracted using a 1.0 mL syringe (Korean Vaccine Co., LTD, Seoul, Korea), filtered through a 0.2-μm syringe filter (MFS-25, Advantec MFS, Inc.) and exposed to air to prevent further reduction of nitrite by Fe(II) (Sørensen & Thorling, 1991). Filtered samples were centrifuged, and the supernatant was withdrawn for nitrate and nitrite analyses. Nitrate and nitrite were determined by ion chromatography (Dionex, CA) equipped with IonPac AG14 guard and AS14 analytical columns (Thermo Scientific, Sunnyvale, CA). Nitrate and nitrite were separated using isocratic conditions consisting of 3.5 mM Na2CO3/1 mM NaHCO3 for 15 min. The injection volume was 10 μL, and the flow rate was 1.2 mL min−1. Furthermore, N2O and NO were quantified by gas chromatography coupled mass spectrometry (GC-MS) directly from the culture headspace. Pyruvate was quantified by high-performance liquid chromatography (HPLC), which was equipped with a photodiode array (PDA) detector (Varian, Walnut Creek, CA) and a Aminex HPX-87H ion exclusion column (Bio-Rad, Hercules, CA). The mobile phase was 5 mM sulfuric acid with a flow rate of 0.6 mL min−1 for 30 min. Ten microliter of each sample was injected, and the UV detection was performed at 210 nm.
Analyses of bacterial and mineralogical morphologies
During mineralogical analyses, precipitated mineral residues were collected from the culture medium using syringes at 10 days of incubation and washed three times with deionized water. The washed minerals were dried in the anoxic glove box. X-ray diffraction (XRD) analysis was performed using a Rigaku D/MAX Ultima III high resolution XRD meter (Rigaku, Tokyo, Japan) equipped with monochromatic high-intensity CuK α radiation. The generator was operated at 40 kV and 40 mA. The samples were scanned between 2θ = 10° and 70° at a scan speed 1° min−1. Scanning electron microscopic (SEM, XL30-FEG, Philips, Eindhoven, The Netherlands) and transmission electron microscopic (TEM, JEOL JEM-2100, Tokyo, Japan) analyses were performed with samples to ascertain bacterial and mineralogical morphologies. Samples were prepared as described previously by Schadler et al.(2008). One microliter of culture was withdrawn anoxically with sterile syringes at a selected time, centrifuged at 9000 g for 3 min, and washed with distilled water to remove medium salt. For SEM analyses, samples were fixed with 2% glutaraldehyde and 4% formaldehyde in 20 mM KH2PO4 and 80 mM Na2HPO4 buffer solution for 20 min at room temperature. After fixation, the samples were washed three times in phosphate-buffered saline (PBS). Ten microliter of PBS suspended samples were deposited onto silicon wafers, dried in anoxic chamber for 12 h. The grid was washed with PBS for 10 min and immersed into a dehydration dilution series of distilled water. The prepared samples for SEM analyses were coated with a thin layer of Au/Pd [90/10% (w/w)]. The coating thickness was c. 10 nm. For TEM analyses, 10 μL of the samples fixed with 3% glutaraldehyde for 2 h at 4 °C, centrifuged for a few minutes at 3637 g and, subsequently, washed three times in distilled water. The fixed samples were stained in 1% osmium tetroxide (OsO4) for 3 h at 4 °C and placed onto carbon-coated 200-mesh copper grids. The images were obtained at 200 kV using a JEOL JEM-JEM-2100 high resolution TEM (JEOL, Tokyo, Japan). The cross-sections were prepared by ultramicrotomy. The cross-sectioned specimens were taken from fixed cells as mentioned above and progressively embedded in epoxy resin (Epoxy, Fluka Chemika, St. Louis, MO). Cross-sections with a nominal thickness of 70 nm were prepared with an EM-UC6 ultramicrotome (LEICA, Wetzlar, Germany) using a diamond knife. After deposition on a copper grid, they were stained with uranyl acetate [2% (w/v)] and lead citrate (2 g L−1).
Results and discussion
Mixotrophic Fe(II) oxidation by S. natans strain DSM 6575T
In the previous study, Gaudy & Wolfe (1961) reported that iron-oxidizing bacteria, S. natans strain DSM 6575T, grew as a single cell in the presence of both 0.5% of glucose and peptone, and S. natans also showed filamentous growth with sheath formation in the presence of both 0.1% of glucose and peptone (Supporting Information, Fig. S1a and b). However, no additional factors that regulate filamentous growth with sheath formation have been described (Pellegrin et al., 1999). In our result, it was observed that single rod cells of S. natans strain DSM 6575T were dominant rather than filamentous long-chained cells in the basal medium with pyruvate and nitrate under anoxic conditions (Fig. S1c). It is suggested that bacterial culture condition such as either the presence of nitrate or anoxic condition can affect the bacterial morphology of S. natans.
Furthermore, Pellegrin et al. (1999) confirmed that microaerobic Fe(II)-oxidizing and sheathed filamentous S. natans strain DSM 6575T harbored nitrate reductase. The amino acid sequence of nitrate reductase from strain DSM 6575T showed 75.7%, 62.3%, 21% and 20.2% identities to those from Leptothrix cholodnii SP-6 (accession number YP001791710), Acidovorax delafieldil (accession number WP005798151), Paracoccus denitrificans PD1222 (accession number YP918478), and Pseudomonas sp. G-179 (accession number AAC79443) (Fig. S2), respectively. The sequence comparison suggests that nitrate reductase from nitrate-dependent Fe(II) oxidizing bacteria could be quite different structurally from denitrifying bacteria, P. denitrificans and Pseudomonas sp.
In this study, strain DSM 6575T almost completely consumed the provided pyruvate with decreasing from 2 mM to c. 0.02–0.04 mM by 10 days of incubation in bacterial medium containing either nitrate or nitrate and Fe(II) during 10 days of incubation with a similar metabolizing trend for pyruvate (Fig. 1). However, the amount of consumed pyruvate in the bacterial medium containing both nitrate and Fe(II) as an electron acceptor and donor, respectively, was slightly higher than that in the bacterial medium containing nitrate alone as an electron acceptor (Fig. 1), and the rate of pyruvate consumption for the first 2 days in the presence of both Fe(II) and nitrate was higher than that in the presence of nitrate only, suggesting physiological effect of Fe(II) on the initial anaerobic metabolism of pyruvate. Based on the results, strain DSM 6575T was able to grow heterotrophically with pyruvate and also mixotrophically with pyruvate and Fe(II) by utilizing nitrate as an electron acceptor under anoxic conditions. In addition, strain DSM 6575T hardly oxidized Fe(II) in the absence of pyruvate (Fig. 2a), similar to other bacteria previously described (Straub et al., 1996, 2004; Benz et al., 1998; Lack et al., 2002). Strain DSM 6575T was not able to utilize ferrous iron as a sole electron donor, that is, they need an organic cosubstrate such as pyruvate for both growth and Fe(II) oxidation. Muehe et al. (2009) demonstrated that mixotrophic oxidation of ferrous iron with cosubstrate, acetate, enhanced growth yields with acetate alone (12.5 g dry mass mol−1 acetate) by about 1.4 g dry mass mol−1 Fe(II) and contributes to the energy metabolism of bacteria. Considering the fact that lithoautotrophical growth of the pure culture with both Fe(II) and nitrate was reported to be weak, with hardly two doublings in one cultivation period (Weber et al., 2006a ,b, c,2006b), mixotrophic Fe(II) oxidation with pyruvate is the preferred process for nitrate-dependent Fe(II) oxidation in most environments (Straub & Buchholz-Cleven, 1998; Hauck et al., 2001). Indeed, when both nitrate (4 mM) and pyruvate (2 mM) were present in the bacterial culture as an electron acceptor and as an organic substrate, respectively, strain DSM 6575T almost oxidized the dissolved Fe(II, 4 mM) within 10 days of incubation while consuming only 0.5 mM for the first 2 days (Fig. a). This two-phase Fe(II) oxidation pattern was also previously reported with Acidovorax strain BoFeN1 (Klueglein & Kappler, 2013; Klueglein et al., 2014), Pseudogulbenkiania strain 2002 (Weber et al., 2006a ,b, c,2006b), P. denitrificans ATCC 19367, and P. denitrificans Pd 1222 (Klueglein et al., 2014). The decrease of Fe(II) in bacterial medium without either nitrate, pyruvate (Fig. 2a), or bacterial inoculation (Fig. 2b) was not observed. As mentioned before, strain DSM 6575T barely oxidized Fe(II) in the absence of pyruvate (Fig. 2a), indicating that strain DSM 6575T was likely to depend on the presence of pyruvate for the oxidation of Fe(II). As strain DSM 6575T oxidized Fe(II) in the bacterial culture containing nitrate and pyruvate during incubation, the provided nitrate (4 mM) was also decreased to c. 0. 3 mM with a small amount of nitrite production in the range of 0.9–1.0 mM at day 10 (Fig. 3). In addition, strain DSM 6575T consumed more nitrate in the bacterial culture containing pyruvate in the presence of Fe(II) than in the absence of Fe(II) during 10 days of incubation (Fig. 3a). It is assumed that addition of Fe(II) in the presence of pyruvate is likely to provide another electron sources to strain DSM 6575T for the assimilatory or dissimilatory nitrate utilization with more consumption of pyruvate under anoxic conditions. It should be noted that nitrite accumulation in bacterial culture started at day 1 and reached the maximum concentration, c. 1.0 mM after 6 days (Fig. 3b). Neither decrease of nitrate nor increase of nitrite was observed in the control experiment without bacterial inoculation (Fig. S3). Nitrite was not formed at bacterial culture in the absence of either Fe(II) or pyruvate (Fig. 3b). The accumulation of nitrite in bacterial culture of nitrate-dependent Fe(II) oxidation reaction has been also observed in previous studies (Kappler et al., 2005; Larese-Casanova et al., 2010; Chakraborty et al., 2011). The exact reason that nitrite is accumulated in the mixotrophic Fe(II) oxidation culture has been remained an enigma (Picardal, 2012). However, there is possibility that precipitation of Fe(III) mineral on the nitrite reductase through abiotic Fe(II) oxidation by biogenic nitrite causes inhibition of the nitrite reductase by mineral deposition and leads to nitrite accumulation (Miot et al., 2011; Picardal, 2012). However, further reduced forms of nitrogen species, N2O and NO, in bacterial cultures amended with dissolved Fe(II), pyruvate, and nitrate, were not detected in the culture headspace (data not shown).
Fig 1.
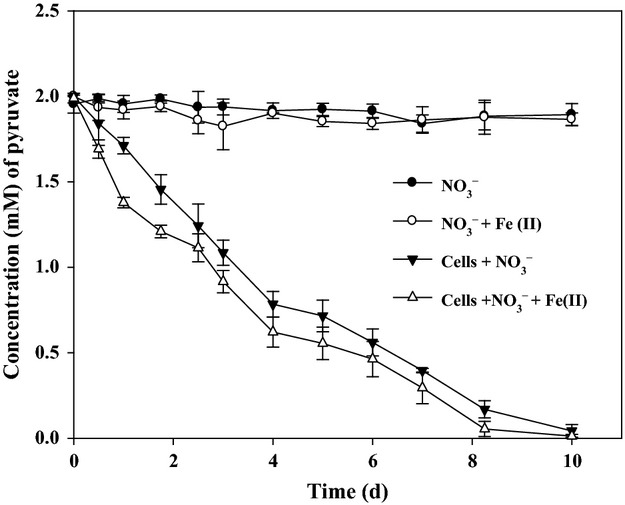
Concentration of consumed pyruvate by Sphaerotilus natans strain DSM 6575T with 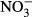 alone as electron acceptor (▾), and with nitrate and Fe(II) (▵) in anaerobic medium, and no bacterial inoculation with nitrate (●), and with nitrate and Fe(II) (○) as control experiments. The error bars indicate standard deviation calculated from three independent parallels. The absence of error bars indicates that the error was smaller than the symbol size.
alone as electron acceptor (▾), and with nitrate and Fe(II) (▵) in anaerobic medium, and no bacterial inoculation with nitrate (●), and with nitrate and Fe(II) (○) as control experiments. The error bars indicate standard deviation calculated from three independent parallels. The absence of error bars indicates that the error was smaller than the symbol size.
Fig 2.
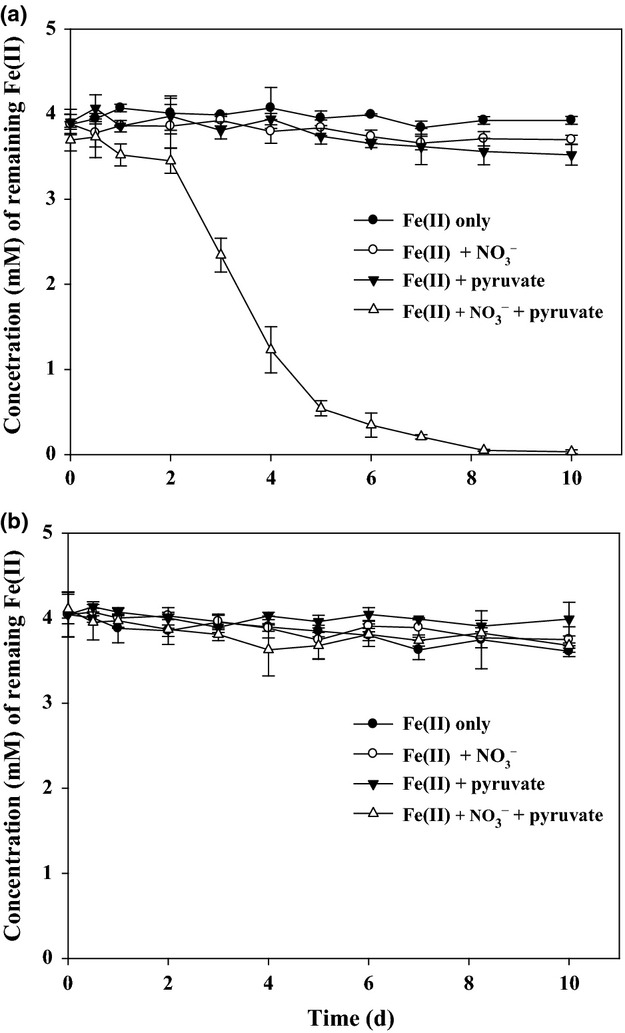
Concentration of remaining Fe(II) in the bacterial culture of Sphaerotilus natans DSM 6575T (a) and control experiment without bacterial inoculation (b). The bacterial culture and control experiment were incubated with Fe(II) (●), with Fe(II) and nitrate (○), with Fe(II) and pyruvate (▾), and with Fe(II), nitrate, and pyruvate (▵), respectively. The error bars indicate standard deviation calculated from three independent parallels. The absence of error bars indicates that the error was smaller than the symbol size.
Fig 3.
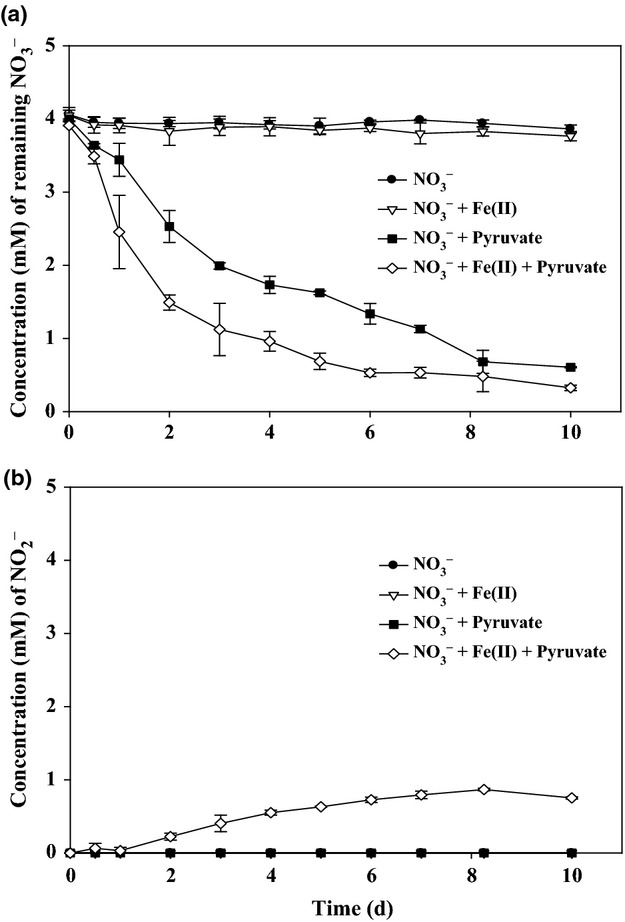
Concentration of remaining nitrate (a) and present nitrite (b) in the bacterial culture of Sphaerotilus natans strain DSM 6575T. The bacterial culture was incubated with nitrate alone (●), with nitrate and Fe(II) (▵), with nitrate and pyruvate (■), and with nitrate, Fe(II), and pyruvate (Δ), respectively. The error bars indicate standard deviation calculated from three independent parallels. The absence of error bars indicates that the error was smaller than the symbol size.
It has been recognized that abiotic oxidation of Fe(II) by nitrate at neutral pH occurs at high temperature (75 °C) (Buresh & Moraghan, 1976) or in the presence of green rust (Hansen et al., 1996). Therefore, the abiotic oxidation of Fe(II) by nitrate in this study is negligible. Indeed, it has been known that Fe(II) oxidation by nitrite is more rapid than by nitrate (Picardal, 2012). The question is whether the observed Fe(II) oxidation was an enzymatic reaction or chemical reaction by nitrite produced from the nitrate reduction. Klueglein et al. (2014) reported recently that Fe(II) oxidation is a nitrite-driven, indirect mechanism during heterotrophic denitrification of Acidovorax strain BoFeN1, Pseudogulbenkiania strain 2002, P. denitrificans ATCC 19367, and P. denitrificans Pd 1222. As shown in Figs2 and 3, fast decrease of Fe(II) after 2 days incubation of strain DSM 6575T was correlated with nitrite accumulation found only in bacterial culture with pyruvate and Fe(II), suggesting abiotic Fe(II) oxidation by nitrite produced during mixotrophic denitrification. However, it does not rule out the possibility of inducible enzymatic reactions by different bacteria. No concrete evidences have been revealed for the enzymatic or abiotic reactions for Fe(II) oxidation under denitrifying conditions (Glasauer et al., 2013; Klueglein & Kappler, 2013; Klueglein et al., 2014).
Cell encrustation and Fe(III) mineral formation
SEM image analyses also showed that single cells of strain DSM 6575T encrusted with Fe(III) mineral crusts at their cell surface in the presence of pyruvate and Fe(II) as the electron donor and nitrate as the electron acceptor (Fig. 4). Interestingly, some of the enlarged Fe(III) oxides seemed to show holes or hollowness within the ball-shape structure (see arrows, Fig. 4c). Fe(III) mineral crusts around the cell surfaces were formed as soon as Fe(II) oxidation occurred (Fig. 4a and b), and bacterial cells were completely encrusted with Fe(III) mineral formed via iron oxidation (Figs 4c, d and 5a, c). TEM analyses revealed the images of bacterial cell cross-section showing the cell interior as well as cell–mineral interfaces (Fig. 5c). It is observed that S. natans cells contained iron mineral in the cellular membrane, and the thickness of the iron mineral layer is c. 30–40 nm (Fig. 5c). The thickness of the mineral layer varies among the cells. In addition, the presence of ball-shaped on the surface of the cells as observed in SEM analyses was confirmed (Fig. 5a and c). However, cell encrustation was not observed in the bacterial culture containing both ferrous iron and nitrite instead of nitrate (Fig. 4b). Several studies have been also demonstrated that Fe(III) mineral precipitation by nitrate-reducing Fe(II) oxidizer starts in the periplasm, continuous on the cell surface, and then terminates in the cytoplasm (Miot et al., 2009; Klueglein et al., 2014).
Fig 4.
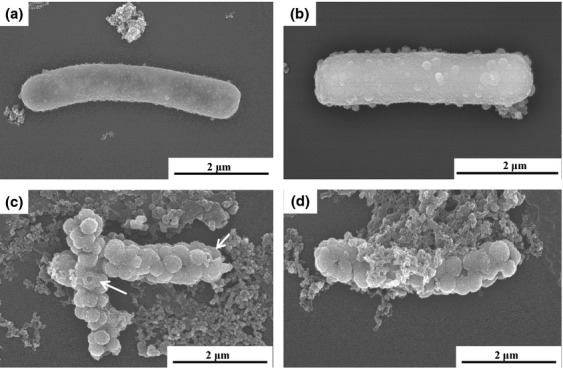
SEM images of Sphaerotilus natans strain DSM 6575T grown in the presence of Fe(II),  , and pyruvate under anaerobic conditions. Samples of S. natans strain DSM 6575T were taken after 1 day (a–b) and 3 days (c–d). The insert (d) shows a close-up image of S. natans strain DSM 6575T with Fe(III) oxide mineral deposition at the cell surface during Fe(II) oxidation under anaerobic conditions.
, and pyruvate under anaerobic conditions. Samples of S. natans strain DSM 6575T were taken after 1 day (a–b) and 3 days (c–d). The insert (d) shows a close-up image of S. natans strain DSM 6575T with Fe(III) oxide mineral deposition at the cell surface during Fe(II) oxidation under anaerobic conditions.
Fig 5.
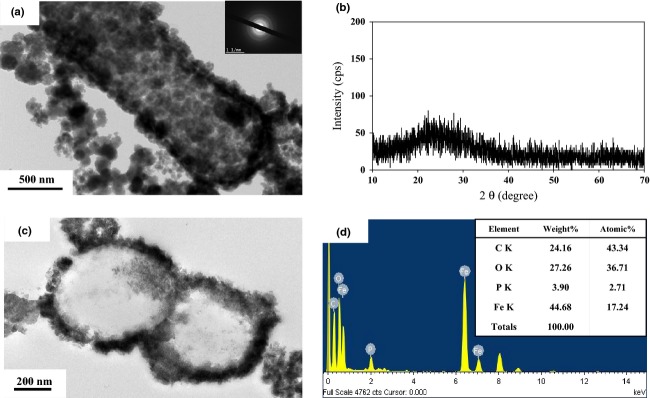
TEM image of Sphaerotilus natans DSM 6575T with inserted SAED pattern (a), XRD pattern of precipitates collected from bacterial cultures after 5 days incubation (b), TEM image of cross-sectioned S. natans strain DSM 6575T (c), and EDS spectrum (d), showing encrustation of the cell surface with amorphous Fe(III) oxide mineral deposition at the cellular membrane structure. The bacterial culture were incubated with pyruvate, Fe(II), and nitrate.
It has been reported that metabolically diverse Fe(II)-oxidizing bacteria were encrusted mainly by crystallized Fe(III) goethite mineral (Emerson & Moyer, 1997; Kappler & Straub, 2005; Schaedler et al., 2009). Partly or fully encrusted bacterial cells have been also observed in natural environments, such as Fe(II)-rich rivers and springs (Benzerara et al., 2008; Preston et al., 2011). Moreover, many studies suggest that the encrustation of bacterial cells by Fe(III) oxide minerals has been considered biosignatures or microfossils resulted from the presence of microbial activity in modern and ancient environments (Banfield et al., 2001; Posth et al., 2008; Cosmidis et al., 2013; Glasauer et al., 2013). However, it is unclear whether the Fe(III) oxide mineral formation encrusting the cell surface is beneficial to the bacterial cells for uptaking or diffusing substrate (Hallberg & Ferris, 2004). In our study, the result of consumed pyruvate by strain DSM 6575T indicates that encrusted cells could still utilize pyruvate (Fig. 1), and therefore, substrate may be transported to the encrusted cells. Previously, the results were reported that encrusted cells were metabolically active (Miot et al., 2009) and even divided (Schaedler et al., 2009). As some of researchers suggested, there are possibilities that cell encrustation can protect cells from UV radiation, predation, and dehydration (Pierson et al., 1993; Phoenix & Konhauser, 2008). Furthermore, Fe(III) oxide mineral crust at the cell surface may play a role in electrons transfer from Fe(II) to the cells via the conductive iron mineral crust, as it was shown in abiotic mineral environment (Schaefer et al., 2011).
In addition, XRD analysis for the Fe(III) oxide minerals formed from the nitrate-dependent Fe(II) oxidation by strain DSM 6575T did not reveal significant signals of crystalline phases (Fig. 5b and d), indicating formation of amorphous or less crystalline Fe(III) oxide minerals. It has been also known that biologically produced amorphous Fe(III) oxide minerals could be an excellent substrate for a diverse Fe(III)-reducing bacteria in environments (Straub & Buchholz-Cleven, 1998; Lovley et al., 2004) and highly reactive to other metal(loids)s such as arsenic, uranium, and organic pollutants (Weber et al., 2001; Borch et al., 2009; Hohmann et al., 2009, 2011; Vaughan & Lloyd, 2011; Hitchcock et al., 2012). Thus, this iron species transformation between Fe(II) and Fe(III) by coupling Fe(II)-oxidizing bacteria with Fe(III)-reducing bacteria in the environments could provide important ecological and environmental implications for understanding biogeochemical cycling of Fe.
On one hand, the abiotic reaction of ferrous iron with nitrite in the basal medium without bacterial culture at neutral pH leaded to nano-sized yellowish precipitates (Fig. S4a) and identified as goethite [XRD Power Diffraction File (PDF) number 81-0462] (Fig. S4b). The recent study demonstrated that nitrite reacts abiotically with aqueous Fe(II), resulting in the formation of green-rust-like minerals, which are further oxidized to form goethite as the final product (Kampschreur et al., 2011). In the recent studies, Pantke et al. (2012) reported the formation of green rust during Fe(II) oxidation by the nitrate-reducing Acidovorax sp. strain BoFeNa, and Klueglein et al. (2014) also identified goethite mineral formed by heterotrophic denitrification of Acidovorax strain BoFeN1, Pseudogulbenkiania strain 2002, P. denitrificans ATCC 19367, and P. denitrificans Pd 1222. However, as previously mentioned, the bacterial culture of strain DSM 6575T in the basal medium containing ferrous iron and nitrate showed reddish precipitates of amorphous or less crystalline Fe(III) oxide minerals (Fig. 5b and d). Considering Fe(II) oxidation under denitrifying conditions and formation of amorphous or less crystalline Fe(III) oxide minerals on the bacterial surface with changing bacterial morphology, we propose that S. natans strain DSM 6575T mediates abiotic Fe(II) oxidation using nitrate as an electron acceptor in the presence of pyruvate and provides inducible enzymatic reaction mechanism for the cell encrustation with amorphous iron oxide minerals on the cell membrane. Further experiments are necessary to identify the corresponding enzymes involved in the iron oxidation reactions and their location in the cells.
In summary, Fe(II)-oxidizing and sheath-forming S. natans strain DSM 6575T was able to oxidize Fe(II) using nitrate as an electron acceptor in the presence of pyruvate and appeared as single cells encrusted by the nanoball-shaped amorphous Fe(III) oxide minerals, which contributes to biogeochemical cycles of Fe and N in anaerobic environment.
Nucleotide sequence accession numbers
This Whole-Genome Shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession AZRA00000000. The version described in this paper is version AZRA01000000.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea (NRF: 2010-0029224), Ministry of Education, Science & Technology, Korea.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Transmission electron microscopic images of Sphaerotilus natans grown as single cells in the presence of 0.5% glucose and 0.5% peptone (a), as filamentous cells in the presence of 0.1% peptone and 0.1% glucose (b) under aerobic condition, and (c) single cells in the basal medium with pyruvate under anaerobic condition.
Fig. S2. Homologies of nitrate reductase of Sphaerotilus natans strain DSM 6575T with other bacterial nitrate reductase.
Fig. S3. Concentration of dissolved nitrate in the control experiment without Sphaerotilus natans strain DSM 6575T inoculation.
Fig. S4. TEM images of goethite formed from abiotic reaction between ferrous iron at 4 mM and nitrite at 1 mM (a) and XRD pattern of abiotically formed goethite from ferrous iron and nitrite (b).
References
- Banfield JF, Moreau JW, Chan CS, Welch SA. Little B. Mineralogical biosignatures and the search for life on Mars. Astrobiology. 2001;1:447–465. doi: 10.1089/153110701753593856. [DOI] [PubMed] [Google Scholar]
- Benz M, Brune A. Schink B. Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria. Arch Microbiol. 1998;169:159–165. doi: 10.1007/s002030050555. [DOI] [PubMed] [Google Scholar]
- Benzerara K, Morin G, Yoon TH, et al. Nanoscale study of As biomineralization in an acid mine drainage system. Geochim Cosmochim Acta. 2008;72:3949–3963. [Google Scholar]
- Benzerara K, Miot J, Morin G, Ona-Nguema G, Skouri-Panet F. Férard C. Significance, mechanisms and environmental implications of microbial biomineralization. Comp Rend Geosci. 2011;343:160–167. [Google Scholar]
- Blake RC. Johnson DB. Environmmental Microbe-Metal Interactions. Washington, DC: ASM Press; 2000. [Google Scholar]
- Blake RC, Shute EA, Greenwood MM, Spencer GH. Ingledew WJ. Enzymes of aerobic respiration on iron. FEMS Microbiol Rev. 1993;11:9–18. doi: 10.1111/j.1574-6976.1993.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Borch T, Kretzschmar R, Kappler A, Cappellen PV, Ginder-Vogel M, Voegelin A. Campbell K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol. 2009;44:15–23. doi: 10.1021/es9026248. [DOI] [PubMed] [Google Scholar]
- Buresh RJ. Moraghan JT. Chemical reduction of nitrate by ferrous iron. J Environ Qual. 1976;5:320–325. [Google Scholar]
- Chakraborty A, Roden EE, Schieber J. Picardal F. Enhanced growth of Acidovorax sp. strain 2AN during nitrate-dependent Fe(II) oxidation in batch and continuous-flow systems. Appl Environ Microbiol. 2011;77:8548–8556. doi: 10.1128/AEM.06214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri SK, Lack JG. Coates JD. Biogenic magnetite formation through anaerobic biooxidation of Fe(II) Appl Environ Microbiol. 2001;67:2844–2848. doi: 10.1128/AEM.67.6.2844-2848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell RM. Schwertmann U. The Iron Oxides: Structure, Properties, Reaction, Occurrences and Uses. Weiheim, Germany: Wiley-VCH; 2004. Introduction to the iron oxides; pp. 1–7. [Google Scholar]
- Cosmidis J, Benzerara K, Gheerbrant E, Estève I, Bouya B. Amaghzaz M. Nanometer-scale characterization of exceptionally preserved bacterial fossils in Paleocene phosphorites from Ouled Abdoun (Morocco) Geobiology. 2013;11:139–153. doi: 10.1111/gbi.12022. [DOI] [PubMed] [Google Scholar]
- Ehrenreich A. Widdel F. Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl Environ Microbiol. 1994;60:4517–4526. doi: 10.1128/aem.60.12.4517-4526.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson D. Environmental Microbe-Metal Interactions. Washington, DC: ASM Press; 2000. [Google Scholar]
- Emerson D. Moyer C. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol. 1997;63:4784–4792. doi: 10.1128/aem.63.12.4784-4792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson D. Revsbech NP. Investigation of an iron-oxidizing microbial mat community located near aarhus, Denmark: field studies. Appl Environ Microbiol. 1994;60:4022–4031. doi: 10.1128/aem.60.11.4022-4031.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson D, Fleming EJ. McBeth JM. Iron-oxidizing bacteria: an environmental and genomic perspective. Annu Rev Microbiol. 2010;64:561–583. doi: 10.1146/annurev.micro.112408.134208. [DOI] [PubMed] [Google Scholar]
- Gaudy E. Wolfe RS. Factors affecting filamentous growth of Sphaerotilus natans. Appl Microbiol. 1961;9:580–584. doi: 10.1128/am.9.6.580-584.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasauer S, Al Mattes. Gehring A. Constraints on the preservation of ferriferous microfossils. Geomicrobiol J. 2013;30:479–489. [Google Scholar]
- Hafenbradl D, Keller M, Dirmeier R, et al. Ferroglobus placidus gen. nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch Microbiol. 1996;166:308–314. doi: 10.1007/s002030050388. [DOI] [PubMed] [Google Scholar]
- Hallberg R. Ferris FG. Biomineralization by Gallionella. Geomicrobiol J. 2004;21:325–330. [Google Scholar]
- Hansen HCB, Koch CB, Nancke-Krogh H, Borggaard OK. Sørensen J. Abiotic nitrate reduction to ammonium: key role of green rust. Environ Sci Technol. 1996;30:2053–2056. [Google Scholar]
- Hauck S, Benz M, Brune A. Schink B. Ferrous iron oxidation by denitrifying bacteria in profundal sediments of a deep lake (Lake Constance) FEMS Microbiol Ecol. 2001;37:127–134. [Google Scholar]
- Hegler F, Posth NR, Jiang J. Kappler A. Physiology of phototrophic iron(II)-oxidizing bacteria: implications for modern and ancient environments. FEMS Microbiol Ecol. 2008;66:250–260. doi: 10.1111/j.1574-6941.2008.00592.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock AP, Obst M, Wang J, Lu YS. Tyliszczak T. Advances in the detection of as in environmental samples using low energy X-ray fluorescence in a scanning transmission X-ray microscope: arsenic immobilization by an Fe(II)-oxidizing freshwater bacteria. Environ Sci Technol. 2012;46:2821–2829. doi: 10.1021/es202238k. [DOI] [PubMed] [Google Scholar]
- Hoeniger JF, Tauschel HD. Stokes JL. The fine structure of Sphaerotilus natans. Can J Microbiol. 1973;19:309–313. doi: 10.1139/m73-051. [DOI] [PubMed] [Google Scholar]
- Hohmann C, Winkler E, Morin G. Kappler A. Anaerobic Fe(II)-oxidizing bacteria show as resistance and immobilize as during Fe(III) mineral precipitation. Environ Sci Technol. 2009;44:94–101. doi: 10.1021/es900708s. [DOI] [PubMed] [Google Scholar]
- Hohmann C, Morin G, Ona-Nguema G, Guigner JM, Brown GE., Jr Kappler A. Molecular-level modes of As binding to Fe(III) (oxyhydr)oxides precipitated by the anaerobic nitrate-reducing Fe(II)-oxidizing Acidovorax sp. strain BoFeN1. Geochim Cosmochim Acta. 2011;75:4699–4712. [Google Scholar]
- Kampschreur MJ, Kleerebezem R, de Vet WW. van Loosdrecht MC. Reduced iron induced nitric oxide and nitrous oxide emission. Water Res. 2011;45:5945–5952. doi: 10.1016/j.watres.2011.08.056. [DOI] [PubMed] [Google Scholar]
- Kappler A. Newman DK. Formation of Fe(III)-minerals by Fe(II)-oxidizing photoautotrophic bacteria. Geochim Cosmochim Acta. 2004;68:1217–1226. [Google Scholar]
- Kappler A. Straub KL. Geomicrobiological cycling of iron. Rev Mineral Geochem. 2005;59:85–108. [Google Scholar]
- Kappler A, Schink B. Newman DK. Fe(III) mineral formation and cell encrustation by the nitrate-dependent Fe(II)-oxidizer strain BoFeN1. Geobiology. 2005;3:235–245. [Google Scholar]
- Klueglein N. Kappler A. Abiotic oxidation of Fe(II) by reactive nitrogen species in cultures of the nitrate-reducing Fe(II) oxidizer Acidovorax sp. BoFeN1 - questioning the existence of enzymatic Fe(II) oxidation. Geobiology. 2013;11:180–190. doi: 10.1111/gbi.12019. [DOI] [PubMed] [Google Scholar]
- Klueglein N, Zeitvogel F, Stierhof YD, Floetenmeyer M, Konhauser KO, Kappler A. Obst M. Potential role of nitrite for abiotic Fe(II) oxidation and cell encrustation during nitrate reduction by denitrifying bacteria. Appl Environ Microbiol. 2014;80:1051–1061. doi: 10.1128/AEM.03277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack JG, Chaudhuri SK, Chakraborty R, Achenbach LA. Coates JD. Anaerobic biooxidation of Fe(II) by Dechlorosoma suillum. Microb Ecol. 2002;43:424–431. doi: 10.1007/s00248-001-1061-1. [DOI] [PubMed] [Google Scholar]
- Larese-Casanova P, Haderlein SB. Kappler A. Biomineralization of lepidocrocite and goethite by nitrate-reducing Fe(II)-oxidizing bacteria: effect of pH, bicarbonate, phosphate, and humic acids. Geochim Cosmochim Acta. 2010;74:3721–3734. [Google Scholar]
- Lovley DR, Holmes DE. Nevin KP. Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol. 2004;49:219–286. doi: 10.1016/S0065-2911(04)49005-5. [DOI] [PubMed] [Google Scholar]
- Miot J, Benzerara K, Morin G, et al. Iron biomineralization by anaerobic neutrophilic iron-oxidizing bacteria. Geochim Cosmochim Acta. 2009;73:696–711. [Google Scholar]
- Miot J, Maclellan K, Benzerara K. Boisset N. Preservation of protein globules and peptidoglycan in the mineralized cell wall of nitrate-reducing, iron(II)-oxidizing bacteria: a cryo-electron microscopy study. Geobiology. 2011;9:459–470. doi: 10.1111/j.1472-4669.2011.00298.x. [DOI] [PubMed] [Google Scholar]
- Muehe EM, Gerhardt S, Schink B. Kappler A. Ecophysiology and the energetic benefit of mixotrophic Fe(II) oxidation by various strains of nitrate-reducing bacteria. FEMS Microbiol Ecol. 2009;70:335–343. doi: 10.1111/j.1574-6941.2009.00755.x. [DOI] [PubMed] [Google Scholar]
- Nealson KH. Saffarini D. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu Rev Microbiol. 1994;48:311–343. doi: 10.1146/annurev.mi.48.100194.001523. [DOI] [PubMed] [Google Scholar]
- Nierman WC. Maglott DR. American Type Culture Collection Catalogue of Bacteria and Bacteriophages. 18. Rockville, MD: American Type Culture Collection; 1989. [Google Scholar]
- Pantke C, Obst M, Benzerara K, Morin G, Ona-Nguema G, Dippon U. Kappler A. Green rust formation during Fe(II) oxidation by the nitrate-reducing Acidovorax sp. strain BoFeN1. Environ Sci Technol. 2012;46:1439–1446. doi: 10.1021/es2016457. [DOI] [PubMed] [Google Scholar]
- Pellegrin V, Juretschko S, Wagner M. Cottenceau G. Morphological and biochemical properties of a Sphaerotilus sp. isolated from paper mill slimes. Appl Environ Microbiol. 1999;65:156–162. doi: 10.1128/aem.65.1.156-162.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix VR. Konhauser KO. Benefits of bacterial biomineralization. Geobiology. 2008;6:303–308. doi: 10.1111/j.1472-4669.2008.00147.x. [DOI] [PubMed] [Google Scholar]
-
Picardal F. Abiotic and microbial interactions during anaerobic transformations of Fe(II) and
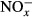 . Front Microbiol. 2012;3:112. doi: 10.3389/fmicb.2012.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
. Front Microbiol. 2012;3:112. doi: 10.3389/fmicb.2012.00112. [DOI] [PMC free article] [PubMed] [Google Scholar] - Pierson BK, Mitchell HK. Ruff-Roberts AL. Chloroflexus aurantiacus and ultraviolet radiation: implications for archean shallow-water stromatolites. Orig Life Evol Biosph. 1993;23:243–260. [Google Scholar]
- Posth NR, Hegler F, Konhauser KO. Kappler A. Alternating Si and Fe deposition caused by temperature fluctuations in Precambrian oceans. Nat Geosci. 2008;1:703–708. [Google Scholar]
- Poulain AJ. Newman DK. Rhodobacter capsulatus catalyzes light-dependent Fe(II) oxidation under anaerobic conditions as a potential detoxification mechanism. Appl Environ Microbiol. 2009;75:6639–6646. doi: 10.1128/AEM.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston LJ, Shuster J, Fernandez-Remolar D, Banerjee NR, Osinski GR. Southam G. The preservation and degradation of filamentous bacteria and biomolecules within iron oxide deposits at Rio Tinto, Spain. Geobiology. 2011;9:233–249. doi: 10.1111/j.1472-4669.2011.00275.x. [DOI] [PubMed] [Google Scholar]
- Schadler S, Burkhardt C. Kappler A. Evaluation of electron microscopic sample preparation methods and imaging techniques for characterization of cell-mineral aggregates. Geomicrobiol J. 2008;25:228–239. [Google Scholar]
- Schaedler S, Burkhardt C, Hegler F, Straub KL, Miot J, Benzerara K. Kappler A. Formation of cell-iron-mineral aggregates by phototrophic and nitrate-reducing anaerobic Fe(II)-oxidizing bacteria. Geomicrobiol J. 2009;26:93–103. [Google Scholar]
- Schaefer MV, Gorski CA. Scherer MM. Spectroscopic evidence for interfacial Fe(II)-Fe(III) electron transfer in a clay mineral. Environ Sci Technol. 2011;45:540–545. doi: 10.1021/es102560m. [DOI] [PubMed] [Google Scholar]
- Seder-Colomina M, Morin G, Benzerara K, Ona-Nguema G, Pernelle J-J, Esposito G. Van Hullebusch ED. Sphaerotilus natans, a neutrophilic iron-related sheath-forming bacterium: perspectives for metal remediation strategies. Geomicrobiol J. 2013;31:64–75. [Google Scholar]
- Sørensen J. Thorling L. Stimulation by lepidocrocite (γ-FeOOH) of Fe(II)-dependent nitrite reduction. Geochim Cosmochim Acta. 1991;55:1289–1294. [Google Scholar]
- Straub KL. Buchholz-Cleven BE. Enumeration and detection of anaerobic ferrous iron-oxidizing, nitrate-reducing bacteria from diverse European sediments. Appl Environ Microbiol. 1998;64:4846–4856. doi: 10.1128/aem.64.12.4846-4856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub KL, Benz M, Schink B. Widdel F. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl Environ Microbiol. 1996;62:1458–1460. doi: 10.1128/aem.62.4.1458-1460.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub KL, Schönhuber WA, Buchholz-Cleven BEE. Schink B. Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiol J. 2004;21:371–378. [Google Scholar]
- Suzuki T, Kanagawa T. Kamagata Y. Identification of a gene essential for sheathed structure formation in Sphaerotilus natans, a filamentous sheathed bacterium. Appl Environ Microbiol. 2002;68:365–371. doi: 10.1128/AEM.68.1.365-371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Nakano F, Nagase T, Iohara K. Koizumi J. Isolation and chemical composition of the sheath of Sphaerotilus natans. Biosci Biotechnol Biochem. 1998;62:1138–1143. doi: 10.1271/bbb.62.1138. [DOI] [PubMed] [Google Scholar]
- Vaughan DJ. Lloyd JR. Mineral-organic-microbe interactions: environmental impacts from molecular to macroscopic scales. Comp Rend Geosci. 2011;343:140–159. [Google Scholar]
- Weber KA, Picardal FW. Roden EE. Microbially catalyzed nitrate-dependent oxidation of biogenic solid-phase Fe(II) compounds. Environ Sci Technol. 2001;35:1644–1650. doi: 10.1021/es0016598. [DOI] [PubMed] [Google Scholar]
- Weber KA, Achenbach LA. Coates JD. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol. 2006a;4:752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
- Weber KA, Pollock J, Cole KA, O'Connor SM, Achenbach LA. Coates JD. Anaerobic nitrate-dependent iron(II) bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002. Appl Environ Microbiol. 2006b;72:686–694. doi: 10.1128/AEM.72.1.686-694.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KA, Urrutia MM, Churchill PF, Kukkadapu RK. Roden EE. Anaerobic redox cycling of iron by freshwater sediment microorganisms. Environ Microbiol. 2006c;8:100–113. doi: 10.1111/j.1462-2920.2005.00873.x. [DOI] [PubMed] [Google Scholar]
- Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B. Schink B. Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature. 1993;362:834–836. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transmission electron microscopic images of Sphaerotilus natans grown as single cells in the presence of 0.5% glucose and 0.5% peptone (a), as filamentous cells in the presence of 0.1% peptone and 0.1% glucose (b) under aerobic condition, and (c) single cells in the basal medium with pyruvate under anaerobic condition.
Fig. S2. Homologies of nitrate reductase of Sphaerotilus natans strain DSM 6575T with other bacterial nitrate reductase.
Fig. S3. Concentration of dissolved nitrate in the control experiment without Sphaerotilus natans strain DSM 6575T inoculation.
Fig. S4. TEM images of goethite formed from abiotic reaction between ferrous iron at 4 mM and nitrite at 1 mM (a) and XRD pattern of abiotically formed goethite from ferrous iron and nitrite (b).


