Abstract
Background
Pazopanib (PZP) may induce prolonged cardiac repolarization and proarrhythmic effects, similarly to other tyrosine kinase inhibitors.
Objectives
To demonstrate PZP-induced prolonged cardiac repolarization and proarrhythmic electrophysiological effects and to investigate possible preventive effects of metoprolol and diltiazem on ECG changes (prolonged QT) in an experimental rat model.
Methods
Twenty-four Sprague-Dawley adult male rats were randomly assigned to 4 groups (n = 6). The first group (normal group) received 4 mL of tap water and the other groups received 100 mg/kg of PZP (Votrient® tablet) perorally, via orogastric tubes. After 3 hours, the following solutions were intraperitoneally administered to the animals: physiological saline solution (SP), to the normal group and to the second group (control-PZP+SP group); 1 mg/kg metoprolol (Beloc, Ampule, AstraZeneca), to the third group (PZP+metoprolol group); and 1mg/kg diltiazem (Diltiazem, Mustafa Nevzat), to the fourth group (PZP+diltiazem group). One hour after, and under anesthesia, QTc was calculated by recording ECG on lead I.
Results
The mean QTc interval values were as follows: normal group, 99.93 ± 3.62 ms; control-PZP+SP group, 131.23 ± 12.21 ms; PZP+metoprolol group, 89.36 ± 3.61 ms; and PZP+diltiazem group, 88.86 ± 4.04 ms. Both PZP+metoprolol and PZP+diltiazem groups had significantly shorter QTc intervals compared to the control-PZP+SP group (p < 0.001).
Conclusion
Both metoprolol and diltiazem prevented PZP-induced QT interval prolongation. These drugs may provide a promising prophylactic strategy for the prolonged QTc interval associated with tyrosine kinase inhibitor use.
Keywords: Arrhythmias, Cardiac / therapy; Pyrimidines / adverse effects; Heart Conduction System / physiology; Heart / physiology
Introduction
Multi-targeted tyrosine kinase inhibitors (TKI) have become important for the treatment of several malignancies in the past few years1. Sorafenib is a Raf kinase inhibitor that inhibits vascular endothelial growth factor receptors 1, 2 and 3 (VEGFr-1, -2, and -3, respectively), platelet-derived growth factor receptor b (PDGFr-b), Fms-like tyrosine kinase-3 (Flt-3), and c-kit. Sunitinib inhibits VEGFr-1, -2, and -3, PDGFr-a and -b, c-kit, Flt-3, colony-stimulating factor type 1, and glial cell line-derived neurotrophic factor (GDNF) receptor (RET)2. Pazopanib is a potent multi-targeted TKI targeting VEGFr, PDGFr, and c-kit2,3. There are lots of ongoing trials with pazopanib in different types of tumors. Pazopanib has shown monotherapy activity in advanced renal cell cancer (RCC) patients and is approved for the treatment of advanced RCC patients4.
While exploring the therapeutic index of TKIs, oncology investigators are confronted with drug adverse effects such as cardiac toxicities5. QT prolongation has drawn attention because of the risk of serious cardiac arrhythmias, such as torsade de pointes, and may be associated with ventricular arrhythmia and sudden cardiac death6.
In several studies, QTc prolongation has been observed with TKIs such as sunitinib7,8, vandetanib7,9,10, dasatinib7, crizotinib11. However there are limited data about QTc prolongation with pazopanib treatment. Metoprolol is a class 2 antiarrhythmic agent according to the Vaughan-Williams classification and a member of the group of drugs called beta-blockers12. Diltiazem is a class 4 antiarrhythmic agent and a member of the non-dihydropyridine group of drugs known as calcium channel blockers12. This study was aimed to demonstrate that pazopanib induced prolonged cardiac repolarization and to investigate the possible corrective effects of metoprolol and diltiazem on electrocardiographic changes in an experimental model.
Methods
After receiving the Animal Ethics Committee's consent, the experiments performed in this study have been carried out according to the rules in the Guide for the Care and Use of Laboratory Animals adopted by National Institutes of Health (USA). Animals were fed ad libitum and housed in pairs in steel cages, in a temperature-controlled environment (22 ± 2 ºC) with 12-h light/dark cycles. The experimental procedures were approved by the Ege University Committee for Animal Research. All animal studies strictly conformed to the animal experiment guidelines of the Committee for Humane Care.
Electrocardiograms (ECG) were recorded on male rats under anesthesia in the prone position. Electrodes consisted of 26-gauge needles placed subcutaneously for 1 cm. Standard limb leads were constructed from electrodes placed at the paws. Rats were anesthetized with a combination of 40 mg/kg of ketamine hydrochloride (Alfamine®, Ege Vet, Alfasan International B.V., Holland) and 4 mg/kg of xylazine hydrochloride (Alfazyne®, Ege Vet, Alfasan International B.V., Holland), intraperitoneally administered. Under anesthesia, ECG was taken in lead I (DI) (Biopac MP 150). Data were evaluated using Biopac Student Lab Pro software, version 3.6.7 (BIOPAC Systems, Inc.), and the parameters were as follows: QT interval, T-wave duration, and heart rate. For the calculation of QTc, Fridericia formula was used.
The rats were randomly assigned to four groups (n = 6). Table 1 shows detailed information about the groups. The first group (normal group) received 4 mL of tap water, while the other groups received 100 mg/kg of pazopanib, perorally, via orogastric tubes. Tablets containing 200 mg of pazopanib (Votrient®, GlaxoSmithKline) were crushed and suspended in tap water to yield a concentration of 10 mg/mL. According to the weight of each rat, suspended drug solution was completed to 4 mL with tap water. Three hours after the oral administration of pazopanib, the animals received intraperitoneally: physiological saline solution (SP), to the normal group and to the second group (control-PZP+SP group); 1 mg/kg of metoprolol (Beloc, Ampule, AstraZeneca), to the third group (PZP+metoprolol group); and 1 mg/kg of diltiazem (Diltiazem, Mustafa Nevzat), to the fourth group (PZP+diltiazem group). One hour after administering those drugs, and under anesthesia, the QTc interval was calculated by recording ECG on lead I. The observer (O.E.) who measured the QT intervals was blind to laboratory data. The QT interval was determined from the onset of the QRS complex to the end of the T wave. Six consecutive beats were evaluated, and the arithmetic means of RR and QT were obtained.
Table 1.
Detailed description of the groups
| Groups | Beginning of the study (orogastric tube) | 3 hour later (intraperitoneal) | 1 hour later |
|---|---|---|---|
| Group 1 (normal group) (n=6) | Tap water | SP | ECG records are taken |
| Group 2 (control-PZP+SP group) (n=6) | PZP | SP | |
| Group 3 (PZP+metoprolol) (n=6) | PZP | Metoprolol | |
| Group (PZP+diltiazem) (n=6) | PZP | Diltiazem |
PZP: pazopanib; SP: physiological saline solution.
Statistical analysis
Results were given as mean ± standard error of the mean (SEM). Shapiro-Wilk test was used for checking normal distributions. All variables had normal distribution. One-Factor Analysis of Variance (ANOVA) was used to assess the groups with normally distributed continuous variables. Bonferroni adjustment for multiple comparisons was performed. Data analyses were performed using the SPSS software, version 15.0, for Windows (Chicago, IL, USA).
A p value < 0.05 was accepted as statistically significant, and a p value < 0.001, as highly statistically significant.
Results
Figure 1 shows the ECGs of all groups, and Table 2 summarizes the QTc intervals of all groups.
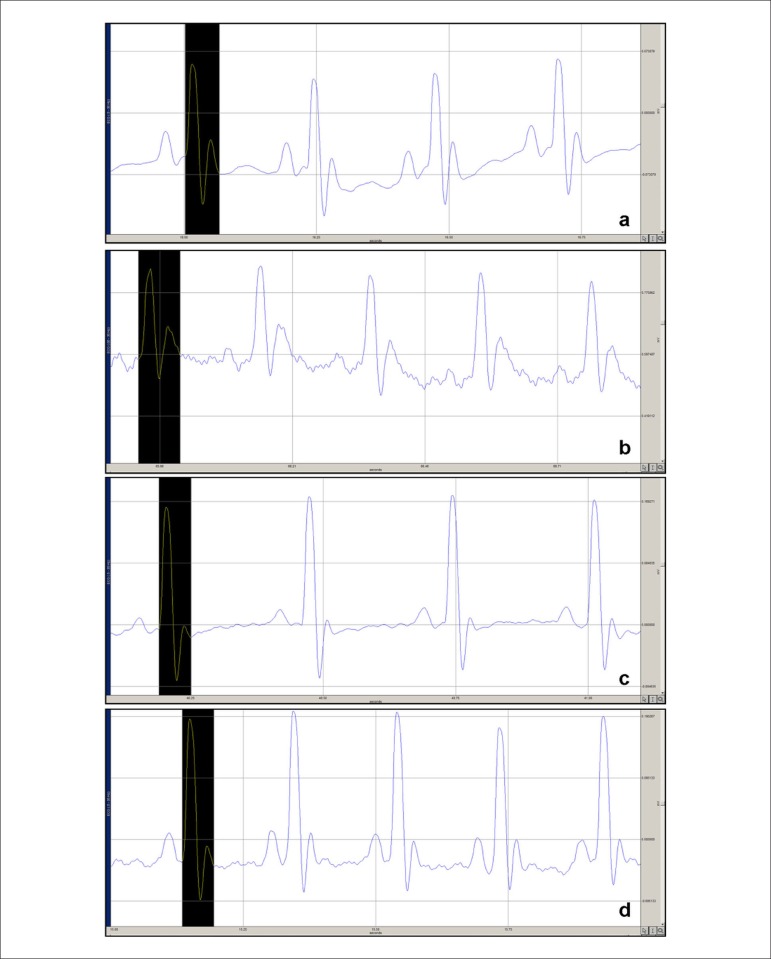
Figure 1.
a) ECG of normal group rats receiving only saline solution without pazopanib (PZP); b, c, d) ECG changes in rats receiving saline solution, metoprolol and diltiazem after 3 hours of PZP administration; b) QT prolongation in control group rats receiving only saline solution after PZP administration; c) QT-interval shortage in the PZP+metoprolol group; and d) QT-interval shortage in the PZP+diltiazem group. QT intervals are shown in the black areas.
Table 2.
The values of QTc intervals of all groups
| Groups | QTc (ms) | Heart rate (beats per minute) | T duration (ms) | QT duration (ms) |
|---|---|---|---|---|
| Normal | 99.93 ± 3.62 | 266 ± 21.69 | 28.33 ± 1.21 | 60.83 ± 0.98 |
| Control-PZP+SP | 131.23 ± 12.21** | 231.33 ± 67.95 | 47 ± 4.09** | 85 ± 9.33** |
| PZP+metoprolol | 89.36 ± 3.61†† | 164.67 ± 27.04† | 29.33 ± 2.73†† | 64 ± 0.89†† |
| PZP+diltiazem | 88.86 ± 4.04†† | 176.67 ± 9.30† | 29.67 ± 1.36†† | 62 ± 1.78†† |
PZP: pazopanib; SP: physiological saline solution.
p < 0.001 control group compared with normal group
p < 0.05 diltiazem or metoprolol group compared with control group
p < 0.001 diltiazem or metoprolol group compared with control group
The QTc interval was significantly longer in the control-PZP+SP group then in the normal group (131.23 ± 12.21 ms vs 99.93 ± 3.62 ms, respectively) (p < 0.001).
Both metoprolol and diltiazem treatments significantly shortened the QTc intervals when compared with that of the control-PZP+SP group (89.36 ± 3.61 ms, 88.86 ± 4.04 vs 131.23 ± 12.21 ms, respectively) (p < 0.001).
Discussion
Several molecular-targeted therapy drugs, such as multi-targeted TKIs, histone deacetylase inhibitors, vascular-disrupting agents, farnesyl protein transferase (FTPase) inhibitors, protein kinase C inhibitors, and Src/Abl kinase inhibitors, have been found to have an effect on the QT interval13.
Long QT syndrome (LQTS) is either an inherited (congenital) or acquired disorder of the electrical system of the heart. Congenital LQTS usually results from disorders in the cardiac membrane sodium or potassium ion channels14. The clinical course of patients with LQTS is variable, owing to incomplete penetrance of QT elongation, which may or may not cause arrhythmias. Patients with LQTS may be classified into three main categories of risk for developing life-threatening cardiac events, such as torsade de pointes and cardiac arrest, as follows: very high risk, high risk, and low risk. Patients at low risk may have just QT prolongation, the risk of life-threatening cardiac events being 0,5%15. The major electrolyte imbalances associated with prolonged QT interval are hypocalcemia, hypophosphatemia, hypokalemia, and hypomagnesemia16. Usually the pharmacologic agents that cause significant QT prolongation have potassium channel blocking effects predominantly affecting the delayed rectifier repolarizing current, IK (which is the sum of two kinetically and pharmacologically distinct types of IK current components: a rapid, IKr, and a slow, IKs, component), which has a major role in the electrical activity of cardiac ventricular myocytes. IKr plays a major role in the termination of the plateau phase of the action potential and in driving cell membrane repolarization17,18. Dysfunction of these channels is determined in certain forms of inherited and acquired LQTS19.
In vitro studies have suggested that most QT-prolonging drugs block the IKr component that is encoded by hERG (human ether-a-go-go-related gene) in humans. Although functional IKr current has been found in many species, such as dogs, guinea pigs, and rabbits, there is very little (if any) functional IKr or hERG-like current in the rat ventricle20. Studies with macrolide antibiotics in rats have shown good agreement between QT-interval prolongation assessments based on rat ECG data and the clinical findings of human beings; however, those results should be carefully assessed and the existence of differences between rats and humans should be kept in mind21.
Cancer patients may be particularly prone to QT prolongation, because many of them (16% to 36%) have baseline ECG abnormalities and use concomitant drugs, such as antiemetics, antifungals or antibiotics, that can enlarge the QT interval7,9. In addition, cancer patients often have symptoms such as nausea, vomiting, diarrhea, and decreased oral intake, which may lead to electrolyte disturbances, placing the patient at risk for QT prolongation7,22.
TKIs have played an important role in the treatment of several malignancies in the past few years and provided a significant improvement in patients' outcome1. The use of small molecule vascular endothelial growth factor receptor-tyrosine kinase inhibitors (VEGFr-TKIs) has shown to increase the risk and the incidence of fatal adverse events (FAEs) in patients being treated for cancer. Compared with 0.7% for controls, the incidence of FAEs associated with the use of VEGFr-TKI was 1.5%. Cardiotoxicity is the second most common cause of FAEs1. The use of TKIs in the past decade has revealed a new spectrum of pro-arrhythmogenic adverse effects, QT-interval prolongation being one the most important ones7,9. QT-interval prolongation has been observed in several trials with the use of molecular-targeted agents, and QT-interval prolongation has drawn attention because of its risk of malignant cardiac arrhythmia and sudden cardiac death23.
QT-interval prolongation has been associated with several multi-targeted TKIs, such as sunitinib, vandetanib, and nilotinib7,9,10,24. In clinical studies, 2% (11/558) of patients receiving pazopanib have experienced QT-interval prolongation. Torsade de pointes has been reported in less than 1% (2/977) of those receiving pazopanib in monotherapy studies. In a randomized clinical trial, 3 of 290 patients receiving pazopanib have had post-baseline QTc-interval values between 500 and 549 ms. None of the patients receiving placebo have had post-baseline QTc-interval values greater than or equal to 500 msec25. Despite these findings French et al26 have conducted a series of detailed experiments examining the potential cardiotoxicity of sunitinib, sorafenib and pazopanib in rodents under dobutamine challenge, and have found no biochemical, mitochondrial, heart rate, ECG or echocardiographic changes in radial and circumferential strain with 12-hour monitoring. However, in a recent clinical study evaluating the effect of repeated oral doses of pazopanib on cardiac conduction in patients with solid tumors, supratherapeutic concentrations were achieved with pazopanib administration over 8 days, which produced a concentration-dependent decrease in heart rate and a concentration-independent prolongation of the QTc interval27.
Medical therapy with beta-blockers is considered to be first-line prophylactic therapy in LQTS. According to current guidelines, there is a class I indication for beta-blockers treatment among patients with a clinical diagnosis of LQTS28,29. Different beta-blockers have similar effectiveness in preventing cardiac events in patients with LQTS30. In a different study, the authors have shown that pretreatment with diltiazem and propranolol reduce the epinephrine-induced QTc-interval prolongation31. In an animal study, the authors have put forward the beneficial effects of calcium-channel blockers in cardiac failure and LQTS30. Furthermore, proarrhythmia and QT-interval prolongation have been predominantly demonstrated with class Ia, class Ic, and class III antiarrhythmic agents32,33. Due to the above-mentioned reasons, we selected metoprolol and diltiazem in our study34.
In the present study, we evaluated pazopanib-induced QT-interval prolongation in an experimental rat model. First, we demonstrated QTc-interval prolongation in rats treated with pazopanib alone. Three hours following drug administration, the control-group rats, which had been given pazopanib and saline solution injection intraperitoneally, showed QTc-interval elongation, thus supporting the previous studies that had shown the association of TKIs and ECG abnormalities7,9. Secondly, this study showed that the QT-prolonging effect of pazopanib can be reverted by some drugs. The PZP+metoprolol and PZP+diltiazem groups did not show QTc-interval elongation compared to the control-PZP+SP group. On the other hand, QTc intervals of both groups were significantly shorter than that of the control group, while similar results were obtained in the PZP+metoprolol and PZP+diltiazem groups. Based on the study results, both metoprolol and diltiazem were considered effective in preventing the QT-prolonging effect of pazopanib.
Treatment with QT-prolonging TKIs is frequently necessary for cancer patients. The therapy risks must be weighed against benefits. Treatment must start with withdrawal of the precipitating medication. Underlying risk factors should be assessed, administration of the possible precipitating drug must be ceased and reversible conditions, such as metabolic abnormalities, must be corrected before drug initiation34. Concurrent use of multiple QT-prolonging drugs should be avoided. Patients should be advised about the proarrhythmic risk. Periodic monitoring with ECG and close follow-up are essential for early detection and treatment in high risk patients with a history of QT-interval prolongation, patients taking antiarrhythmics, or individuals with relevant preexisting disease, such as bradycardia and electrolyte imbalances35.
Our study shows some limitations. First, this is an experimental study on rats. The question of whether or not these experimental results are also relevant to humans in the clinical setting and have therapeutic implications needs to be further investigated. Second, in our study, no ventricular stability test was performed. Because of this limitation, the potential prophylactic effects of the drugs against proarrhythmia cannot be evaluated according to our results. Therefore, different studies with specific models of ventricular stability are needed for the recommendation of these drugs for prophylactic use.
A better knowledge about potential cardiac side effects of pazopanib and the identification of patients at higher risk are required to reduce cardiotoxicity with that agent. In the present study, the preventing effects of metoprolol and diltiazem on pazopanib-induced QT-interval prolongation were evaluated.
In conclusion, both metoprolol and diltiazem significantly shortened pazopanib-induced QT-interval prolongation. Although metoprolol and diltiazem prevented QT-interval prolongation, long-term studies including ventricular stability test in different models can provide evidence for the prophylactic use of those drugs in high risk patients treated with pazopanib.
Footnotes
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conception and design of the research and Obtaining financing: Akman T, Erbas O, Akman L, Yilmaz AU; Acquisition of data, Analysis and interpretation of the data and Statistical analysis: Akman T, Erbas O, Akman L; Writing of the manuscript and Critical revision of the manuscript for intellectual content: Akman T, Yilmaz AU.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any thesis or dissertation work.
References
- 1.Schutz FA, Je Y, Richards CJ, Choueiri TK. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J Clin Oncol. 2012;30(8):871–877. doi: 10.1200/JCO.2011.37.1195. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15(12):4220–4227. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- 3.Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010;28(3):475–480. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- 4.Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. 2010;60(4):222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Heeckeren WJ, Bhakta S, Ortiz J, Duerk J, Cooney MM, Dowlati A, et al. Promise of new vascular-disrupting agents balanced with cardiac toxicity: is it time for oncologists to get to know their cardiologists? J Clin Oncol. 2006;24(10):1485–1488. doi: 10.1200/JCO.2005.04.8801. [DOI] [PubMed] [Google Scholar]
- 6.Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, et al. The potential for QT prolongation and proarrhythmia by non-antiarrhythmic drugs: clinical and regulatory implications. Report on a policy conference of the European Society of Cardiology. Eur Heart J. 2000;21(15):1216–1231. doi: 10.1053/euhj.2000.2249. [DOI] [PubMed] [Google Scholar]
- 7.Strevel EL, Ing DJ, Siu LL. Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol. 2007;25(22):3362–3371. doi: 10.1200/JCO.2006.09.6925. [DOI] [PubMed] [Google Scholar]
- 8.Bello CL, Mulay M, Huang X, Patyna S, Dinolfo M, Levine S, et al. Electrocardiographic characterization of the QTc interval in patients with advanced solid tumors: pharmacokinetic- pharmacodynamic evaluation of sunitinib. Clin Cancer Res. 2009;15(22):7045–7052. doi: 10.1158/1078-0432.CCR-09-1521. [DOI] [PubMed] [Google Scholar]
- 9.Ederhy S, Cohen A, Dufaitre G, Izzedine H, Massard C, Meuleman C, et al. QT interval prolongation among patients treated with angiogenesis inhibitors. Target Oncol. 2009;4(2):89–97. doi: 10.1007/s11523-009-0111-3. [DOI] [PubMed] [Google Scholar]
- 10.Brassard M, Rondeau G. Role of vandetanib in the management of medullary thyroid cancer. Biologics. 2012;6:59–66. doi: 10.2147/BTT.S24220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou SH, Azada M, Dy J, Stiber JA. Asymptomatic profound sinus bradycardia (heart rate = 45) in non-small cell lung cancer patients treated with crizotinib. J Thorac Oncol. 2011;6(12):2135–2137. doi: 10.1097/JTO.0b013e3182307e06. [DOI] [PubMed] [Google Scholar]
- 12.The Sicilian gambit A new approach to the classification of antiarrhythmic drugs based on their actions on arrhythmogenic mechanisms. Task Force of the Working Group on Arrhythmias of the European Society of Cardiology. Circulation. 1991;84(4):1831–1851. doi: 10.1161/01.cir.84.4.1831. [DOI] [PubMed] [Google Scholar]
- 13.Ederhy S, Izzedine H, Massard C, Dufaitre G, Spano JP, Milano G, et al. Cardiac side effects of molecular targeted therapies: towards a better dialogue between oncologists and cardiologists. Crit Rev Oncol Hematol. 2011;80(3):369–379. doi: 10.1016/j.critrevonc.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Neyroud N, Tesson F, Denjoy I, Leibovici M, Donger C, Barhanin J, et al. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet. 1997;15(2):186–189. doi: 10.1038/ng0297-186. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol. 2008;51(24):2291–2300. doi: 10.1016/j.jacc.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 16.Khan IA. Clinical and therapeutic aspects of congenital and acquired long QT syndrome. Am J Med. 2002;112(1):58–66. doi: 10.1016/s0002-9343(01)01011-7. [DOI] [PubMed] [Google Scholar]
- 17.Priebe L, Beuckelmann DJ. Cell swelling causes the action potential duration to shorten in guinea-pig ventricular myocytes by activating IKATP. Pflugers Arch. 1998;436(6):894–898. doi: 10.1007/pl00008087. [DOI] [PubMed] [Google Scholar]
- 18.Tomaselli GF, Marbán E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42(2):270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 19.Sanguinetti MC. Dysfunction of delayed rectifier potassium channels in an inherited cardiac arrhythmia. Ann N Y Acad Sci. 1999;868:406–413. doi: 10.1111/j.1749-6632.1999.tb11302.x. [DOI] [PubMed] [Google Scholar]
- 20.McDermott JS, Salmen HJ, Cox BF, Gintant GA. Importance of species selection in arrythmogenic models of Q-T interval prolongation. Antimicrob Agents Chemother. 2002;46(3):938–939. doi: 10.1128/AAC.46.3.938-939.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtani H, Taninaka C, Hanada E, Kotaki H, Sato H, Sawada Y, Iga T. Comparative pharmacodynamic analysis of Q-T interval prolongation induced by the macrolides clarithromycin, roxithromycin, and azithromycin in rats. Antimicrob Agents Chemother. 2000;44(10):2630–2637. doi: 10.1128/aac.44.10.2630-2637.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusuf SW, Razeghi P, Yeh ET. The diagnosis and management of cardiovascular disease in cancer patients. Curr Probl Cardiol. 2008;33(4):163–196. doi: 10.1016/j.cpcardiol.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, et al. The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications. Report on a Policy Conference of the European Society of Cardiology. Cardiovasc Res. 2000;47(2):219–233. doi: 10.1016/s0008-6363(00)00119-x. [DOI] [PubMed] [Google Scholar]
- 24.Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, et al. Food and drug administration drug approval summary: sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007;12(1):107–113. doi: 10.1634/theoncologist.12-1-107. [DOI] [PubMed] [Google Scholar]
- 25. [Cited in 2014 Jan 10];Highlights of prescribing information. 2009. Votrient US prescribing information. Available from: http://us.gsk.com/products/assets/us_votrient.pdf.
- 26.French KJ, Coatney RW, Renninger JP, Hu CX, Gales TL, Zhao S, et al. Differences in effects on myocardium and mitochondria by angiogenic inhibitors suggest separate mechanisms of cardiotoxicity. Toxicol Pathol. 2010;38(5):691–702. doi: 10.1177/0192623310373775. [DOI] [PubMed] [Google Scholar]
- 27.Heath EI, Infante J, Lewis LD, Luu T, Stephenson J, Tan AR, et al. A randomized, double-blind, placebo-controlled study to evaluate the effect of repeated oral doses of pazopanib on cardiac conduction in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71(3):565–573. doi: 10.1007/s00280-012-2030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. European Heart Rhythm Association Heart Rhythm Society. American College of Cardiology. American Heart Association Task Force. European Society of Cardiology Committee for Practice Guidelines ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) . J Am Coll Cardiol. 2006;48(5):e247–e346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Barsheshet A, Dotsenko O, Goldenberg I. Genotype-specific risk stratification and management of patients with long QT syndrome. Ann Noninvasive Electrocardiol. 2013;18(6):499–509. doi: 10.1111/anec.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz PJ. Pharmacological and non-pharmacological management of the congenital long QT syndrome: the rationale. Pharmacol Ther. 2011;131(1):171–177. doi: 10.1016/j.pharmthera.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darbar D, Smith M, Mörike K, Roden DM. Epinephrine-induced changes in serum potassium and cardiac repolarization and effects of pretreatment with propranolol and diltiazem. Am J Cardiol. 1996;77(15):1351–1355. doi: 10.1016/s0002-9149(96)00204-4. [DOI] [PubMed] [Google Scholar]
- 32.Redfern WS, Carlsson L, Davis AS, Lynch WG, Mackenzie I, Redfern WS, et al. Relationships between preclinical cardiac electrophysiology, clinical QT-interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58(1):32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 33.Stanton MS, Prystowsky EN, Fineberg NS, Miles WM, Zipes DP, Heger JJ. Arrhythmogenic effects of antiarrhythmic drugs: a study of 506 patients treated for ventricular tachycardia or fibrillation. J Am Coll Cardiol. 1989;14(1):209–215. doi: 10.1016/0735-1097(89)90074-0. [DOI] [PubMed] [Google Scholar]
- 34.Shantsila E, Watson T, Lip GY. Drug-induced QT-interval prolongation and proarrhythmic risk in the treatment of atrial arrhythmias. Europace. 2007;9(Suppl 4):iv37–iv44. doi: 10.1093/europace/eum169. [DOI] [PubMed] [Google Scholar]
- 35.Brana I, Tabernero J. Cardiotoxicity. Ann Oncol. 2010;21(7):vii.173–vii.179. doi: 10.1093/annonc/mdq295. [DOI] [PubMed] [Google Scholar]