Abstract
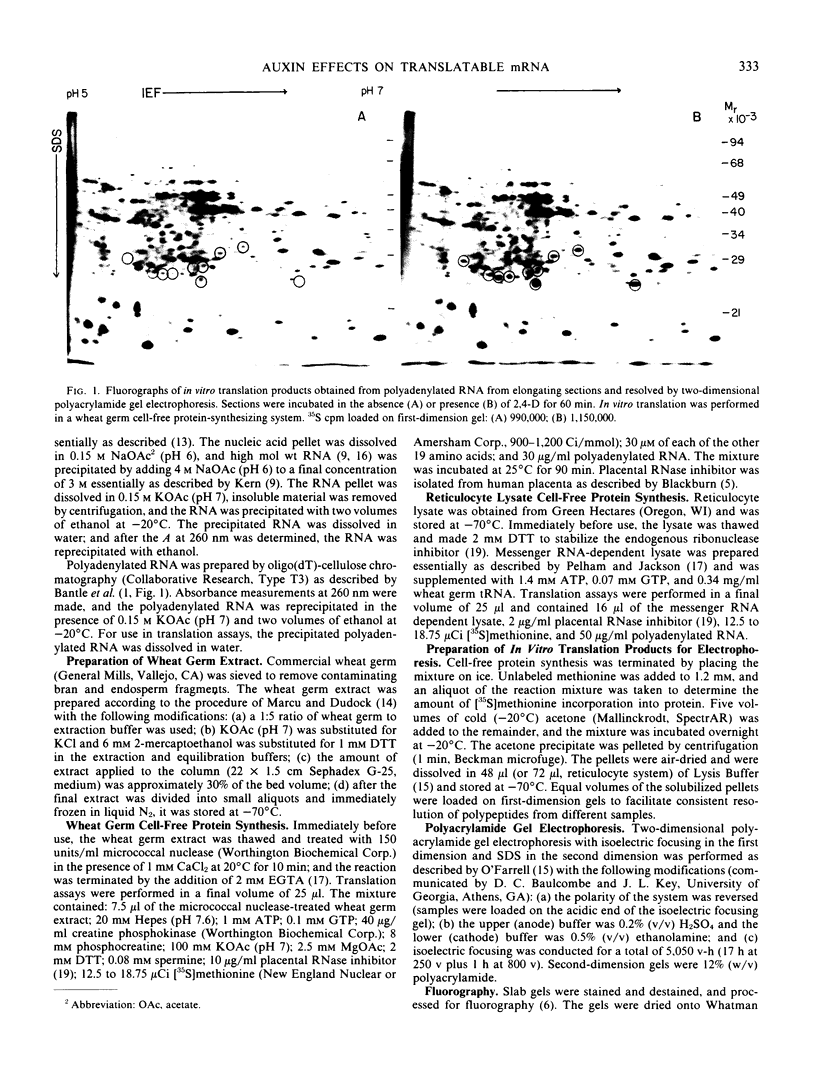
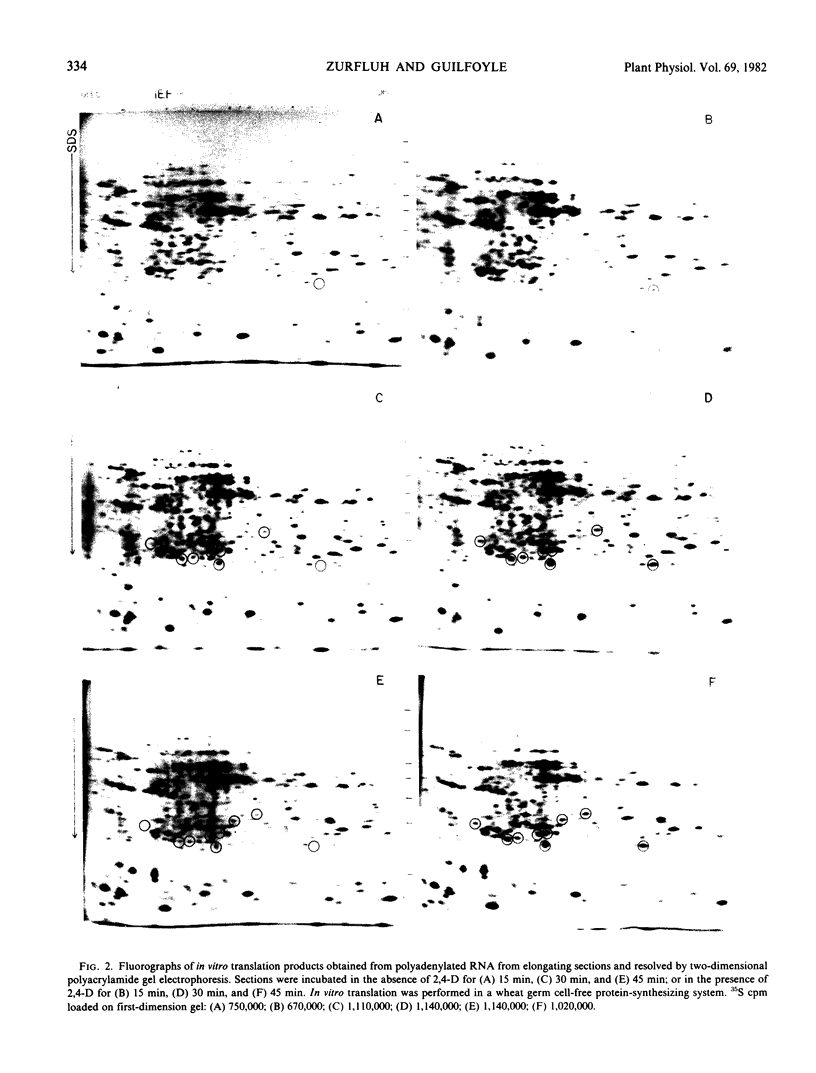
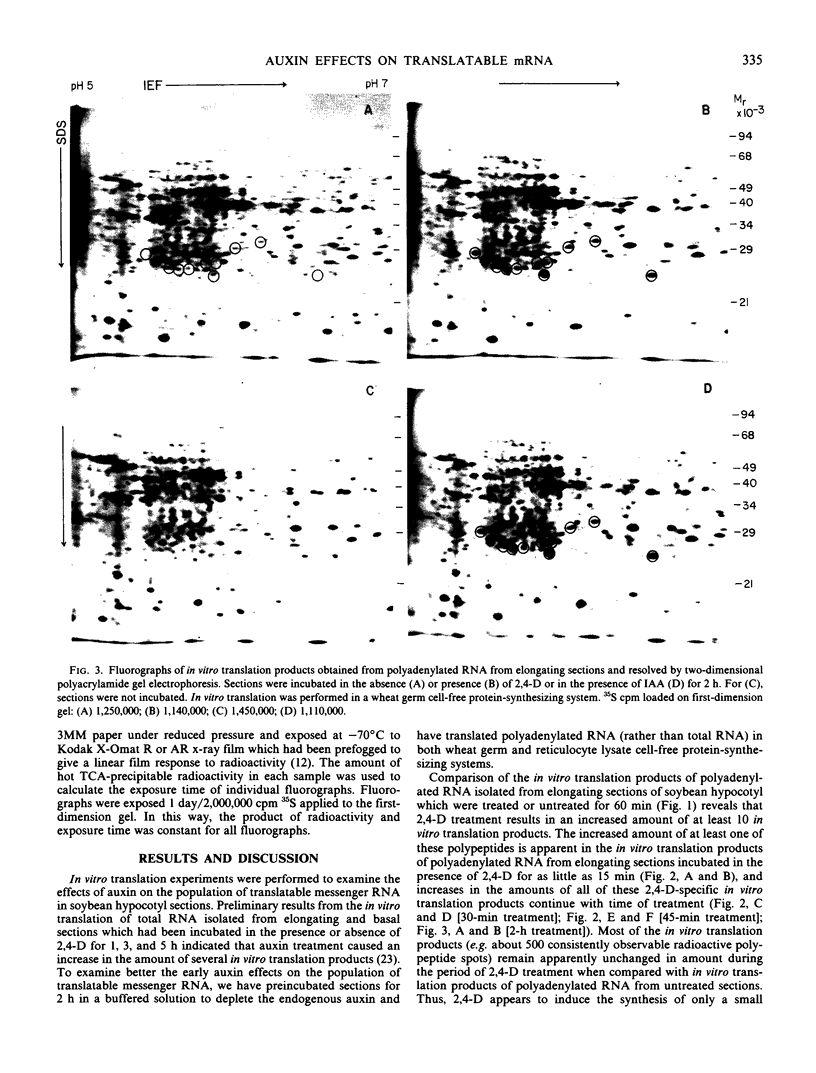
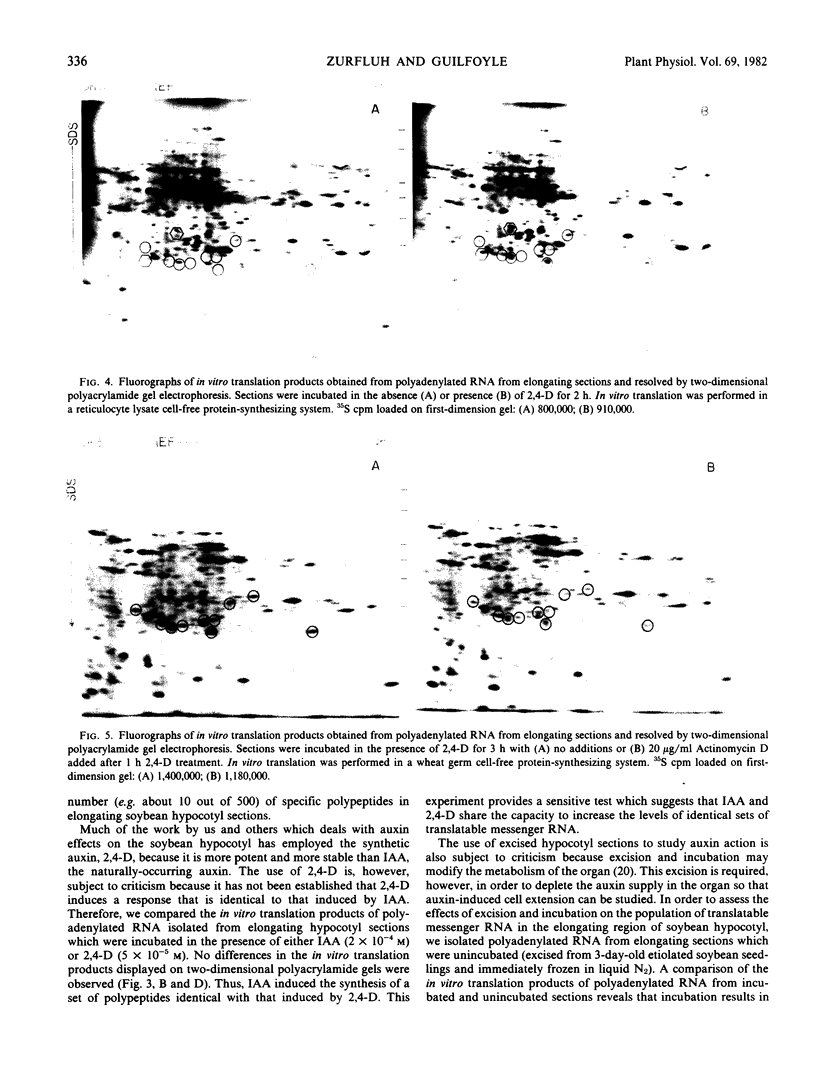
In vitro translation products of polyadenylated RNA from untreated and auxin-treated elongating sections of soybean (Glycine max var. Wayne) hypocotyl were analyzed by two-dimensional polyacrylamide gel electrophoresis. The levels of translatable messenger RNA for at least ten in vitro translation products are increased by auxin treatment. The induction by auxin occurs rapidly (within 15 minutes), and the amounts of the induced in vitro translation products increase with time of auxin treatment. Indoleacetic acid has the same effect on the population of translatable messenger RNA as 2,4-dichlorophenoxyacetic acid. The auxin-induced in vitro translation products disappear rapidly when Actinomycin D is present during the last two hours of a three-hour auxin treatment.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Blackburn P. Ribonuclease inhibitor from human placenta: rapid purification and assay. J Biol Chem. 1979 Dec 25;254(24):12484–12487. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cleland R. E. Kinetics of Hormone-induced H Excretion. Plant Physiol. 1976 Aug;58(2):210–213. doi: 10.1104/pp.58.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Ray P. M. Timing of the auxin response in coleoptiles and its implications regarding auxin action. J Gen Physiol. 1969 Jan;53(1):1–20. doi: 10.1085/jgp.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern H. Fractionation of nucleic acids with special regard to rapidly labeled RNA. Anal Biochem. 1975 Jul;67(1):147–156. doi: 10.1016/0003-2697(75)90282-1. [DOI] [PubMed] [Google Scholar]
- Key J. L., Ingle J. REQUIREMENT FOR THE SYNTHESIS OF DNA-LIKE RNA FOR GROWTH OF EXCISED PLANT TISSUE. Proc Natl Acad Sci U S A. 1964 Dec;52(6):1382–1388. doi: 10.1073/pnas.52.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lizardi P. M., Engelberg A. Rapid isolation of RNA using proteinase K and sodium perchlorate. Anal Biochem. 1979 Sep 15;98(1):116–122. doi: 10.1016/0003-2697(79)90714-0. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Scheele G., Blackburn P. Role of mammalian RNase inhibitor in cell-free protein synthesis. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4898–4902. doi: 10.1073/pnas.76.10.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Anderson J. M., Key J. L. Influence of auxin and incubation on the relative level of polyribosomes in excised soybean hypocotyl. Plant Physiol. 1973 Dec;52(6):608–612. doi: 10.1104/pp.52.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcockson J. The differential precipition of nucleic acids and proteins from aqueous solutions by ethanol. Anal Biochem. 1975 May 26;66(1):64–68. doi: 10.1016/0003-2697(75)90724-1. [DOI] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin- and ethylene-induced changes in the population of translatable messenger RNA in Basal sections and intact soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):338–340. doi: 10.1104/pp.69.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the patterns of protein synthesis in soybean hypocotyl. Proc Natl Acad Sci U S A. 1980 Jan;77(1):357–361. doi: 10.1073/pnas.77.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]