Abstract
Aims
We investigated the relative pharmacokinetics, pharmacodynamics, and safety of the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab following injection at three different sites.
Methods
Sixty healthy subjects (39 male, 21 female; age 20–45 years) were randomized to receive a single subcutaneous injection of alirocumab 75 mg via 1-mL prefilled pen into the abdomen, upper arm, or thigh (NCT01785329). Subjects were followed for 85 days ± 2 days following study drug administration. Pharmacokinetic (PK) parameters for the systemic exposure of alirocumab were calculated, and levels of free PCSK9 were assessed. Percentage changes from baseline in LDL-C were compared between injection site groups using linear mixed-effects models.
Results
Alirocumab concentration–time profiles were similar, and free PCSK9 levels were reduced to approximately zero between Day 3 and Day 4 postinjection in all groups. LDL-C levels reached nadir on Day 15 postinjection in all groups with mean percentage reductions of 48.4% (abdomen), 39.5% (upper arm), and 45.6% (thigh) at this time point. A similar effect on LDL-C levels was seen across the entire time course of the study at all three injection sites. Treatment-emergent adverse events were experienced by 8/20 (abdomen), 11/20 (upper arm), and 13/20 (thigh) subjects. There were 2 mild/transient injection site reactions. There were no serious adverse events.
Discussion
A single subcutaneous administration of alirocumab 75 mg via prefilled pen was well tolerated with similar pharmacokinetics and pharmacodynamics when injected into the abdomen, upper arm, or thigh.
Conclusion
These results suggest that alirocumab can be interchangeably injected in the abdomen, upper arm, or thigh.
Keywords: Alirocumab, Cholesterol, Low-density lipoprotein, Pharmacodynamics, Pharmacokinetics, Proprotein convertase subtilisin/kexin type 9
Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a protease that mediates degradation of low-density lipoprotein (LDL) receptors 1. By its effect of increasing the numbers of LDL receptors, inhibition of PCSK9 is being investigated as a means of reducing levels of LDL cholesterol (LDL-C). Alirocumab is a fully human monoclonal antibody that specifically binds to and inhibits PCSK9. In Phase 2 studies, alirocumab administered every 2 weeks at a dose of 150 mg reduced LDL-C by up to 72% when combined with statins ± ezetimibe, with the most common treatment-emergent adverse event (TEAE) being transient injection site reactions of mild intensity and short duration 2–4. In these studies, all patients received alirocumab injections in the abdomen; however, patients may prefer to use different injection sites. Here, we report the relative pharmacokinetics (PK), pharmacodynamics (PD), and safety of alirocumab after single subcutaneous (SC) administration of 75 mg into the abdomen, upper arm, and thigh of healthy subjects.
Methods
Study Design and Population
This was an open-label, randomized, Phase 1 study conducted in healthy subjects aged 18–45 years with LDL-C levels >95 mg/dL (2.46 mmol/L) not receiving background lipid-lowering therapy. The study was conducted at the Hammersmith Medicines Research Clinical Research Unit in London, UK (NCT01785329). The protocol was approved by the Scotland A Research Ethics Committee, Edinburgh, Scotland, and written informed consent was obtained from all participants.
Subjects were randomized to one of the three parallel groups and received a single 75 mg dose of alirocumab SC via 1-mL prefilled pen at one of the three distinct sites (abdomen, upper arm, and thigh) in the morning on Day 1. Samples for PK and PD analyses (including free PCSK9 and LDL-C assessments) were collected following a 10-h fast predose on Day 1, and at various time points up to Day 85 (±2 days, end of the study).
The primary objective was to compare the relative PK of a single SC dose of alirocumab 75 mg administered at three different injection sites in healthy subjects. Additional objectives included assessments of the effect of a single SC dose of alirocumab on serum LDL-C, other lipid parameters, free PCSK9 levels, and safety.
Alirocumab and free PCSK9 serum concentrations were determined using validated enzyme-linked immunosorbent assays with lower limits of quantification (LLOQ) of 78 and 31.2 ng/mL, respectively. PK parameters for the systemic exposure of alirocumab, calculated using noncompartmental methods, included maximum serum concentration (Cmax), area under the serum concentration versus time curve (AUC), and AUC from time zero to time of last concentration above LLOQ (AUClast). LDL-C was calculated using the Friedewald formula 5. Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs), apolipoprotein (apo) B, and apoA1 were measured directly.
Safety assessments included TEAEs, especially local tolerability (injection site reactions). TEAEs were defined as any AE occurring from the time of alirocumab administration up to the end of the study visit.
Statistical Analyses
A sample size of 20 subjects per group was calculated to be sufficient to obtain an estimate for the ratio of PK parameters between groups with a maximum imprecision of 19.7% and 90% assurance in terms of the 90% confidence interval (CI), and assuming a maximum standard deviation (SD) of 0.35 for log-transformed PK parameters based on previous experience with alirocumab.
PK parameters were log-transformed prior to statistical analysis with PKDMS (PKDMS version 2.0 incorporating WinNonlin Professional, version 5.2.1; Pharsight [now Certara, St. Louis, MO, USA]) and SAS® (version 9.2 on Windows platform; SAS Institute, Cary, NC, USA). Relative PK of systemic exposure between injection sites was assessed using a linear fixed-effects model with terms for injection site, gender, and weight as covariate. Ratios of geometric means for Cmax, AUClast, and AUC were obtained by computing estimates and 90% CIs for the differences between injection sites means within the linear mixed-effects model framework, then converting to ratios by antilog transformation. Percentage changes from baseline for each PD parameter were compared between each injection site group at each time point using a linear mixed-effects model (SAS Proc Mixed®; SAS Institute) to obtain P-values for the interaction effect between injection site and PD parameter. Safety data were analyzed using descriptive statistics.
Results
Subjects
In total, 60 subjects were randomized (20 per group), and all completed the study. Baseline characteristics, including mean LDL-C and free PCSK9 levels, were similar across the three groups (Table1).
Table 1.
Baseline demographics and subject characteristics
| Alirocumab 75 mg SC | |||
|---|---|---|---|
| Abdomen (n = 20) | Upper arm (n = 20) | Thigh (n = 20) | |
| Age, years | 34.4 (7.5) | 30.7 (5.3) | 29.7 (6.3) |
| Male gender, n (%) | 17 (85%) | 10 (50%) | 12 (60%) |
| Race, n (%) | |||
| Caucasian/white | 12 (60%) | 11 (55%) | 13 (65%) |
| Black | 2 (10%) | 4 (20%) | 2 (10%) |
| Asian/Oriental | 5 (25%) | 4 (20%) | 5 (25%) |
| Other | 1 (5%) | 1 (5%) | 0 |
| BMI < 30 kg/m2, n (%) | 20 (100%) | 20 (100%) | 20 (100%) |
| LDL-C, mg/dL | 131.1 (27.5) | 129.2 (26.7) | 121.0 (16.6) |
| HDL-C, mg/dL | 43.7 (7.3) | 56.1 (14.7) | 49.5 (11.6) |
| Non-HDL-C, mg/dL | 150.8 (32.1) | 148.9 (29.4) | 141.1 (20.1) |
| Total cholesterol, mg/dL | 194.5 (32.1) | 204.9 (33.6) | 190.6 (18.6) |
| Triglycerides, mg/dL | 97.4 (17.7–194.9) | 88.6 (35.4–230.3) | 75.3 (26.6–292.3) |
| Free PCSK9, ng/mL | 150.0 (52.8) | 149.4 (53.9) | 160.2 (57.3) |
Values are mean (standard deviation), unless otherwise stated.
BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; SC, subcutaneous.
Relative Pharmacokinetics
Alirocumab serum concentration–time profiles were similar among the three injection sites, with Cmax of 8.18, 6.77, and 7.13 mg/L and AUC of 129, 130, and 115 mg day/L for the abdomen, upper arm, and thigh groups, respectively (Figure1A). The ratios of point estimates between upper arm versus abdomen injection site groups showed a decrease of 21% for Cmax and 8% for AUC and AUClast (Table2). Comparing thigh versus abdomen, a difference of 12% was observed for Cmax and 16% for AUC and AUClast. For upper arm versus thigh, a 10% difference was observed for Cmax (90% CI: 0.76–1.06), whereas a 9% greater difference was observed for AUC and AUClast.
Figure 1.
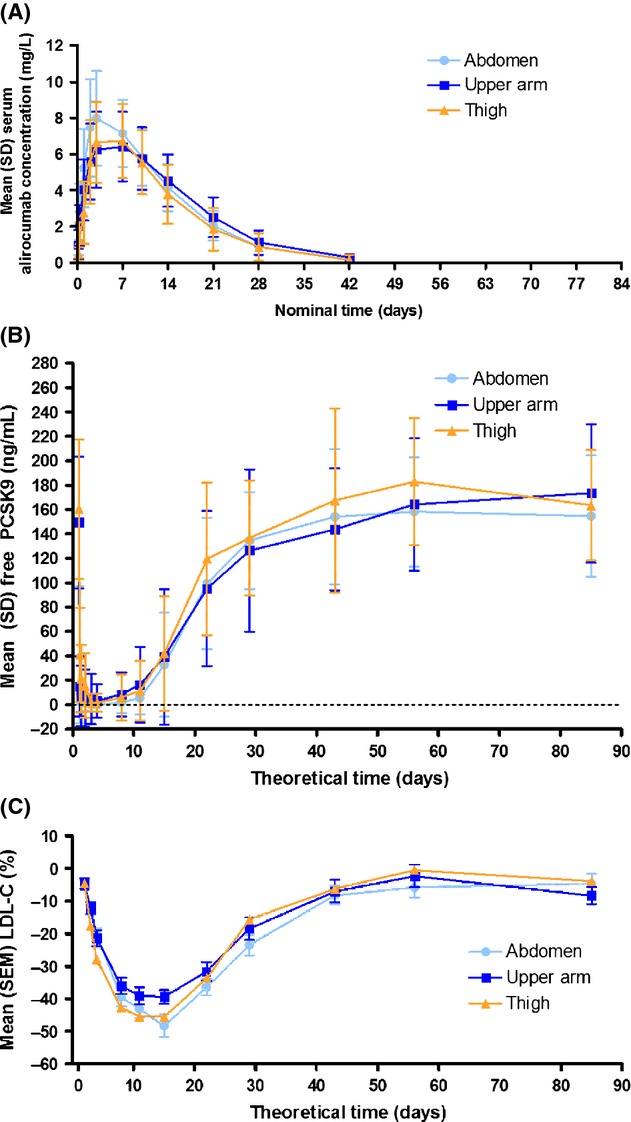
Mean (SD) alirocumab concentration (A), free PCSK9 levels (B), and percentage change in LDL-C from baseline (C) after subcutaneous administration of alirocumab 75 mg. LDL-C, low-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; SEM, standard error of the mean; SD, standard deviation.
Table 2.
Pairwise comparisons of geometric mean ratios of pharmacokinetic parameters between injection site groups
| Parameter | Injection sites compared | Ratios | |
|---|---|---|---|
| Point estimate | 90% CI | ||
| Cmax | Upper arm versus abdomen | 0.79 | 0.66–0.93 |
| Upper arm versus thigh | 0.90 | 0.76–1.06 | |
| Thigh versus abdomen | 0.88 | 0.74–1.04 | |
| AUC | Upper arm versus abdomen | 0.92 | 0.78–1.09 |
| Upper arm versus thigh | 1.09 | 0.93–1.28 | |
| Thigh versus abdomen | 0.84 | 0.72–0.99 | |
| AUClast | Upper arm versus abdomen | 0.92 | 0.77–1.08 |
| Upper arm versus thigh | 1.09 | 0.93–1.29 | |
| Thigh versus abdomen | 0.84 | 0.71–0.99 | |
AUC, area under the serum concentration versus curve from time zero to infinity (AUC); AUClast, area under the serum concentration versus curve from time zero to time of last concentration above lower limit of quantification; Cmax, maximum concentration; CI, confidence interval.
Median time to reach Cmax (tmax) was 2.96, 6.95, and 3.06 days in the abdomen, upper arm, and thigh, respectively (Table3). Despite the higher median tmax value for the upper arm, with high variability being observed for the distribution of tmax between the treatment groups, the time–course curves for upper arm and thigh were very similar (Figure1A). Elimination of alirocumab resulted in mean residence time of 11.6–13.5 days and mean half-life of 5.77–6.66 days.
Table 3.
Pharmacokinetic parameters of alirocumab following injection of a single 75 mg dose into three different injection sites
| Alirocumab 75 mg SC | |||
|---|---|---|---|
| Abdomen (n = 20) | Upper arm (n = 20) | Thigh (n = 20) | |
| Cmax, mg/L | 8.18 (2.51) | 6.77 (2.02) | 7.13 (2.21) |
| tmax, days; median (range) | 2.96 (1.95–7.01) | 6.95 (1.96–10.08) | 3.06 (2.15–8.11) |
| t1/2z, days | 6.03 (1.11) | 6.66 (0.97) | 5.77 (1.59) |
| AUC, mg day/L | 129 (35.7) | 130 (42.0) | 115 (44.4) |
| CL/F, L/day | 0.63 (0.18) | 0.63 (0.20) | 0.78 (0.31) |
| Vss/F, L | 7.28 (2.46) | 8.54 (3.40) | 8.92 (4.66) |
| MRT, days | 11.6 (1.83) | 13.5 (2.35) | 11.9 (2.49) |
Values are mean (SD), unless otherwise stated.
Cmax, maximum concentration; tmax, time to reaching Cmax; t1/2z, half-life; AUC, area under the serum concentration versus time curve; CL/F, clearance relative to bioavailability; Vss/F, distribution volume at the steady state relative to bioavailability; MRT, mean residence time; SC, subcutaneous.
Pharmacodynamics
Maximal reduction of mean free PCSK9 was observed between Day 3 and Day 4 in all groups, with mean values at zero or close to zero (Figure1B). After this suppression, serum concentrations of free PCSK9 started to gradually increase and returned within the baseline range by the end of the study.
The PD effects of alirocumab on LDL-C (Figure1C) were similar for all three injection site groups. LDL-C declined reaching a nadir on Day 15 in all groups. At this time point, the percentage decrease in LDL-C was 48.4% in the abdomen group, 39.5% in the upper arm group, and 45.6% in the thigh group (Figure1C). The effect on LDL-C levels was similar across the entire time course at all three injection sites (P-value of the effect of injection site groups = 0.403). There was a trend for a slightly smaller PD effect in the upper arm group observed at nadir values for LDL-C, but not for the overall time course. The PD effects of alirocumab on apoB and non-HDL-C were consistent with LDL-C (Supporting information Figure S1).
Safety
TEAEs were experienced in 8 (40%), 11 (55%), and 13 (65%) subjects in the abdomen, upper arm, and thigh groups, respectively. The most common TEAEs in all groups were nasopharyngitis, which occurred in two (10%), one (5%), and six (30%) subjects, and headache in two (10%), four (20%), and five (25%) subjects in the abdomen, upper arm, and thigh groups, respectively (Table4). Only two local injection site reactions were reported (pain and discoloration); both occurred in the thigh group and were transient and of mild intensity. There were no serious AEs (SAEs) or TEAEs of severe intensity.
Table 4.
TEAEs occurring in ≥2 subjects in any group (as defined by primary system organ class [SOC] and MedDRA preferred term [PT], safety population)
| SOC PT | Alirocumab 75 mg SC (%) | ||
|---|---|---|---|
| Abdomen (n = 20) | Upper arm (n = 20) | Thigh (n = 20) | |
| Any TEAE | 8 (40) | 11 (55) | 13 (65) |
| Infections and infestations | 3 (15) | 4 (20) | 7 (35) |
| Nasopharyngitis | 2 (10) | 1 (5) | 6 (30) |
| Nervous system disorders | 3 (15) | 4 (20) | 7 (35) |
| Headache | 2 (10) | 4 (20) | 5 (25) |
| Reproductive system and breast disorders | 1 (5) | 2 (10) | 0 |
| Dysmenorrhea | 1 (5) | 2 (10) | 0 |
| Injury, poisoning, and procedural complications | 0 | 1 (5) | 3 (15) |
| Limb injury | 0 | 0 | 2 (10) |
An AE is considered as treatment-emergent if it occurred from the time of alirocumab administration up to the EOS visit (inclusive). The two reported injection site AEs were recorded under separate preferred terms (pain and discoloration, respectively), hence do not appear in the table.
TEAE, treatment-emergent adverse event; SC, subcutaneous.
Discussion
The concentration–time profiles for alirocumab after a single SC injection of 75 mg into the abdomen, upper arm, and thigh were similar in this population of healthy subjects. There was a slight trend for lower exposure in the upper arm and thigh compared with the abdomen. The observed mean half-life of 5.8–6.7 days was consistent with previous estimates of 5.6–8.8 days with single ascending SC doses of alirocumab 6.
Subcutaneously administered alirocumab rapidly bound to and reduced circulating free PCSK9, reaching a nadir close to zero between Day 3 and Day 4 in all injection site groups. This was followed by a decrease in LDL-C with maximal reduction on Day 15 in all groups. The dynamics between a single alirocumab 75 mg dose, free PCSK9, and LDL-C observed in this study at each injection site are in agreement with the findings of a single ascending dose study in healthy subjects in which a SC injection of alirocumab into the abdomen resulted in reductions in free PCSK9 levels within 3 days of dosing and peak reductions in LDL-C 8–15 days after dosing 7,8. Additionally, in a Phase 3 monotherapy study, alirocumab 75 mg every 2 weeks produced sustained LDL-C reductions over 12 weeks of treatment (least square mean reduction of 53.2% from baseline at Week 12) 9,10.
During the study, no SAEs or TEAEs of severe intensity were reported, as expected based on the data observed in Phase 2 and Phase 3 studies to date 2,3,10,4. A prefilled pen was used to deliver the single alirocumab 75 mg dose as a 1-mL SC injection. Injection site reactions were infrequent, with only two reports of mild and transient events in the group of subjects receiving the injection in the thigh.
Overall, a single administration of alirocumab 75 mg by SC route delivered via prefilled pen into the abdomen, upper arm, or thigh was well tolerated and presented similar PK and PD profiles regardless of injection site. Our findings suggest that alirocumab could be interchangeably injected in the abdomen, upper arm, or thigh offering patients' flexibility in choice of injection site.
Acknowledgments
The authors wish to acknowledge the contribution of Pascale Lewanczyk and are also grateful to François Falda-Buscaiot and Yann-Joel Beyer for their assistance with operational aspects of the study.
Funding source
This work was funded by Sanofi and Regeneron. Sanofi was responsible for the study design, data collection, and data analysis. Medical writing support was provided by Melanie Jones of Prime Medica Ltd (Knutsford, Cheshire, UK), funded by Sanofi and Regeneron. Responsibility for opinions, conclusions, and interpretation of data lies with the authors.
Conflict of Interest
C.L., T.P., F.P., A.B., J.R., and C.H. are employees of Sanofi. W.J.S. is an employee of Regeneron.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Percentage change from baseline after administration of alirocumab 75 mg in (A) total cholesterol, (B) Non-HDL-C, (C) apoB, (D) HDL-C, (E), apoA1, (F) triglycerides.
References
- 1.Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 2.McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–2353. doi: 10.1016/j.jacc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367:1891–1900. doi: 10.1056/NEJMoa1201832. [DOI] [PubMed] [Google Scholar]
- 4.Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- 5.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 6.Roth EM, Diller P. Alirocumab for hyperlipidemia: physiology of PCSK9 inhibition, pharmacodynamics and Phase I and II clinical trial results of a PCSK9 monoclonal antibody. Future Cardiol. 2014;10:183–199. doi: 10.2217/fca.13.107. [DOI] [PubMed] [Google Scholar]
- 7.McKenney JM, Swergold GA, DiCoccio AT, et al. Dynamics between the monoclonal antibody alirocumab (SAR236553/REGN727), Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) and low-density lipoprotein cholesterol (LDL-C) levels.. 81st European Atherosclerosis Society Congress,; Lyon, France. 2013. [Google Scholar]
- 8.Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–1118. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- 9.Farnier M, Kastelein JJ, Roth E, et al. Relationship between alirocumab, PCSK9, and LDL-C levels: results from the ODYSSEY MONO Phase 3 trial of alirocumab 75 mg every 2 weeks.. 82nd European Atherosclerosis Society Congress,; Madrid, Spain. 2014. [Google Scholar]
- 10.Roth EM, Taskinen MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesteremia: results of a 24-week, double-blind, randomized Phase 3 trial. Int J Cardiol. 2014;176:55–61. doi: 10.1016/j.ijcard.2014.06.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percentage change from baseline after administration of alirocumab 75 mg in (A) total cholesterol, (B) Non-HDL-C, (C) apoB, (D) HDL-C, (E), apoA1, (F) triglycerides.


