ABSTRACT
Exposure to faces is known to shape and change the face processing system; however, no study has yet documented infants' natural daily first‐hand exposure to faces. One‐ and three‐month‐old infants' visual experience was recorded through head‐mounted cameras. The video recordings were coded for faces to determine: (1) How often are infants exposed to faces? (2) To what type of faces are they exposed? and (3) Do frequently encountered face types reflect infants' typical pattern of perceptual narrowing? As hypothesized, infants spent a large proportion of their time (25%) exposed to faces; these faces were primarily female (70%), own‐race (96%), and adult‐age (81%). Infants were exposed to more individual exemplars of female, own‐race, and adult‐age faces than to male, other‐race, and child‐ or older‐adult‐age faces. Each exposure to own‐race faces was longer than to other‐race faces. There were no differences in exposure duration related to the gender or age of the face. Previous research has found that the face types frequently experienced by our participants are preferred over and more successfully recognized than other face types. The patterns of face exposure revealed in the current study coincide with the known trajectory of perceptual narrowing seen later in infancy.© 2013 The Authors. Developmental Psychobiology Published by Wiley Periodicals, Inc. Dev Psychobiol 56: 249–261, 2014.
Keywords: perceptual narrowing, face perception, development, infancy, exposure to faces, head‐mounted camera, other‐race effect, other‐age effect
INTRODUCTION
Infants are born with the potential to adapt to their new world and to adjust their sensory‐perceptual systems to fit their environment (Huttenlocher, 2002). As infants learn what is relevant in their world, they improve their ability to perceive relevant differences and show a concomitant lack of improvement or decrement in their ability to perceive irrelevant differences. Like directing a beam of light from diffusely illuminating a room to high‐lighting a single item, as generalization declines, specialization emerges. This experience‐driven change in perceptual ability during infancy has been termed perceptual narrowing. Perceptual narrowing is evident in multiple areas of learning within the first year of life, including in speech perception (Kuhl et al., 2006), cross‐modal perception (Pons, Lewkowicz, Soto‐Faraco, & Sebastian‐Galles, 2009), and the perception of music (Hannon & Trehub, 2005).
Perceptual narrowing is also evident in the domain of face perception (Pascalis, de Haan, & Nelson, 2002). Previous research has demonstrated that early experience shapes the development of the perceptual skills and underlying neural architecture necessary to utilize this highly important feature of the infant's social world. Infants begin with a face processing system that predisposes them to prefer faces over other visual stimuli (Johnson, Dziurawiec, Ellis, & Morton, 1991; Mondloch et al., 1999). Building on this preference, infants show the ability to discriminate between faces very early in life: 4‐day‐old infants discriminate and prefer their mother's face to the face of a stranger (Pascalis, de Schonen, Morton, De Ruelle, & Fabre‐Grenet, 1995). This preference for the mother's face is dose‐dependent; infants who receive more exposure to their mother's face during their first hours after birth show a greater preference for their mother's face (Bushnell, 2001).
By 3 months of age, infants begin to demonstrate systematic preferences for particular types of faces. Three‐month‐old‐infants prefer female faces over male faces (Quinn, Yahr, Kuhn, Slater, & Pascalis, 2002), own‐race faces over other‐race faces (Kelly et al., 2005), and own‐species faces over other‐species faces (DiGiorgio, Méary, Pascalis, & Simion, 2012). At 7 months of age, infants show a preference for parent‐ and infant‐age faces over child‐age faces (Sanefuji, Ohgami, & Hashiya, 2005). These preferences seem to be shaped by the particular experiences infants are receiving, although no study has yet documented infant experience. For example, Quinn et al. (2002) demonstrated that the typical preference for female faces over male faces is reversed when the infant has a male primary caregiver. Although infants display preferences for certain face types by 3 months of age, young infants are generally equally facile at discriminating among faces from multiple face categories, even those face categories with which they have little experience. For example, at 3 months of age infants are able to discriminate between faces of races to which they have had minimal exposure (Kelly et al., 2007) and at 6 months of age they can discriminate between faces of species to which they have never been exposed (e.g., monkey faces; Pascalis et al., 2005).
By comparison, adults are less able or unable to discriminate other‐race faces (Hayward, Rhodes, & Schwaninger, 2008) and other‐species faces (Dufour, Coleman, Campbell, Petit, & Pascalis, 2004; Pascalis et al., 2002). As infants age, the early, broad tuning of the perceptual system narrows progressively. By approximately 9 months of age, infants no longer show the ability to discriminate between the types of faces with which they have had no or little experience: 9‐month‐old infants no longer show discrimination between other‐species (Pascalis et al., 2005) and other‐race (Kelly et al., 2007) faces when tested with the same procedures on which they were successful at 6 months. Additionally, studies using event‐related potentials (ERPs) reveal that in 9‐month‐old infants the brain processes own‐race and own‐species faces differently than other‐race and other‐species faces (Balas, Westerlund, Hung, & Nelson, 2011; Scott, Shannon, & Nelson, 2006). This pattern of gradual behavioral and neurological specialization for “own”‐type faces presumably reflects the experiences with faces that infants accumulate across the first year of life. Of yet, however, it is unclear how much natural, daily exposure infants receive to own‐ and other‐face types in the first year, whether there are large or small differences in exposure, and how well these reflect typical patterns of narrowing.
Although perceptual narrowing is observed in the first year of life, the system is not inflexible; both experimentally induced and natural changes in the environment can alter the type of faces for which we show proficiency. For example, infants who received regular exposure to a picture book containing individually labeled other‐species (monkey) faces from 6 to 9 months retained their ability to discriminate and developed neural specialization for monkey faces (Scott & Monesson, 2009, 2010). Here, experimental training kept the perceptual window for discriminating other‐species faces open. Natural experience has also been shown to re‐open the perceptual window after infancy. For example, Sangrigoli, Pallier, Argenti, Ventureyra, and de Schonen (2005) demonstrated that Korean children adopted into French families between 3 and 9 years of age showed a reversal in the other‐race effect; that is, these children became better able to discriminate faces from their current culture (Caucasian faces) relative to their ability to discriminate faces from their previous home (Korean faces) (Sangrigoli et al., 2005). In adulthood, experience allows adults to become more proficient with face types that were previously poorly discriminated. For example, adults can develop facility with newborn (Macchi Cassia, Picozzi, Kuefner, & Casati, 2009) or child (Harrison & Hole, 2009) faces through daily at‐work exposure to these types of faces.
Since experience exerts a large influence on the trajectory of perceptual narrowing, it has been hypothesized that experience is what is ultimately driving the development of the face processing system (Scott, Pascalis, & Nelson, 2007). All experience is not equal, with individuation of faces being key to “tune” the face processing system (Scott & Monesson, 2009). From this perspective, perceptual narrowing reflects adaptation to salient perceptual inputs received from the environment. Therefore, understanding the perceptual inputs received by infants is key to understanding the perceptual abilities and patterns of narrowing displayed by infants. To examine whether the patterns of preference and perceptual narrowing reflect the environment in which infants develop, two studies have documented infants' early experiences and found support for an environmental basis for both preference and perceptual narrowing. Researchers monitoring infant–parent interaction after birth found a positive correlation between the amount of exposure to the mother's face and the preference for mother (Bushnell, 2001). Additionally, through parent report of infants' natural daily exposure to different face types, Rennels and Simmons (2008) found that infants receive the most exposure to female, parent‐age, and parent‐race faces, reflecting infants' later patterns of ability in face discrimination.
These early studies documenting the face types seen by infants represent a valuable first step in quantifying and qualifying infants' early exposure to faces. However, recent work documenting the differences between adult and child perspectives has called into question whether an adult perspective can accurately portray the visual experience of a child (Smith, Yu, & Pereira, 2009). Smith and coworkers' recent series of studies documenting the first‐person perspective of adults and children clearly show that the visual experiences of child and parent in the same interactive context differ significantly. While a parent views the world more globally, children view objects more locally (Smith et al., 2009). For example, while playing with their parents, children spend very little time looking at their parent and much more time examining single items up‐close so that these items are large in the child's field of view. In contrast, parents spend much more time looking at their child and monitoring the entire scene in order to guide the interaction with their child, and spend very little time with single items in their field of view (Yoshida & Smith, 2008). Finally, and most importantly, it is only the child perspective that is predictive of learning in these parent‐child interactions, not the parent perspective (Yurovsky, Smith, & Yu, 2013). Based on these findings, it seems critical to document the typical daily face exposure of the infant from the infant's perspective. Therefore, only observations collected from the infant perspective can establish definitively whether perceptual narrowing tracks the exposure to faces received during the first year of life. Fortunately, technological advances have made it possible to document the infant's natural world from a first‐person infant perspective.
Here, we provide the first documentation of 1‐ and 3‐month‐old infants' natural daily exposure to faces recorded from the perspective of the infant, through the use of a head‐mounted camera. This novel method of capturing infants' visual worlds was used to answer three questions: (1) How often are infants exposed to faces? (2) To what types of faces are they exposed? and (3) Do the faces types to which they are exposed reflect the perceptual narrowing seen later in the first year of life? We hypothesized that (1) infants would spend a large proportion of their time exposed to faces, (2) they would be exposed primarily to female, own‐race, parent‐age faces, and (3) this pattern of exposure would be consistent with infants' abilities after perceptual narrowing.
METHOD
Participants
Fourteen 1‐month‐old (8 female) and 16 3‐month‐old (7 female) infant participants were recruited from a database of parents who were interested in participating in developmental research studies. We chose to test 1‐ and 3‐month‐old infants to determine if face experience is biased both before any behavioral markers of perceptual narrowing are evident (i.e., at 1 month of age) and before differences in discrimination ability but after perceptual preferences for some face types have emerged (i.e., at 3 months of age).
The average age at first visit of the 1‐month‐old group was 38 days (Range: 27–53 days). The average age at first visit of the 3‐month‐old group was 98 days (Range: 90–118 days). Infants in the 1‐month‐old group were Caucasian (9 infants), Asian (2 infants), Black‐Caucasian (1 infant), Southeast Asian (1 infant), and Southeast Asian‐Caucasian (1 infant). Infants in the 3‐month‐old group were Caucasian (10 infants), Asian‐Caucasian (2 infants), Southeast Asian (1 infants), Asian (1 infant), Southeast Asian‐Caucasian (1 infant), and Black‐Caucasian (1 infant). One‐month‐old infants spent an average of 7 hr awake per day (M = 7.00 hr, Range: 3.5–12 hr) while 3‐month‐old infants spent an average of nearly 9 hr awake per day (M = 8 hr and 55 min, Range: 6.5–16 hr). All parents were of the same racial background as their infant (for infants with two listed races, each parent belongs to one of the two listed races). Parents of all of the infants were adults between the ages of 20 and 45. Nearly all parents reported that their infants had a female primary caregiver (their mother; 29 infants) and one family reported that caretaking responsibilities for their 1‐month‐old infant were shared equally by a male and female primary caregiver (the mother and father).
Procedure
Each family was first visited in their home where parents completed a demographics questionnaire, discussed privacy issues related to video recording with a hidden camera, and were shown how to operate the video camera. If the infant was awake, parents turned the camera on, mounted the camera on a headband, and placed the camera on their infants' head, with the experimenter providing guidance and feedback as necessary. If the infant was not awake, parents turned the camera on and mounted the camera on the headband, with the experimenter going over in detail how to place the camera on their infant's head.
Parents were asked to place the camera on their infant's head whenever their infant was awake and alert and to remove the camera if the infant became fussy, fell asleep, or the parent felt that the camera should be removed. To facilitate parent and infant participation in this study, parents were not given a minimum amount of time that the infant was required to wear the camera, nor were parents given a schedule for when to place the camera on the infant's head. Parents were asked to keep the camera for 2 weeks. On average, parents kept the camera for 14 days (Range: 4–21 days) in the 1‐month‐old group and 13 days (Range: 6–21 days) in the 3‐month‐old group, after which either the experimenter visited the family in their home to retrieve the camera or the parents came to Ryerson University to return the camera. All parents completed a final questionnaire documenting their experience with the camera. Parents received a copy of the video recorded from their infant's perspective and $25 for their participation.
A total of 44 hr and 47 min (M = 1 hr and 30 min per participant, Range: 17 min to 4 hr and 22 min) of video were recorded from the infants' perspectives over an average of 13 days (Range: 4–21 days). One‐month‐olds contributed a total of 19 hr and 43 min (M = 1 hr and 25 min per participant, Range: 19 min to 4 hr and 44 min) of infant‐perspective video over an average of 14 days (Range: 4–21 days). Three‐month‐olds contributed a total of 25 hr and 3 min (M = 1 hr and 34 min per participant, Range: 17 min to 4 hr and 22 min) of infant‐perspective video over an average of 13 days (Range: 6–21 days).
Places the infant participant was likely to visit during their participation in the study was assessed during the initial interview with parents, in the context of a conversation about privacy concerns related to recording. All families stated that the infant spent most of their time at home and all except for three families said that they would be visiting at least one other location (M = 1.85 non‐home locations, Range: 0–4 non‐home locations). Prior to each recording, parents completed a brief privacy questionnaire to remind them of privacy issues related to recording; as part of this questionnaire they reported the location in which recording began and ended. The questionnaire data revealed that all parents reported recording at home and most reported recording in at least two non‐home locations (M = 2.00 non‐home locations, Range: 0–7 non‐home locations). One‐month‐olds typically were recorded in at least one other location (M = 1.72 non‐home locations, Range: 0–7 non‐home locations) while 3‐month‐olds typically were recorded in at least two other locations (M = 2.23 non‐home locations, Range: 0–5 non‐home locations). The three families who recorded only at home had reported, in the initial interview, that they would not be leaving the house with the infant except for doctor's appointments, due to cultural tradition (two families) and sibling illness (one family).
Equipment
A commercially available 4.7‐cm‐diameter DVR spy‐camera was used to record video. The camera had a smiley‐face printed on it; the camera looked similar to a happy‐face pin or button (see Fig. 1). This spy‐camera was chosen because it is small, lightweight, and designed to be inconspicuous (viz. it does not look like a camera). The smiley‐face camera, specifically, was chosen because it looks very much like an accessory designed for an infant; “cutesy” infant clothing often includes large smiling faces. The camera was worn on the infant's head clipped to a fuzzy elasticized headband. To ensure that the aperture of the camera sat above the bridge of the infant's nose and in‐line with their eyebrows, the camera was worn upside down. As a consequence, the smiley‐face was oriented upside‐down. The camera captured 29 frames per second and provided image resolution of 2048 × 1536 pixels. Video was recorded direct, in .AVI format, to a 16 GB microSD memory card. While the camera recorded both video and audio, only the video was used for the current study. Parents were aware that the audio would not be used.
Figure 1.

Three‐month‐old infant wearing the happy‐face camera mounted on a headband. The lens of the camera is in the left eye of the happy‐face. The camera was worn upside‐down to ensure that the lens is nearer to the infant's eyes, in line with their eyebrows. Printed with permission of parent, Dr. M. C. Moulson.
Coding and Reliability
All videos were coded second‐by‐second for faces of people viewed in person by highly trained coders. For each face, coders documented age, gender, and ethnicity. Non‐human faces (e.g., dog faces) and faces viewed on media (e.g., in photographs or on television) were not included in the analysis. All coders received at least 2 hr of orientation and 2 hr of one‐on‐one training with the experimenter, studied a 40‐page coding guide, had at least 40 hr of video coding experience coding three training videos, and achieved at least 85% reliability on all variables of interest on the last (28‐min) training video. The ages, genders, and races were known for some faces in the first video and all of the faces in the second training video. Therefore, the coders could be evaluated on how well they estimated these variables. Coders for whom reliability on one of these variables was below 85% received specific training on the variable they found difficult to estimate.
All video recordings were spot‐checked for video coding accuracy by a lead coder with over 100 hr of coding experience. Spot‐checking was done to ensure that no faces were missed because a coder was focusing on another face that was present at the same time [viz. inattentional blindness as described by Simons and Chabris (1999)]. If a random spot‐check found a face that was not included on the coding sheet for a coded video, that video was re‐coded from start to finish. The video was then spot‐checked again. Videos were spot‐checked and re‐coded until spot‐checking found no missed faces. No video was re‐coded more than twice due to missed faces. All coders were made aware of the age, race, and gender of the family members of the infant participant.
Ten percent of all participant videos (4 hr and 43 min) were coded for all measures by a second coder. None of the coders were aware if a video was going to be coded by a second coder or if they were the second coder. The video coding results produced by the first person to code the video were included in the results. The results produced by the second person to code the video were used only as a measure of inter‐rater reliability. There was high inter‐rater reliability for total amount exposure to all faces (α = .998), female faces (α = .996), other‐race faces (α = .998), and other‐age faces (α = .989). Considered a second way, raters' mean level of agreement per participant was 94% for face exposure (Range: 75–100%), 93% for female face exposure (Range: 82–100%), 96% for own‐race face exposure (Range: 83–100%), and 95% for adult‐age face exposure (83–100%). That there was such a high degree of agreement amongst the coders is partially attributable to the fact that most infants saw a very restricted range of faces and coders were aware of the age, gender, and ethnicity of the faces most commonly seen by each infant, those of the infant's family members.
RESULTS
We expected that infants' experiences with faces would be highly variable and that our data would reflect this high degree of variability. We examined adherence to the assumptions of parametric statistical tests in the overall dataset and between the 1‐ and 3‐month‐olds' datasets for the following variables: overall exposure to faces, exposure to female faces, exposure to own‐race faces, and exposure to adult‐age faces. All variables violated at least one assumption and most violated multiple assumptions of parametric statistical tests. Accordingly, all statistical tests reported below are non‐parametric tests.
Exposure to Real Faces
Of the total amount of video recorded, 11 hr and 24 min (25%) contained faces of humans who were physically present in the infant's environment. One‐month‐olds were exposed to faces 25% of the time, and 3‐month‐olds were exposed to faces 26% of the time. A Mann–Whitney test of the difference in exposure to faces in 1‐month‐olds (Mdn = 20%) and 3‐month‐olds (Mdn = 27%) was not statistically significant, U = 109.00, z = −.125, p = .918, r = .02.
To capture a measure of the individual faces to which infants are exposed that are potentially individuated, we calculated the average number of faces of each type present during each video. Since all interactions do not provide equal opportunity to individuate faces, we excluded videos in which infants were unlikely to individuate the majority of faces present (i.e., visual environments that contained more than 20 faces per video—e.g., at the mall, walking down a busy street). Eight videos (two 1‐month‐old videos and six 3‐month‐old videos), with an average of 115 faces per video (M = 115.25, Range: 23–315 faces per video), were excluded from subsequent analyses of the number of individual faces per video. Overall, infants were exposed to an average of 2 faces (M = 2.19, Mdn = 2.00, Range: 1–19) per video. Both 1‐ and 3‐month‐olds were exposed to an average of two faces (1‐month‐olds: M = 1.92, Mdn = 1.97, Range: 1–19; 3‐month‐olds: M = 2.40, Mdn = 2.00, Range: 1–10) per video, which was not significantly different, Mann–Whitney test, U = 71.50, z = −1.695, p = .092, r = −.31.
On average, based on all videos (i.e., not excluding videos with 20 or more faces), each face appeared in the infant's field of view for 4 s (M = 4.12 s per face, Range: 1.89–8.90 s per face). This was true for both 1‐month‐olds (M = 3.95 s per face, Range: 1.89–8.90 s per face, Mdn = 3.72 s per face) and 3‐month‐olds (M = 4.27 s per face, Range: 2.44–6.15 s per face, Mdn = 4.30 s per face), with no significant difference between the two age groups as confirmed by a Mann–Whitney test, U = 84.00, z = −1.164, p = .257, r = .21.
Exposure to Own‐Race Faces
We predicted that infants would be primarily exposed to faces of their own race due to high levels of contact with their primary caregiver and immediate family, as reported by Rennels and Simmons (2008). This was confirmed. Infants spent an overwhelming majority of their time exposed to own‐race faces (M = 96%, Range: 70–100%). Infants' exposure to own‐race faces (Mdn = 100%) was significantly greater than their exposure to other‐race faces (Mdn = 0%), as confirmed by a Wilcoxon signed‐ranks related‐samples test, T = 0, Z = −4.910, exact significance p < .001, r = .90. That infants were exposed to faces of their own race almost exclusively was true for both 1‐month‐olds (M = 96%, Range: 84–100%) and 3‐month‐olds (M = 96%, Range: 70–100%). The majority of infants from each age group (8 1‐month‐olds and 10 3‐month‐olds) were exposed exclusively to own‐race faces (see Fig. 2). A Mann–Whitney test confirmed that there was no significant difference between 1‐month‐olds' (Mdn = 100%) and 3‐month‐olds' (Mdn = 100%) exposure to own‐race faces, U = 108.00, z = −.19, p = .868, r = −.03.
Figure 2.
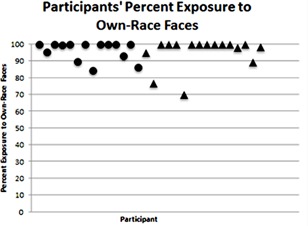
Each participant's percent exposure to own‐race faces. Each point represents one participant. Circles represent 1‐month‐old infant participants and triangles represent 3‐month‐old infant participants. Infants are ordered by age, from youngest to oldest.
Infants were exposed to significantly more individual own‐race faces (M = 2.00, Mdn = 2.00, Range: 0–15) than individual other‐race face (M = .25, Mdn = .00, Range: 0–4) per video, as confirmed by a Wilcoxon signed‐ranks paired‐samples test, T = 1, Z = −4.406, p < .001, r = −.80. Both 1‐month‐olds and 3‐month‐olds were exposed to more individual own‐race faces (M = 1.86, Range: 0–15; and M = 2.12, Range: 0–8, respectively) than individual other‐race faces (M = .04; Range: 0–4; and M = .43, Range: 0–4, respectively) per video. A Mann–Whitney test confirmed that there was no significant difference between 1‐month‐olds' (Mdn = 1.93 individual faces) and 3‐month‐olds' (Mdn = 2.00 individual faces) per video exposure to individual own‐race faces, U = 76.00, z = −1.511, p = .135, r = −.28.
On average, infants were exposed to each own‐race face for significantly longer per face (M = 4.12 s, Mdn = 4.00 s, Range: 1.89–9.09 s) than each other‐race face (M = 1.43 s, Mdn = .00 s, Range: 0–11.50 s), as confirmed by a Wilcoxon signed‐ranks related‐samples test, T = 3, Z = −4.001, exact significance p < .001, r = .73. The difference remained even when infants with no exposure to other‐race faces were excluded (M = 4.49 s per own‐race face, Range: 2.94–9.09 s per own‐race face; M = 3.57 s per other‐race face, Range: 1.52–11.50 s per own‐race face).
The greater length of time exposed to each own‐ versus other‐race face was true both for 1‐month‐olds (M = 4.06 s, Range: 1.89–9.09 s per own‐race face; M = 1.56 s, Range: 0–11.50 s per other‐race face) and for 3‐month‐olds (M = 4.18 s, Range: 2.44–6.15 s per own‐race face; M = 4.18 s, Range: 0–5.87 s per other‐race face). A Mann–Whitney test confirmed that there was no significant difference between 1‐month‐olds' (Mdn = 3.94 s per face) and 3‐month‐olds' (Mdn = 4.12 s per face) exposure to own‐race faces, U = 95.00, z = −0.707, p = .498, r = −.13.
Exposure to Female Faces
Since nearly all infants (n = 29) had a female primary caregiver, we predicted that infants would spend more time exposed to female faces than to male faces. As expected, we found that infants spent more time exposed to female than to male faces, with female faces accounting for 70% of time spent with faces across both age groups (Range: 7–100%). A Wilcoxon signed‐rank related‐samples test confirmed that infants are exposed to significantly more female (Mdn = 76%) than male (Mdn = 24%) faces, T = 5, z = −3.469, p < .001, r = .63. Exposure to female faces was also significantly different than a chance level of 50%, as confirmed by a Wilcoxon signed rank test, T = 5, Z = 3.754, p < .001, r = .69.
That infants were exposed to primarily female faces was true for both 1‐month‐old infants (M = 73%, Range: 30–100%) and 3‐month‐old infants (M = 67%, Range: 7–100%). One 3‐month‐old and one 1‐month‐old were exposed exclusively to female faces (see Fig. 3). A Mann–Whitney test confirmed that there was no significant difference between 1‐month‐olds' (Mdn = 79%) and 3‐month‐olds' (Mdn = 73%) exposure to female faces, U = 89.50, z = −.935, p = .361, r = −.17.
Figure 3.
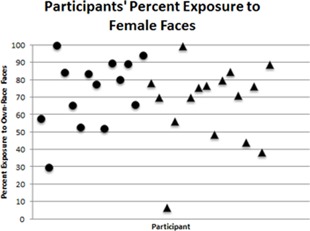
Each participant's percent exposure to female faces. Each point represents one participant. Circles represent 1‐month‐old infant participants and triangles represent 3‐month‐old infant participants. Infants are ordered by age, from youngest to oldest.
Since there was large variability in infants' exposure to female faces, we examined the data of infants at both the high (100% exposure to female faces) and low (less than 50% exposure to female faces) ends of the range to determine what factors might have influenced these levels of exposure. Two infants received 100% exposure to female faces. Both of these infants were female. One 1‐month‐old spent 100% of her time exposed to female faces. During the infant's participation in this study, the father was working in a different province. For this family, this was typical; the father worked away from home for several months per year. One 3‐month‐olds spent 100% of her time exposed to female faces. The parents of this infant had separated and the father was not living in the home at the time of the study. The mother reported that the father had infrequent contact with the infant (2 days per month).
Five infants received less than 50% exposure to female faces. One male 3‐month‐old was exposed to only 7% female faces. Although this infant had a female primary caregiver (the mother), he spent a large amount of time exposed to his own face in mirrors (93% of his total time exposed to faces came from exposure to his own face). The mother reported, and the video confirmed, that the infant viewed himself in mirrors while on his play mat, while in his car seat, and while being carried by his mother. The mother reported that he found this engaging and that she would put him in front of a mirror when he was distressed, since seeing himself would often soothe him. If this participant's exposure to his own face is excluded, then exposure to female faces accounted for 96% of all remaining face exposure. One male 1‐month‐old was exposed to only 30% female faces. The parents of this infant reported sharing parenting responsibilities equally. One 3‐month‐old male infant was exposed to 39% female faces. This infant had an older male sibling and spent 50% of his time exposed to young faces (including both his own and that of his brother). One 3‐month‐old female infant was exposed to 44% female faces. This infant had an older male sibling with whom she spent 24% of her time. A second 3‐month‐old female infant was exposed to 49% female faces, but parental report did not clarify why this infant received lower‐than‐expected exposure to female faces.
Since the infants with the lowest three scores for female face exposure were male and all of the infants with very high female face exposure (100%) were female, we examined the data to determine whether there was a systematic difference between male and female infants' exposure to male and female faces. Irrespective of age, we found that female infants' exposure to female faces was higher (M = 73%) than was male infants' (M = 65%). However, a Mann–Whitney test revealed no significant difference between females' (Mdn = 76%) and males' (Mdn = 73%) exposure to female faces, U = 93, z = −.809, p = .430, r = .15.
Infants were exposed to significantly more individual female faces (M = 1.40; Mdn = 1.31, Range: .93–3.00) than individual male faces (M = .83; Mdn = .74, Range: 0–5) per video, as confirmed by a Wilcoxon signed‐ranks paired‐samples test, T = 2, Z = −3.577, p < .001, r = −.65. One‐month‐olds and 3‐month‐olds were both exposed to more individual female faces (M = 1.31, Range: .93–2.25; and M = 1.48, Range: 0–5, respectively) than individual male faces (M = .64, Range: 1.00–3.00; and M = .98, Range: 0–5, respectively) per video. A Mann–Whitney test confirmed that there was no significant difference between 1‐month‐olds' (Mdn = 1.24 individual faces) and 3‐month‐olds' (Mdn = 1.48 individual faces) per‐video exposure to female faces, U = 90.50, z = −.900, p = .379, r = −.16.
On average, infants were exposed to each female face (M = 3.94 s, Mdn = 3.91 s, Range: 2.03–9.88 s per face) and each male face (M = 4.15 s, Mdn = 3.83 s, Range: 0–10.81 s per face) for approximately the same amount of time, 4 s. The difference between genders was not significant, as confirmed by a Wilcoxon signed‐rank related‐samples test, T = 0, Z = −.072, p = .952, r = .01. One‐month‐olds and 3‐month‐olds spent similar amounts of time exposed to each female face (M = 3.79 s, Range: 2.03–9.88 s per face; and M = 4.06 s, Range: 2.31–6.64 s per face, respectively) as each male face (M = 1.56 s, Range: 0–11.50 s per face; and M = 4.52 s, Range: 0–10.81 s per face, respectively). A Mann–Whitney test confirmed that there was no significant difference between 1‐month‐olds' (Mdn = 3.13 s per face) and 3‐month‐olds' (Mdn = 4.25 s per face) exposure to female faces, U = 80.00, z = −1.330, p = .193, r = −.24.
Exposure to Adult‐Age Faces
We predicted that infants would spend the majority of their time exposed to their caregivers; thus, we expected that infants would be exposed primarily to adult‐age faces (aged 20–49 years). The data confirmed this prediction. Infants spent the majority of their time exposed to adult‐age faces (M = 81%, Range: 7–100%). There was a statistically significant difference between adult‐age (Mdn = 92%) and not‐adult‐age (all faces not 20–49 years old) (Mdn = 10%) face exposure, as confirmed by a Wilcoxon signed‐ranks paired‐samples test, T = 3, Z = −4.228, p < .001, r = −.77.
One‐month‐old infants spent 91% of their time exposed to adult‐age faces (Range: 61–100%), with 2 of 14 infants spending 100% of their time exposed to adult‐age faces (see Fig. 4). Three‐month‐old infants spent 73% of their time exposed to adult‐age faces (Range: 7–100%), with 3 of 13 infants spending 100% of their time exposed to adult‐age faces. A Mann–Whitney test confirmed that there was no significant difference between 1‐month‐olds' (Mdn = 94%) and 3‐month‐olds (Mdn = 80%) exposure to adult‐age faces, U = 89.50, z = −.935, p = .361, r = −.17.
Figure 4.
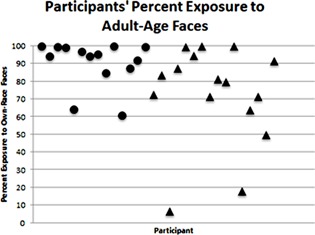
Each participant's percent exposure to adult‐age faces. Each point represents one participant. Circles represent 1‐month‐old infant participants and triangles represent 3‐month‐old infant participants. Infants are ordered by age, from youngest to oldest.
Infants were exposed to an average of 1–2 individual adult‐age faces (M = 1.45, Range:0–13) and less than 1 individual not‐adult‐age face (M = .71, Range: 0–6) per video. The difference between per‐video exposure to adult‐age (Mdn = 1.50 individual faces) and not‐adult‐age (Mdn = .50 individual faces) faces was statistically significant, as confirmed by a Wilcoxon signed‐ranks paired‐samples test, T = 4, Z = −3.380, p < .001, r = −.62. One‐ and three‐month‐olds were exposed to similar numbers of individual adult‐age (M = 1.55, Range: 0–13; and M = 1.36, Range: 0–6, respectively) and not‐adult‐age (M = .39, Range: 0–6; and M = .97, Range: 0–5, respectively) faces per video. A Mann–Whitney test confirmed that there was no significant difference between 1‐month‐olds' (Mdn = 1.57) and 3‐month‐olds' (Mdn = 1.38) per‐video exposure to adult‐age faces, U = 97.00, z = −.625, p = .544, r = −.11.
On average, infants' per face length of exposure to each adult‐age face (M = 3.98 s, Mdn = 3.87 s, Range: .00–9.17 s) was not significantly different than their per face length of exposure to each not‐adult‐age face (M = 3.68 s, Mdn = 3.17 s, Range: .00–12.00 s), as confirmed by a Wilcoxon signed‐ranks paired‐samples test, T = 0, Z = −.792, p = .440, r = .14. One‐ and three‐month‐olds had similar per face length of exposure to each individual adult‐age face (M = 4.06 s, Range: 1.89–9.17 s; and M = 3.90 s, Range: .00–6.10 s, respectively) and not‐adult‐age‐face (M = 3.77 s, Range: .00–12.00 s; and M = 3.60 s, Range: .00–7.03 s). A Mann–Whitney test confirmed that there was no significant difference between 1‐month‐olds' (Mdn = 3.68 s) and 3‐month‐olds (Mdn = 4.09 s per face) exposure to adult‐age faces, U = 103.00, z = −.374, p = .728, r = −.07.
DISCUSSION
The current study is the first to document infants' natural daily exposure to faces from the perspective of the infant. Consistent with theoretical perspectives that emphasize the role of learning in the development of face perception (e.g., Gauthier & Tarr, 2002; Nelson, 2001), our results reveal that faces are a common and frequent part of the infant visual world. One‐month‐olds spent 25% of their recorded waking hours exposed to faces. Three‐month‐olds spent 26% of their recorded waking hours exposed to faces. Given this massive exposure to faces, it is not surprising that infants quickly become proficient with this class of visual stimuli. Our results are congruent with previous studies that have attempted to document face exposure in infancy (e.g., Rennels & Simmons, 2008), but goes beyond previous research in two ways. First, by documenting infants' exposure to faces from a first‐person perspective, we can be certain that the results reported here reflect the infant's true experience with faces, something that is not possible from results collected from the adult's perspective (Smith et al., 2009). Second, by documenting infants' exposure to faces at 1 and 3 months of age, the current study characterizes the very early experiences that shape later perceptual development. Infants at 1 and 3 months of age have not undergone perceptual narrowing of any kind, but by 3 months they begin showing preferences for faces of certain categories (e.g., own‐race, Kelly et al., 2005), a potential precursor to perceptual narrowing. By examining these two age groups, this study can characterize the influences that may be driving preference and later differential ability.
The current data represent the normal, daily experiences of our infant participants. The video data collected were rich and highly variable. Most was filmed at the family home, representing the environment in which parents reported that infants spent the most time, and captured activities such as playing with parents, playing with siblings, watching parents go about household chores (cooking, cleaning), being changed, and being fed. The video data also included outside‐of‐the‐home destinations and activities, such as mommy‐and‐me groups, library reading groups, shopping, walking outside (in a stroller, car‐seat, sling, chest‐carrier, etc.), grandparents' and friends' homes, father's workplace, sibling's dance recital, and dinner in a restaurant. The activities in which infants and caregivers engaged during these videos included times during which they were not interacting (e.g., infant passively watching a mobile, with the caregiver not visible), intermittent interactions (e.g., the caregiver engaged in a discussion with a friend or pushing a pram in a shopping mall while regularly pulling faces at their infant), and involved interactions (e.g., reading a book to, playing with objects with, introducing new people to, engaging in song‐and‐dance games with, feeding, and changing the infant). Analyzing how particular types of activities influence infants' visual experiences would be a rich area for future research.
While it is possible that these data may not be fully representative of infant's experiences due to parents selecting when to record and due to the camera potentially influencing the behavior of the people being recorded, we believe that our study captures the typical daily experiences of our participants for several reasons. First, the videos captured do reflect the locations in which, in the initial interview, parents anticipated being or visiting; the parents who anticipated recording only at home did record only at home, while parents who expected to be attending non‐home locations with their infants did record at non‐home locations. Second, the experiences and activities recorded varied widely and included age‐typical interactions for 1‐ and 3‐month‐old infants (e.g., feeding, changing, playing). Third, while it is difficult to assess whether adults changed their behavior during their interaction with an infant wearing a camera, we suspect that any alterations in adult behavior were minimal. This is primarily because, unless they were informed about the study, most adults would not be aware that the infant was wearing a camera. The smiley‐face spy camera was selected for this reason—it is very small and is similar to typical infant accessories. Finally, some parents spontaneously reported when they were returning the camera that, during recording, they and their family would forget that the infant was wearing a camera; candid sections of video seem to corroborate these statements.
Infants not only receive a large amount of exposure to faces, they also receive a disproportionate amount of exposure to some face types and little or no exposure to other face types. This disproportionate exposure to particular face types manifests in the total proportion of time spent exposed to those face types, the number of individual exemplars of those face types seen, and the amount of time spent with each individual exemplar of those face types. Not unexpectedly, this disproportionate exposure reflects the makeup of each infant's home environment rather than the broader community in which the infants live.
Own‐race faces were the most commonly experienced face types (96% of all faces) despite infants' being of a variety of different backgrounds (i.e., own‐race represents Caucasian, Asian, Southeast Asian, Asian and Caucasian, Southeast Asian and Caucasian, and Black and Caucasian). This is similar to the 92% exposure to own‐race faces reported by Rennels and Simmons (2008). Eighteen of 30 infants (8 1‐month‐olds and 10 3‐month‐olds) were exposed exclusively to own‐race faces. Our infant participants were, on average, more than 25 times more likely to be exposed to the face of an own‐race individual than the face of an other‐race individual (M = .07 individual other‐race faces and M = 1.88 individual own‐race faces per video) and, if exposed to an other‐race face, they spent less time exposed to that particular face (M = 1.43 and 4.12 s per face, respectively). All of the infants who participated in this study lived in metropolitan Toronto, a highly multicultural city with a diverse population. The largest visible racial group is Caucasians, who account for only 42% of the population (Statistics Canada, 2006). Against this backdrop of diversity, it is even more striking that infants were exposed nearly exclusively to own‐race faces.
This predominant exposure to own‐race faces in early infancy parallels the pattern of perceptual narrowing for other‐race faces that has been well documented in previous studies. By 3 months of age, infants demonstrate a preference for own‐race faces (Kelly et al., 2007); by 6 months of age, infants demonstrate reduced ability to discriminate between faces in certain other‐race categories (Kelly et al., 2009); and by 9 months of age, when tested with the same procedure, infants demonstrate behavioral evidence of perceptual narrowing, maintaining only the ability to discriminate among faces belonging to their own racial group (Kelly et al., 2009). Infants show a similar pattern of perceptual narrowing for other‐species faces, with 6‐month‐olds showing equal facility discriminating human and monkey faces, and 9‐month‐olds showing diminished ability to discriminate monkey faces (Pascalis et al., 2002).
Previous work has demonstrated that it is possible to keep the perceptual window for processing “other” faces open between 6 and 9 months of age. Scott and Monesson (2009) exposed 6‐month‐old infants to a picture book containing six other‐species faces (i.e., monkey faces) for 10 min per day over the first 2 weeks of the study, then with decreasing frequency thereafter. This limited exposure to monkey faces was sufficient to keep the perceptual window open, such that infants who received this exposure were still able to discriminate monkey faces at 9 months of age. Limited exposure to other‐race faces has also been found to keep the perceptual window open for these types of faces in infants trained between the ages of 6–9 months (Heron‐Delaney et al., 2011) and in infants trained for three weeks between the ages of 8–10 months (Anzures et al., 2012). Therefore, the perceptual narrowing for other‐race faces found in 9‐month‐old infants (Kelly et al., 2009) suggests that infants receive a dearth of exposure to other‐race faces. Indeed, our results support that supposition in three ways: (1) infants in the current study received nearly exclusive exposure to own‐race faces, (2) they were exposed to more own‐race individuals, and (3) they spent more time per face exposed to these highly‐available own‐race faces.
Infants were primarily exposed to female faces (70% of all face exposure was female face exposure). Based on this differential exposure, we suggest that gender may be an area in which perceptual narrowing operates early and reflects infants' disproportionate exposure to female faces. Although gender has not typically been considered a domain of perceptual narrowing, the development of infant preference for and superior recognition of female faces (Quinn et al., 2002) mirrors early preference for and discrimination of own‐race faces (Kelly et al., 2007, 2005). If perceptual narrowing is considered a progressive process, then early preference for the face‐types with which infants have received the most experience might reflect early tuning of the perceptual system to the most common face type—that is, preference for the face types with which infants have the most experience may be an early marker of experience‐based perceptual narrowing.
In the case of gender, it is surprising that infants do not show equal ability with both male and female faces (Fagan, 1976; Quinn et al., 2002), given that our data demonstrate a significant amount of exposure to male faces (on average, 30% of the faces seen by infants are male faces) and no significant difference in length of exposure per face. The key difference appears to be that there are simply fewer male faces available in infants' visual worlds. From Scott and Monesson's (2009) training study, it is clear that the perceptual window for ‘other’ faces can be held open with surprisingly little exposure, as long as the exposure involves individuation of face exemplars. However, it is still uncertain what minimum number of exemplars or minimum amount of time is required for infants to maintain facility with “other” face categories, and it is possible that the male face exposure received by most infants does not meet the criteria. Our data suggests that, on average, infants did see significantly more, nearly twice as many, individual female faces than male faces (M = 1.37 and .73 faces per video, respectively). If infants receive exposure to very few male faces (e.g., only father), it is possible that infants' exposure to male faces does not individuate those male faces, that there are too few individual male face exemplars, or infants' attention is not being equally captured by male and female faces. All of these factors could potentially lead to decreased facility with male faces than female faces. Directly linking natural exposure to male faces to early preferences for female over male and later discrimination of female and male faces would help to resolve this issue. An analysis of how males and females may interact differently with infants in natural situations, in combination with eye‐tracking data capturing infant visual attention during these interactions, may also yield insight into what factors drive differential tuning of the perceptual system to male and female faces.
Infants were primarily exposed to adult‐age faces (81% of all face exposure was to adult‐age faces). This level of exposure suggests a third area in which perceptual narrowing may be operating. Due to a lack of research that has directly investigated this question in infants, it is unclear whether infants prefer adult‐age faces and/or show superior discrimination of adult‐age faces over other‐age faces. One study compared 7‐month‐olds' preference for infant, child, and adult faces as measured by visual preference and behavioral response (i.e., table‐banging) (Sanefuji et al., 2005). They found only a small visual preference for infant over child faces and increased table‐banging in response to both adult and infant faces. In childhood, the evidence is mixed: there is evidence for both a perceptual bias for child‐age faces (i.e., children show superior recognition for faces within two years of their chronological age compared to younger and older faces; Hills & Lewis, 2011), and a perceptual bias for adult‐age faces (i.e., children show superior recognition for adult faces compared to children's faces; Macchi Cassia, Pisacane, & Gava, 2012). There is also a clear adult‐age bias in adulthood, as adults are better able to recognize adult faces than children's faces (Anastasi & Rhodes, 2006).
Macchi Cassia (2011) hypothesizes that an “other‐age effect,” similar to the other‐race effect, will be found in infancy. Based on our data, if total exposure time is driving the bias, we predict that this will manifest as an adult‐age bias in infancy, with 3‐month‐old infants preferring adult‐age faces, and that this adult‐age bias will be slightly more robust than the preference for female faces (e.g., Quinn et al., 2002) and less robust than the preference for other‐race faces (e.g., Kelly et al., 2007), due to exposure to adult‐age faces (81%) being higher than exposure to female faces (70%) but lower than exposure to own‐race faces (96%) in the current study. At 9 months, infants should also demonstrate a reduced ability to discriminate child‐age or infant‐age faces. In other words, the perceptual window for processing faces of all different ages would narrow as it does for other‐race and male faces. If individual exemplars or exposure time per face, however, is key to perceptual narrowing, then the majority of infants should show no adult‐age bias provided that they are receiving, similar to our participants, exposure to equal numbers of adult‐age and not‐adult age individuals and spending an equivalent amount of time exposed to both of these face types on a per face basis. Future research should examine preferences for and discrimination ability with adult‐age versus infant‐, child‐, and older‐adult‐age faces in 3‐, 6‐, and 9‐month‐old infants in order to explore these predictions.
While the pattern of exposure to different face types was consistent with our predictions, there were differences between the groups and individual differences among infants. Despite similar proportions of time spent exposed to faces (26% and 25%, respectively), since 3‐month‐old infant participants spent more time awake (M = 8 hr and 55 min awake per day) than 1‐month‐old participants (M = 7 hr awake per day), their absolute level of exposure was different. It is unclear how these differences in exposure may influence infants' learning at each age; specifically, it is currently unknown what feature(s) of face exposure drives perceptual narrowing (i.e., is it the proportion of time exposed to particular face types or is there some minimum level of exposure that ensures facility with a particular face type). As depicted in Figures 2, 3, 4, while most infants were exposed primarily to adult‐age, own‐race, female faces, some infants were exposed to a more heterogeneous sampling of face types. While we have suggested that patterns of homogeneous face exposure are congruent with known patterns of perceptual narrowing, the current study cannot speak directly to how this exposure influences infants' later abilities. This open question leads to a new line of inquiry that can be investigated using the powerful first‐person perspective methodology used in the current study: the relations between individual differences in early experience and individual differences in later perceptual ability. Within individual differences, a longitudinal study would allow for an investigation of the stability or variability in these differences within an individual. A second opportunity afforded by this methodology is the examination of cultural differences in early experiences and, similarly, how these relate to later perceptual ability.
A first‐person perspective provides researchers with the opportunity to understand whether individual infants or cultural populations of infants who receive more own‐race, adult‐age, or female face exposure show greater reductions in the ability to discriminate the “other” face types. It also allows for the description of other factors, beyond mere exposure, that may influence infants' later performance. For example, Yurovsky Fricker, Yu, and Smith (2013) have documented that greater proximity and fewer competing visual stimuli in the visual field facilitate learning. Scott and Monesson (2009) have reported that individuation of exemplars maintains perceptual discrimination while mere exposure does not. Exploring relations between particular aspects of face exposure and variations in perceptual ability through the use of the first‐person perspective methodology can lead to an increased understanding of the precise facets of exposure that drive perceptual narrowing in the domain of face perception. Expanding beyond the domain of face perception, understanding early, natural, daily experiences of infants would permit a better understanding of factors that influence later development in multiple diverse areas, such as motor or language development.
Overall, our results suggest that the exposure to faces received by infants at 1 and 3 months of age mirrors the pattern of perceptual narrowing across three different face characteristics—race, gender, and age. Though this study did not directly examine the relationship between exposure and ability, it offers a powerful tool that can be used to quantify natural, daily, face exposure for future studies directly assessing this relationship. Infants spend one‐quarter (25%) of their waking hours exposed to faces. This massive exposure does not provide equal representation of all face types; rather, young infants see primarily female, adult‐age, own‐race faces. This study is the first to document the quantity and quality of infants' natural daily face exposure from the infant's perspective, and offers strong support for the idea that experience drives the development of the face processing system.
NOTES
We thank Ameera Ali, Andrea Andrei, Nina Arcon, Ajani Asokumar, Rafael Baguinan, Pavanpreet Bhardwaj, Jamie Lynn Juarez, Alyssa Gagnon, Yumna Gulzar, Zoe Kornhauser, Andrea Kusec, Shara Nauth, Bessie Orfanakos, Angelica Rojas, Kristina Safar, Nirosini Thanabalasingam, Vaunam Venkadasalam, and Lan (Mary) Wei for their dedication, commitment, and hours of hard work helping to code the data. We thank Dr. Benjamin Balas for his input during the preparation of this manuscript. This research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada to M. Moulson.
REFERENCES
- Anastasi J. S., & Rhodes, M. G. (2006). Evidence for an own‐age bias in face recognition. North American Journal of Psychology, 8(2), 237–252. [Google Scholar]
- Anzures, G. , Wheeler, A. , Quinn, P. C. , Pascalis, O. , Slater, A. M. , Heron‐Delaney, M. , … Lee, K. (2012). Brief daily exposure to Asian females reverses perceptual narrowing for Asian faces in Caucasian infants. Journal of Experimental Child Psychology, 112(4), 484–495. doi: 10.1016/j.jecp.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balas, B. , Westerlund, A. , Hung, C. , & Nelson, C. A. III. (2011). Shape, color, and the other‐race effect in the infant brain. Developmental Science, 14(4), 892–900. doi: 10.1111/j.1467‐7687.2011.01039.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell, I. W. R. (2001) Mother's face recognition in newborn infants: Learning and memory. Infant and Child Development, 10, 67–74. doi: 10.1002/icd.248 [Google Scholar]
- DiGiorgio, E. , Méary, D. , Pascalis, O. , & Simion, F. (2012). The face perception system becomes species‐specific at three months: An eye‐tracking study. International Journal of Behavioral Development, 37(2), 95–99. doi: 10.1177/0165025412465362 [Google Scholar]
- Dufour, V. , Coleman, M. , Campbell, R. , Petit, O. , & Pascalis, O. (2004). On the species‐specificity of face recognition in human adults. Current Psychology of Cognition, 22(3), 315–333. [Google Scholar]
- Fagan, J. F. (1976). Infants' recognition of invariant features of faces. Child Development, 47, 627–638. Stable URL: http://www.jstor.org/stable/1128177. [Google Scholar]
- Gauthier I., & Tarr, M. J. (2002). Unraveling mechanisms for expert object recognition: Bridging brain activity and behaviour. Journal of Experimental Psychology: Human Perception and Performance, 28(2), 431–446. doi: 10.1.1.20.5290 [DOI] [PubMed] [Google Scholar]
- Hannon E. E., & Trehub, S. E. (2005). Metrical categories in infancy and adulthood. Psychological Science, 16, 48–55. doi: 10.1111/j.0956‐7976.2005.00779.x [DOI] [PubMed] [Google Scholar]
- Harrison V., & Hole, G. J. (2009). Evidence for a contact‐based explanation of the own‐age bias in face recognition. Psychonomic Bulletin & Review, 16, 264–269. doi: 10.3758/PBR.16.2.264 [DOI] [PubMed] [Google Scholar]
- Hayward, W. G. , Rhodes, G. , & Schwaninger, A. (2008). An own‐race advantage for components as well as configurations in face recognition. Cognition, 106, 1017–1027. doi: 10.1016/j.cognition.2007.04.002 [DOI] [PubMed] [Google Scholar]
- Heron‐Delaney, M. , Anzures, G. , Herberg, J. S. , Quinn, P. C. , Slater, A. M. , Tanaka, J. W. , … Pascalis, O. (2011). Perceptual training prevents the emergence of the other race effect during infancy. PLoS ONE, 6(5), e19858. doi: 10.1371/journal.pone.0019858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills P. J., & Lewis, M. B. (2011). The own‐age face recognition bias in children and adults. Quarterly Journal of Experimental Psychology, 64(1), 17–23. doi: 10.1080/17470218.2010.537926 [DOI] [PubMed] [Google Scholar]
- Huttenlocher, P. R. (2002) Neural plasticity: The effects of environment on the development of the cerebral cortex. Cambridge, MA: Harvard University Press. [Google Scholar]
- Johnson, M. H. , Dziurawiec, S. , Ellis, H. , & Morton, J. (1991). Newborns' preferential tracking of face‐like stimuli and its subsequent decline. Cognition, 40, 1–19. doi: 10.1016/0010‐0277(91)90045‐6 [DOI] [PubMed] [Google Scholar]
- Kelly, D. J. , Liu., S. , Lee., K. , Quinn, P. C. , Pascalis, O. , Slater, A. M. , & Ge, L. (2009). Development of the other‐race effect during infancy: Evidence towards universality? Journal of Experimental Child Psychology, 104, 105–114. doi: 10.1016/j.jecp.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, D. J. , Quinn, P. C. , Slater, A. M. , Lee, K. , Ge, L. , & Pascalis, O. (2007). The other‐race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science, 18, 1084–1089. doi: 10.1111/j.1467‐9280.2007.02029.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, D. J. , Quinn, P. C. , Slater, A. M. , Lee, K. , Gibson, A. , Smith, M. , Ge, L. , & Pascalis, O. (2005). Three‐month‐olds, but not newborns, prefer own‐race faces. Developmental Science, 8, F31–F36. doi: 10.1111/j.1467‐7687.2005.0434a.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl, P. K. , Stevens, E. , Hayashi, A. , Deguchi, T. , Kiritani, S. , & Iverson, P. (2006). Infants show a facilitation effect for native language phonetic perception between 6 and 12 months. Developmental Science, 9(2), F13–F21. doi: 10.1111/j.1467‐7687.2006.00468.x [DOI] [PubMed] [Google Scholar]
- Macchi Cassia, V. M. (2011) Age biases in face processing: The effects of experience across development. British Journal of Psychology, 4, 816–882. doi: 10.1111/j.2044‐8295.2011.02046.x [DOI] [PubMed] [Google Scholar]
- Macchi Cassia, V. , Picozzi, M. , Kuefner, D. , & Casati, M. (2009). Why mix‐ups don't happen in the nursery. Evidence for an experience‐based interpretation of the other‐age effect. The Quarterly Journal of Experimental Psychology, 62, 1099–1107. doi: 10.1080/17470210802617654 [DOI] [PubMed] [Google Scholar]
- Macchi Cassia, V. , Pisacane, A. , & Gava, L. (2012). No own‐age bias in 3‐year‐old children: More evidence for the role of early experience in building face‐processing biases. Journal of Experimental Child Psychology, 113(3), 372–382. doi: 10.1016/j.jecp.2012.06.014 [DOI] [PubMed] [Google Scholar]
- Mondloch, C. J. , Lewis, T. L. , Budreau, D. R. , Maurer, D. , Dannemiller, J. L. , Stephens, B. R. , & Kleiner‐Gathercoal, K. A. (1999). Face perception during early infancy. Psychological Science, 10, 419–422. doi: 10.1111/1467‐9280.00179 [Google Scholar]
- Nelson, C. A. (2001) The development and neural bases of face recognition. Infant and Child Development, 10, 3–18. doi: 10.1.1.130.8912 [Google Scholar]
- Pascalis, O. , de Haan, M. , & Nelson, C. A. (2002). Is face processing species‐specific during the first year of life? Science, 296, 1321–1323. doi: 10.1126/science.1070223 [DOI] [PubMed] [Google Scholar]
- Pascalis, O. , de Schonen, S. , Morton, J. , Deruelle, C. , & Fabre‐Grenet, M. (1995). Mother's face recognition by neonates: A replication and an extension. Infant Behaviour and Development, 18, 79–85. doi: 10.1016/0163‐6383(95)90009‐8 [Google Scholar]
- Pascalis, O. , Scott, L. S. , Kelly, D. J. , Shannon, R. W. , Nicholson, E. , Coleman, M. , & Nelson, C. A. (2005). Plasticity of face processing in infancy. Proceedings of the National Academy of Sciences of the United States of America, 102(14), 5297–5300. doi: 10.1073/pnas.0406627102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons, F. , Lewkowicz, D. J. , Soto‐Faraco, S. , & Sebastián‐Gallés, N. (2009). Narrowing of intersensory speech perception in infancy. Proceedings of the National Academy of Sciences of the United States of America, 106(26), 10598–10602. doi: 10.1073/pnas.0904134106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, P. C. , Yahr, J. , Kuhn, A. , Slater, A. M. , & Pascalis, O. (2002). Representation of the gender of human faces by infants: A preference for female. Perception, 31(9), 1109–1121. doi: 10.1068/p3331 [DOI] [PubMed] [Google Scholar]
- Rennels J. L., & Simmons, R. E. (2008). Facial experience during the first year. Infant Behavioral Development, 31(4), 665–678. doi: 10.1016/j.infbeh.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanefuji, W. , Ohgami, H. , & Hashiya, K. (2005). Infants' preference for infants and adults. Proceedings of 2005 4th IEEE International Conference on Development and Learning, 93–95. doi: 10.1109/DEVLRN.2005.1490950
- Sangrigoli, S. , Pallier, C. , Argenti, A.‐M. , Ventureyra, V. A. G. , & deSchonen, S. (2005). Reversibility of the other‐race effect in face recognition during childhood. Psychological Science, 16(6), 440–444. doi: 10.1111/j.0956‐7976.2005.01554.x [DOI] [PubMed] [Google Scholar]
- Scott, L. S. , & Monesson, A. (2010). Experience dependent neural specialization during infancy. Neuropsychologia, 48, 1857–1861. doi: 10.1016/j.neuropsychologia.2010.02.008 [DOI] [PubMed] [Google Scholar]
- Scott L. S., & Monesson, A. (2009). The origin of biases in face perception. Psychological Science, 20, 676–680. doi: 10.1111/j.1467‐9280.2009.02348.x [DOI] [PubMed] [Google Scholar]
- Scott, L. S. , Pascalis, O. , & Nelson, C. A. (2007). A domain‐general theory of the development of perceptual discrimination. Current Directions in Psychological Science, 16, 197–201. doi: 10.1111/j.1467‐8721.2007.00503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, L. S. , Shannon, R. W. , & Nelson, C. A. (2006). Neural correlates of human and monkey face processing in 9‐month‐old infants. Infancy, 10(2), 171–186. doi: 10.1207/s15327078in1002_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons D. J., & Chabris, C. F. (1999). Gorillas in our midst: Sustained inattentional blindness for dynamic events. Perception, 28(9), 1059–1074. doi: 10.1068/p2952 [DOI] [PubMed] [Google Scholar]
- Smith, L. B. , Yu, C. , & Pereira, A. F. (2009). Not your mother's view: The dynamics of toddler visual experience. Developmental Science, 14(1), 9–17. doi: 10.1111/j.1467‐7687.2009.00947.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada . (2006). Visible minority population by census metropolitan area: 2006 Census: Kingston, Peterborough, Oshawa, Toronto, Hamilton. Retrieved from http://www.statcan.gc.ca/tables‐tableaux/sum‐som/l01/cst01/demo53c‐eng.htm.
- Yoshida H., & Smith, L. B. (2008). What's in view for toddlers? Using a head camera to study visual experience. Infancy, 13(3), 229–248. doi: 10.1080/15250000802004437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurovsky, D. , Fricker, D. C. , Yu, C. , & Smith, L. B. (2013). The role of partial knowledge in statistical word learning. Psychonomic Bulletin and Review. Advance online publication. doi: 10.3758/s13423‐013‐0443‐y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurovsky, D. , Smith, L. B. , & Yu, C. (2013). Statistical word learning at scale: The baby's view is better. Developmental Science, 16(6), 959–966. doi: 10.1111/desc.12036 [DOI] [PMC free article] [PubMed] [Google Scholar]


