Abstract
Background
We aimed to study the association between cytomegalovirus (CMV) infection and hypertension in Kazakh and Han populations from Xinjiang Province, China.
Material/Methods
We analyzed data on 800 Kazakhs (467 hypertension patients and 333 healthy control participants) and 800 Hans (482 hypertension patients and 318 healthy control participants) aged 18–84 years old. ELISA and real-time quantitative PCR coupled with restriction fragment length polymorphism analysis were applied for determining CMV infection and glycoprotein B (gB) genotypes, respectively.
Results
Serologic evidence of CMV infection was obtained for 95.4% and 90.1% of the Kazakhs and Hans, respectively. The CMV seroprevalence rates among the Kazakh and Han participants with hypertension were 96.8% and 89.8%, respectively. Multiple logistic regression analyses revealed statistically significant independent associations between CMV seropositivity and hypertension in Kazakh males and between CMV antibody titers and hypertension in Hans; significant relationships also existed between CMV antibody titers and blood pressure in Hans. In Kazakhs, 3 CMV gB genotypes were identified: gB2 and genotype mixtures gB1+gB2 and gB2+gB3. In Hans, 4 CMV gB genotypes were identified: gB1, gB2, gB1+gB2, and gB2+gB3. Of the 4 studied genotypes, gB2+gB3 showed a significant independent association with hypertension in Kazakh females.
Conclusions
CMV infection is associated with essential hypertension in Kazakh males and Hans in Xinjiang. CMV seropositivity is associated with hypertension in Kazakh males, and CMV antibody titers are associated with blood pressure and hypertension in Han males and females. Moreover, the CMV gB2+gB3 genotype mixture is associated independently with essential hypertension in Kazakh females.
MeSH Keywords: Cytomegalovirus, Genotype, Hypertension, Kazakh and Han Chinese
Background
Essential hypertension (EH) is the most common form of hypertension [1]; it accounts for 90–95% of hypertension and affects >1 billion adults worldwide [2]. Hypertension is recognized as a disease that is caused by a combination of environmental and genetic factors [3]; however, the precise cause of hypertension is unknown, and effective early prevention and treatment for the disease are not available [4].
Human cytomegalovirus (CMV), which contains a large double-stranded DNA genome, is a member of the beta-herpesvirus group [5]. Like all herpesviruses, CMV undergoes latency and reactivation in the host. Primary infection with CMV is typically asymptomatic and self-limiting in immunocompetent hosts, but it results in the development of a lifelong carrier status with periodic reactivation and shedding of the virus from mucosal sites [6]. CMV is best recognized for causing congenital infection and severe opportunistic disease in immunocompromised people [7], and the virus is widespread in populations worldwide: Sero-epidemiological studies have shown that in developed countries, >50% of adults present evidence of past CMV infection [8]. In China, 48% of the population is CMV seropositive, with the seroprevalence in females (54.60%) being considerably higher than that in males (41.58%) [9]. Furthermore, severe or acute disease might be induced in immunocompromised hosts, including AIDS patients and transplant recipients, as a result of the reactivation of latent CMV [10, 11].
CMV glycoprotein B (gB), which is the most highly enriched viral envelope glycoprotein, exhibits extremely strong immunogenicity [12]. Based on the sequence variation in the UL55 gene that encodes gB, 4 gB genotypes of CMV have been identified (gB1–gB4) [13], and a fifth genotype (gB5) has also been occasionally detected [14]; evidence has been presented suggesting genotype-dependent differences in CMV virulence [15]. Previous studies have demonstrated that these distinct genotypes are not associated in a statistically significant manner with specific clinical presentations or organ-system involvements in infants with clinically suspected CMV disease [16]. However, whether specific gB genotypes are associated with an increased risk of hypertension remains unclear.
Previous studies have reported numerous cases of cardiovascular diseases (CVDs) caused by CMV infection, including myocarditis, atherosclerosis, and coronary artery disease [17–20]. Moreover, emerging evidence indicates that CMV infection might be associated with hypertension [21–24]. However, the association between hypertension and CMV remains unclear in the Chinese Kazakh population. The incidence of hypertension in Kazakhs ranks among the top 5 rates measured for the 56 ethnic groups recognized in China [25]. In this study, we focused on an isolated Kazakh community located in northeastern Xinjiang. The genetic homogeneity and geographic stability of this population, coupled with the shared exposure to common environmental variables, provided a highly favorable opportunity for examining genetic influences on hypertension. Therefore, we investigated the association of CMV and hypertension in relation to other cardiovascular risk factors in a large-scale, community-based study on this Chinese Kazakh population. Furthermore, in order to reveal how racial diversity affects the association of CMV infection and hypertension, we recruited 800 Han Chinese from the same geographic area. Our study is, to our knowledge, the first on this research topic to be conducted in a Kazakh population.
Material and Methods
Participants and data collection
In this case-controlled study, from 2009 to 2012, we selected Kazakh and Han Chinese from rural communities in the Boertonggu countryside of Shawan region in Xinjiang Uygur Autonomous Region, China. The study protocol was approved by the ethics committee of Medical College of Shihezi University, and all participants provided written informed consent. The participants also provided written consent for their blood samples to be retained for future research, such as for use in measuring levels of CMV-specific IgGs. All participants were instructed to complete a detailed investigation questionnaire and subject themselves to essential auxiliary examinations and clinical diagnosis. We obtained the details of the Kazakh and Han Chinese populations from the local government. Overall, we included 2370 people (age: 18–84 years) in the investigation.
We used the 1999 WHO/ISH Hypertension Guidelines for diagnosing hypertension, which is defined as systolic BP (SBP) ≥140 mmHg (18.7 kPa) and/or diastolic BP (DBP) ≥90 mmHg (12.0 kPa). Blood pressure was measured after the participants had rested for 15 min, and the measurement was repeated 3 times. During the investigation, we recorded the measurements of the following cardiovascular risk factors: body mass index (BMI), waist-hip ratio (WHR), SBP, DBP, mean arterial pressure (MAP), fasting blood sugar (FBS), total cholesterol (TC), serum low-density lipoprotein cholesterol (LDL-C), serum high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), alcohol consumption, vegetable intake, and smoking habit. Overnight fasting blood (10 mL) was drawn and processed (centrifuged, separated, frozen, and packaged in a −80°C freezer) and used for measuring plasma levels of CMV infection. Blood cells were prepared for DNA extraction and detection of CMV gB genotypes. We excluded participants for whom data were not available on smoking, BMI, waist circumference, WHR, blood pressure, alcohol consumption, and vegetable intake. Therefore, in total, 2300 participants were included in the study. From this pool, we chose, using random cluster sampling, 800 Kazakhs (467 hypertension patients, 218 males and 249 females; and 333 healthy participants, 118 males and 215 females) and 800 Hans (482 hypertension patients, 218 males and 264 females; and 318 healthy participants, 124 males and 194 females). People with heart disease or renal or endocrinological diseases that cause secondary hypertension were excluded from this study.
Enzyme-linked immunosorbent assay
We tested for anti-CMV IgGs by using a commercially available ELISA kit (CMV Diagnostic Kit, Haitai Biotech Inc., Zhuhai, China) according to the manufacturer’s instructions; IgGs were measured successfully in all 1600 participants. Among Kazakhs, 37 participants were seronegative (antibody titer <1.1) and 763 were seropositive (≥1.1), and among Hans, 79 were seronegative and 721 were seropositive. According to the manufacturer, the test sensitivity was 99.9% and the specificity was 99.8%. Thus, ELISA values of ≥1.1 and <1.1 U were considered positive and negative results, respectively. The optical density obtained from the ELISA assay is reported as an approximate measure of the antibody titer; a low and positive optical density reflects a low antibody titer, whereas a high optical density reflects a high antibody titer.
DNA extraction and qPCR detection
Genomic DNA was purified from the participants’ whole blood samples by using a QIAGEN kit (QIAGEN Inc., Valencia, CA) according to the manufacturer’s instructions. CMV-DNA was amplified using a CMV-DNA kit (DaAn Co, Guangzhou, China), with the PCR primers used for this assay being derived from the immediate early (IE) gene. PCR amplification was performed using these steps: initial denaturation at 93°C for 2 min, followed by 10 cycles of 93°C for 45 s and 55°C for 1 min, and, lastly, 30 cycles of 93°C for 30 s and 33°C for 45 s; we used a 7300 Real-Time PCR system (Applied Biosystems, Singapore). Real-time fluorescence measurements were recorded, and the threshold cycle (CT) value for each sample was calculated by determining the point at which the fluorescence exceeded a threshold limit. The plasmid pGEM-IE, which contains the IE gene, was included as a positive control in all steps, from extraction to quantitation, and a negative control (distilled water) was also included to verify the absence of contamination. A standard graph was constructed, the CT values obtained for the clinical samples were plotted on the standard curve, and the copy number was calculated. Samples were defined as positive when the CT values exceeded 30 cycles.
CMV gB genotyping through PCR-RFLP analysis
The CMV gB genotype was determined using nested PCR and restriction fragment length polymorphism (RFLP) analysis, applied as described by Chou and Dennison [13]. A total of 141 CMV-DNA-positive genomic DNA samples were obtained from the participants included in the study (107 from Kazakhs, 34 from Hans). Nested PCR was performed using external primers (sense: 5′-GGCATCAAGCAAAAATCT-3′, antisense: 5′-CAGTTGACCGTACTGCAC-3′; product: 482–488 bp) and internal primers (sense: 5′-TGGAACTGGAACGTTTGGC-3′, antisense: 5′-GAAACGCGCGGCAATCGG-3′; product: 299–305 bp). DNA amplification was initiated using a 50-μL PCR mixture containing 2 μL of extracted DNA and 1 pmol of each primer. The first round of PCR amplification was performed in a thermal cycler (2720 Thermal Cycler, Applied Biosystems) under these conditions: denaturation at 95°C for 5 min, followed by amplification for 35 cycles (95°C for 30 s, 55°C for 45 s, 72°C for 60 s), and then an extension for 10 min at 72°C at the end of the reaction. The second round of PCR differed from the first in that annealing was performed at 60°C. The amplified gB products were digested with HinfI and RsaI (TaKaRa, Dalian, China) at 37°C for 12 h and separated by electrophoresing them at 100 V in 2.5% agarose gels. From the results of this analysis, 4 gB genotypes could be distinguished based on their distinct patterns of fragment lengths [13].
Statistical analysis
A t test was used for normally distributed variables, the Mann-Whitney U test was used for skewed variables, and the χ2 test was used for categorized variables. Logistic and multiple regressions were used to determine the association between CMV infection and risk factors for blood pressure and hypertension among the 2 sexes and ethnicities. Furthermore, binary logistic regression models were used for assessing the association between CMV gB genotype and hypertension (as a dependent variable) in the Kazakh and Han Chinese participants after adjustment for other confounders and stratification for sex and ethnicity. P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS Version 20.0 (IBM Corporation, Armonk, NY).
Results
We evaluated 800 Kazakhs and 800 Hans (18–84 years old) for associations of CMV infection and hypertension and determined that among them, 763 Kazakhs (95.4%) and 721 Hans (90.1%) had CMV infection and 467 Kazakhs (59.2%) and 482 Hans (60.1%) had hypertension. Among the 467 and 482 hypertensive Kazakh and Han participants, 452 Kazakhs and 433 Hans had CMV infection (96.8% and 89.8%). In the tables, we present the relevant anthropometric information, including cardiovascular risk factors, biochemical measurements, and prevalence of CMV seropositivity, CMV IgG titers, and CMV gB genotypes.
Table 1 lists the characteristics of all 1600 participants according to the CMV seropositivity status. Hans with CMV infection were older (P<0.001) and more likely to be females (P<0.001) when compared with Han participants without CMV infection. Among Hans, the frequency of drinking alcohol was significantly lower and BMI was higher (both P<0.001) in participants with CMV infection than in participants without CMV infection. Among Kazakhs, higher SBP (P=0.052), DBP (P=0.021), and MAP (P=0.017) and a lower frequency of vegetable intake were measured in participants with CMV infection than in those without CMV infection.
Table 1.
Clinical characteristics of the 1600 Kazakh and Han study participants with and without serologic evidence of CMV infection.
| Characteristics | Kazakh participants (n=800) | Han participants (n=800) | ||||
|---|---|---|---|---|---|---|
| CMV infection | No CMV infection | P value | CMV infection | No CMV infection | P value | |
| n | 763 | 37 | 721 | 79 | ||
| Age (years) | 45.04 (12.95) | 43.24 (11.66) | 0.500 | 46.49 (15.29) | 42.10 (15.25) | 0.027* |
| Males, % | 41.70 | 48.60 | 0.401 | 40.10 | 67.10 | <0.001* |
| Smokers, % daily | 23.10 | 10.80 | 0.081 | 22.90 | 24.10 | 0.815 |
| Alcohol, % daily | 22.40 | 10.80 | 0.096 | 26.60 | 46.80 | <0.001* |
| vegetable, % daily | 88.90 | 100.0 | 0.032* | 97.5 | 100.0 | 0.155 |
| BMI (kg/m2) | 26.22 (5.00) | 25.05 (2.99) | 0.237 | 25.56 (4.27) | 24.31 (4.07) | <0.001* |
| WHR | 0.88 (0.07) | 0.87 (0.07) | 0.434 | 0.90 (0.06) | 0.90 (0.05) | 0.251 |
| Hypertension, % | 59.2 | 40.5 | 0.024* | 60.1 | 62.0 | 0.734 |
| SBP (mmHg) | 136.49 (26.32) | 130.72 (31.82) | 0.052 | 132.27 (22.68) | 137.44 (34.72) | 0.942 |
| DBP (mmHg) | 89.62 (16.26) | 84.58 (21.22) | 0.021* | 87.22 (10.80) | 87.90 (15.47) | 0.894 |
| MAP (mmHg) | 105.24 (18.67) | 99.96 (21.79) | 0.017* | 102.90 (13.38) | 104.41 (16.77) | 0.385 |
| FBS (mmol/L) | 5.82 (1.28) | 5.84 (1.10) | 0.706 | 5.85 (1.09) | 5.95 (1.52) | 0.596 |
| TC (mmol/L) | 4.97 (1.10) | 4.89 (1.38) | 0.691 | 4.63 (1.06) | 4.47 (0.87) | 0.094 |
| TG (mmol/L) | 1.16 (1.04) | 1.13 (0.86) | 0.925 | 1.51 (1.23) | 1.46 (1.20) | 0.629 |
| LDL-C (mmol/L) | 1.48 (0.41) | 1.43 (0.42) | 0.881 | 1.41 (0.70) | 1.37 (0.29) | 0.559 |
| HDL-C (mmol/L) | 3.16 (1.01) | 3.04 (1.27) | 0.354 | 3.03 (0.96) | 2.89 (0.71) | 0.104 |
Values are expressed as means or percentages (SD). BMI – body mass index; WHR – waist-hip ratio; SBP – systolic blood pressure; DBP – diastolic blood pressure; MAP – mean arterial blood pressure; FBS – fasting blood sugar; CMV – cytomegalovirus; TC – plasma total cholesterol; TG – triglyceride; LDL-C – low-density lipoprotein cholesterol; HDL-C – high-density lipoprotein cholesterol.
Statistically significant difference (P<0.05) present between various clinical and biochemical indices measured for participants with and without CMV infection in the Kazakh and Han populations.
Table 2 presents the characteristics of the Kazakh and Han participants according to their hypertension status. Compared with Kazakh participants without hypertension, those with hypertension exhibited a higher frequency of drinking alcohol (P<0.001) and lower vegetable intake (P = 0.008) and had lower HDL-C (P=0.001). Moreover, hypertensive Kazakh participants were more likely to be females than males (P=0.001). Compared with participants without hypertension, both Kazakh and Han participants with hypertension were older (P<0.001) and had higher levels of other traditional CVD risk factors (BMI, P<0.001; WHR, P<0.001; FBS, P=0.004; TC, P<0.001; TG, P<0.001; LDL-C, P<0.001). The prevalence of CMV seropositivity was higher in hypertension groups than in control groups among Kazakh participants (P=0.024) but not Han participants (P=0.734). However, in Hans, CMV IgG titers were significantly lower (P<0.001) in participants with hypertension than in those without hypertension.
Table 2.
Clinical characteristics of 1600 Kazakh and Han participants with and without hypertension.
| Characteristics | Kazakh participantsa (n=800) | Han participantsb (n=800) | ||||
|---|---|---|---|---|---|---|
| Hypertension | No hypertension | P value | Hypertension | No hypertension | P value | |
| n | 467 | 333 | 482 | 318 | ||
| Age (years) | 49.01 (11.91) | 39.28 (12.06) | <0.001* | 47.90 (15.83) | 43.26 (14.11) | <0.001* |
| Males, % | 46.70 | 35.40 | 0.001* | 45.20 | 39.00 | 0.081 |
| Smokers, % daily | 23.80 | 20.70 | 0.309 | 23.20 | 22.60 | 0.845 |
| Alcohol, % daily | 25.30 | 17.10 | 0.006* | 27.10 | 30.80 | 0.253 |
| vegetable, % daily | 86.90 | 92.80 | 0.008* | 97.50 | 98.10 | 0.574 |
| BMI (kg/m2) | 27.41 (4.65) | 24.42 (4.78) | <0.001* | 26.14 (4.80) | 24.36 (3.01) | <0.001* |
| WHR | 0.89 (0.07) | 0.86 (0.06) | <0.001* | 0.91 (0.06) | 0.88 (0.06) | <0.001* |
| SBP (mmHg) | 151.76 (22.87) | 114.43 (12.38) | <0.001* | 148.01 (20.64) | 114.23 (11.43) | <0.001* |
| DBP (mmHg) | 98.81 (14.20) | 76.17 (7.79) | <0.001* | 93.20 (9.71) | 78.32 (6.86) | <0.001* |
| MBP (mmHg) | 116.46 (15.60) | 88.92 (8.30) | <0.001* | 111.47 (9.97) | 90.29 (7.36) | <0.001* |
| FBS (mmol/L) | 5.97 (1.42) | 5.62 (0.98) | 0.004* | 6.00 (1.20) | 5.64 (1.01) | 0.004* |
| TC (mmol/L) | 5.14 (1.15) | 4.73 (1.03) | <0.001* | 4.81 (1.10) | 4.31 (0.88) | <0.001* |
| TG (mmol/L) | 1.36 (1.21) | 0.88 (0.60) | <0.001* | 1.65 (1.25) | 1.30 (1.15) | <0.001* |
| LDL-C (mmol/L) | 3.28 (1.07) | 2.98 (0.92) | <0.001* | 3.21 (0.97) | 2.72 (0.81) | <0.001* |
| HDL-C (mmol/L) | 1.45 (0.42) | 1.53 (0.39) | 0.001* | 1.43 (0.82) | 1.37 (0.34) | 0.693 |
| CMV seropositivity, % | 96.80 | 93.40 | 0.024* | 89.80 | 90.60 | 0.734 |
| CMV IgG antibody titers | 3.15 (0.95) | 3.08 (1.08) | 0.651 | 2.25 (0.76) | 2.46 (0.76) | <0.001* |
| Single genotype, % | ||||||
| gB1 | – | – | – | 0.4 | 2.2 | 0.019* |
| gB2 | 5.60 | 4.20 | 0.383 | 1.2 | 0.6 | 0.392 |
| Genotype mixtures, % | ||||||
| gB1+gB2 | 7.30 | 5.10 | 0.214 | 0.8 | 1.9 | 0.188 |
| gB2+gB3 | 2.10 | 1.80 | 0.735 | 1.0 | 0.6 | 0.544 |
Values are expressed as means or percentages (SD). Hypertension was defined according to the World Health Organization guidelines. BMI – body mass index; WHR – waist-hip ratio; SBP – systolic blood pressure; DBP – diastolic blood pressure; MAP – mean arterial blood pressure; FBS – fasting blood sugar; CMV – cytomegalovirus; TC – plasma total cholesterol; TG – triglyceride; LDL-C – low-density lipoprotein cholesterol; HDL-C – high-density lipoprotein cholesterol; ‘–’ – not applicable.
In Kazakh participants, 3 CMV gB genotypes were identified (single genotype: gB2; genotype mixtures: gB1+gB2 and gB2+gB3).
In Han participants, 4 CMV gB genotypes were identified (gB1, gB2, gB1+gB2, and gB2+gB3).
Statistically significant difference (P<0.05) present between various clinical and biochemical indices measured for participants with and without hypertension in the 2 populations.
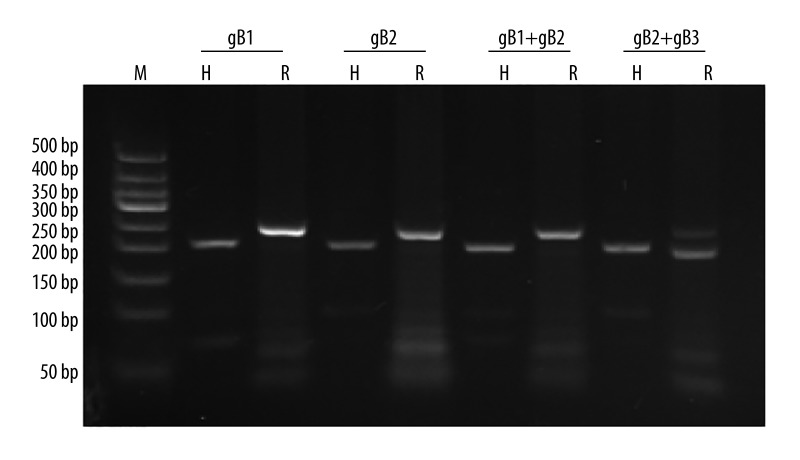
Because the prevalence of CMV infection was high in hypertensive participants in our study cohort (96.8% and 89.8% in Kazakh and Han hypertensive participants, respectively), we examined whether the CMV gB genotypes differed between hypertensive and control (healthy) participants. Using qPCR assays, we detected CMV DNA in 70 of the 467 blood samples obtained from Kazakh hypertensive participants and in 37 of the 333 control samples (15.0% vs. 11.1%; P=0.112). Moreover, we detected CMV DNA in 17 of the 482 blood samples collected from Han hypertensive participants and in 17 of the 318 control samples (3.5% vs. 5.3%; P=0.212). The restriction-digest patterns that distinguish the gB genotypes 1–2 and the mixtures of genotypes are shown in Figure 1. In Kazakhs, 3 CMV gB genotypes were identified, gB2 and the genotype mixtures gB1+gB2 and gB2+gB3. In Hans, 4 CMV gB genotypes were identified, gB1, gB2, gB1+gB2, and gB2+gB3. The prevalence of gB1 in Han participants with hypertension was lower than that in Han participants without hypertension (0.4% vs. 2.2%; P=0.019).
Figure 1.
RFLP analysis of the PCR-amplified glycoprotein B gene sequences. The products were digested by Hinf I (H) and Rsa I (R), respectively. M=50-bp DNA ladder marker (50 bp,100 bp, 150 bp, 200 bp, 250 bp, 300 bp, 350 bp, 400 bp, 500 bp).
Table 3 shows the association between CMV seropositivity and blood pressure as a continuous variable among Kazakhs and Hans. In Kazakh males, CMV seropositivity was significantly associated with DBP or MAP before adjustment (P=0.023). However, in both Kazakh and Han participants, CMV seropositivity was not associated with blood pressure in either males or females in any of the models.
Table 3.
Association of CMV seropositivity with blood pressure in Kazakh and Han males and females.
| Systolic blood pressure | Diastolic blood pressure | Mean arterial pressure | |||||
|---|---|---|---|---|---|---|---|
| B | P value | B | P value | B | P value | ||
| Kazakh participants | Males (n=336) | ||||||
| Unadjusted | 11.539 | 0.051 | 8.933 | 0.023* | 9.802 | 0.023* | |
| Model 1 | 6.614 | 0.229 | 5.014 | 0.162 | 5.547 | 0.159 | |
| Model 2 | 5.921 | 0.235 | 4.809 | 0.173 | 5.18 | 0.166 | |
| Females (n=464) | |||||||
| Unadjusted | 0.891 | 0.892 | 1.766 | 0.646 | 1.475 | 0.746 | |
| Model 1 | 2.192 | 0.716 | 2.174 | 0.54 | 2.18 | 0.600 | |
| Model 2 | 1.378 | 0.807 | 1.826 | 0.595 | 1.677 | 0.671 | |
| Han participants | Males (n=342) | ||||||
| Unadjusted | −2.809 | 0.427 | 0.379 | 0.832 | −0.683 | 0.730 | |
| Model 1 | −4.743 | 0.202 | −2.290 | 0.204 | −3.108 | 0.124 | |
| Model 2 | −6.483 | 0.079 | −1.950 | 0.283 | −3.461 | 0.089 | |
| Females (n=458) | |||||||
| Unadjusted | −1.420 | 0.774 | −1.454 | 0.508 | −1.442 | 0.612 | |
| Model 1 | 1.177 | 0.79 | −1.455 | 0.504 | −0.577 | 0.829 | |
| Model 2 | 0.325 | 0.938 | −1.597 | 0.463 | −1.173 | 0.653 | |
Model 1: adjusted for smoking, alcohol consumption, vegetable intake, BMI, WHR, FBS, TC, TG, HDL-C, and LDL-C. Model 2: further adjusted for age.
Table 4 shows the results of multiple logistic regression analysis of hypertension. In this analysis, CMV seropositivity was associated with hypertension in Kazakh males before adjustment (OR=4, 95% CI=1.46–10.95, P=0.004). Models 1 and 2 show the logistic regression analysis performed after adjusting for covariates: The association between CMV seropositivity and hypertension in Kazakh males was attenuated after adjusting for smoking, alcohol consumption, vegetable intake, BMI, WHR, FBS, TC, TG, HDL-C, and LDL-C (OR=4.78, 95% CI=1.52–15.00, P=0.007), and the association remained significant after further adjustment for age (OR=6.78, 95% CI=1.82–25.23, P=0.004). However, CMV seropositivity was not significantly associated with hypertension in either Kazakh females or Hans in any of the models.
Table 4.
Association of CMV seropositivity with hypertension in Kazakhs and Hans.
| Kazakh participants | Han participants | |||||
|---|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Unadjusted | Model 1 | Model 2 | |
| Males | ||||||
| OR | 4.00 | 4.78 | 6.78 | 1.08 | 0.78 | 0.83 |
| 95% CI | 1.46–10.95 | 1.52–15.00 | 1.82–25.23 | 0.59–1.98 | 0.39–1.56 | 0.41–1.69 |
| P value | 0.004* | 0.007* | 0.004* | 0.808 | 0.477 | 0.603 |
| Females | ||||||
| OR | 1.3 | 1.51 | 1.43 | 0.84 | 1.25 | 1.21 |
| 95% CI | 0.52–3.26 | 0.55–4.13 | 0.52–3.96 | 0.37–1.90 | 0.49–3.18 | 0.47–3.10 |
| P value | 0.574 | 0.426 | 0.488 | 0.679 | 0.644 | 0.695 |
Model 1: adjusted for smoking, alcohol consumption, vegetable intake, BMI, WHR, FBS, TC, TG, HDL-C, and LDL-C. Model 2: further adjusted for age.
Table 5 shows the association between antibody titers obtained from the CMV-specific IgG assay and blood pressure as a continuous variable among Kazakhs and Hans. In Han males and females, CMV antibody titers were not associated with SBP, DBP, and MAP before adjustment, but these associations were significant after adjusting for smoking, alcohol consumption, vegetable intake, BMI, WHR, FBS, TC, TG, HDL-C, and LDL-C (males: SBP, P=0.019; DBP, P=0.011; MAP, P=0.003; females: DBP, P=0.017; MAP, P=0.019), except in the case of SBP in Han females (P=0.057). Moreover, after further adjustment for age, significant relationships remained between CMV antibody titers and blood pressure (males: SBP, P=0.004; DBP, P=0.019; MAP, P=0.002; females: SBP, P=0.025; DBP, P=0.015; MAP, P=0.011). By contrast, in Kazakhs, CMV antibody titers were not significantly associated with blood pressure in any of the models.
Table 5.
Association of CMV antibody titers with blood pressure in Kazakhs and Hans.
| Systolic blood pressure | Diastolic blood pressure | Mean arterial pressure | |||||
|---|---|---|---|---|---|---|---|
| B | P value | B | P value | B | P value | ||
| Kazakh participants | Males (n=336) | ||||||
| Unadjusted | −0.445 | 0.725 | 1.133 | 0.179 | 0.607 | 0.513 | |
| Model 1 | −0.636 | 0.603 | 0.470 | 0.557 | 0.101 | 0.908 | |
| Model 2 | −0.801 | 0.471 | 0.421 | 0.592 | 0.014 | 0.987 | |
| Females (n=464) | |||||||
| Unadjusted | 0.005 | 0.997 | 0.747 | 0.351 | 0.500 | 0.599 | |
| Model 1 | −0.284 | 0.823 | 0.387 | 0.605 | 0.163 | 0.852 | |
| Model 2 | −0.282 | 0.812 | 0.388 | 0.592 | 0.164 | 0.843 | |
| Han participants | Males (n=342) | ||||||
| Unadjusted | −2.833 | 0.064 | −0.901 | 0.244 | −1.545 | 0.071 | |
| Model 1 | −3.689 | 0.019* | −1.937 | 0.011* | −2.521 | 0.003* | |
| Model 2 | −4.502 | 0.004* | −1.805 | 0.019* | −2.704 | 0.002* | |
| Females (n=458) | |||||||
| Unadjusted | −2.064 | 0.224 | −1.48 | 0.05 | −1.675 | 0.087 | |
| Model 1 | −2.926 | 0.057 | −1.796 | 0.017* | −2.172 | 0.019* | |
| Model 2 | −3.251 | 0.025* | −1.827 | 0.015* | −2.302 | 0.011* | |
Model 1: adjusted for smoking, alcohol consumption, vegetable intake, BMI, WHR, FBS, TC, TG, HDL-C, and LDL-C. Model 2: further adjusted for age.
Table 6 shows the association of CMV antibody titers with hypertension in Kazakh and Han participants. In Han males and females, CMV antibody titers were associated with hypertension in all models (before adjustment: males: OR=0.68, 95% CI=0.51–0.90, P=0.007; females: OR=0.72, 95% CI=0.85–1.31, P=0.024; after adjustment for smoking, alcohol consumption, vegetable intake, BMI, WHR, FBS, TC, TG, HDL-C, and LDL-C: males: OR=0.54, 95% CI=0.39–0.75, P<0.001; females: OR=0.62, 95% CI=0.44–0.88, P=0.007; after further adjusting for age: males: OR=0.55, 95% CI=0.40–0.76, P<0.001; females: OR=0.61, 95% CI=0.43–0.87, P=0.006).
Table 6.
Association of CMV antibody titers with hypertension in Kazakhs and Hans.
| Kazakh participants | Han participants | |||||
|---|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Unadjusted | Model 1 | Model 2 | |
| Males | ||||||
| OR | 1.18 | 1.24 | 1.31 | 0.68 | 0.54 | 0.55 |
| 95% CI | 0.95–1.45 | 0.96–1.60 | 0.98–1.74 | 0.51–0.90 | 0.39–0.75 | 0.40–0.76 |
| P value | 0.130 | 0.104 | 0.065 | 0.007* | <0.001* | <0.001* |
| Females | ||||||
| OR | 1.07 | 1.06 | 1.05 | 0.72 | 0.62 | 0.61 |
| 95% CI | 0.88–1.30 | 0.86–1.31 | 0.85–1.31 | 0.85–1.31 | 0.44–0.88 | 0.43–0.87 |
| P value | 0.493 | 0.594 | 0.647 | 0.024* | 0.007* | 0.006* |
Model 1: adjusted for smoking, alcohol consumption, vegetable intake, BMI, WHR, FBS, TC, TG, HDL-C, and LDL-C. Model 2: further adjusted for age.
Table 7 shows the association between CMV gB genotypes and hypertension. In Kazakhs and Hans, no significant relationships were detected between single genotypes or mixtures of genotypes and hypertension, except in the case of the single genotype gB2 in Kazakh males before adjustment for confounding factors (OR=4.59, 95% CI=1.04–20.34, P=0.039). In Kazakh males and in Hans, no significant relationships were detected between single genotypes or mixtures of genotypes and hypertension after adjustments. However, in Kazakh females, after adjusting for smoking, alcohol consumption, vegetable intake, BMI, WHR, FBS, TC, TG, HDL-C, and LDL-C, the gB2+gB3 genotype mixture was borderline associated with hypertension (OR=5.49, 95% CI=0.97–31.06, P=0.054), and the association was significant after further adjustment for age (OR=7.55, 95% CI=1.33–43.01, P=0.023).
Table 7.
Hypertension in Kazakhs and Hans: prediction based on CMV single genotypes and mixtures of genotypes by using multivariable odds ratios and their 95% confidence intervals.
| Unadjusted | Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | ||
| Kazakh participants | Males | |||||||||
| Single genotype | ||||||||||
| gB2 | 4.59 | 1.04–20.34 | 0.039* | 1.63 | 0.30–8.94 | 0.575 | 1.82 | 0.31–10.58 | 0.508 | |
| Genotype mixtures | ||||||||||
| gB1+gB2 | 1.48 | 0.56–3.89 | 0.495 | 0.68 | 0.17–2.67 | 0.573 | 1.27 | 0.28–5.86 | 0.761 | |
| gB2+gB3 | 0.26 | 0.05–1.46 | 0.189 | 0.19 | 0.02–1.56 | 0.122 | 0.18 | 0.02–1.61 | 0.124 | |
| Females | ||||||||||
| Single genotype | ||||||||||
| gB2 | 0.708 | 0.30–1.67 | 0.513 | 0.78 | 0.31–1.93 | 0.591 | 0.88 | 0.33–2.35 | 0.81 | |
| Genotype mixtures | ||||||||||
| gB1+gB2 | 1.45 | 0.67–3.13 | 0.443 | 1.16 | 0.49–2.76 | 0.733 | 1.14 | 0.46–2.77 | 0.782 | |
| gB2+gB3 | 3.54 | 0.74–16.83 | 0.115 | 5.49 | 0.97–31.06 | 0.054 | 7.55 | 1.33–43.01 | 0.023* | |
| Han participantsa | Males | |||||||||
| Genotype mixtures | ||||||||||
| gB1+gB2 | 0.37 | 0.06–2.27 | 0.357 | 0.42 | 0.07–2.67 | 0.358 | 0.42 | 0.07–2.71 | 0.364 | |
| gB2+gB3 | 0.57 | 0.04–9.14 | 1.000 | 0.25 | 0.01–4.89 | 0.363 | 0.26 | 0.01–4.98 | 0.368 | |
| Females | ||||||||||
| Single genotype | ||||||||||
| gB1 | 0.29 | 0.06–1.50 | 0.140 | 0.20 | 0.03–1.30 | 0.092 | 0.19 | 0.03–1.22 | 0.080 | |
| Genotype mixtures | ||||||||||
| gB1+gB2 | 0.486 | 0.08–2.94 | 0.655 | 0.37 | 0.05–2.60 | 0.319 | 0.38 | 0.06–2.60 | 0.322 | |
| gB2+gB3 | 2.97 | 0.33–26.78 | 0.401 | 5.53 | 0.43–71.42 | 0.190 | 5.63 | 0.45–71.02 | 0.181 | |
Model 1: adjusted for smoking, alcohol consumption, vegetable intake, BMI, WHR, FBS, TC, TG, HDL-C, and LDL-C. Model 2: further adjusted for age.
Because the sample sizes were extremely small, we did not analyze the association of gB1 with hypertension in Han males (n=0) and of gB2 with hypertension in Han males and females (n=6 and n=0).
Discussion
To our knowledge, this is the first study to demonstrate a link between serological evidence of CMV exposure and EH risk in a community sample of 1600 people (800 Kazakhs and 800 Hans) from Xinjiang Province, China. We used a CMV-specific IgG assay and determined that the CMV seroprevalence rates in Kazakh and Han participants with hypertension were 96.8% and 89.8%. Although several recent reports have indicated a role of CMV in hypertension, few studies have reported CMV seroprevalence in hypertensive China minorities. Our results are similar to those of a cross-sectional study of 194 hypertensive and 97 healthy Chinese, in which the CMV seroprevalence in the hypertensive participants was measured to be 94.33% [21]. CMV seroprevalence is recognized to be >90% in certain risk groups such as HIV-infected patients [8]. Therefore, we hypothesize that a weak general health condition associated with hypertension might have led to an increased risk of human CMV infection in Kazakh and Han hypertensive participants. Epidemiological data obtained in 2009 on 3740 people in Ji’nan City, Shandong Province, China, showed that human CMV seroprevalence was 48.3% in adults [9]. Our sero-epidemiological survey showed that between 2009 and 2012, the overall infection rates of human CMV in Kazakh and Han adults were 95.4% and 90.1%, respectively, which are higher than that reported for the people from Ji’nan City in eastern China. However, it is unclear whether this difference was caused by the disparities in geographical areas, socioeconomic populations, sample sizes, age ranges, and races/ethnicities covered in these studies.
CMV infection has been widely reported to be associated with hypertension. Regardless of the approach used, both negative and positive associations have been reported. Recently, in a Chinese cohort, Li et al. [21] showed that plasma CMV DNA copy number but not CMV seropositivity was associated with hypertension. However, in this study, we determined that in Kazakh males but not females, CMV seropositivity was associated independently in a statistically significant manner with hypertension after adjusting for smoking, alcohol consumption, vegetable intake, BMI, WHR, FBS, TC, TG, HDL-C, LDL-C, and age. Our finding also differs from the result of the United States National Health and Nutrition Examination Survey 1999–2002; in that study cohort, CMV seropositivity was associated with hypertension in females before adjustment, but the association was not significant after adjusting for age [23]. A previous study involving 1074 females and 857 males (aged 24–39 years), the Cardiovascular Risk in Young Finns study, showed that CMV antibody titers were independent determinants for SBP and DBP elevation and flow-mediated dilation in young males, but not in females [22]. By contrast, our results demonstrated that in both Han males and females (aged 18–84 years), CMV antibody titers were significantly associated independently with hypertension after adjusting for the aforementioned confounding hypertension risk factors. A similar trend also appeared in the association between CMV antibody titers and blood pressure (SBP, DBP, and MAP) in Hans.
Although CMV infections have been reported in association with hypertension in various types of studies, little is known about CMV’s role as a potential risk factor for hypertension. Furthermore, the specific underlying pathophysiological mechanisms that link CMV infection with EH remain to be elucidated [21–24,26]. However, numerous studies have provided key insights into the pathogenic mechanism underlying this association. Landmesser et al. [27] reported that CMV infections can result in increased levels of oxidative stress and inflammation, which have been linked to endothelial damage in EH [28]. In an animal study, CMV infection was shown to reduce nitric oxide (NO) bioavailability in endothelial cells and activate nicotinamide adenine dinucleotide phosphate oxidase, which resulted in arteriolar dysfunction [29]. Recent evidence indicates that CMV infection impairs endothelial NO synthase (eNOS) function and causes endothelial damage [30]. Furthermore, in relation to oxidative stress, CMV infection of smooth muscle cells was shown to generate reactive oxygen species through a cyclooxygenase-2-dependent pathway, and activation of the transcription factor nuclear factor-kappa B was shown to induce endothelial cell dysfunction and vascular inflammation [31].
In this study, CMV seroprevalence was higher and vegetable intake was lower among hypertensive Kazakhs than among Kazakh participants without hypertension. However, this was not observed in Hans. Currently, no information is available in the literature that can explain this difference. We speculate that CMV seroprevalence in hypertensive Kazakhs might have been increased in the following manner. An eNOS cofactor such as folacin might be decreased in hypertensive Kazakh Chinese. Kazakhs include few vegetables in their diet, which results in a low folacin uptake. Because folacin functions as an essential cofactor, its deficiency might contribute to injury under conditions of inflammation-driven oxidative stress in the vascular wall [32,33]. Alternatively, CMV can activate the p38-mitogen-activated protein kinase signaling pathway, and the activation of this or other signaling pathways might cause the inhibition of eNOS and a reduction of NO production, leading to endothelial dysfunction [34–36]. Both folacin deficiency and CMV infection accelerate the development of CVDs.
A key mechanism through which CMV might be associated with hypertension could involve the renin-angiotensin system (RAS) [37]. An in vivo experimental study showed that CMV infection increased arterial pressure, and further that it stimulated the expression of renin in a dose-dependent manner in both kidney cells and blood vessel endothelial cells and increased angiotensin II (Ang II) levels in blood and arterial tissues [26]. In the Cardiovascular Risk in Young Finns study, Haarala et al. [22] found that high CMV antibody titers were associated directly with SBP and DBP and inversely with brachial artery flow-mediated dilation in young males; the researchers speculated that RAS activation might mediate these associations. High CMV antibody titers could potentially indicate frequent reactivation of CMV or reinfection with new strains of CMV, which can strengthen the immunity in these infected people. CMV activity might result in increased RAS activation and thus lead to arterial constriction through the influence of Ang II.
A relationship between CMV antibody titers and hypertension has been reported only in a few studies, and the findings have not been consistent. In this study, we determined that in Hans, CMV antibody titers remained significantly associated independently with blood pressure (SBP, DBP, and MAP) after adjusting for confounding hypertension risk factors. However, an unexpected finding was that a low CMV antibody titer was associated with hypertension in Hans. We speculate that this low CMV antibody titer in the hypertensive participants might be related to their immunocompromised state; this is because, in immunocompetent people, infection with human CMV is typically asymptomatic. By contrast, repeated CMV infections cause substantial morbidity and mortality in immunocompromised patients, and once patients are immunocompromised, CMV can be reactivated [38,39]. Enhanced expression of the pro-inflammatory cytokines renin and Ang II underlies the pathogenic mechanism that causes repeated CMV infections to increase arterial intima-media thickness and vascular endothelial dysfunction, which results in an increase of arterial blood pressure. These phenomena can be observed in the blood vessels of mice infected with mouse CMV [26]. However, our findings contrast those of Haarala et al. [22], and the discrepancies between the results of the Cardiovascular Risk in Young Finns study and our study might be caused by the difference in the age ranges of the participants included, 24–39 and 18–84 years in the Haarala et al. study and our study, respectively; this suggests that the humoral immune status in hypertension patients declines as they age.
The immunological status of patients can influence the clinical outcome of CMV infection, and distinct virus strains have been proposed to exhibit varying virulence [15,40]. In this study, the genotype mixture gB2+gB3 was associated independently with hypertension in Kazakh females after adjusting for confounding hypertension risk factors. Previous studies showed that the protein encoded by the gB gene of CMV is not only a major target for neutralizing antibodies, but also a molecule that is critical for the entry of virions into cells, transmission of infection from cell to cell, and the fusion of infected cells [41,42]. CMV infection triggers the proliferation of one type of T lymphocytes, CD8+ cytotoxic T lymphocytes; these lymphocytes participate in the alteration of vascular tone encountered in hypertension induced by Ang II, a potent vasoconstrictor [43–45]. Therefore, T cells might serve as a link between CMV infection and hypertension. Furthermore, considering these functions of gB, the most direct explanation for the preferential tropism of gB2 and gB3 for T lymphocytes is that the gB proteins differ in their ability to support the penetration of virions into T lymphocytes [46]. This could explain why the gB2+gB3 genotype mixture was associated independently with hypertension in Kazakh females. Thus, these findings suggest that a unique synergy of this coinfection, rather than infection by viruses of a single genotype, functions in the development of hypertension. However, no genotypes were associated independently with hypertension in Hans. Currently, we cannot explain the observed race-related differences in our results, but they might potentially be related to the lower percentage of CMV DNA detected in Hans than in Kazakhs.
The role of CMV as a risk factor for hypertension remains controversial. Several previous studies have investigated the role of CMV seropositivity as a risk factor for hypertension [23,24], but few studies have examined the roles of CMV antibody titers and CMV gB genotypes. With regard to the conflicting results obtained in previous human studies, we suggest that to elucidate the role of CMV in hypertension, a complex approach, involving the use of a panel of new atherosclerotic risk factors, is required. In this study, we comprehensively investigated the relationship between CMV and hypertension by focusing on how 3 specific aspects, CMV seropositivity, CMV antibody titers, and the pathogenic genotype of CMV gB, affected hypertension in Kazakhs and Hans. CMV seropositivity was significantly associated independently with hypertension in Kazakh males, but not in Hans, whereas CMV antibody titers were associated independently with hypertension in Hans, but not in Kazakhs. The genotype mixture gB2+gB3 was associated independently with EH in Kazakh females but not males. These differences might arise because of dissimilarities in socioeconomic factors, dietary habits, lifestyles, or CMV infection leading to distinct immune responses and subclinical manifestations in Kazakhs and Hans.
Conclusions
CMV infection is associated with hypertension in Kazakh males and Hans in Xinjiang Province. The gB2+gB3 genotype CMV coinfection is associated with an increased risk of hypertension in Kazakh females. Thus, controlling CMV infection to restrict the development of hypertension might provide a new strategy for preventing CVDs associated with CMV infection in Kazakhs and Hans. However, to further confirm our findings, additional studies must be conducted in which the sample population sizes are increased and the mechanisms of hypertension development in Kazakhs and Hans are investigated.
Footnotes
Conflicts of interest
No conflicts of interest exist in this study.
Source of support: This project was supported by the Joint Funds of the National Natural Science Foundation of China (No.U1403123) and the Ministry of Major Science & Technology of Shihezi University (No.gxjs2013-zdgg04, No.gxjs2013-zdgg04-1, No.gxjs2013-zdgg04-2, No.gxjs2013-zdgg04-3, and No.gxjs2013-zdgg04-4)
References
- 1.Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003;139:761–76. doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–86. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.Kuneš J, Zicha J. The interaction of genetic and environmental factors in the etiology of hypertension. Physiol Res. 2009;58:S33–41. doi: 10.33549/physiolres.931913. [DOI] [PubMed] [Google Scholar]
- 4.Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370:591–603. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- 5.Dolan A, Cunningham C, Hector RD, et al. Genetic content of wild-type human cytomegalovirus. J Gen Virol. 2004;85:1301–12. doi: 10.1099/vir.0.79888-0. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi MK, Wills MR, Patrick Sissons J, Carmichael AJ. Human cytomegalovirus-specific immunity following haemopoietic stem cell transplantation. Blood Rev. 2003;17:259–64. doi: 10.1016/s0268-960x(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 7.Ho M. The history of cytomegalovirus and its diseases. Med Microbiol Immun. 2008;197:65–73. doi: 10.1007/s00430-007-0066-x. [DOI] [PubMed] [Google Scholar]
- 8.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50:1439–47. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao P, Ma D, Xue F, et al. Seroprevalence and risk factors of human cytomegalovirus infection in the eastern Chinese population. Arch Virol. 2009;154:561–64. doi: 10.1007/s00705-009-0339-3. [DOI] [PubMed] [Google Scholar]
- 10.Cheung TW, Teich SA. Cytomegalovirus infection in patients with HIV infection. Mt Sinai J Med. 1999;66(2):113–24. [PubMed] [Google Scholar]
- 11.Rowshani AT, Bemelman FJ, van Leeuwen EM, et al. Clinical and immunologic aspects of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2005;79:381–86. doi: 10.1097/01.tp.0000148239.00384.f0. [DOI] [PubMed] [Google Scholar]
- 12.Bale JF, Miner L, Petheram SJ. Congenital cytomegalovirus infection. Curr Treat Option Ne. 2002;4:225–30. doi: 10.1007/s11940-002-0039-8. [DOI] [PubMed] [Google Scholar]
- 13.Chou S, Dennison KM. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J Infect Dis. 1991;163:1229–34. doi: 10.1093/infdis/163.6.1229. [DOI] [PubMed] [Google Scholar]
- 14.Shepp D, Match M, Lipson S, Pergolizzi R. A fifth human cytomegalovirus glycoprotein B genotype. Res Virol. 1998;149:109–14. doi: 10.1016/s0923-2516(98)80086-1. [DOI] [PubMed] [Google Scholar]
- 15.Brown JM, Kaneshima H, Mocarski ES. Dramatic interstrain differences in the replication of human cytomegalovirus in SCID-hu mice. J Infect Dis. 1995;171:1599–603. doi: 10.1093/infdis/171.6.1599. [DOI] [PubMed] [Google Scholar]
- 16.Mewara A, Mishra B, Kanta Ratho R, Kumar P. Cytomegalovirus glycoprotein B gene polymorphism and its association with clinical presentations in infants. Southeast Asian J Trop Med Public Health. 2009;40:759–64. [PubMed] [Google Scholar]
- 17.Simanek AM, Dowd JB, Pawelec G, et al. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One. 2011;6:e16103. doi: 10.1371/journal.pone.0016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kytö V, Vuorinen T, Saukko P, et al. Cytomegalovirus infection of the heart is common in patients with fatal myocarditis. Clin Infect Dis. 2005;40:683–88. doi: 10.1086/427804. [DOI] [PubMed] [Google Scholar]
- 19.Popović M, Smiljanić K, Dobutović B, et al. Human cytomegalovirus infection and atherothrombosis. J Thromb Thrombolysis. 2012;33:160–72. doi: 10.1007/s11239-011-0662-x. [DOI] [PubMed] [Google Scholar]
- 20.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–36. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Zhu J, Zhang W, et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation. 2011;124:175–84. doi: 10.1161/CIRCULATIONAHA.110.012237. [DOI] [PubMed] [Google Scholar]
- 22.Haarala A, Kähönen M, Lehtimäki T, et al. Relation of high cytomegalovirus antibody titres to blood pressure and brachial artery flow-mediated dilation in young men: the Cardiovascular Risk in Young Finns Study. Clin Exp Immunol. 2012;167:309–16. doi: 10.1111/j.1365-2249.2011.04513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Samaranayake NR, Ong KL, et al. Is human cytomegalovirus infection associated with hypertension? The United States National Health and Nutrition Examination Survey 1999–2002. PLoS One. 2012;7:e39760. doi: 10.1371/journal.pone.0039760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vahdat K, Pourbehi MR, Ostovar A, et al. Association of pathogen burden and hypertension: the Persian Gulf Healthy Heart Study. Am J Hypertens. 2013;26:1140–47. doi: 10.1093/ajh/hpt083. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Wang H, Yan Z, et al. Ethnic disparities in the clustering of risk factors for cardiovascular disease among the Kazakh, Uygur, Mongolian and Han populations of Xinjiang: a cross-sectional study. BMC Public Health. 2012;12:499. doi: 10.1186/1471-2458-12-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng J, Ke Q, Jin Z, et al. Cytomegalovirus infection causes an increase of arterial blood pressure. PLoS Pathog. 2009;5:e1000427. doi: 10.1371/journal.ppat.1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landmesser U, Cai H, Dikalov S, et al. Role of p47phox in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–15. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chobanian AV, Alexander RW. Exacerbation of atherosclerosis by hypertension: potential mechanisms and clinical implications. Arch Intern Med. 1996;156:1952–56. [PubMed] [Google Scholar]
- 29.Leskov IL, Whitsett J, Vasquez-Vivar J, Stokes KY. NAD (P) H oxidase and eNOS play differential roles in cytomegalovirus infection-induced microvascular dysfunction. Free Radical Bio Med. 2011;51:2300–8. doi: 10.1016/j.freeradbiomed.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Yang Y, Yang X, Cai J. Human cytomegalovirus infection is a novel etiology for essential hypertension. Med Hypotheses. 2011;76:682–84. doi: 10.1016/j.mehy.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 31.Collins T. Endothelial nuclear factor-kappa B and the initiation of the atherosclerotic lesion. Lab Invest. 1993;68:499–508. [PubMed] [Google Scholar]
- 32.Munteanu A, Zingg J-M. Cellular, molecular and clinical aspects of vitamin E on atherosclerosis prevention. Mol Aspects Med. 2007;28:538–90. doi: 10.1016/j.mam.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Shirodaria C, Antoniades C, Lee J, et al. Global Improvement of Vascular Function and Redox State With Low-Dose Folic Acid Implications for Folate Therapy in Patients With Coronary Artery Disease. Circulation. 2007;115:2262–70. doi: 10.1161/CIRCULATIONAHA.106.679084. [DOI] [PubMed] [Google Scholar]
- 34.Shen YH, Zhang L, Utama B, et al. Human cytomegalovirus inhibits Akt-mediated eNOS activation through upregulating PTEN (phosphatase and tensin homolog deleted on chromosome 10) Cardiovasc Res. 2006;69:502–11. doi: 10.1016/j.cardiores.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Weis M, Kledal TN, Lin KY, et al. Cytomegalovirus infection impairs the nitric oxide synthase pathway role of asymmetric dimethylarginine in transplant arteriosclerosis. Circulation. 2004;109:500–5. doi: 10.1161/01.CIR.0000109692.16004.AF. [DOI] [PubMed] [Google Scholar]
- 36.Grahame-Clarke C, Chan NN, Andrew D, et al. Human cytomegalovirus seropositivity is associated with impaired vascular function. Circulation. 2003;108:678–83. doi: 10.1161/01.CIR.0000084505.54603.C7. [DOI] [PubMed] [Google Scholar]
- 37.Sachetelli S, Liu Q, Zhang S, et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69:1016–23. doi: 10.1038/sj.ki.5000210. [DOI] [PubMed] [Google Scholar]
- 38.Landolfo S, Gariglio M, Gribaudo G, Lembo D. The human cytomegalovirus. Pharmacol Therapeut. 2003;98:269–97. doi: 10.1016/s0163-7258(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 39.Pass R. Cytomegalovirus. Fields Virology. 2001;2:2675–705. [Google Scholar]
- 40.Fries BC, Chon S, Boeckh M, Torok-Storb B. Frequency distribution of cytomegalovirus envelope glycoprotein genotypes in bone marrow transplant recipients. J Infect Dis. 1994;169:769–74. doi: 10.1093/infdis/169.4.769. [DOI] [PubMed] [Google Scholar]
- 41.Cranage M, Kouzarides T, Bankier A, et al. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986;5:3057–63. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navarro D, Paz P, Tugizov S, et al. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology. 1993;197:143–58. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- 43.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II – induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–60. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–79. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuhrmann S, Streitz M, Reinke P, et al. T cell response to the cytomegalovirus major capsid protein (UL86) is dominated by helper cells with a large polyfunctional component and diverse epitope recognition. J Infect Dis. 2008;197:1455–58. doi: 10.1086/587692. [DOI] [PubMed] [Google Scholar]
- 46.Meyer-König U, Vogelberg C, Bongarts A, et al. Glycoprotein B genotype correlates with cell tropism in vivo of human cytomegalovirus infection. J Med Virol. 1998;55:75–81. [PubMed] [Google Scholar]