Abstract
Objectives
Cost-effectiveness of percutaneous coronary intervention (PCI) using drug-eluting stents (DES), and coronary artery bypass surgery (CABG) was analyzed in patients with multivessel coronary artery disease over a 5-year follow-up.
Background
DES implantation reducing revascularization rate and associated costs might be attractive for health economics as compared to CABG.
Methods
Consecutive patients with multivessel DES-PCI (n = 114, 3.3 ± 1.2 DES/patient) or CABG (n = 85, 2.7 ± 0.9 grafts/patient) were included prospectively. Primary endpoint was cost-benefit of multivessel DES-PCI over CABG, and the incremental cost-effectiveness ratio (ICER) was calculated. Secondary endpoint was the incidence of major adverse cardiac and cerebrovascular events (MACCE), including acute myocardial infarction (AMI), all-cause death, revascularization, and stroke.
Results
Despite multiple uses for DES, in-hospital costs were significantly less for PCI than CABG, with 4551 €/patient difference between the groups. At 5-years, the overall costs remained higher for CABG patients (mean difference 5400 € between groups). Cost-effectiveness planes including all patients or subgroups of elderly patients, diabetic patients, or Syntax score >32 indicated that CABG is a more effective, more costly treatment mode for multivessel disease. At the 5-year follow-up, a higher incidence of MACCE (37.7% vs. 25.8%; log rank P = 0.048) and a trend towards more AMI/death/stroke (25.4% vs. 21.2%, log rank P = 0.359) was observed in PCI as compared to CABG. ICER indicated 45615 € or 126683 € to prevent one MACCE or AMI/death/stroke if CABG is performed.
Conclusions
Cost-effectiveness analysis of DES-PCI vs. CABG demonstrated that CABG is the most effective, but most costly, treatment for preventing MACCE in patients with multivessel disease. © 2014 Wiley Periodicals, Inc.
Keywords: coronary artery bypass surgery, percutaneous coronary intervention, drug-eluting stent, cost-benefit, follow-up study
INTRODUCTION
Cost-effectiveness studies comparing percutaneous coronary angioplasty (PTCA) with percutaneous coronary intervention (PCI) to either bare-metal stents (BMS) or drug-eluting stents (DES) have shown that the use of DES in single lesions or patients with low-moderate risk of restenosis cannot be justified because the substantial additional costs do not offer significant additional benefits compared to currently available BMS unless the price of DES is substantially reduced 1–5. Another economic consideration for the use of DES is the treatment of multivessel coronary artery disease (CAD) as DES reduces the need for repeat revascularization and associated combined safety and efficacy clinical endpoints 6–8, therefore it may be more cost-effective than coronary artery bypass surgery (CABG) 9–11. Health-economic investigations 11,12 considering the resources and costs have shown a short-term (in-hospital) economic advantage of the use of DES as compared to CABG, as personal and material resources for CABG cost more, even if multiple use of DES augment multivessel DES-PCI costs. In the first couple of years, the financial benefit of DES vs. CABG decreases and CABG continues to accrue substantial life-year and quality of life advantages without any further additional cost.
Most of the studies comparing DES-PCI and CABG have been short or of intermediate follow-up (up to 3 years) and provided no long-term cost-effectiveness data. The length of follow-up is crucial when comparing the cost-effectiveness of CABG and stents/DES because bypass graft degeneration usually starts 4–5 years after surgery, leading to an increase in the number of repeat target vessel revascularization (TVR) procedures for the grafts 13; while late stent thrombosis and the long-term safety issues of DES are still a matter of debate. Bearing in mind that the DES market has changed dynamically with new DES designs, coating substances, and technologies, with successional financial sequel in health economics, cost-effectiveness analyses comparing CABG with PCI require continuous update. Furthermore, CABG and PCI are not interchangeable procedures, requiring individual decisions on the type of action. Accordingly, up to now, besides several nonrandomized comparative studies there are still limited number of prospective randomized long-term studies (SYNTAX and FREEDOM study) using drug-eluting stent for PCI in multivessel disease.
Considering all of these hurdles, the aim of our study was to elucidate the long-term national cost-effectiveness of CABG vs. PCI with DES in patients with multivessel CAD, comparing the costs and incidence of adverse events during follow-up. The present analysis reports the cost-utility and clinical results in the first 5 years after the index intervention, with additional sub-analyses of high-risk patient subsets, such as diabetes mellitus, elderly patients, and high Syntax score.
MATERIALS AND METHODS
Study Design
The present study is a prospective nonrandomized single-center Austrian registry including 199 consecutive patients with multivessel disease requiring multivessel coronary intervention using either PCI with TAXUS Express stent (paclitaxel-eluting DES, Boston Scientific, Natick, MA) or CABG. The study protocol complies with the Declaration of Helsinki and was approved by the local Ethics Committee of the Medical University of Vienna (ClinicalTrials.gov: NCT01199419).
Patient Population
Between May 2004 and March 2006, 199 consecutive patients with symptomatic coronary multivessel disease and predictable availability for long-term (up to 10 years) follow-up were prospectively enrolled in the study. The patients gave their written consent for revascularization and inclusion in the study before and after revascularization, respectively. The decision of multivessel PCI with DES (n = 114 patients) or CABG (n = 85 patients) was made by cardiologists on the basis of clinical and angiographic assessments and considering the individual patient's decision and clinical condition. In the case of complex coronary lesions, a heart team comprising cardiac surgeons and clinical, noninterventional, and interventional cardiologists made a recommendation for the revascularization procedure; an individual decision was made based on the coronary anatomy with the feasibility to achieve complete revascularization, the individual's cardiac function, the urgency of the need for revascularization with an assumption of the risk of the percutaneous or surgical procedure, compliance with antiplatelet therapy, age, prior cardiac surgery, and comorbidities, such as diabetes mellitus, pulmonary dysfunction, and renal dysfunction.
The inclusion criteria were symptomatic two or three-vessel CAD requiring percutaneous or surgical multivessel repair with the aim of complete revascularization; age >18 years; clinical presentation (stable or unstable angina) or signs of myocardial ischemia and ≥50% stenosis of each lesion; and availability for long-term (10 years) follow-up (i.e., willingness to participate and no life-time-limiting concomitant disease).
The exclusion criteria were ST-segment elevation acute myocardial infarction (<48 h); concomitant heart valve or other cardiac surgery; contraindications to clopidogrel, aspirin, heparin, or taxol; pregnancy, lack of protection against pregnancy, or breast-feeding during the study; hemorrhagic diathesis; and platelet count <100,000/ml3.
Study Endpoints
The primary outcome measure was the cost-effectiveness of PCI with TAXUS stents as compared to CABG in patients with multivessel CAD during the in-hospital phase and at 5 years. The secondary outcome measures included the composite major adverse cardiac and cerebrovascular event (MACCE, including all-cause death, nonfatal acute myocardial infarction/AMI/, TVR, and stroke) during the in-hospital phase, at 6 and 12 months, and at 2, 3, 4, and 5 years, and the total costs during the in-hospital phase, at 6 and 12 months, and at 2, 3, 4, and 5 years.
If a patient suffered from several concomitant adverse coronary events (e.g., AMI with subsequent TVR and death), the most serious event was considered the end point. In addition, the date of hospitalization related to the index procedure, days in the intensive care unit, number of blood transfusions, number of implantations of pacemaker (PM), automatic implantable cardioverter defibrillator (AICD), and intraaortic balloon pump (IABP) were recorded.
Coronary Procedure With Multivessel PCI or CABG
Patients receiving PCI were pretreated with clopidogrel (300–600 mg loading dose and 75 mg maintenance dose) and aspirin (250 mg loading dose and 100 mg maintenance dose). Taxus stents were implanted in each lesion. CABG was treated as an elective surgical procedure, with extra-corporal circulation (Online Supporting Information).
Data Collection
Demographic data included age, gender, and atherosclerotic risk factors, such as diabetes mellitus, hypercholesterolaemia, hypertension, and smoking, unstable angina (UA), non-ST-segment elevation myocardial infarction (NSTEMI) at clinical presentation and previous AMI (Online Supporting Information).
Calculation of Costs
The total expense of the procedure, cardiac rehabilitation, and further hospitalization during follow-up were calculated using information about the coronary care unit, ward, and personal and material cost from the Cost Unit Department of the General Hospital Vienna. The cost of PCI was obtained from the medical history by considering all interventions. The total expenses covered the materials used throughout the interventions (e.g., catheters, guide wires, dilatation balloons, and stents), drugs, contrast medium, the costs for medical staff, such as physicians, nurses, and technicians, and the costs for clinical follow-up, repeat revascularization, and dual antiplatelet therapy (DAPT) following the index procedure and TVR.
The cost of CABG covered the materials used throughout the procedures, drugs, the costs for medical staff, such as surgeons, nurses, and technicians, and the costs incurred for peri- and postinterventional complications (e.g., bleeding complications, blood transfusions, antibiotics, use of IABP) and hospitalization. In the case of reinterventions, the financial burden was calculated according to the model above.
Unit costs were based on estimates provided by the General Hospital of Vienna. Outpatient services or noncardiac related hospitalization were not included in this study, as they were considered to be similar in patients with multivessel CAD, irrespective of the treatment strategy. The costs present the direct medical costs.
Definitions
Definitions are presented in the On-line Supporting Information.
Statistical Analysis
Results for continuous variables are expressed as mean ± SD; in the case of skewed distributions, median [quartiles] values are given. Categorical variables are expressed as percentages. Continuous outcome variables are compared between treatment groups using the nonparametric Wilcoxon rank sum test.
Two different survival endpoints were evaluated for the statistical analysis; MACCE-free survival considered the first MACCE event of any kind as the endpoint, whereas AMI and/or stroke and/or death-free survival considered only AMI/stroke/death as the clinical hard endpoint. The Kaplan–Meier method was used to estimate MACCE-free and AMI/stroke/death-free survival. The log-rank test was used for comparisons of event-free survival between the treatment groups (DES-PCI vs. CABG).
A propensity score analysis was performed to test the role of patient selection in determining the outcome (Online Supporting Information).
The cost-effectiveness analysis was based on data for patients with a follow-up of at least 5 years. Complete costs within the first 5 years (±3 months) were calculated for each year and compared between treatment groups using the Wilcoxon rank sum test. Effectiveness was evaluated as the probability of surviving without any event for 5 years (±3 months) considering MACCE-free survival and AMI/stroke/death-free survival separately. Differences in effectiveness were tested for significance using the chi-square test. The incremental cost-effectiveness ratio (ICER) was calculated as the difference in cost between the two treatment groups related to the difference in effectiveness, and provided with the Fieller 95% confidence interval 14.
The number of patients needed to treat (NNT) with CABG rather than PCI to prevent one MACCE or AMI/stroke/death event was calculated. The results of the cost-effectiveness analyses are illustrated in cost-effectiveness planes. To illustrate the joint distribution of the differences in costs and effectiveness between the two treatment groups, 25,000 bootstrap replications of the observed data were produced and the results presented graphically. Arbitrarily, a willingness to pay 40,000 and 80,000 € was considered to be cost effective to prevent one MACCE and AMI/death/stroke.
As no sample size calculation for cost-effectiveness exists, power calculation could not be performed.
Significant differences between the groups were considered if P < 0.05. The SAS 9.2 software (SAS Institute, 2002–2008, Cary, NC) and R package ICEinger were used for statistical analyses.
RESULTS
Baseline Clinical Characteristics
The mean age, male gender, cardiac history, and prevalence of risk factors were similar in the two groups (Table1). Syntax score was higher in patients undergoing CABG. Propensity score analysis revealed a skewed distribution of the individual scores in both groups, with an expected significant difference between the groups (P = 0.034) unless Syntax score was excluded (P = 0.643; Supporting Information Fig. 1). Supporting Information Table1 lists the baseline parameters of patients with and without diabetes mellitus.
Table 1.
Demographic Characteristics of Patients with Multivessel Disease and Treated with Either Coronary Artery Bypass Graft Surgery (CABG) or Percutaneous Coronary Intervention (PCI)
| CABG (n = 85) | PCI (n = 114) | |
|---|---|---|
| Age (years) | 66 ± 10 | 65 ± 12 |
| Age ≥65 years | 41 (48.2%) | 50 (43.9%) |
| Male gender | 70 (82.4%) | 90 (78.9%) |
| Risk factors | ||
| Diabetes mellitus | 39 (45.9%) | 42 (36.8%) |
| Hypertension | 62 (72.9%) | 88 (77.2%) |
| Hyperlipidaemia | 61 (71.8%) | 87 (76.3%) |
| Smoking | 26 (30.6%) | 33 (28.9%) |
| Cardiac history | ||
| Previous myocardial infarction | 23 (27.1%) | 28 (24.6%) |
| UA/NSTEMI at clinical presentation | 17 (20.0%) | 23 (20.2%) |
| Number of diseased vessels | 2.72 ± 0.50 | 2.54 ± 0.50 |
| Left ventricular ejection fraction | 56.7 ± 7.7% | 57.2 ± 9.5% |
| Number of implanted grafts/DES | 2.67 ± 0.85 | 3.29 ± 1.20 |
| Syntax score | 29.3 ± 9.9 | 24.2 ± 8.5a |
| Syntax score >32 | 25 (29.4%) | 17 (14.7%)a |
| Procedure data | ||
| Duration of hospitalization (days) | 13 [10,17; 7–74] | 6 [3,9; 2–38]a |
| Duration of intensive care unit (days) | 2 [1,4; 1–27] | 0 [0,0; 0–23]a |
| Number of blood transfusions (units) | 0 [0,0; 0–8] | 0 [0,0; 0–2]a |
| Number of PM-Impl. | 1 (1.2%) | 0 (0%) |
| Number of AICD-Impl. | 0 (0%) | 1 (0.88%) |
| Number of IABP-Impl. | 1 (1.2%) | 1 (0.88%) |
UA/NSTEMI: unstable angina/non-ST-segment elevation myocardial infarction; PM: pacemaker; AICD: automatic implantable cardioverter defibrillator; IABP: intraaortic balloon pump. Data are mean ± SD or median [quartiles; ranges].
P < 0.05 between the groups.
Figure 1.
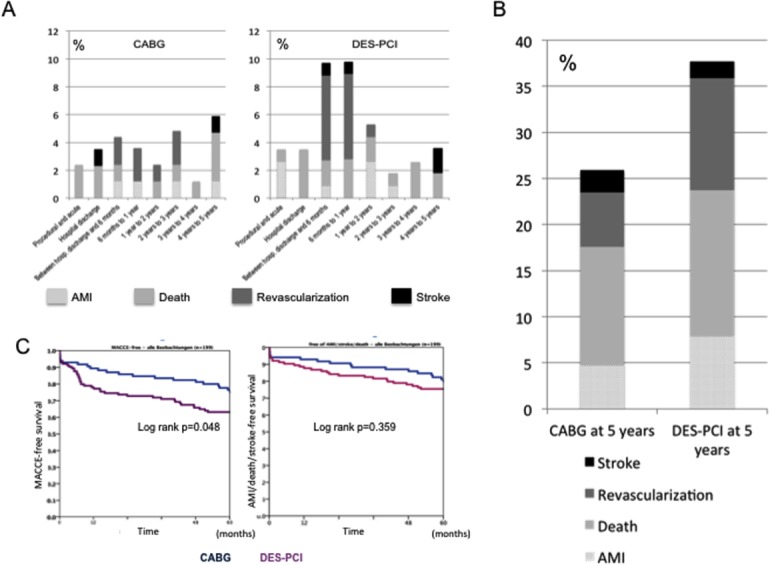
Incidence of adverse events in the coronary artery bypass graft surgery (CABG) and drug-eluting stent-percutaneous coronary intervention (DES-PCI) groups. (A) Detailed events in the groups. (B) Cumulative 5-year events in the groups. (C) Major adverse cardiac and cerebrovascular event (MACCE)-free survival rate (left) and acute myocardial infarction (AMI) and/or all cause death and/or stroke-free survival rate (right). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Procedure Data and In-Hospital Follow-Up
The number of diseased and treated vessels was similar in both groups (Table1). The duration of hospitalization and treatment in the intensive care unit was longer and the number of blood transfusion units higher in the CABG group as compared to the PCI group. No difference was found between the groups regarding the necessity of implantation of PM, AICD, or IABP.
Follow-Up Events
Procedure-related complication occurred in 2.4% and 3.7% of patients in the CABG and DES-PCI groups, respectively (Fig. 1). During the first 6 months of follow-up, 3 graft closures occurred in the CABG group, while 8 patients underwent TVR in the DES-PCI group (Fig. 1).
Later follow-up resulted in more favorable outcomes for CABG patients, with rare occurrence of events up to 5 years, whereas repeat TVR occurred more frequently (without very late stent thrombosis) in the PCI group, resulting in a significantly worse 5-year MACCE-free survival as compared to CABG (Fig. 1). During the 5-year follow-up, no differences were recorded in the prevalence of all-cause death (12.9% vs. 15.8%), AMI (4.7% vs. 7.9%), and stroke (2.4% vs. 1.8%), resulting in a 5-year incidence of combined death, AMI, and stroke of 21.2% vs. 25.4% (P > 0.05) in the CABG and PCI groups, respectively.
Cost-Effectiveness
The in-hospital cost was significantly lower in PCI patients compared to CABG patients (Table2), and remained lower after one year, mainly due to the postoperative rehabilitation costs for CABG patients. Although TVR was performed more often in the PCI group, the total cost at 5 years remained significantly lower in the PCI group.
Table 2.
Mean Differences in Cost and Follow-up (FUP) of Patients with Multivessel Disease Treated with Either Coronary Artery Bypass Graft Surgery (CABG) or Percutaneous Coronary Intervention (PCI) with Drug-Eluting Stents (DES)
| Mean difference between CABG and DES-PCI | |
|---|---|
| In-hospital costs | 4551 €a |
| Cumulative costs up to 6 months | 5510 €a |
| Cumulative costs up to 1 year | 5077 €a |
| Cumulative costs up to 2-year FUP | 4882 €a |
| Cumulative costs up to 3-year FUP | 5188 €a |
| Cumulative costs up to 4-year FUP | 5068 €a |
| Cumulative costs up to 5-year FUP | 5400 €a |
P < 0.05 (Wilcoxon-test).
At 5 years, no meaningful change in cost difference (5400 €/patient) was observed between the groups. Because of the higher rate of MACCE-free survival for CABG as compared to PCI, performing 8.4 CABG instead of PCI would result in one additional case of MACCE-free survival (Table3). The ICER indicated an additional amount of 45 615 € for each additional MACCE-free patient treated with CABG instead of PCI.
Table 3.
Incremental Cost-Efficacy Ratio (ICER) and Number Need to Treat (NNT) at 5-year Follow-up for Coronary Bypass Graft Surgery (CABG) or Percutaneous Coronary Intervention (PCI) with a Solely Drug-Eluting Stent to Save 1 MACCE-Free or 1 AMI/Death/Stroke-Free Life
| Cost differences between PCI and CABG (€) | ICER (€) | NNT | |
|---|---|---|---|
| All patients | 5400 | ||
| MACCE-free | 45,615 | 8.4 | |
| AMI/death/stroke-free | 126,683 | 23.5 | |
| Diabetes mellitus | 5472 | ||
| MACCE | 114,903 | 21 | |
| AMI/death/stroke | 186,718 | 34.1 | |
| Age > 65 years | 3258 | ||
| MACCE | 22,111 | 6.8 | |
| AMI/death/stroke | 42,805 | 13.1 | |
| Syntax score >32 | 4838 | ||
| MACCE | 19,397 | 4 | |
| AMI/death/stroke | 89,397 | 18.5 | |
| Syntax score ≤32 | 5439 | ||
| MACCE | 54,119 | 9.9 | |
| AMI/death/stroke | 114,709 | 21.1 |
Considering AMI and/or death and/or stroke as clinical hard endpoint events, the difference in event-free survival was smaller (78.8% for CABG vs. 74.5% for PCI; P > 0.05). Accordingly, performing 23.5 CABG instead of PCI would generate one additional event-free patient. The ICER indicated that, for each additional AMI/death/stroke-free patient treated by CABG, the cost would increase by 126 683 €.
Results of the bootstrap replications (Fig. 2) clearly show that CABG is the “more effective, more costly” treatment mode for patients with multivessel disease. For the endpoint AMI/death/stroke-free survival, the distribution of estimated ICERs shifted partially to the “less effective” quadrant, reflecting the unclear benefit of CABG as compared to PCI with respect to AMI/death/stroke-free survival.
Figure 2.
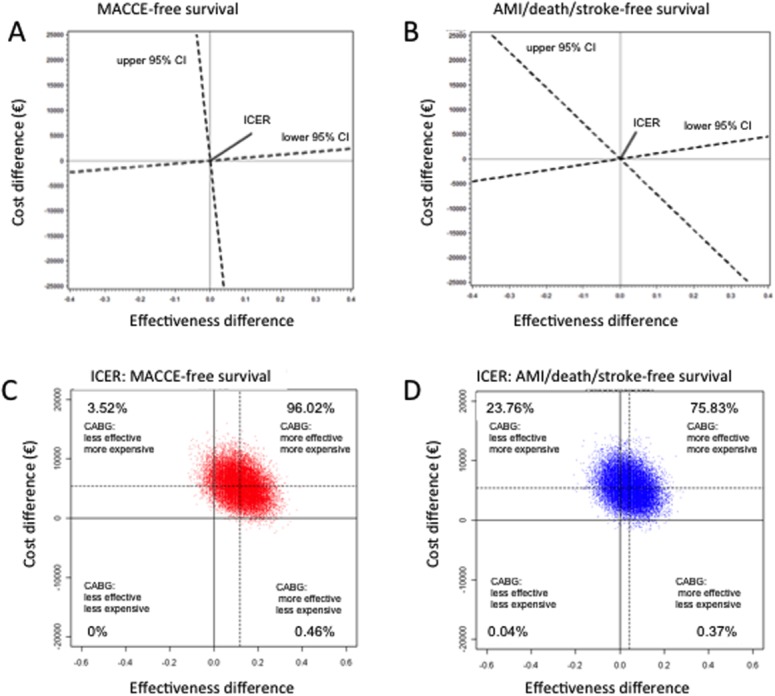
Effectiveness, cost differences, and incremental cost-effectiveness ratio (ICER) of CABG and DES-PCI. (A) Differences regarding major adverse cardiac and cerebrovascular events (MACCE). The x-axis describes the difference in effectiveness (Δe), with quadrants to the right of the y-axis representing the region where CABG is associated with a gain in effectiveness compared to PCI. The y-axis describes the cost difference (Δc), with quadrants above the x-axis representing the region where CABG is associated with an increase in costs compared to PCI. The slope of the line connecting the point (,) with the origin (0, 0) equals the estimated ICER. The slopes of the dashed lines represent the 95% confidence limits for the estimated ICERs. The dashed line represents the ICER and its 95% confidence interval of MACCE exhibits a trend towards cost-effectiveness favoring CABG (right upper quadrant). (B) Differences regarding AMI/death/stroke. The x- and y-axes and slope are similar to (A). The dashed line representing the ICER and its 95% confidence interval of AMI/Death exhibits cost-effectiveness favoring CABG, but the 95% confidence interval slips into the range favoring multivessel PCI (left upper quadrant). (C) Results of the bootstrap replications illustrate the distribution of the estimated ICERs in cost-effectiveness planes, where each point in the plane represents the estimated ICER of one bootstrap sample. The distributions of the estimated ICERs for the efficacy endpoint MACCE-free survival are predominantly in the “more effective, more expensive” quadrant of the figure (right upper quadrant). (D) The distribution of the estimated ICERs for the efficacy endpoint AMI/death/stroke-free survival shift to the “less effective” (left upper) quadrant, reflecting the unclear benefit of CABG as compared to PCI with respect to AMI/death/stroke-free survival. Percentages are the frequencies of samples in each quadrant. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
With a willingness to pay 80,000 €, CABG is cost-effective with respect to MACCE-free survival, but not AMI/death/stroke. On the other hand, considering the willingness to pay 40,000 €, CABG is not cost-effective with respect to both endpoints (Fig. 3).
Figure 3.
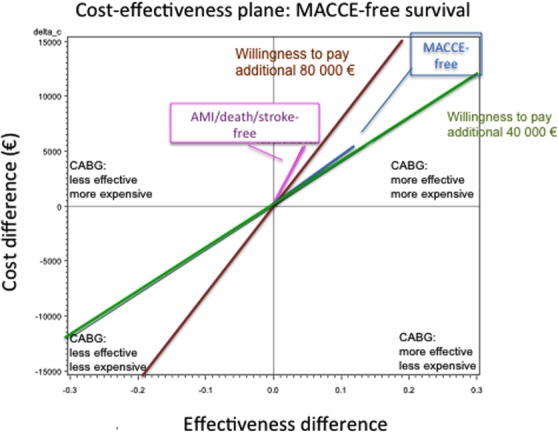
Cost effectiveness plane and willingness to pay more money for major adverse cardiac and cerebrovascular events (MACCE)-free and acute myocardial infarction (AMI)/death/stroke-free survival. The x- and y-axes are the same as Fig. 2. Two reference lines in the plot represent two different levels of willingness to pay to prevent one event. The amounts of 40,000 and 80,000 € were chosen arbitrarily. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Sub-Analysis of High-Risk Groups
Sub-analyses revealed a nonsignificant benefit of CABG over PCI in diabetic (45.9% and 36.8% patients in CABG and PCI groups, respectively) or older (≥65 years of age) patients (48.2% and 36.8% patients in CABG and PCI groups, respectively) in regards to MACCE, but a significantly better outcome for patients with a Syntax score >32 (29.4% and 14.7% patients in CABG and PCI groups, respectively) (Table1) (Fig. 4). Additional details are given in the On-line Supporting Information and Supporting Information Fig. 2.
Figure 4.
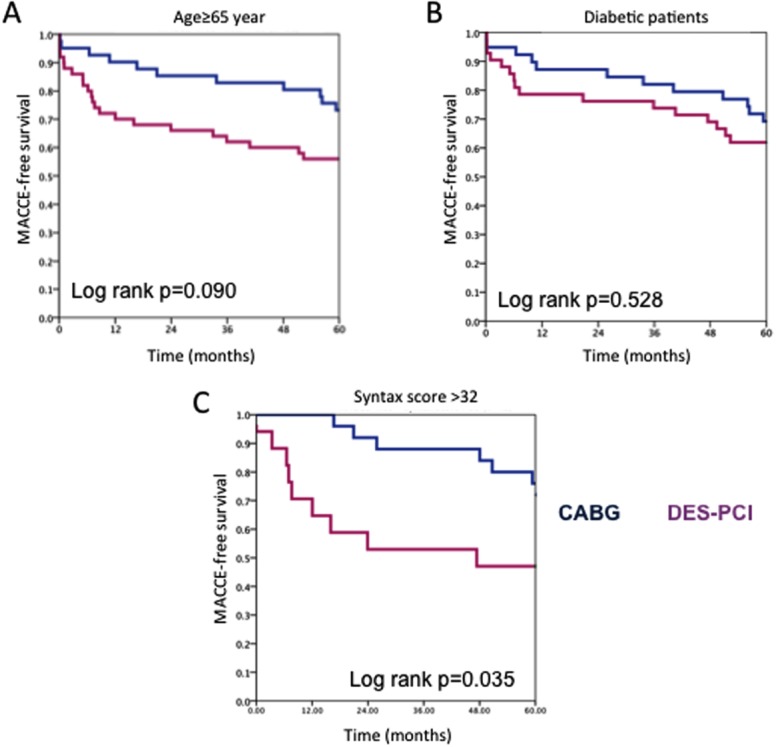
Kaplan–Meier major adverse cardiac and cerebrovascular events (MACCE)-free survival plot of subgroups of patients with multivessel coronary artery disease. (A) Aged ≥ 65 years, (B) With diabetes mellitus, (C) With Syntax score >32. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Our cost-effectiveness analysis demonstrated that CABG is a more effective, but significantly more costly treatment for patients with multivessel CAD within the first 5 years. Multiple DES implantations led to an increase in the in-hospital costs balanced by a relatively low TVR rate. The incremental cost-effectiveness analysis revealed that 8.4 patients should undergo CABG instead of PCI in order to rescue one patient from MACCE, and this would require an additional 5400 €/patient. However, regarding irreversible clinical endpoints (i.e., death, stroke, or AMI), the 5-year clinical follow-up revealed similar outcomes for multivessel PCI and CABG. Angiographic complex lesions (Syntax score >32) justified CABG over multivessel DES-PCI, leading to significantly better clinical outcomes for an additional 4838 €/patient.
Comparison With Literature Data
Up to now only few cost-effectiveness analyses have been conducted for patients undergoing CABG vs. stenting 11,15,16. The ARTS-I and SOS comparing PCI with BMS to CABG clearly favored CABG in terms of clinical and economic endpoints 2–4. In contrast, the ARTS-II and SYNTAX trials (DES-PCI vs. CABG) showed a step-wise narrowing of the cost-difference between the two treatments at 3 and 1-year 2,16,17. In the ARTS II study, the cost difference between the CABG and DES-PCI modes was 3042, 2930, and 2725 € for the primary intervention, at 1 and 3 years, respectively. The 1-year Syntax cost-effectiveness calculation revealed an in-hospital cost difference of 5693 USD between DES-PCI and CABG, and the difference narrowed to 3590 USD/patient at 1 year 16,17. The randomized multicenetr FREEDOM trial reported the 5-year clinical and cost-effectiveness data for diabetic patients, showing an in-hospital cost difference of 8600 USD/patient between CABG and DES-PCI patients, which decreased to 3600 USD at 5 years 11. Similar to the published SYNTAX and FREEDOM trials, our study revealed a mean initial difference of 5400 € between the two treatment arms, but the difference did not decrease considerably during the 5-year follow-up, indicating a marginal economic impact of repeat TVR of DES implantation in the PCI group.
We added the non-negligible rehabilitation costs to the cost of CABG, and we calculated the overall cost of PCI, including the cost of 1-year DAPT and additional DAPT following each repeat TVR. Similar to our study, the ARTS-II and FREEDOM trials calculated the 1-year cost of DAPT to the global costs 11,18 and stated that DAPT due to multivessel DES implantation considerably influenced PCI costs.
Adverse Events Requiring Cost
The main reason for an increased cost of CABG is not the surgical procedure itself, but longer hospitalization and blood transfusions, in contrast with the increased cost of PCI because of use of multiple DES. In addition, the cost of treating relatively rare adverse events in patients with CABG is similar to that of patients with PCI; therefore, the cost differences did not narrow during the 5-year follow-up in our study.
The clinical events (death, stroke, and AMI) were similar in both groups at any time point. Stroke occurrence was not dominant in patients undergoing CABG, probably due to DAPT increasing stroke rate in the PCI group.
The reduced costs from fewer TVR (which is further reduced by using the currently cheaper DES) seem to balance the higher initial costs of DES, reducing the long-term clinical and economic advantages of CABG. Moreover, TVR belongs to the so-called “soft” adverse events, as no evidence has shown that it influences mortality 19,20.
Subgroup Analysis
Diabetic patients undergoing PCI experienced marginally worse long-term outcomes as compared to CABG patients, but they were not charged more. Older patients did not have a significantly worse outcome as compared to younger patients, with approximately similar costs as the entire study population. Patients with a high Syntax score (>32) do benefit from CABG, with no conspicuous increase in cost.
Clinical Paradox in Cost Calculations
A patient who dies early or at home during the follow-up does not incur additional costs in the health system and might obscure the economic analysis. In contrast, any hospital admission due to STEMI, acute heart failure, or stroke, or any attempt to save a life, shifts the cost-effectiveness in the negative, even if the life was saved.
Limitations
The major limitations of our study are the lack of randomization and the limited number of patients, which raises statistical consideration. However, at the time the study began, multivessel stenting with DES was regarded as an “off-label” indication, with unknown incidence of late stent thrombosis and lack of guidelines. Therefore, no power calculation was made based on secondary endpoints. Currently, no sample size calculation exists for cost-effectiveness, which would be required for the primary endpoint of cost calculation. The number of included patients was determined a priori considering the availability of patient follow-up data in the following 5 years, which was a strong limiting factor for inclusion of patients suffering also from other concomitant disorders. Accordingly, inclusion of patients fulfilling all inclusion- and having no exclusion criteria was impeded. However, all living patients could be controlled clinically during the study period, reaching a follow-up rate of 100%, which strengthens our study regarding the assessment of the clinical outcomes. Furthermore, based on the anatomic complexity of the coronary lesions, only 2–12% of patients with multivessel CAD can be randomized 20 to CABG or PCI. A single-center experience reported a final inclusion rate of 9.7% for “all-comers” in the SYNTAX trial 21, emphasizing the bias; the majority of patients must be excluded from CABG-PCI randomization for ethical reasons. Our medical team's decision of CABG or PCI was based on the relevant guidelines (updated ACCF/AHA, ESC guidelines) (On-line Refs.2–3) at the time when the Syntax scoring system was not available. For this understandable reason, the propensity score analysis revealed significant differences between the groups (similar to the recently published comparative registry) 22 if Syntax score was retrospectively included in the analysis. Apart from the difference in Syntax score, which is restricted to coronary morphology 23, the propensity score analysis resulted in clinical comparability of the PCI and CABG groups.
In addition to listing all major events, we also calculated the composite MACCE as a secondary endpoint in order to allow better comparisons with other published trials, such as SYNTAX or ARTS, even if this composite end point includes both the safety and efficacy variables, resulting in substantial heterogeneity in definitions 24. However, the SYNTAX score has been validated for the occurrence of MACCE 10,25.
We did not perform quality of life analysis or a subgroup analysis of left-main patients, and our data is based on the Austrian healthcare system. Therefore, our cost-effectiveness analysis is only partially comparable to that of SYNTAX or other trials.
Patients were included in the present study between 2004 and 2006, with procedures using somewhat obsolete techniques in the current praxis. Second and third generation DES replaced the first generation DES. However, millions of patients worldwide currently live with implanted intracoronary Taxus stents, and a long-term clinical outcome analysis provides important information for these patients.
We do not report the absolute value of the costs considering the confidentiality of the hospital financial management. However, the differences in the costs of DES-PCI and CABG in this 5-year trial are comparable to the costs reported in the SYNTAX (1-year), ARTS II (3-year), and FREEDOM (5-year, diabetics) studies.
CONCLUSION
Our study demonstrates that multivessel stenting solely with DES is considerably less resource-intensive than CABG during 5 years post index procedure, but it is still associated with a significantly higher rate of adverse events as compared to CABG. Use of DES for multiple stenting does not narrow the cost difference between CABG and PCI because of the balance of rare CABG events with the cost of repeat revascularization with DES.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Berger PB, Velianou JL, Aslanidou Vlachos H, Feit F, Jacobs AK, Faxon DP, Attubato M, Keller N, Stadius ML, Weiner BH, Williams DO, Detre KM. Investigators BARI. Survival following coronary angioplasty versus coronary artery bypass surgery in anatomic subsets in which coronary artery bypass surgery improves survival compared with medical therapy. Results from the Bypass Angioplasty Revascularization Investigation (BARI) J Am Coll Cardiol. 2001;38:1440–1449. doi: 10.1016/s0735-1097(01)01571-6. [DOI] [PubMed] [Google Scholar]
- 2.Legrand VMG, Serruys PW, Unger F, van Hout BA, Vrolix MC, Fransen GM, Nielsen TT, Paulsen PK, Gomes RS, de Queiroz e Melo JM, Neves JP, Lindeboom W, Backx B ; Arterial Revascularization Therapy Study (ARTS) Investigators. Three-year outcome after coronary stenting versus bypass surgery for the treatment of multivessel disease. Circulation. 2004;109:1114–1120. doi: 10.1161/01.CIR.0000118504.61212.4B. [DOI] [PubMed] [Google Scholar]
- 3.Macaya C, García-García HM, Colombo A, Morice MC, Legrand V, Kuck KH, Sheiban I, Suttorp MJ, Carrie D, Vrolix M, Wittebols K, Stoll HP, Donohoe D, Bressers M, Serruys PW. One-year results of coronary revascularization in diabetic patients with multivessel coronary artery disease.Sirolimus stent vs. coronary artery bypass surgery and bare metal stent: Insights from ARTS-II and ARTS-I. EuroIntervention. 2006;2:69–76. [PubMed] [Google Scholar]
- 4.The SoS Investigators. Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery Trial): A randomised trial. Lancet. 2002;360:965–970. doi: 10.1016/S0140-6736(02)11078-6. [DOI] [PubMed] [Google Scholar]
- 5.Yock CA, Boothroyd DB, Owens DK, Garber AM, Hlatky MA. Cost-effectiveness of bypass surgery versus stenting in patients with multivessel coronary artery disease. Am J Med. 2003;115:382–389. doi: 10.1016/s0002-9343(03)00296-1. [DOI] [PubMed] [Google Scholar]
- 6.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnàr F, Falotico R. Study Group RAVEL. Randomized study with the sirolimus-coated BX Velocity balloon-expandable stent in the treatment of patients with de novo native coronary artery lesions. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 7.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME. Investigators TAXUS-IV. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 8.Eisenstein EL, Leon MB, Kandzari DE, Mauri L, Edwards R, Kong DF, Cowper PA, Anstrom KJ. ENDEAVOR III Investigators. Long-term clinical and economic analysis of the Endeavor zotarolimus-eluting stent versus the cypher sirolimus-eluting stent: 3-year results from the ENDEAVOR III trial (Randomized controlled trial of the Medtronic Endeavor drug [ABT-578]-eluting coronary stent system versus the Cypher sirolimus-eluting coronary stent system in de novo native coronary artery lesions) JACC Cardiovasc Interv. 2009;2:1199–1207. doi: 10.1016/j.jcin.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Serruys PW, Onuma Y, Garg S, Vranckx P, De Bruyne B, Morice MC, Colombo A, Macaya C, Richardt G, Fajadet J, Hamm C, Schuijer M, Rademaker T, Wittebols K, Stoll HP. ARTS II Investigators. 5-year clinical outcomes of the ARTS II (Arterial Revascularization Therapies Study II) of the sirolimus-eluting stent in the treatment of patients with multivessel de novo coronary artery lesions. J Am Coll Cardiol. 2010;55:1093–1101. doi: 10.1016/j.jacc.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 10.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr F. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 11.Magnuson EA, Farkouh ME, Fuster V, Wang K, Vilain K, Li H, Appelwick J, Muratov V, Sleeper LA, Boineau R, Abdallah M, Cohen DJ ; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes mellitus and multivessel coronary artery disease: Results from the FREEDOM trial. Circulation. 2013;127:820–831. doi: 10.1161/CIRCULATIONAHA.112.147488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, Mujica Mota R, Walley T, Bagust A. Drug-eluting stents: A systematic review and economic evaluation. Health Technol Assess. 2007;11:iii–xi. doi: 10.3310/hta11460. [DOI] [PubMed] [Google Scholar]
- 13.Goldman S, Sethi GK, Holman W, Thai H, McFalls E, Ward HB, Kelly RF, Rhenman B, Tobler GH, Bakaeen FG, Huh J, Soltero E, Moursi M, Haime M, Crittenden M, Kasirajan V, Ratliff M, Pett S, Irimpen A, Gunnar W, Thomas D, Fremes S, Moritz T, Reda D, Harrison L, Wagner TH, Wang Y, Planting L, Miller M, Rodriguez Y, Juneman E, Morrison D, Pierce MK, Kreamer S, Shih MC, Lee K. Radial artery grafts vs saphenous vein grafts in coronary artery bypass surgery: A randomized trial. JAMA. 2011;305:167–174. doi: 10.1001/jama.2010.1976. [DOI] [PubMed] [Google Scholar]
- 14.Willan AR. Briggs AH. Statistical Analyses of Cost-effectiveness Data. Chichester: Wiley; 2006. [Google Scholar]
- 15.Cohen DJ, Lavelle TA, Van Hout B, Li H, Lei Y, Robertus K, Pinto D, Magnuson EA, McGarry TF, Lucas SK, Horwitz PA, Henry CA, Serruys PW, Mohr FW, Kappetein AP. Economic outcomes of percutaneous coronary intervention with drug-eluting stents versus bypass surgery for patients with left main or three-vessel coronary artery disease: One-year results from the SYNTAX trial. Catheter Cardiovasc Interv. 2012;79:198–209. doi: 10.1002/ccd.23147. [DOI] [PubMed] [Google Scholar]
- 16.Birim O, Bogers AJ, Kappetein AP. Cost effectiveness of coronary revascularisation. EuroIntervention. 2010;5:763–767. doi: 10.4244/eijv5i7a128. [DOI] [PubMed] [Google Scholar]
- 17.Filion KB, Roy AM, Baboushkin T, Rinfret S, Eisenberg MJ. Cost-effectiveness of drug-eluting stents including the economic impact of late stent thrombosis. Am J Cardiol. 2009;103:338–344. doi: 10.1016/j.amjcard.2008.09.086. [DOI] [PubMed] [Google Scholar]
- 18.Groeneveld PW, Suh JJ, Matta MA. The costs and quality-of-life outcomes of drug-eluting coronary stents: A systematic review. J Interv Cardiol. 2007;20:1–9. doi: 10.1111/j.1540-8183.2007.00214.x. [DOI] [PubMed] [Google Scholar]
- 19.Gyöngyösi M, Christ G, Lang I, Kreiner G, Sochor H, Probst P, Neunteufl T, Badr-Eslam R, Winkler S, Nyolczas N, Posa A, Leisch F, Karnik R, Siostrzonek P, Harb S, Heigert M, Zenker G, Benzer W, Bonner G, Kaider A, Glogar D. 2-year results of the AUTAX (Austrian Multivessel TAXUS-Stent) registry. JACC Cardiovasc Intervent. 2009;2:718–727. doi: 10.1016/j.jcin.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman SN, TenBrook JA, Wolf MP, Pauker SG, Salem DN, Wong JB. A meta-analysis of randomized controlled trials comparing coronary artery bypass graft with percutaneous transluminal coronary angioplasty: One- to eight-year outcomes. J Am Coll Cardiol. 2003;41:1293–1304. doi: 10.1016/s0735-1097(03)00157-8. [DOI] [PubMed] [Google Scholar]
- 21.Rastan AJ, Boudriot E, Falk V, Kappetein AP, Borger MA, Serruys PW, Schuler G, Mohr FW. Frequency and pattern of de-novo three-vessel and left main coronary artery disease; insights from single center enrolment in the SYNTAX study. Eur J Cardiothorac Surg. 2008;34:376–382. doi: 10.1016/j.ejcts.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Weintraub WS, Grau-Sepulveda MV, Weiss JM, O'Brien SM, Peterson ED, Kolm P, Zhang Z, Klein LW, Shaw RE, McKay C, Ritzenthaler LL, Popma JJ, Messenger JC, Shahian DM, Grover FL, Mayer JE, Shewan CM, Garratt KN, Moussa ID, Dangas GD, Edwards FH. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–1476. doi: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen SL, Chen JP, Mintz G, Xu B, Kan J, Ye F, Zhang J, Sun X, Xu Y, Jiang Q, Zhang A, Stone GW. Comparison between the NERS (New Risk Stratification) score and the SYNTAX (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) score in outcome prediction for unprotected left main stenting. JACC Cardiovasc Interv. 2010;3:632–641. doi: 10.1016/j.jcin.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Kip KE, Hollabaugh K, Marroquin OC, Williams DO. The problem with composite end points in cardiovascular studies: The story of major adverse cardiac events and percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:701–707. doi: 10.1016/j.jacc.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 25.Kim YH, Park DW, Kim WJ, Lee JY, Yun SC, Kang SJ, Lee SW, Lee CW, Park SW, Park SJ. Validation of SYNTAX (Synergy between PCI with Taxus and Cardiac Surgery) score for prediction of outcomes after unprotected left main coronary revascularization. JACC Cardiovasc Interv. 2010;3:612–623. doi: 10.1016/j.jcin.2010.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


