Abstract
Background
Parascaris univalens is an ascaridoid nematode of equids. Little is known about its epidemiology and population genetics in domestic and wild horse populations. PCR-based methods are suited to support studies in these areas, provided that reliable genetic markers are used. Recent studies have shown that mitochondrial (mt) genomic markers are applicable in such methods, but no such markers have been defined for P. univalens.
Methods
Mt genome regions were amplified from total genomic DNA isolated from P. univalens eggs by long-PCR and sequenced using Illumina technology. The mt genome was assembled and annotated using an established bioinformatic pipeline. Amino acid sequences inferred from all protein-encoding genes of the mt genomes were compared with those from other ascaridoid nematodes, and concatenated sequences were subjected to phylogenetic analysis by Bayesian inference.
Results
The circular mt genome was 13,920 bp in length and contained two ribosomal RNA, 12 protein-coding and 22 transfer RNA genes, consistent with those of other ascaridoids. Phylogenetic analysis of the concatenated amino acid sequence data for the 12 mt proteins showed that P. univalens was most closely related to Ascaris lumbricoides and A. suum, to the exclusion of other ascaridoids.
Conclusions
This mt genome representing P. univalens now provides a rich source of genetic markers for future studies of the genetics and epidemiology of this parasite and its congener, P. equorum. This focus is significant, given that there is no published information on the specific prevalence and distribution of P. univalens infection in domestic and wild horse populations.
Electronic supplementary material
The online version of this article (doi:10.1186/1756-3305-7-428) contains supplementary material, which is available to authorized users.
Keywords: Parascaris univalens (Nematoda: Ascaridida), Mitochondrial genome, Genetic markers, Epidemiology, Population genetics
Background
Parasitic worms of the gastrointestinal tracts of equids cause diseases of major veterinary importance. For instance, Parascaris is a large, parasitic nematode of the small intestine and has a direct life cycle [1, 2]. Infective eggs (each containing a third-stage larva, L3) are ingested by the equid, hatch in the intestine, L3s undergo liver and lung (hepato-pulmonary) migration, moult to fourth-stage larvae, are swallowed and then establish in the small intestine where they mature, mate and reproduce; female worms lay millions of eggs, which pass in the faeces into the environment [1, 2]. Infection with large numbers of adult worms, particularly in foals, can cause colic associated with enteritis and/or intestinal impaction/obstruction, weight loss and anorexia [2, 3]. Migrating larval stages can also cause hepatitis and pneumonitis, associated respiratory disorders (mild signs of coughing and nasal discharge) and secondary bacterial infections [2, 3]. Foals are particularly susceptible to infection and are clinically most affected, but immunity usually develops by the age of 6–12 months [2], such that infections are eliminated from older horses, unless there is a problem with immunosuppression or immunodeficiency.
Parascariasis, the disease caused by Parascaris, is treated using anthelmintics such as benzimidazoles (fenbendazole, febantel, oxfendazole and oxibendazole), macrocyclic lactones (including ivermectin and moxidectin), piperazine or pyrantel. As a high frequency of anthelmintic treatment is considered to be a key factor for the selection of drug resistance, this approach is not sustainable in relation to drug efficacy. Accordingly, in the past decade resistance against commonly used anthelmintics [4] has been reported in Parascaris [5–13], but it is not yet clear which species of Parascaris is involved in this resistance.
Presently, two species of Parascaris are recognised, namely P. univalens and P. equorum. Classical, cytological techniques can be used to identify P. univalens and distinguish it from P. equorum; the former nematode has two chromosomes, whereas the latter has four [14–18]. Chromosomal banding patterns studied during the gonial metaphase have shown that P. univalens chromosomes contain only terminal heterochromatin, whereas P. equorum chromosomes also contain intercalary heterochromatin [15]. Although chromosomal differences allow their specific identification, in most, if not all, parasitological and epidemiological studies of equine parasites conducted to date, the specific status of Parascaris was not verified. The assumption has been that P. equorum is the only or the dominant species of Parascaris. However, some recent results from a cytological study of Parascaris from domestic horses in northern Germany have shown that P. univalens has a higher prevalence than previously expected (G. von Samson-Himmelstjerna et al., unpublished findings). Indeed, P. equorum was hardly found. This raises questions about the prevalence and clinical relevance of P. univalens as well as drug resistance in this species in countries around the world.
While cytological analysis is a useful method for specific identification and differentiation, it would be desirable to have available genomic markers for PCR-based analyses of genetic variation within Parascaris (at any stage of development) as well as the specific diagnosis of infection. Recent studies have shown that mitochondrial (mt) genomic markers are suited for this purpose [19–22]. Although mt genomes have been published for numerous ascaridoids, including A. suum, A. lumbricoides, Ascaridia columbae, As. galli, Baylisascaris procyonis, B. transfuga, B. ailuri, B. schroederi, Anisakis simplex, Contracaecum osculatum, C. rudolphii B, Cucullanus robustus, Toxocara canis, T. cati, T. malaysiensis and Toxascaris leonina [23–33], this is not the case for Parascaris. Therefore, defining a mt genome for P. univalens could provide a rich source of markers to underpin detailed investigations of the genetic composition of Parascaris populations in domestic and wild horses around the world. The aim of the present study was to utilize a next-generation sequencing-based approach for the characterisation of the mt genome of P. univalens from Switzerland as a foundation for such future investigations.
Methods
Parasite and genomic DNA isolation
In 1999, eggs of P. univalens were collected from an adult female specimen of Parascaris from the small intestine from a domesticated horse. This horse was slaughtered for meat in an approved abattoir in Fribourg, Switzerland, and the worms were provided to one of the authors by a registered veterinarian. This work was approved under the Scientific Procedures Premises License for the Faculty of Science, University of Fribourg. The specific identity of the worm was based on cytological examination [15] of the eggs taken from the uterus of this female worm. Total genomic DNA was purified from eggs by sodium dodecyl-sulphate/proteinase K treatment, phenol/chloroform extraction and ethanol precipitation and purified over a spin column (Wizard Clean-Up, Promega) [34].
Long-PCR, sequencing, mt genome assembly and annotation
Using each of the primer pairs MH39F-MH38R and MH5F-MH40R [35], two regions of the entire mt genome (of ~5 and 10 kb, respectively) were amplified by long-range PCR (BD Advantage 2, BD Biosciences) from 50 ng of genomic DNA [35]. The cycling conditions (in a 2720 thermal cycler, Applied Biosystems) were: one cycle at 95°C for 1 min (initial denaturation), followed by 35 cycles of 95°C for 15 s (denaturation), 53°C for 15 s (~5 kb region) or 55 for 15 s (~10 kb region) (annealing) and 62°C for 5 min (~5 kb region) or 68°C for 6 min (~10 kb region) (extension), followed by a final elongation at 62°C or 68°C for 5 min [35]. Amplicons were treated with shrimp alkaline phosphatase and exonuclease I [36], and DNA was quantified spectrophotometrically.
Amplicons were sequenced as part of a multi-species multi-sample Illumina HiSeq run [37], yielding partial contigs and also as pooled amplicons on a dedicated MiSeq run, yielding a complete mt genome. For the MiSeq run following agarose electrophoretic analysis, each amplicon was quantified fluorometrically using a Qubit 2.0 fluorometer (Invitrogen), pooled in equimolar ratios and prepared for paired-end sequencing on 1/10th Illumina MiSeq flow cell (v.3.0 chemistry; 2x 300 bp) using TruSeq nano DNA preparation kits [38]. Partial contigs from a previous mixed-sample HiSeq run (see [37] for details), including the present target amplicons, were used to map reference assemblies. Briefly, Trimmomatic [39] was used to trim the Illumina HiSeq reads. All complete nematode mt genomes available from the GenBank database were downloaded and protein-coding sequences extracted using Biopython [40]. Reads were interrogated using the GenBank protein sequences (each gene separately) employing usearch v.6.1 [41]. Matches were assembled into contigs (separately for each gene) using Mira v 4. [42]; these contigs were used as starters for mapping-extension using usearch to find reads, and Mira to enable assembly and further extension. The program CAP3 [43] was used to join contigs together. MiSeq reads were trimmed using the program Geneious (v.6.1.8, Biomatters) from both ends of each read, allowing a maximum of one ambiguous base and an error probability limit of 0.05, thus maximising the sequence length, whilst minimising the overall error to no more than 1 uncalled base. Previously, assembled contigs (above) were used to seed a high stringency mapping assembly in Geneious; one iteration at no mismatch, with no gaps allowed. Once matched to P. univalens, contigs were extended for a further 25 iterations using default (low sensitivity) assembly options, with no gaps allowed, minimum read overlap of 25 bp, minimum overlap identity of 90% and maximum mismatches set to 2% per read, to allow for assembly errors in the reference contigs. Resultant contigs were examined for overlapping regions and assembled into a complete mt genome. The mt genome (GenBank accession no. KM067271) was annotated using an established bioinformatic pipeline [22].
Analyses of sequence data
The sequence was compared with the mt genome sequences of A. suum using the program Clustal X [44], and the circular map was drawn using the program MacVector v.9.5 (http://www.macvector.com/index.html). Amino acid sequences, translation initiation and termination codons, codon usage and transfer RNA (tRNA or trn) genes and non-coding regions were predicted using established approaches [22]. The structure and organisation of the mt genome of P. univalens was then compared with those of other ascaridoid nematodes A. suum (NC_001327); A. lumbricoides (NC_016198); As. columbae (NC_021643); As. galli (NC_021642); An. simplex (KC965056); B. procyonis (NC_016200); B. transfuga (NC_015924); B. ailuri (NC_015925); B. schroederi (NC_015927); C. osculatum (KC965057); C. rudolphii B (NC_014870); Cu. robustus (NC_016128); T. canis (NC_010690); T. cati (NC_010773); T. malaysiensis (NC_010527); To. leonina (NC_023504) [23–33].
Phylogenetic analysis of concatenated amino acid sequence datasets
For phylogenetic analysis, amino acid sequences conceptually translated from individual protein-encoding genes of P. univalens and 16 reference mt genomes (Table 1) were aligned using MUSCLE, ensuring accurate alignment of homologous characters. Finally, all aligned blocks of sequences were concatenated and the entire alignment verified again by eye. These data were then subjected to phylogenetic analysis using Bayesian inference (BI). BI analysis was conducted using MrBayes v.3.2.2 [45] with a mixed amino acid substitution model [46] using four rate categories approximating a Γ distribution, four chains and 200,000 generations, sampling every 100th generation; the first 200 generations were removed from the analysis as burn-in.
Table 1.
Details of the mitochondrial genome sequence determined in this study and reference sequences used for analyses
| Species | Accession No. | Mt genome size | Host and geographical origins | Reference |
|---|---|---|---|---|
| Parascaris univalens | KM067271 | 13920 | Horse (Equus caballus), Switzerland | This study |
| Anisakis simplex (s.s.) | KC965056 | 13926 | Fish (Clupea harengus), Poland | [32] |
| Ascaridia columbae | NC_021643 | 13931 | Pigeon (Columba livia), China | [30] |
| Ascaridia galli | NC_021642 | 13977 | Chicken (Gallus gallus), China | [30] |
| Ascaris lumbricoides | HQ704900 | 14303 | Human (Homo sapiens), China | [33] |
| Ascaris suum | HQ704901 | 14311 | Pig (Sus scrofa), China | [33] |
| Contracaecum osculatum | KC965057 | 13823 | Fish, (Gadus morhua), Poland | [32] |
| Contracaecum rudolphi B | FJ905109 | 14022 | Cormorant (Phalacrocorax carbo), China | [29] |
| Cucullanus robustus | NC_016128 | 13972 | Conger eel (Conger myriaster), Korea | [26] |
| Baylisascaris ailuri | NC_015925 | 14657 | Red panda (Ailurus fulgens), China | [27] |
| Baylisascaris procyonis | JF951366 | 14781 | Raccoon (Procyon lotor), China | [28] |
| Baylisascaris schroederi | NC_015927 | 14778 | Giant panda (Ailuropoda melanoleuca), China | [27] |
| Baylisascaris transfuga | NC_015924 | 14898 | Polar bear (Ursus maritimus), China | [27] |
| Toxocara canis | NC_010690 | 14322 | Dog (Canis lupus), China | [25] |
| Toxocara cati | NC_010773 | 14029 | Cat (Felis cati), China | [25] |
| Toxocara malaysiensis | NC_010527 | 14266 | Cat (Felis cati), China | [25] |
| Toxascaris leonina | NC_023504 | 14310 | Dog (Canis lupus), Australia | [31] |
Results and discussion
Features and organisation of the mt genome
The circular mt genome of P. univalens (Figure 1) was 13,920 bp in length (GenBank accession number KM067271) and contained 36 genes: 12 protein-coding genes (adenosine triphosphatase subunit 6 [atp6], the cytochrome c oxidase subunits 1, 2 and 3 [cox1-cox3], cytochrome b (cytb) and the nicotinamide dehydrogenase subunits 1–6 [nad1-nad6 and nad4L]), 22 tRNA genes (two coding for leucine and two coding for serine) and the small [rrnS] and large [rrnL] subunits of rRNA. Each protein-coding gene had an open reading frame (ORF), and all genes were located on the same strand and transcribed in the same direction (5′ to 3′) (Figure 1), consistent with the mt genomes of other secernentean nematodes characterised to date [20–22]. The gene arrangement (GA) for the mt genome of P. univalens was consistent with GA2 [47]. This gene arrangement has been reported previously for other ascaridoids; also, like other members of this nematode group, the AT-rich region for P. univalens was located between rrnS and nad1, flanked (5′) by the genes trnS (UCN) and (3′) by trnN and trnY (Figure 1) [20].
Figure 1.
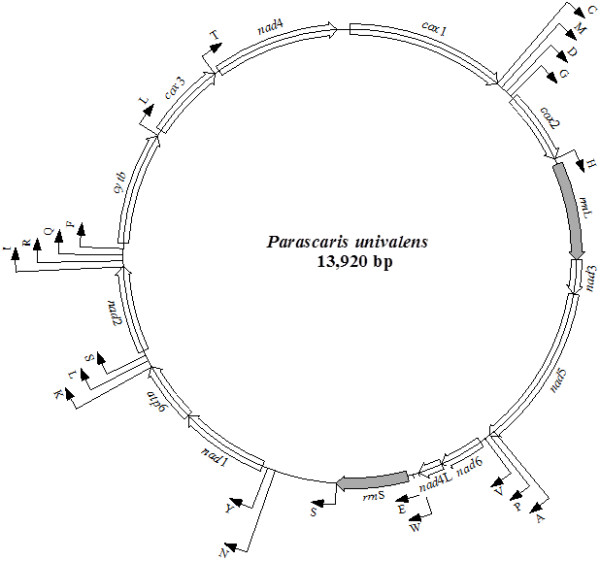
Schematic representation of the circular mt genome of Parascaris univalens. All 12 protein-coding genes and the large and small subunits of the rRNA genes are indicated in italics. Each tRNA gene is identified by its one letter abbreviation. The direction of transcription is indicated by arrows.
Nucleotide contents and codon usage
The coding strand of the mt genome sequence of P. univalens consisted of 19.1% A, 7.5% C, 22.12% G and 51.2% T (Table 2). Though AT-rich (78.1% AT), the sequence had a slightly lower AT content than has been reported for other nematode species (~70-80%; [20]); the AT-rich region was 828 bp in length (Table 2). In the protein-coding genes, the AT-contents varied from 65.5% (cox1) to 76.0% (nad4L), with the overall ranking (increasing richness) of cox1, cox2, cox3, cytb, nad3, nad1, atp6, nad5, nad4, nad6, nad2, followed by nad4L. To date, studies of secernentean nematodes [20, 21] have shown that the cytochrome c oxidase genes tend to have the lowest AT-contents. Although the overall AT-content of the mt genome sequence of P. univalens was ~0.65-2.25% less than that of other ascaridoids studied to date [24, 25, 27, 28], there was no appreciable impact on the relative amino acid codon usage in the protein-coding genes. As has been reported for other secernentean nematodes [20, 21], the usage in the protein-coding genes favoured codons with many A or T residues (e.g., 14.1% were TTT [phenylalanine]) over those with many C or G residues (e.g., none were CGA [arginine]).
Table 2.
Nucleotide composition 1 (%) for the entire or regions/genes of the mitochondrial genome of Parascaris univalens
| Mitochondrial gene/region | Length (bp) | A | C | G | T | AT |
|---|---|---|---|---|---|---|
| atp6 | 604 | 17.7 | 7.1 | 22.7 | 52.5 | 70.2 |
| cox1 | 1,570 | 18.9 | 10.1 | 24.34 | 46.6 | 65.5 |
| cox2 | 724 | 21.0 | 8.6 | 25.7 | 44.7 | 65.7 |
| cox3 | 772 | 18.1 | 9.5 | 21.4 | 51.0 | 69.1 |
| cytb | 1,108 | 19.0 | 9.0 | 21.8 | 50.1 | 69.1 |
| nad1 | 880 | 19.3 | 8.3 | 21.9 | 50.5 | 69.8 |
| nad2 | 859 | 19.3 | 5.7 | 19.8 | 55.2 | 74.5 |
| nad3 | 340 | 17.1 | 4.1 | 26.2 | 52.6 | 69.7 |
| nad4 | 1,240 | 18.9 | 10.0 | 19.0 | 52.1 | 71.0 |
| nad4L | 238 | 21.8 | 2.9 | 21.0 | 54.2 | 76.0 |
| nad5 | 1,540 | 19.7 | 7.3 | 22.3 | 50.5 | 70.2 |
| nad6 | 439 | 18.0 | 7.7 | 19.6 | 54.7 | 72.7 |
| rrnL | 961 | 26.4 | 6.3 | 19.5 | 47.9 | 74.3 |
| rrnS | 702 | 30.5 | 9.5 | 20.8 | 39.2 | 69.7 |
| AT-rich | 828 | 38.6 | 9.9 | 11.8 | 39.5 | 78.1 |
| Genome | 13,920 | 21.7 | 8.0 | 21.4 | 48.9 | 70.6 |
1Lengths and A + T contents (%) of the sequences of the 12 protein-coding genes, the large and small ribosomal RNA genes, the AT-rich region and of the entire mitochondrial genome of P. univalens.
All but the two serine tRNAs (AGN and UCN) had a predicted secondary structure containing a DHU arm and loop and a TV-replacement loop instead of the TψC arm and loop. As reported previously for secernentean nematodes [20], the two serine tRNAs each contained the TψC arm and loop but lacked the DHU arm and loop. The rrnL and rrnS genes were 961 and 702 bp in length. The AT-content of the sequences of rrnL, rrnS and the AT-rich (“control”) region were 74.3%, 69.7% and 78.1%, respectively. The relatively low AT-richness exhibited in the mt genome of P. univalens was pronounced for the rRNA genes. The AT-content of the rrnL sequence was 2.5% less compared with, for example, A. suum (76.8%) [23]. The AT-content of the rrnS sequence of P. univalens was 2.3% less than that reported for A. suum (71.9%) [23].
Comparative analysis with other ascaridoids
Pairwise comparisons were made among the amino acid sequences inferred from individual protein-coding genes and the nucleotide sequences of the rRNA genes in the P. univalens mt genome with those representing 16 other ascaridoid nematodes (Table 3). The amino acid sequence similarities in individual inferred proteins ranged from 76.8% (CYTB) to 96% (COX3) between P. univalens and A. suum, and from 76.7% (NAD2) to 92% (COX2) between P. univalens and An. simplex. The amino acid sequence similarities between P. univalens and individual species of ascaridoids included here (A. suum, A. lumbricoides, Ascaridia columbae, As. galli, B. procyonis, B. transfuga, B. ailuri, B. schroederi, An. simplex, C. osculatum, C. rudolphii B, Cu. robustus T. canis, T. cati, T. malaysiensis and To. leonina; cf. Table 1) ranged from 41.8% (ATP6) to 97.5% (COX2), respectively. The nucleotide sequence similarities (Table 3) in rrnS and rrnL were 60.4-84.5% and 54.6-84.9% between P. univalens and other ascaridoids, respectively (Table 3). Additional file 1: Table S1 lists initiation and termination codons for protein-coding genes as well as the lengths of the amino acid sequences encoded in P. univalens compared with other ascaridoids (cf. Table 1).
Table 3.
Pairwise comparison 1 of the amino acid (aa) sequences of the 12 protein-encoding mitochondrial genes and in the nucleotide sequence of each of the two ribosomal genes
| aa/rRNA | Al | As | Asc | Asg | Ans | Ba | Bp | Bs | Bt | Co | Cr | Cur | Tcan | Tcat | Tm | Tol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATP6 | 83.42 | 84.42 | 50.75 | 48.74 | 78.89 | 84.42 | 84.92 | 82.91 | 83.92 | 77.89 | 79.39 | 58.8 | 81.91 | 80.4 | 80.9 | 81.91 |
| COX1 | 93.54 | 92.97 | 92.59 | 92.78 | 91.44 | 93.73 | 92.97 | 93.92 | 93.92 | 92.40 | 92.5 | 85.25 | 84.41 | 83.65 | 86.69 | 90.11 |
| COX2 | 91.18 | 91.18 | 81.51 | 84.03 | 92.02 | 91.18 | 91.18 | 91.60 | 91.60 | 91.18 | 92.2 | 77.49 | 89.08 | 89.50 | 97.48 | 97.48 |
| COX3 | 90.00 | 96.00 | 77.00 | 79.00 | 91.00 | 88.00 | 90.00 | 90.00 | 90.00 | 91.00 | 89.8 | 69.41 | 81.00 | 90.00 | 90.00 | 96.00 |
| CYTB | 80.80 | 76.80 | 74.13 | 71.20 | 78.13 | 79.47 | 78.40 | 78.13 | 77.87 | 77.60 | 73.35 | 68.95 | 60.00 | 60.53 | 90.93 | 91.47 |
| NAD1 | 89.00 | 94.00 | 71.00 | 73.00 | 88.00 | 88.00 | 84.00 | 84.00 | 88.00 | 88.00 | 85.86 | 70 | 84.00 | 86.00 | 84.00 | 94.00 |
| NAD2 | 74.91 | 77.03 | 53.71 | 54.06 | 76.68 | 73.85 | 77.39 | 78.80 | 76.68 | 77.74 | 69.04 | 41.78 | 72.79 | 84.10 | 89.75 | 78.45 |
| NAD3 | 88.29 | 86.49 | 64.86 | 66.67 | 86.49 | 86.49 | 89.19 | 88.29 | 87.39 | 86.49 | 81.08 | 64.54 | 85.59 | 88.29 | 88.29 | 88.29 |
| NAD4 | 82.48 | 82.73 | 82.00 | 81.75 | 80.78 | 81.02 | 76.89 | 82.48 | 80.78 | 82.00 | 73.59 | 63.57 | 67.40 | 67.64 | 80.05 | 81.51 |
| NAD4L | 89.61 | 88.31 | 58.44 | 54.55 | 84.42 | 89.61 | 90.91 | 90.91 | 90.91 | 89.61 | 88.31 | 68.83 | 87.01 | 84.42 | 85.71 | 83.12 |
| NAD5 | 83.00 | 82.00 | 66.00 | 66.00 | 80.00 | 84.00 | 85.00 | 85.00 | 83.00 | 83.00 | 74.19 | 62.62 | 80.00 | 90.00 | 92.00 | 82.00 |
| NAD6 | 79.00 | 80.00 | 52.00 | 52.00 | 70.00 | 79.00 | 78.00 | 77.00 | 77.00 | 72.00 | 68.05 | 59.72 | 74.00 | 76.00 | 75.00 | 75.00 |
| rrnL | 84.91 | 84.79 | 54.66 | 60.46 | 71.40 | 78.04 | 78.88 | 78.25 | 77.73 | 69.00 | 69.3 | 59.6 | 72.51 | 70.99 | 72.36 | 78.85 |
| rrnS | 84.00 | 84.45 | 63.39 | 64.05 | 76.14 | 82.45 | 83.71 | 81.41 | 82.42 | 74.00 | 75.9 | 60.4 | 75.32 | 75.14 | 75.72 | 82.71 |
1Percentage of similarity in the amino acid sequences inferred from the 12 protein-coding genes and in the nucleotide sequence of each of the two ribosomal genes (rrnL and rrnS) upon pairwise comparison between Parascaris univalens and selected ascaridoid nematodes, including Ascaris lumbricoides (Al), Ascaris suum (As), Ascaridia columbae (Asc), Ascaridia galli (Asg), Anisakis simplex (Ans), Baylisascaris ailuri (Ba), Baylisascaris procyonis (Bp), Baylisascaris schroederi (Bs), Baylisascaris transfuga (Bt), Contracaecum osculatum (Co), Contracaecum rudolphii B (Cr), Cucullanus robustus (Cur), Toxocara canis (Tcan), Toxocara cati (Tcat), Toxocara malaysiensis (Tmal), Toxascaris leonina (Tol).
Subsequently, we undertook a phylogenetic analysis of the concatenated amino acid sequence data set representing P. univalens and reference sequences for selected ascaridoids (Figure 2). This analysis showed a robust estimate of interrelationships of P. univalens with selected ascaridoid nematodes, with each node strongly supported by a posterior probability (pp) value of 1.00 (see Figure 2). The analysis revealed that P. univalens grouped separately from A. suum and A. lumbricoides with absolute support (Figure 2). Within the monophyletic clade, As. galli and As. columbae grouped together to the exclusion of other species, which grouped together – here, T. canis, T. cati and T. malaysiensis grouped together, as did B. ailuri, B. procyonis, B. schroederi and B. transfuga, to the exclusion of To. leonina; C. osculatum and C. rudolphii B grouped together, to the exclusion of An. simplex.
Figure 2.
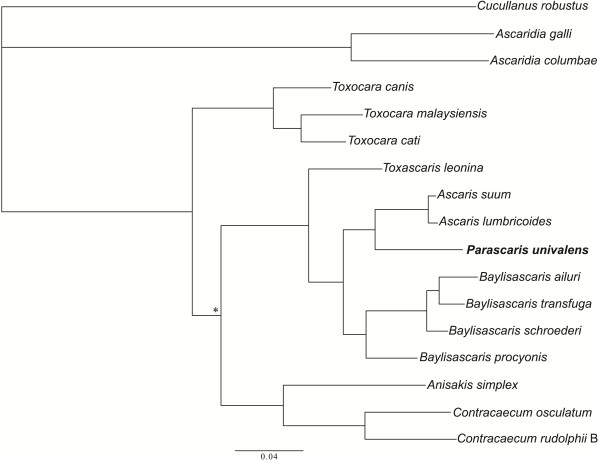
Genetic relationship of Parascaris univalens with other ascaridoid nematodes. Concatenated amino acid sequence data for all protein-encoding mitochondrial genes of P. univalens (bold) and other ascaridoids were subjected to Bayesian inference analysis. All nodes are supported by pp = 1.00, except that marked with an asterisk (pp = 0.98).
Significance and implications
There is considerable significance in the use of mt DNA markers for investigating the genetic make-up of Parascaris populations, particularly given that there are no morphological features that allow the specific identification of most developmental stages. In nematodes, mt DNA is proposed to be maternally inherited, and is usually more variable in sequence within a species than nuclear ribosomal DNA [19, 20]. The complete mt genome of P. univalens characterised here provides a likely foundation for assessing the extent of genetic variation within and between P. univalens and P. equorum populations, and might allow the definition of markers for specific PCR-based identification/differentiation of these nematodes at any stage of development.
Oligonucleotide primers could be selectively designed to conserved regions flanking “variable tracts” in the mt genome considered to be most informative following mt sequencing from a relatively small number of individuals identified as P. univalens and P. equorum by cytological analysis (cf. [15]). Using such primers, PCR-coupled single-strand conformation polymorphism (SSCP) analysis [34] might be employed to screen large numbers of Parascaris individuals representing different populations, and samples representing the spectrum of haplotypic variability could then be selected for subsequent sequencing and analyses. As the two internal transcribed spacers (ITS-1 and ITS-2) of nuclear ribosomal DNA usually provide species-level identification of closely and distantly related ascaridoids [19, 48–50], comparative genetic analyses using these spacers would also be informative. Such approaches have been applied, for example, to study the genetic make-up of the Ascaris populations in humans and pigs in six provinces in China [51, 52]; like A. suum and A. lumbricoides, P. univalens and P. equorum are recognised to be very closely related taxa [53, 54], and based on early observations, there is some evidence that P. univalens and P. equorum might hybridise but produce infertile offspring [15]. Having available reliable markers and molecular tools might not only enable studies of the biology and epidemiology of these parasites, but also investigations into anthelmintic resistance in P. univalens and P. equorum, in combination with conventional faecal egg count reduction testing (FECRT) [55, 56], as well as single nucleotide polymorphism (SNP) analyses of genes inferred to be involved in such resistance [57, 58].
Conclusions
The mt genome of P. univalens provides a rich source of genetic markers for future studies of the population genetics and epidemiology of Parascaris in horses. It sets the scene particularly for large-scale genetic studies of Parascaris from domestic and wild horses around the world. This focus is significant, given that there is no published information on the prevalence and distribution of P. univalens in domestic and wild horses or anthelmintic resistance in this species.
Electronic supplementary material
Additional file 1: Table S1: Comparison of the mt genome of Parascaris univalens with those of other ascaridoid nematodes. (DOCX 39 KB)
Acknowledgements
We thank Kevin Hopkins (NHM) for running the MiSeq samples. This study was supported by Early Career Researcher (ECR) and Collaborative Research grants from The University of Melbourne (AJ) and the Australian Research Council (RBG), BBSRC (BB/H023534/1; DTJL and PGF) and the NHM Disease Initiative (DTJL and AGB). RBG thanks the Alexander von Humboldt Foundation, Germany, for supporting academic visits to Berlin to develop aspects of this and other papers with GvSH.
Abbreviations
- atp6
adenosine triphosphatase subunit 6
- BI
Bayesian inference
- cox
cytochrome c subunit
- cytb
cytochrome b subunit
- ITS-2
second internal transcribed spacer
- mt
mitochondrial
- nad
nicotinamide dehydrogenase subunit
- ORF
open reading frame
- rDNA
nuclear ribosomal DNA
- rrnL
large subunit of the ribosomal gene
- rrnS
small subunit of the ribosomal gene
- tRNA
transfer RNA.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FM undertook the karyotyping of the nematode used in this study and identified it as P. univalens. AJ produced the amplicons, DJTL carried out the sequencing. NM undertook bioinformatic analyses, and AJ and RBG interpreted the data. RBG, AJ and GvSH wrote the manuscript with input from all other coauthors. All authors read commented on and approved the final version of the manuscript.
Contributor Information
Abdul Jabbar, Email: jabbara@unimelb.edu.au.
D Timothy J Littlewood, Email: t.littlewood@nhm.ac.uk.
Namitha Mohandas, Email: namigrt@gmail.com.
Andrew G Briscoe, Email: a.briscoe@nhm.ac.uk.
Peter G Foster, Email: p.foster@nhm.ac.uk.
Fritz Müller, Email: fritz.mueller@unifr.ch.
Georg von Samson-Himmelstjerna, Email: samson.georg@fu-berlin.de.
Aaron R Jex, Email: ajex@unimelb.edu.au.
Robin B Gasser, Email: robinbg@unimelb.edu.au.
References
- 1.Clayton HM, Duncan JL. The migration and development of Parascaris equorum in the horse. Int J Parasitol. 1979;9:285–292. doi: 10.1016/0020-7519(79)90076-6. [DOI] [PubMed] [Google Scholar]
- 2.Clayton HM. Ascarids: recent advances. Vet Clin North Am Equine Pract. 1986;2:313–328. doi: 10.1016/s0749-0739(17)30718-6. [DOI] [PubMed] [Google Scholar]
- 3.Orr JP. Perforated duodenal ulcer in a foal. Vet Rec. 1972;90:571. doi: 10.1136/vr.90.20.571. [DOI] [PubMed] [Google Scholar]
- 4.Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Boersema JH, Eysker M, Nas JW. Apparent resistance of Parascaris equorum to macrocylic lactones. Vet Rec. 2002;150:279–281. doi: 10.1136/vr.150.9.279. [DOI] [PubMed] [Google Scholar]
- 6.Craig TM, Diamond PL, Ferwerda NS, Thompson JA. Evidence of ivermectin resistance by Parascaris equorum on a Texas horse farm. J Eq Vet Sci. 2007;27:67–71. doi: 10.1016/j.jevs.2006.12.002. [DOI] [Google Scholar]
- 7.Schougaard H, Nielsen MK. Apparent ivermectin resistance of Parascaris equorum in foals in Denmark. Vet Rec. 2007;160:439–440. doi: 10.1136/vr.160.13.439. [DOI] [PubMed] [Google Scholar]
- 8.von Samson-Himmelstjerna G, Fritzen B, Demeler J, Schürmann S, Rohn K, Schnieder T, Epe C. Cases of reduced cyathostomin egg-reappearance period and failure of Parascaris equorum egg count reduction following ivermectin treatment as well as survey on pyrantel efficacy on German horse farms. Vet Parasitol. 2007;144:74–80. doi: 10.1016/j.vetpar.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 9.Slocombe JO, de Gannes RV, Lake MC. Macrocyclic lactone resistant Parascaris equorum on stud farms in Canada and effectiveness of fenbendazole and pyrantel pamoate. Vet Parasitol. 2007;145:371–376. doi: 10.1016/j.vetpar.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Lyons ET, Tolliver SC, Ionita M, Collins SS. Evaluation of parasiticidal activity of fenbendazole, ivermectin, oxibendazole, and pyrantel pamoate in horse foals with emphasis on ascarids (Parascaris equorum) in field studies on five farms in Central Kentucky in 2007. Parasitol Res. 2008;103:287–291. doi: 10.1007/s00436-008-0966-8. [DOI] [PubMed] [Google Scholar]
- 11.Laugier C, Sevin C, Ménard S, Maillard K. Prevalence of Parascaris equorum infection in foals on French stud farms and first report of ivermectin-resistant P. equorum populations in France. Vet Parasitol. 2012;188:185–189. doi: 10.1016/j.vetpar.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Bishop RM, Scott I, Gee EK, Rogers CW, Pomroy WE, Mayhew IG. Sub-optimal efficacy of ivermectin against Parascaris equorum in foals on three thoroughbred stud farms in the Manawatu region of New Zealand. NZ Vet J. 2014;62:91–95. doi: 10.1080/00480169.2013.843146. [DOI] [PubMed] [Google Scholar]
- 13.Relf VE, Lester HE, Morgan ER, Hodgkinson JE, Matthews JB. Anthelmintic efficacy on UK thoroughbred stud farms. Int J Parasitol. 2014;44:507–514. doi: 10.1016/j.ijpara.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Biocca E, Nascetti G, Iori A, Costantini R, Bullini L. Descrizione di Parascaris univalens, parassita degli equini, e suo differenziamento da Parascaris equorum. Acc Naz Lincei Rend Cl Sc Fis Mat Nat Ser VIII. 1978;65:133–141. [Google Scholar]
- 15.Goday C, Pimpinelli S. Chromosome organization and heterochromatin elimination in Parascaris. Science. 1984;224:411–413. doi: 10.1126/science.224.4647.411. [DOI] [PubMed] [Google Scholar]
- 16.Goday C, Pimpinelli S. Cytological analysis of chromosomes in the two species Parascaris univalens and P. equorum. Chromosoma. 1986;94:1–10. doi: 10.1007/BF00293524. [DOI] [Google Scholar]
- 17.Bullini L, Nascetti G, Ciafre S, Rumore F, Biocca E. Ricerche cariologiche ed elettroforetiche su Parascaris univalens e Parascaris equorum. Acc Naz Lincei Rend Cl Sc Fis Mat Nat. 1978;65:151–156. [Google Scholar]
- 18.Boveri T. Über Differenzierung der Zellkerne während der Furchung des Eis von Ascaris megacephala. Anat Anz. 1887;2:688–693. [Google Scholar]
- 19.Gasser RB. Molecular tools – advances, opportunities and prospects. Vet Parasitol. 2006;136:69–89. doi: 10.1016/j.vetpar.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Hu M, Gasser RB. Mitochondrial genomes of parasitic nematodes - progress and perspectives. Trends Parasitol. 2006;22:78–84. doi: 10.1016/j.pt.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Jex AR, Littlewood DT, Gasser RB. Towards next-generation sequencing of mitochondrial genomes - focus on parasitic worms of animals and biotechnological implications. Biotechnol Adv. 2010;28:151–159. doi: 10.1016/j.biotechadv.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Jex AR, Hall RS, Littlewood DT, Gasser RB. An integrated pipeline for next-generation sequencing and annotation of mitochondrial genomes. Nucleic Acids Res. 2010;38:522–533. doi: 10.1093/nar/gkp883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okimoto R, Macfarlane JL, Clary DO, Wolstenholme DR. The mitochondrial genomes of two nematodes, Caenorhabditis elegans and Ascaris suum. Genetics. 1992;130:471–498. doi: 10.1093/genetics/130.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jex AR, Waeschenbach A, Littlewood DT, Hu M, Gasser RB. The mitochondrial genome of Toxocara canis. PLoS Negl Trop Dis. 2008;2:e273. doi: 10.1371/journal.pntd.0000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li MW, Lin RQ, Song HQ, Wu XY, Zhu XQ. The complete mitochondrial genomes for three Toxocara species of human and animal health significance. BMC Genomics. 2008;9:224. doi: 10.1186/1471-2164-9-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JK, Sultana T, Lee SH, Kang S, Kim HK, Min GS, Eom KS, Nadler SA. Monophyly of clade III nematodes is not supported by phylogenetic analysis of complete mitochondrial genome sequences. BMC Genomics. 2011;12:392. doi: 10.1186/1471-2164-12-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Y, Zhang Z, Wang C, Lan J, Li Y, Chen Z, Fu Y, Nie H, Yan N, Gu X, Wang S, Peng X, Yang G. Complete mitochondrial genomes of Baylisascaris schroederi, Baylisascaris ailuri and Baylisascaris transfuga from giant panda, red panda and polar bear. Gene. 2011;482:59–67. doi: 10.1016/j.gene.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Xie Y, Zhang Z, Niu L, Wang Q, Wang C, Lan J, Deng J, Fu Y, Nie H, Yan N, Yang D, Hao G, Gu X, Wang S, Peng X, Yang G. The mitochondrial genome of Baylisascaris procyonis. PLoS One. 2011;6:e27066. doi: 10.1371/journal.pone.0027066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin RQ, Liu GH, Zhang Y, D’Amelio S, Zhou DH, Yuan ZG, Zou FC, Song HQ, Zhu XQ. Contracaecum rudolphii B: gene content, arrangement and composition of its complete mitochondrial genome compared with Anisakis simplex s.l. Exp Parasitol. 2012;130:135–140. doi: 10.1016/j.exppara.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Liu GH, Shao R, Li JY, Zhou DH, Li H, Zhu XQ. The complete mitochondrial genomes of three parasitic nematodes of birds: a unique gene order and insights into nematode phylogeny. BMC Genomics. 2013;14:414. doi: 10.1186/1471-2164-14-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu GH, Zhou DH, Zhao L, Xiong RC, Liang JY, Zhu XQ. The complete mitochondrial genome of Toxascaris leonina: comparison with other closely related species and phylogenetic implications. Infect Genet Evol. 2014;21:329–333. doi: 10.1016/j.meegid.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Mohandas N, Jabbar A, Podolska M, Zhu XQ, Littlewood DT, Jex AR, Gasser RB. Mitochondrial genomes of Anisakis simplex and Contracaecum osculatum (sensu stricto)–comparisons with selected nematodes. Infect Genet Evol. 2014;21:452–462. doi: 10.1016/j.meegid.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Park YC, Kim W, Park JK. The complete mitochondrial genome of human parasitic roundworm, Ascaris lumbricoides. Mitochondrial DNA. 2011;22:91–93. doi: 10.3109/19401736.2011.624608. [DOI] [PubMed] [Google Scholar]
- 34.Gasser RB, Hu M, Chilton NB, Campbell BE, Jex AR, Otranto D, Cafarchia C, Beveridge I, Zhu X. Single-strand conformation polymorphism (SSCP) for the analysis of genetic variation. Nature Protoc. 2006;1:3121–3128. doi: 10.1038/nprot.2006.485. [DOI] [PubMed] [Google Scholar]
- 35.Hu M, Jex AR, Campbell BE, Gasser RB. Long PCR amplification of the entire mitochondrial genome from individual helminths for direct sequencing. Nat Protoc. 2007;2:2339–2344. doi: 10.1038/nprot.2007.358. [DOI] [PubMed] [Google Scholar]
- 36.Werle E, Schneider C, Renner M, Völker M, Fiehn W. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res. 1994;22:4354–4355. doi: 10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd RE, Foster PG, Guille M, Littlewood DTJ. Next generation sequencing and analysis of Xenopus mitogenomes. BMC Genomics. 2012;13:496. doi: 10.1186/1471-2164-13-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams ST, Foster PG, Littlewood DT. The complete mitochondrial genome of a turbinid vetigastropod from MiSeq Illumina sequencing of genomic DNA and steps towards a resolved gastropod phylogeny. Gene. 2014;533:38–47. doi: 10.1016/j.gene.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Bolger AM, Lohse M, Usadel B: T. Bioinformatics. 2014. A flexible trimmer for Illumina sequence data; p. btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cock PJA, Antao T, Chang JT, Chapman BA, Cox CJ, Dalke A, Friedberg I, Hamelryck T, Kauff F, Wilczynski B, de Hoon MJ. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics. 2009;25:1422–1423. doi: 10.1093/bioinformatics/btp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 42.Chevreux B, Wetter T, Suhai S. Genome sequence assembly using trace signals and additional sequence information. Comput Sci Biol. 1999;99:45. [Google Scholar]
- 43.Huang X, Madan A. CAP3: A DNA sequence assembly program. Genome Res. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;8:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 46.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 47.Hu M, Chilton NB, Gasser RB. The mitochondrial genome of Strongyloides stercoralis (Rhabditida) – idiosyncratic gene order, and evolutionary implications. Int J Parasitol. 2003;33:1393–1408. doi: 10.1016/S0020-7519(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 48.Zhu XQ, Gasser RB, Chilton NB, Jacobs DE. Molecular approaches for studying ascaridoid nematodes with zoonotic potential, with an emphasis on Toxocara species. J Helminthol. 2001;75:101–108. [PubMed] [Google Scholar]
- 49.Gasser RB. A perfect time to harness advanced advanced molecular technologies to explore Toxocara. Vet Parasitol. 2013;193:353–364. doi: 10.1016/j.vetpar.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 50.Zhu XQ, Chilton NB, Jacobs DE, Boes J, Gasser RB. Characterisation of Ascaris from human and pig hosts by nuclear ribosomal DNA sequences. Int J Parasitol. 1999;29:469–478. doi: 10.1016/S0020-7519(98)00226-4. [DOI] [PubMed] [Google Scholar]
- 51.Peng W, Keng Y, Hu M, Zhou X, Gasser RB. Mutation scanning-coupled analysis of haplotypic variability in mitochondrial DNA regions reveals low gene flow between human and porcine Ascaris in endemic regions of China. Electrophoresis. 2005;22:4317–4326. doi: 10.1002/elps.200500276. [DOI] [PubMed] [Google Scholar]
- 52.Peng W, Yuan K, Hu M, Gasser RB. Recent insights into the epidemiology and genetics of Ascaris in China using molecular tools. Parasitology. 2007;134:325–330. doi: 10.1017/S0031182006001521. [DOI] [PubMed] [Google Scholar]
- 53.Criscione CD, Anderson JD, Sudimack D, Peng W, Jha B, Williams-Blangero S, Anderson TJ. Disentangling hybridization and host colonization in parasitic roundworms of humans and pigs. Proc Biol Sci. 2007;274:2669–2677. doi: 10.1098/rspb.2007.0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Betson M, Nejsum P, Bendall RP, Deb RM, Stothard JR. J Infect Dis. 2014. Molecular epidemiology of ascariasis: A global perspective on the transmission dynamics of Ascaris in people and pigs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen MK, Fritzen B, Duncan JL, Guillot J, Eysker M, Dorchies P, Laugier C, Beugnet F, Meana A, Lussot-Kervern I, von Samson-Himmelstjerna G. Practical aspects of equine parasite control: a review based upon a workshop discussion consensus. Equine Vet J. 2010;42:460–468. doi: 10.1111/j.2042-3306.2010.00065.x. [DOI] [PubMed] [Google Scholar]
- 56.Nielsen MK, Reinemeyer CR, Donecker JM, Leathwick DM, Marchiondo AA, Kaplan RM. Anthelmintic resistance in equine parasites - current evidence and knowledge gaps. Vet Parasitol. 2013;204:55–63. doi: 10.1016/j.vetpar.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 57.von Samson-Himmelstjerna G. Anthelmintic resistance in equine parasites - detection, potential clinical relevance and implications for control. Vet Parasitol. 2012;185:2–8. doi: 10.1016/j.vetpar.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 58.Janssen IJ, Krücken J, Demeler J, Basiaga M, Kornaś S, von Samson-Himmelstjerna G. Genetic variants and increased expression of Parascaris equorum P-glycoprotein-11 in populations with decreased ivermectin susceptibility. PLoS One. 2013;8:e61635. doi: 10.1371/journal.pone.0061635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1: Comparison of the mt genome of Parascaris univalens with those of other ascaridoid nematodes. (DOCX 39 KB)


