Abstract
Based on studies that extend back to the 1920s, regression and stabilization of atherosclerosis in humans has gone from just a dream to one that is achievable. Review of the literature indicates that the successful attempts at regression generally applied robust measures to improve plasma lipoprotein profiles. Examples include extensive lowering of plasma concentrations of atherogenic apolipoprotein B and enhancement of reverse cholesterol transport from atheromata to the liver. Possible mechanisms responsible for lesion shrinkage include decreased retention of atherogenic apolipoprotein B within the arterial wall, efflux of cholesterol and other toxic lipids from plaques, emigration of lesional foam cells out of the arterial wall, and influx of healthy phagocytes that remove necrotic debris as well as other components of the plaque. However, currently available clinical agents cause less dramatic changes in plasma lipoprotein levels, and thereby fail to stop most cardiovascular events. There is, therefore, a clear need for preclinical and clinical testing of new agents expected to facilitate atherosclerosis regression with the hope that additional mechanistic insights will allow further progress.
Keywords: atherosclerosis, regression, macrophages, HDL, CCR7
INTRODUCTION
Atherosclerosis, a chronic inflammatory disease that occurs within the artery wall, is one of the underlying causes of vascular complications such as myocardial infarction, stroke, and peripheral vascular disease. Atherogenesis is a process that occurs over many years with the initiation phase being the subendothelial accumulation of apolipoprotein B-containing lipoproteins (ApoB). These particles undergo modifications, including oxidation and hydrolysis, leading to the activation of endothelial cells. These cells secrete chemoattactants called chemokines that interact with specific receptors expressed on monocytes essentially “recruiting” the cells into the lesion. The monocytes then roll along the endothelial cells via interactions of specific selectins, [i.e., P-selectin glycoprotein ligand-1 (PSGL-1)] with attachment being mediated by monocyte integrins such as very late antigen-4 (VLA-4) and lymphocyte function-associated antigen 1 (LFA-1) to the respective endothelial ligands vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). Once attached, a process called diapedesis occurs by which monocytes enter the subendothelial space. Having accessed the subendothelial space, recruited monocytes differentiate into macrophages, a process driven by interactions with the extracellular matrix (ECM) and cytokines, including macrophage colony-stimulating factor and members of the tumor necrosis factor family. The uptake of oxidized LDL by the macrophages occurs via scavenger receptors, notably the type A scavenger receptor (SRA) and CD36, a member of the type B family. The cholesteryl esters of the apoB particles that are ingested are hydrolyzed into free cholesterol, which occurs in late endosomes. The free cholesterol is then delivered to the endoplasmic reticulum (ER) where it is re-esterified by acyl-CoA: cholesterol ester transferase (ACAT). It is this process that leads to the macrophages having the “foamy” appearance. It is well-known that macrophages contribute to formation of the necrotic core and fibrous cap thinning that characterizes the vulnerable plaque. How do these macrophages ultimately contribute to the vulnerable plaque? Macrophage-derived matrix metalloproteinases (MMPs) are a family of proteins that can degrade various types of ECM and hence promote rupture. Moreover, once activated, certain MMPs can activate other ones. Studies have shown a temporal and spatial correlation between the presence of macrophages in rupture-prone shoulder regions of plaques, thinning of the fibrous cap in these regions, and local accumulation of activated MMPs. Another potential mechanism of how macrophages may promote plaque thinning and increase vulnerability is via causing smooth muscle cell (SMC) apoptosis. Vulnerable plaques show evidence of SMC death and decreased numbers of SMCs. Even after plaque rupture, the macrophage continues to play a role as it secretes prothrombotic tissue factor thereby accelerating thrombus formation. 1–8
The idea that human atheromata can regress at all has met considerable resistance over the decades.1–3 Resistance to the idea of lesion regression has been due to the fact that advanced atheromata in humans and in animal models contain components that give an impression of permanence, such as necrosis, calcification and fibrosis. Furthermore, numerous theories have been proposed to explain atherogenesis that included processes thought to be difficult, if not impossible, to reverse including injury,6 oxidation,7 and cellular transformations resembling carcinogenesis.8 In this review, data will be presented that demonstrate that indeed changes in the plaque environment can stabilize and regress even advanced lesions.
PLAQUE REGRESSION-EVIDENCE FROM ANIMAL STUDIES
Regression of atherosclerosis-is it possible?
In the 1920s, Anichkov and colleagues reported that switching cholesterol-fed rabbits to low-fat chow over 2–3 years resulted in arterial lesions becoming more fibrous with a reduced lipid content,9 which from a modern perspective suggests plaque stabilization.10–11 To our knowledge, however, the first prospective, interventional study demonstrating substantial shrinkage of atherosclerotic lesions was performed in cholesterol-fed rabbits and reported in 1957.12 The dietary regimen raised total plasma cholesterol to around 26 mmol/l (~1,000 mg/dl) and induced widespread lesions involving around 90% of the aorta. To mobilize tissue stores of cholesterol, animals received intravenous bolus injections of phosphatidylcholine (PC). After less than a week and a half of treatment, the remaining plaques were scattered and far less severe than initially, and three-quarters of arterial cholesterol stores had been removed.
Over the next 20 years, similar arterial benefits from injections of dispersed phospholipids were reported by a number of groups using a variety of atherosclerotic animal models, including primates.4 Given the heavy reliance of atherosclerosis research on animal models, it is surprising that these impressive, reproducible results were largely ignored, even in numerous historical reviews of regression.1,3,5, 9,13,14
The concept of regression gained support with a short-term study in squirrel monkeys by Maruffo and Portman,15 and more-extensive work by Armstrong and colleagues. The latter reported that advanced arterial lesions in cholesterol-fed Rhesus monkeys underwent shrinkage and remodeling during long-term follow-up when their diet was switched to low-fat or linoleate-rich diets.13,16 The cholesterol-feeding induction period lasted 17 months, producing widespread coronary lesions, with fibrosis, cellular breakdown, intracellular and extracellular lipid accumulation, and 60% luminal narrowing. The subsequent regression period lasted 40 months, bringing total plasma cholesterol values down to approximately 3.6 mmol/l (~140 mg/dl) and resulting in the loss of approximately two-thirds of coronary artery cholesterol, substantial reduction in necrosis, some improvement in extracellular lipid levels and fibrosis, and substantial lesion shrinkage so that only 20% luminal narrowing remained.13,16 Further work by Wissler and Vesselinovich as well as Malinow confirmed and extended these findings.9,14 Three decades ago, in an overview of this work, Armstrong concluded that “In the primate the answer is clear: all grades of induced lesions studied to date improve … the primate lesion shows amazing metabolic responsiveness: some extracellular as well as intracellular lipid is depleted, there is resolution of necrotic lesions, crystalline lipid tends to diminish slowly, and fibroplasia is eventually contained.” 13
Regression of advanced lesions in cholesterol-fed swine after reversion to a chow diet demonstrated an important sequence of events.17 Histologic examination of atheromata from these animals immediately after the high-cholesterol induction phase showed hallmarks of complex plaques, including necrosis and calcification. The regression regimen reduced total plasma cholesterol to approximately 1.8 mmol/l (~70 mg/dl), implying an even lower LDL-cholesterol level. Interestingly, the early phase of regression showed loss of foam cells from the lesions, and an increase in non-foam-cell macrophages around areas of necrosis. Long term, the necrotic areas virtually disappeared, indicating removal of the material by a flux of functioning, healthy phagocytes.17
To revive the long-neglected finding of rapid atherosclerosis regression after injections of dispersed phospholipids, Williams and colleagues sought to determine the underlying mechanism of action.4,18 Aqueous dispersions of PC spontaneously form vesicular structures called liposomes. Initially, cholesterol-free PC liposomes remain intact in the circulation 19 and can mobilize cholesterol from tissues in vivo 19,20–22 by acting as high-capacity sinks into which endogenous HDL cholesterol shuttles lipid.4,23–24 Bolus injections of PC liposomes rapidly restore normal macrovascular and microvascular endothelial function in hyperlipidemic animals,22 remove lipid from advanced plaques in rabbits in vivo,25 and rapidly mobilize tissue cholesterol in vivo in humans.26 Importantly, the optimum liposomal size (~120 nm) has been achieved in animal model studies, which allows these particles to gradually deliver their cholesterol to the liver without suppressing hepatic LDL receptor expression or raising plasma concentrations of LDL cholesterol.21,27
Eventually, in 1976 success in atherosclerosis regression was also achieved in rabbits following reversion to normal-chow diet in combination with hypolipidemic and other agents.9 Decades later, a series of studies achieved shrinkage of atheromata in rabbits with injections of HDL or HDL-like apolipoprotein A–I (apoA-I) and PC disks.28,29 Interestingly, a lipid-lowering regimen in rabbit, however, was found to diminish local proteolytic and prothrombotic factors in the artery wall, again consistent with remodeling of atheromata into a more stable phenotype.30
Unlike humans, mice have a naturally high plasma HDL:LDL ratio, providing a strong intrinsic resistance to atherosclerosis. Drastic manipulations of plasma lipoproteins are required, therefore, to induce arterial lipoprotein accumulation and sequelae. A revolution in murine atherosclerosis research began in the 1980s when Breslow and colleagues began applying transgenic techniques to create mice that were models of human lipoprotein metabolism.31,32 With the emerging technique of gene inactivation through homologous recombination (‘knock out’), came the ability to recreate important aspects of human lipid metabolism in mice. Most mouse models of atherosclerosis are derived from two basic models: the apolipoprotein E (apoE)-null (apoE−/−) mouse 33,34 and the LDL receptor-null (LDLR−/−) mouse.35 In these models, the normally low plasma apoB levels are increased to atherogenic levels by eliminating either a ligand (apoE−/−) or a receptor (LDLR−/−) for lipoprotein clearance. Feeding these modified mice with a cholesterol-enriched and fat-enriched diet (Western diet; WD) increased plasma apoB levels to an even greater degree, resulting in accelerated plaque formation in the major arteries.
Gene transfer was the first strategy used to achieve plaque regression in mice. For example, injection of LDLR−/− mice that had developed fatty streak lesions after a 5-week WD, with an adenoviral vector containing cDNA encoding human apoA-I caused a significant increase in HDL-cholesterol level and, importantly, regression of fatty streak lesions at a sampling point four weeks later.36 The ability of HDL-like particles to rapidly remodel plaques in mice was shown by infusion of apoA-IMilano/PC complexes, a variant of apolipoprotein A–I identified in individuals who exhibit very low HDL cholesterol levels. Infusion of this complex reduced foam cell content in arterial lesions in apoE−/− mice within 48 hours.37 This finding was corroborated by a specific transplantation model that we reported in 2001,38 described later. Although another HDL protein, apolipoprotein M, has been overexpressed in mice to retard plaque progression,39 evaluation of its role in regression has not yet been reported.
Another major target of gene transfer to achieve regression in mice is hepatic overexpression of apoE, which increases the clearance of plasma atherogenic lipoproteins through receptors in the liver for LDL35 and for postprandial lipoprotein remnants.40–43 Following successful transient reduction of atherosclerosis progression in apoE−/− mice with short-term adenoviral-mediated expression of apoE,44 a number of laboratories capitalized on the greater duration of apoE expression afforded by ‘second-generation’ viral vectors.45 For example, in LDLR−/− mice fed a WD for 14 weeks to develop plaques rich in foam cells (~50% macrophage content), increased expression of apoE resulted in considerable plaque regression, despite having no discernable effect on fasting plasma lipoprotein levels.46 These findings were attributed in part to the entry of expressed apoE into the vessel wall, consistent with other studies;47–50 however, another plausible mechanism is that expressed apoE might have also improved clearance of atherogenic lipoproteins in the postprandial state.
Transplantation model of atherosclerosis regression
To further explore cellular and molecular mechanisms of atherosclerosis regression in murine models, we and others have developed new approaches to rapidly induce robust improvements in the plaque environment and trigger lesion remodeling and regression. Our study group developed the technique of transplanting a segment of plaque-containing aorta from a (WD-fed) hyperlipidemic apoE−/− mouse (i.e. an extremely pro-atherogenic milieu consisting of high plasma apoB levels and low HDL-cholesterol levels), into a wild-type recipient (i.e. rapidly normalizing the lipoprotein environment, which is sustainable indefinitely). This approach allows analysis of plaques of any degree of complexity.
We found that transplanting early lesions51–52 or advanced, complicated plaques into wild-type recipients substantially reduced foam cell content and increased the number of smooth muscle cells, particularly in the cap, which is consistent with plaque stabilization and regression.53–54 The loss of foam cells from early lesions was surprisingly rapid, with large decreases evident as early as three days post-transplantation (Figure 1).51–52 With advanced lesions, all features regressed after nine weeks, including necrosis, cholesterol clefts and fibrosis.53–54
Figure 1. Regression of plaques in the mouse transplantation model.
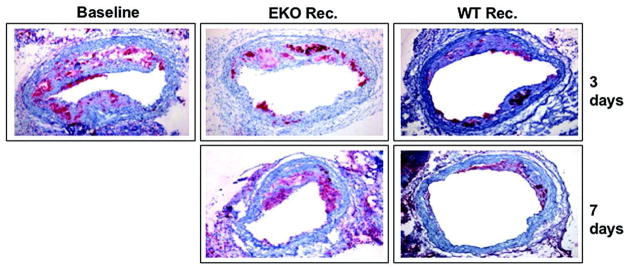
ApoE−/− mice were fed a Western diet for 16 weeks to develop advanced atherosclerosis. Aortic arches from these mice were either harvested and analyzed by histochemical methods, or they were transplanted into apoE−/− (‘progression’) or wild-type (‘regression’) recipient mice. Three or seven days later, the same analyses were performed. Shown are the histochemical results for the foam-cell marker CD68 (red). The pictures show the immunostaining of representative aortic lesions in cross section. The virtual absence of foam cells can be seen in the ‘regression’ group. In contrast with the ‘regression’ results, the ‘progression’ group showed persistence of foam cells.
By using the transplantation model, we characterized cellular and molecular features of the regressing plaque. An early question we sought to answer concerned the fate of the disappearing foam cells—was their disappearance due to apoptosis and phagocytosis by newly recruited macrophages, or emigration? Interestingly, we found that the rapid loss of foam cells was largely accounted for by their emigration into regional and systemic lymph nodes. Furthermore, we found that the wild-type milieu provoked foam cells to display markers characteristic of both macrophages and, surprisingly, dendritic cells, which enabled emigration.51,52,55–59
Using laser microdissection to remove foam cells from regressing and non-regressing plaques,60 analyses revealed the presence of mRNA for CCR7,52 chemokine (C–C motif) receptor 7, which is required for dendritic cell emigration.61 Interestingly, injection of wild-type recipient animals with antibodies against the two CCR7 ligands, CCL19 and CCL21, inhibited the majority of foam cells from emigrating from the aortic transplant lesions—establishing a functional role for CCR7 in regression.52
In addition, mRNA concentrations of several well-known proteins implicated in atherothrombosis, such as vascular cell adhesion protein-1 (VCAM-1), monocyte chemotactic protein 1 (MCP-1) and tissue factor, are decreased in foam cells during regression. In addition, the level of mRNA for the nuclear oxysterol liver X receptor [alpha] (LXRα)—known to be induced in vitro by oxidized sterols62,63—significantly increased in vivo, as did its anti-atherogenic target ATP-binding cassette 1 (ABCA-1).52 Intriguingly, systemic administration of an LXR agonist caused lesion regression in LDLR−/− mice,64 although the concomitant development of fatty liver has dampened enthusiasm for this approach in humans.65 Interestingly, we discovered that LXR activation in macrophages promoted regression in vivo and was dependent on CCR7 expression.66 It is unlikely that regression of atherosclerosis occurs only through one mechanism. A recent report showed that netrin-1, a neuroimmune guidance cue, was secreted by macrophages in human and mouse atheroma, where it inactivated the migration of macrophages toward chemokines (such as CCL19, ligand for CCR7) linked to their egress from plaques.67 These findings suggest that inhibition of netrin-1 may be one method of inducing regression of atherosclerosis. Overall, these findings indicate that regression does not simply comprise of the events leading to lesion progression in reverse order; instead it involves specific cellular and molecular pathways that eventually mobilize all pathologic components of the plaque.
HDL and plaque regression
At least three plasma parameters are changed in the transplantation model when regression was observed: (1) non-HDL levels decreased; (2) HDL levels were restored from ~33% of normal to wild type levels; (3) apoE was now present. For the purpose of this review, we will focus on the HDL change. To selectively test this as a regression factor, we adopted the transplant approach by using as recipients human apoAI transgenic/apoE−/− mice (hAI/EKO) or apoAI−/− mice. 68–69 Briefly, plaque-bearing aortic arches from apoE−/− mice (low HDL-C, high non-HDL-C) were transplanted into recipient mice with differing levels of HDL-C and non-HDL-C: C57BL/6 mice (normal HDL-C, low non-HDL-C), apoAI−/− mice (low HDL-C, low non-HDL-C), or hAI/EKO mice (normal HDL-C, high non-HDL-C). Remarkably, despite persistent elevated non-HDL-C in hAI/EKO recipients, plaque CD68(+) cell content decreased by >50% by one week after transplantation, whereas there was little change in apoAI−/− recipient mice despite hypolipidemia. Interestingly, the decreased content of plaque CD68+ cells was associated with their emigration and induction of their chemokine receptor CCR7. 70 These data are consistent with a recent meta-analysis of clinical studies in which it was shown that atherosclerosis regression (assessed by IVUS) after LDL lowering was most likely to be achieved when HDL was also significantly increased. 71
The induction of CCR7 is also likely related to changes in the sterol content of foam cells when they are placed in a regression environment, given that its promoter has a putative sterol regulatory element (SRE). This idea is in agreement with a report that demonstrated that loading THP-1 human monocytes with oxidized LDL suppresses the expression of this gene. 72 Notably, we have found that statins, potent regulators of SRE-dependent transcription can induce CCR7 expression in vivo and promote regression via emigration of CD68+ cells in a CCR7 dependent manner 73. Recently, it was reported that both atorvastatin and rosuvastatin can promote regression of atherosclerosis as assessed by IVUS. 74 Our data, therefore, suggest that activation of the CCR7 pathway may be one contributing mechanism.
Another aspect of interest has been the effect of HDL on the inflammatory state of CD68+ cells in plaques. A number of benefits from this can be envisioned such as a reduced production of monocyte attracting chemokines and plaque “healing” by macrophages prodded to become tissue re-modelers (M2 macrophages). There are multiple reasons for HDL to have anti-inflammatory effects on plaques, including the anti-oxidant properties of its enzymatic and non-enzymatic components, the ability to remove normal and toxic lipid species from cells, and the dampening of TLR signaling by regulating plasma membrane cholesterol content 3,75. It is important to note that in CD68+ cells laser-captured from the plaques, normalization of HDL-C led to decreased expression of inflammatory factors and enrichment of markers of the M2 macrophage state. 70,76 Macrophage heterogeneity in human atherosclerotic plaques is widely recognized, with both M1 (activated) and M2 markers being detectable in lesions 77,78 but little is known about the factors that regulate M2 marker expression in plaques in vivo.
Cholesterol homeostasis has also recently been investigated with microRNAs (miRNA), which are small endogenous non–protein-coding RNAs that are posttranscriptional regulators of genes involved in physiological processes. MiR-33, an intronic miRNA located within the gene encoding sterol-regulatory element binding protein-2, inhibits hepatic expression of both ABCA-1 and ABCG-1, reducing HDL-C concentrations, as well as ABCA-1 expression in macrophages, thus resulting in decreased cholesterol efflux. Interestingly, enrichment of M2 markers in plaque CD68+ cells was observed in LDLR−/− mice treated with an antagamir of miR-33. 79 The treated mice also exhibited plaque regression (fewer macrophages). The therapeutic potential of miR-33 antagmirs to cause similar benefits in people was suggested by plasma levels of HDL being raised in treated non-human primates.80 Thus, antagonism of miR-33 may represent a novel approach to enhancing macrophage cholesterol efflux and raising HDL-C levels in the future.
Recently, Voight and colleagues 81 reported, using mendelian randomisation, that some genetic mechanisms (i.e. endothelial lipase polymorphisms) that raise plasma HDL cholesterol do not seem to lower risk of myocardial infarction. These data potentially challenge the concept that raising of plasma HDL cholesterol will uniformly translate into reductions in risk of myocardial infarction. However, it is important to note that these results should not lead one to abandon the concept that HDL is beneficial but rather may indicate that it is time to alter the HDL hypothesis- it’s not the quantity of HDL but rather the quality or functionality that is critical. We need clinical trials that have HDL function as an endpoint rather than simply the level.
EVIDENCE FROM CLINICAL STUDIES
Statins, Niacin, HDL, and CETP Inhibitors
The first prospective, interventional study to demonstrate plaque regression in humans was in the mid-1960s, in which approximately 10% of patients (n = 31) treated with niacin showed improved femoral angiograms.82 Larger trials of lipid lowering have since shown angiographic evidence of regression; however, though statistically significant, the effects were surprisingly small, particularly in light of large reductions in clinical events.1, 3,83 This ‘angiographic paradox’ was resolved with the realization that lipid-rich, vulnerable plaques have a central role in acute coronary syndromes. A vulnerable plaque is characterized by being small, causing less than 50% occlusion, and being full of intracellular and extracellular lipid, rich in macrophages and tissue factor, with low concentrations of smooth muscle cells, and with only a thin fibrous cap under an intact endothelial layer.10–11,83–84 Rupture of a vulnerable plaque provokes the formation of a robust local clot, and hence vessel occlusion and acute infarction.85 Lipid lowering, which promoted measurable shrinkage of angiographically prominent but presumably stable lesions, probably had a greater impact on risk reduction by the remodeling and stabilization of small, rupture-prone lesions.83–84 Regression studies in animal models strongly support this interpretation, given that macrophage content, a key hallmark of instability, can be rapidly corrected with robust improvements in the plaque lipoprotein environment.
In order to track potentially more important changes in plaque composition, to avoid the confounding effects of lesion remodeling on lumen size, arterial wall imaging is required. Recent human trials have switched from quantitative angiography, which images only the vascular lumen, to techniques that image plaque calcium (e.g. electron-beam CT) and plaque volume (e.g. intravascular ultrasonography; IVUS). A retrospective analysis found that aggressive LDL-cholesterol lowering with statins correlated significantly with reduction in coronary calcium-volume score by electron-beam CT, indicating that coronary artery calcifications can shrink.86 In the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) study 87 and A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID),88 patients with acute coronary syndromes were treated for over a year with high-dose statins and evaluated by IVUS. The REVERSAL trial compared the high-dose statin therapy with a conventional, less-potent statin regimen. During 18 months of treatment, patients treated with the conventional regimen exhibited statistically significant progression of atheroma volume (+2.7%), despite achieving average LDL-cholesterol levels of 2.8 mmol/l (110 mg/dl) and, therefore, meeting the then-current Adult Treatment Panel III goal.89 By contrast, the high-dose statin group experienced no significant progression of atheroma volume (average LDL-cholesterol level, 2 mmol/l [79 mg/dl]). Importantly, analysis across the treatment groups found that LDL reduction exceeding approximately 50% was associated with a decrease in atheroma volume. In ASTEROID, all patients received the same high-dose therapy for 24 months, and IVUS findings pretreatment and posttreatment were compared. During treatment, LDL cholesterol dropped to 1.6 mmol/l (60.8 mg/dl), and atheroma volume shrank by a median of 6.8%. Thus, in both of these studies, extensive LDL-cholesterol lowering for extended periods caused established plaques to shrink. The greater efficacy seen in ASTEROID could be explained by the lower median LDL-cholesterol level, but also by the longer treatment period and higher HDL cholesterol levels achieved than those in REVERSAL. As in earlier angiographic studies, we believe that these reductions in plaque volume are accompanied by favorable alterations in plaque biology, a theory which is further supported by evidence that robust plasma LDL lowering to 1.0–1.6 mmol/l or below (≤40–60 mg/dl) is associated with further reductions in cardiovascular events.90
In addition to the pre-clinical studies reviewed above, there are a limited number of human studies in which HDL levels have been manipulated by infusion, and the effects on plaques assessed. In the first 91, patients at high risk for cardiovascular disease were infused with either an artificial form of HDL (apoAI milano/phospholipid complexes) or saline (placebo) once a week for 5 weeks. By intravascular ultrasound (IVUS), there was a significant reduction in atheroma volume (−4.2%) in the combined (high and low dose) treatment group, though no dose response was observed of a higher vs. lower dose of the artificial HDL. There was no significant difference in atheroma volume compared to the placebo group, but the study was not powered for a direct comparison. In the second infusion study, high-risk patients received 4 weekly infusion with reconstituted HDL (rHDL; containing wild type apoAI) or saline (placebo). 92 Similar to the previous study, there was a significant decrease in atheroma volume (−3.4%) (as assessed by IVUS) after treatment with rHDL compared to baseline, but not compared to placebo (which the study was not powered for). However, the rHDL group had statistically significant improvements in plaque characterization index and in a coronary stenosis score on quantitative coronary angiography compared to the placebo group. In the third infusion trial 93, a single dose of reconstituted human HDL was infused into patients undergoing femoral atherectomies, with the procedure performed 5–7 days later. Compared to the control group (receiving saline solution), in the excised plaque samples in the HDL infusion group, macrophage activation state (i.e. diminished VCAM-1 expression) as well as cell size (due to diminished lipid content) were reduced.
In addition to the aforementioned meta-analysis of statin trials in which the relationships among LDL, HDL, and plaque regression were analyzed, there are also a number of other drug studies in which effects on plaques were ascribed to the raising of HDL levels. This includes the VA-HIT study, in which coronary events were reduced by 11% with gemfibrozil for every 5-mg/dL increase in HDL-C. 94 In another series of studies (“ARBITER” 95–98), high-risk patients were placed on either statins or statins plus niacin. Over a 18–24 month observation periods, carotid intimal-medial thickness (cIMT) measurements were obtained as a surrogate for coronary artery plaque burden. As expected, when niacin was part of the treatment, HDL-C levels were increased (by 18.4%), and the authors attributed the improvement in cIMT particularly to this change. It is important to note that niacin does more than just raise HDL-C levels; it also decreases plasma triglyceride levels, makes LDL size increase, and possesses anti-inflammatory properties all of which have the potential to limit plaque progression. 99–101 These pleiotropic effects obviously confound the interpretation of both the ARBITER and another statin-niacin clinical trial- the HATS study. 102 In the latter study, the addition of niacin to statin treatment resulted not only in a reduction in coronary artery stenosis, but also in events. The encouraging results with niacin, however, were recently called into question by the early termination of the AIM-HIGH study, which failed to show a benefit in the treatment group. 103 This study has been criticized, however, as being underpowered and for the fact that both the treatment group and the control group in the study received statin therapy, making additional benefits harder to detect, as well as for the placebo that the control patients received was actually a low dose of niacin. 104
Recently, cholesteryl ester transfer protein (CETP) inhibitors have been investigated as pharmacological agents to raise HDL levels. Surprisingly, torcetrapib, the first CETP inhibitor tested in a clinical trial, increased the all-cause mortality and cardiovascular events, which led to the premature ending of the ILLUMINATE trial. 105 Subsequent studies indicated that the observed off-target effects of torcetrapib (increased blood pressure and low serum potassium by stimulation of aldosterone production) were rather molecule specific, unrelated to CETP inhibition and thereby might have overshadowed the beneficial effects of the raised HDL-C levels. Importantly, posthoc analysis of ILLUMINATE showed that subjects with greater increases of HDL-C or apoAI levels had a lower rate of major cardiovascular events within the torcetrapib group. 106 Despite the general failure of torcetrapib, in the posthoc analysis of the ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation) study, regression of coronary atherosclerosis (as assessed by IVUS) was observed in patients who achieved the highest HDL-C levels with torcetrapib treatment. 107 In vitro studies showed an improved functionality of HDL-C particles under CETP inhibition, as HDL-C isolated from patients treated with torcetrapib and anacetrapib exhibited an increased ability to promote cholesterol efflux from macrophages. 108–109 Indeed, the CETP inhibitors anacetrapib, dalcetrapib and evacetrapib increase HDL-C levels between 30–138%, and have not shown the off-target effects of torcetrapib in recent clinical phase II trials, confirming the premise of a non-class related toxicity of torcetrapib. 110–113 Thus, raising HDL-C by CETP inhibition or modulation remains a potential therapeutic approach for atherosclerotic cardiovascular disease. Large clinical outcome trials were initiated for dalcetrapib (dal-OUTCOMES) and anacetrapib (REVEAL) including a total of approximatley 45,000 patients. Surprisingly, in May 2012 Roche stopped the complete dal-HEART program for dalcetrapib after an interims analysis of dal-OUTCOMES due to a lack of clinically meaningful efficacy. The failure of dal-OUTCOMES might have been a result of the rather moderate increases in HDL-C levels (30%) and minor impact on LDL-C levels induced by dalcetrapib, a fate that does not necessarily apply for anacetrapib which has been shown to increase HDL-C levels by 138% accompanied by more robust reductions in LDL-C levels. 114 Whether the failure of dal-OUTCOMES challenges the benefits of raising HDL-C, in general, or rather the underlying mechanisms of how HDL-C is to be raised will be answered by the phase III study with anacetrapib which is expected over the next few years.
Novel Imaging modalities
While IVUS has provided important coronary anatomic information, there is still a need for imaging modalities that provide more details. Optical coherence tomography (OCT) has revolutionized intracoronary imaging. The unprecedented spatial resolution of this technique (15 μm) provides unique insights on the microstructure of the coronary wall. Currently, OCT is increasingly used in clinical practice and also constitutes an emerging, highly robust, research tool. OCT allows detailed visualization of atherosclerotic plaques and provides reliable information on plaque composition (lipid, fibrous, calcified). Importantly, OCT is the only technique allowing accurate measurements of the thickness of the fibrous cap, a classical marker of plaque vulnerability, and readily detects thin-cap fibroatheromas. In patients with acute coronary syndromes, plaque ruptures, with associated red or white thrombus, are nicely identified. 115
The lipid core is an important plaque component and its relationship with macrophages and the vulnerable plaque has been established in animal models. Near-infrared spectroscopy (NIRS) is a technique that can identify the lipid core burden in the coronary arteries. It works by light of discrete wavelengths from a laser being directed onto the tissue sample via glass fibers. Light scattered from the samples is then collected in fibers and launched into a spectrometer. The plot of signal intensity as a function of wavelength is subsequently used to develop chemometric models to discriminate lipid-cores from non-atherosclerotic tissue.116
Ideally, it is the early detection and characterization of atherosclerotic lesions susceptible to sudden rupture and thrombosis that need to be identified. Plaque development has been extensively studied using MRI (magnetic resonance imaging) in animal models of rapidly progressing atherosclerosis. MRI permits the accurate assessment of atherosclerotic plaque burden and the differentiation between the lipid and fibrous content of individual plaques, thus providing a non-invasive approach to serially monitor the evolution of individual plaques. In addition, 18F-FDG PET (positron emission tomography) is a relatively new non-invasive tool for inflammation functional imaging. Low spatial resolution is now compensated by co-registration with CT or MRI. One can envision having novel contrast agents that target specific plaque components or diverse set of molecules within the plaque which would elucidate the changes at the cellular and molecular levels during plaque progression and regression. We have demonstrated the feasibility of this concept in a study in which the detection of macrophages using a nanoparticulate contrast agent was achieved. The above has important implications as pharmaceutical companies are looking for early surrogate markers that could be evaluated in a small number of patients to predict the beneficial effects of new drugs on atherosclerotic plaques before moving to costly clinical trials with a large number of patients. 117–119
CONCLUSION
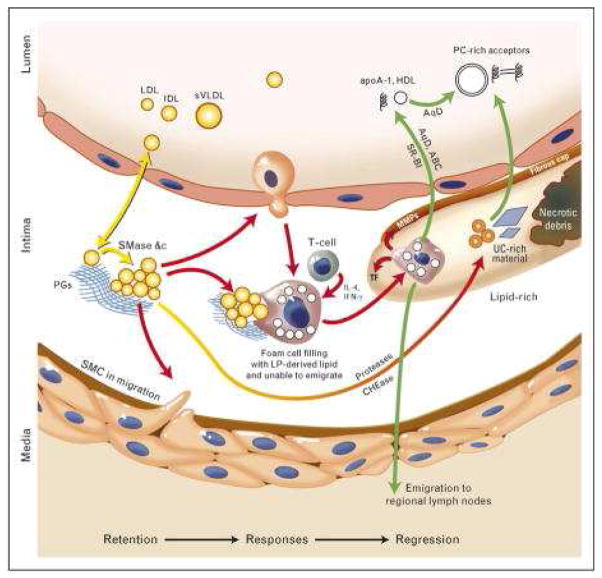
The crucial event in atherosclerosis initiation is the retention, or trapping, of apolipoprotein-B (apoB)-containing lipoproteins within the arterial wall; this process leads to local responses to this retained material, including a maladaptive infiltrate of macrophages that consume the retained lipoproteins but then fail to emigrate. Regression (i.e. shrinkage and healing) of advanced, complex atherosclerotic plaques has been clearly documented in animals, and plausible evidence supports its occurrence in humans as well. Data has shown that plaque regression requires robust improvements in the plaque environment, specifically large reductions in plasma concentrations of apoB-lipoproteins and large increases in the reverse transport of lipids out of the plaque for disposal. Furthermore, it is important to note that regression is not merely a rewinding of progression, but instead involves a coordinated series of events such as emigration of the macrophage infiltrate, followed by the initiation of a stream of healthy, normally functioning phagocytes that mobilize necrotic debris and all other components of advanced plaques (Figure 2).
Figure 2. Retention, responses and regression.
Arrows are color-coded to indicate crucial mechanisms in the retention of atherogenic apolipoprotein B within the arterial wall, the key initiating step in atherogenesis (yellow); local responses to the retained and modified lipoproteins that lead to plaque growth and evolution (red); and then regression of all plaque components after robust improvements in the plasma lipoprotein profile, such as increased concentrations of natural and artificial mediators of reverse lipid transport (green). LDL, low-density lipoprotein; IDL, intermediate density lipoprotein; sVLDL, small VLDL; PGs, proteoglycans; SMase, sphingomyelinase; SMC, smooth muscle cell; LpL, lipoprotein lipase; TF, tissue factor; MMPs, matrix metalloproteinases; UC, unesterified cholesterol; AqD, aqueous diffusion; ABC, ATP binding cassette transporters; SR-BI, scavenger receptor B–I; HDL, high-density lipoprotein; PC, phosphatidylcholine.
For regression of atheromata to become a realistic therapeutic goal, clinicians must be provided with tools that extensively change plasma lipoprotein concentrations and plaque biology while avoiding adverse effects. To date, the animal and human studies that achieved plaque regression required large reductions in plasma levels of apoB, sometimes combined with brisk enhancements in reverse cholesterol transport. Unfortunately, most patients who take statins, for example, will not achieve and sustain the dramatically low LDL-cholesterol levels seen in chow-fed nonhuman primates. Efforts to explore other strategies that lower apoB levels are currently underway (i.e., PCSK9 inhibitors). Experimental agents designed to accelerate reverse cholesterol transport from plaques, into the liver include PC liposomes, apoA-I/PC complexes, and apoA-I mimetic peptides. Other small molecules have been investigated pre-clinically for their potential to enhance HDL-cholesterol levels and reverse lipid transport, such as agonists for LXR and peroxisome proliferator-activated receptors.
On the basis of experimental data summarized above, we expect that the best regression results will be observed when plasma LDL-cholesterol concentrations are reduced and HDL cholesterol function in reverse lipid transport is enhanced. Indeed, years of work has demonstrated that the plaque and its components are dynamic. Most recently, by performing microarrays, we have discovered that regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome with preferential expression of genes that reduce cellular adhesion, enhance cellular motility, and overall act to suppress inflammation.120 Additional strategies, such as specific induction of pro-emigrant molecules to provoke foam cells to leave the arterial wall (for example via CCR7), should attract pharmaceutical interest. Furthermore, there is a need for clinical trials that use the imaging modalities described above to identify the specific effects of novel agents on plaque components rather than just atheroma size. In conclusion, we provide evidence that the plaque is dynamic and depending on the conditions macrophages, which play a crucial role in atherogenesis, can exit the lesions, proving that indeed regression is possible. However, there is still much work to be done and ultimately, the insights gained will lead to new therapeutic targets against cardiovascular disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blankenhorn DH, Hodis HN. George Lyman Duff Memorial Lecture. Arterial imaging and atherosclerosis reversal. Arterioscler Thromb. 1994;14:177–92. doi: 10.1161/01.atv.14.2.177. [DOI] [PubMed] [Google Scholar]
- 2.Schell WD, Myers JN. Regression of atherosclerosis: a review. Prog Cardiovasc Dis. 1997;39:483–96. doi: 10.1016/s0033-0620(97)80041-2. [DOI] [PubMed] [Google Scholar]
- 3.Stein Y, Stein O. Does therapeutic intervention achieve slowing of progression or bona fide regression of atherosclerotic lesions? Arterioscler Thromb Vasc Biol. 2001;21:183–8. doi: 10.1161/01.atv.21.2.183. [DOI] [PubMed] [Google Scholar]
- 4.Williams KJ, et al. Intravenously administered lecithin liposomes: a synthetic antiatherogenic lipid particle. Perspect Biol Med. 1984;27:417–31. doi: 10.1353/pbm.1984.0031. [DOI] [PubMed] [Google Scholar]
- 5.Ross R, et al. Response to injury and atherogenesis. Am J Pathol. 1977;86:675–84. [PMC free article] [PubMed] [Google Scholar]
- 6.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 7.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 8.Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973;70:1753–6. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wissler RW, Vesselinovitch D. Studies of regression of advanced atherosclerosis in experimental animals and man. Ann N Y Acad Sci. 1976;275:363–78. doi: 10.1111/j.1749-6632.1976.tb43368.x. [DOI] [PubMed] [Google Scholar]
- 10.Constantinides P. Coronary thrombosis linked to fissure in atherosclerotic vessel wall. Journal of the Amerrican Medical Association. 1964;188 (Suppl):35–37. doi: 10.1001/jama.1964.03060320157049. [DOI] [PubMed] [Google Scholar]
- 11.Davies MJ, et al. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993;69:377–81. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman M, et al. Resolution of aortic atherosclerotic infiltration in the rabbit by phosphatide infusion. Proc Soc Exp Biol Med. 1957;95:586–8. doi: 10.3181/00379727-95-23300. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong ML. Evidence of regression of atherosclerosis in primates and man. Postgrad Med J. 1976;52:456–61. doi: 10.1136/pgmj.52.609.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malinow MR. Experimental models of atherosclerosis regression. Atherosclerosis. 1983;48:105–18. doi: 10.1016/0021-9150(83)90097-7. [DOI] [PubMed] [Google Scholar]
- 15.Maruffo CA, Portman OW. Nutritional control of coronary artery atherosclerosis in the squirrel monkey. J Atheroscler Res. 1968;8:237–47. doi: 10.1016/s0368-1319(68)80060-2. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong ML, et al. Regression of coronary atheromatosis in rhesus monkeys. Circ Res. 1970;27:59–67. doi: 10.1161/01.res.27.1.59. [DOI] [PubMed] [Google Scholar]
- 17.Daoud AS, et al. Sequential morphologic studies of regression of advanced atherosclerosis. Arch Pathol Lab Med. 1981;105:233–9. [PubMed] [Google Scholar]
- 18.Williams KJ, Tabas I. Lipoprotein retention--and clues for atheroma regression. Arterioscler Thromb Vasc Biol. 2005;25:1536–40. doi: 10.1161/01.ATV.0000174795.62387.d3. [DOI] [PubMed] [Google Scholar]
- 19.Williams KJ, Scanu AM. Uptake of endogenous cholesterol by a synthetic lipoprotein. Biochim Biophys Acta. 1986;875:183–94. doi: 10.1016/0005-2760(86)90167-0. [DOI] [PubMed] [Google Scholar]
- 20.Williams KJ, et al. Low density lipoprotein receptor-independent hepatic uptake of a synthetic, cholesterol-scavenging lipoprotein: implications for the treatment of receptor-deficient atherosclerosis. Proc Natl Acad Sci U S A. 1988;85:242–6. doi: 10.1073/pnas.85.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigueza WV, et al. Large versus small unilamellar vesicles mediate reverse cholesterol transport in vivo into two distinct hepatic metabolic pools. Implications for the treatment of atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:2132–9. doi: 10.1161/01.atv.17.10.2132. [DOI] [PubMed] [Google Scholar]
- 22.Williams KJ, et al. Rapid restoration of normal endothelial functions in genetically hyperlipidemic mice by a synthetic mediator of reverse lipid transport. Arterioscler Thromb Vasc Biol. 2000;20:1033–9. doi: 10.1161/01.atv.20.4.1033. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigueza WV, et al. Remodeling and shuttling. Mechanisms for the synergistic effects between different acceptor particles in the mobilization of cellular cholesterol. Arterioscler Thromb Vasc Biol. 1997;17:383–93. doi: 10.1161/01.atv.17.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams KJ, et al. Structural and metabolic consequences of liposome-lipoprotein interactions. Adv Drug Deliv Rev. 1998;32:31–43. doi: 10.1016/s0169-409x(97)00130-0. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigueza WV, et al. Cholesterol mobilization and regression of atheroma in cholesterol-fed rabbits induced by large unilamellar vesicles. Biochim Biophys Acta. 1998;1368:306–20. doi: 10.1016/s0005-2736(97)00198-3. [DOI] [PubMed] [Google Scholar]
- 26.Rader DJ, et al. Infusiion of large unilamellar vesicles (ETC-588) mobilize unesterified cholesterol in a dose-dependent fashion in healthy volunteers. Arterioscler Thromb Vasc Biol. 2002;22:a-53. [Google Scholar]
- 27.Williams KJ Office, U. P., editor . US: 1998. [Google Scholar]
- 28.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–41. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyazaki A, et al. Intravenous injection of rabbit apolipoprotein A–I inhibits the progression of atherosclerosis in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 1995;15:1882–8. doi: 10.1161/01.atv.15.11.1882. [DOI] [PubMed] [Google Scholar]
- 30.Aikawa M, Libby P. Lipid lowering reduces proteolytic and prothrombotic potential in rabbit atheroma. Ann N Y Acad Sci. 2000;902:140–52. doi: 10.1111/j.1749-6632.2000.tb06309.x. [DOI] [PubMed] [Google Scholar]
- 31.Paigen B, et al. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57:65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 32.Walsh A, et al. High levels of human apolipoprotein A–I in transgenic mice result in increased plasma levels of small high density lipoprotein (HDL) particles comparable to human HDL3. J Biol Chem. 1989;264:6488–94. [PubMed] [Google Scholar]
- 33.Plump AS, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–53. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 34.Zhang SH, et al. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–71. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 35.Ishibashi S, et al. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–93. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tangirala RK, et al. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A–I in mice. Circulation. 1999;100:1816–22. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 37.Shah PK, et al. High-dose recombinant apolipoprotein A–I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 2001;103:3047–50. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- 38.Rong JX, et al. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–52. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 39.Wolfrum C, Poy MN, Stoffel M. Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nat Med. 2005;11:418–22. doi: 10.1038/nm1211. [DOI] [PubMed] [Google Scholar]
- 40.Williams KJ, et al. Mechanisms by which lipoprotein lipase alters cellular metabolism of lipoprotein(a), low density lipoprotein, and nascent lipoproteins. Roles for low density lipoprotein receptors and heparan sulfate proteoglycans. J Biol Chem. 1992;267:13284–92. [PubMed] [Google Scholar]
- 41.Ji ZS, et al. Role of heparan sulfate proteoglycans in the binding and uptake of apolipoprotein E-enriched remnant lipoproteins by cultured cells. J Biol Chem. 1993;268:10160–7. [PubMed] [Google Scholar]
- 42.Fuki IV, et al. The syndecan family of proteoglycans. Novel receptors mediating internalization of atherogenic lipoproteins in vitro. J Clin Invest. 1997;100:1611–22. doi: 10.1172/JCI119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams KJ, et al. Loss of heparan N-sulfotransferase in diabetic liver: role of angiotensin II. Diabetes. 2005;54:1116–22. doi: 10.2337/diabetes.54.4.1116. [DOI] [PubMed] [Google Scholar]
- 44.Kashyap VS, et al. Apolipoprotein E deficiency in mice: gene replacement and prevention of atherosclerosis using adenovirus vectors. J Clin Invest. 1995;96:1612–20. doi: 10.1172/JCI118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsukamoto K, Smith P, Glick JM, Rader DJ. Liver-directed gene transfer and prolonged expression of three major human ApoE isoforms in ApoE-deficient mice. J Clin Invest. 1997;100:107–14. doi: 10.1172/JCI119501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tangirala RK, et al. Reduction of isoprostanes and regression of advanced atherosclerosis by apolipoprotein E. J Biol Chem. 2001;276:261–6. doi: 10.1074/jbc.M003324200. [DOI] [PubMed] [Google Scholar]
- 47.Thorngate FE, Rudel LL, Walzem RL, Williams DL. Low levels of extrahepatic nonmacrophage ApoE inhibit atherosclerosis without correcting hypercholesterolemia in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:1939–45. doi: 10.1161/01.atv.20.8.1939. [DOI] [PubMed] [Google Scholar]
- 48.Wientgen H, et al. Subphysiologic apolipoprotein E (ApoE) plasma levels inhibit neointimal formation after arterial injury in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1460–5. doi: 10.1161/01.ATV.0000134297.61979.3c. [DOI] [PubMed] [Google Scholar]
- 49.Harris JD, et al. Acute regression of advanced and retardation of early aortic atheroma in immunocompetent apolipoprotein-E (apoE) deficient mice by administration of a second generation [E1(-), E3(-), polymerase(−)] adenovirus vector expressing human apoE. Hum Mol Genet. 2002;11:43–58. doi: 10.1093/hmg/11.1.43. [DOI] [PubMed] [Google Scholar]
- 50.Rosenfeld ME, et al. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–92. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- 51.Llodra J, et al. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–84. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trogan E, et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–6. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reis ED, et al. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein E-deficient mouse in a novel transplantation model. J Vasc Surg. 2001;34:541–7. doi: 10.1067/mva.2001.115963. [DOI] [PubMed] [Google Scholar]
- 54.Trogan E, et al. Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arterioscler Thromb Vasc Biol. 2004;24:1714–9. doi: 10.1161/01.ATV.0000139313.69015.1c. [DOI] [PubMed] [Google Scholar]
- 55.Angeli V, et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–74. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Wallet MA, Sen P, Tisch R. Immunoregulation of dendritic cells. Clin Med Res. 2005;3:166–75. doi: 10.3121/cmr.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 58.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 59.Trogan E, et al. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2002;99:2234–9. doi: 10.1073/pnas.042683999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trogan E, Fisher EA. Laser capture microdissection for analysis of macrophage gene expression from atherosclerotic lesions. Methods Mol Biol. 2005;293:221–31. doi: 10.1385/1-59259-853-6:221. [DOI] [PubMed] [Google Scholar]
- 61.Forster R, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 62.Lehmann JM, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–40. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 63.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–70. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 64.Levin N, et al. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol. 2005;25:135–42. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- 65.Beaven SW, Tontonoz P. Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu Rev Med. 2006;57:313–29. doi: 10.1146/annurev.med.57.121304.131428. [DOI] [PubMed] [Google Scholar]
- 66.Feig JE, et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Gils JM, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rong JX, et al. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104(20):2447–52. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 69.Feig JE, et al. ApoAI is required for the regression of atherosclerosis and is a potent regulator of plaque monocyte-derived emigration and inflammatory state. Arterioscler Thromb Vasc Biol. 2009;29(7):e13. [Google Scholar]
- 70.Feig JE, et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108(17):7166–71. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nicholls SJ, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. Jama. 2007;297(5):499–508. doi: 10.1001/jama.297.5.499. [DOI] [PubMed] [Google Scholar]
- 72.Damas JK, et al. Enhanced expression of the homeostatic chemokines CCL19 and CCL21 in clinical and experimental atherosclerosis: possible pathogenic role in plaque destabilization. Arterioscler Thromb Vasc Biol. 2007;27(3):614–20. doi: 10.1161/01.ATV.0000255581.38523.7c. [DOI] [PubMed] [Google Scholar]
- 73.Feig JE, et al. Statins Promote the Regression of Atherosclerosis via Activation of the CCR7-Dependent Emigration Pathway in Macrophages. PLoS One. 2011;6(12):e28534. doi: 10.1371/journal.pone.0028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicholls SJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078–87. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 75.Zhu X, et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283(34):22930–41. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feig JE, et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123(9):989–98. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bouhlel MA, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6(2):137–43. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 78.Chinetti-Gbaguidi G, et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARgamma and LXRalpha pathways. Circ Res. 2011;108(8):985–95. doi: 10.1161/CIRCRESAHA.110.233775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rayner KJ, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121(7):2921–31. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rayner KJ, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478(7369):404–7. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voight BF, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ost CR, Stenson S. Regression of peripheral atherosclerosis during therapy with high doses of nicotinic acid. Scand J Clin Lab Invest Suppl. 1967;99:241–5. [PubMed] [Google Scholar]
- 83.Brown BG, Zhao XQ, Sacco DE, Albers JJ. Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation. 1993;87:1781–91. doi: 10.1161/01.cir.87.6.1781. [DOI] [PubMed] [Google Scholar]
- 84.Farmer JA, Gotto AM., Jr Dyslipidemia and the vulnerable plaque. Prog Cardiovasc Dis. 2002;44:415–28. doi: 10.1053/pcad.2002.123474. [DOI] [PubMed] [Google Scholar]
- 85.Stary HC, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–31. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 86.Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998;339:1972–8. doi: 10.1056/NEJM199812313392703. [DOI] [PubMed] [Google Scholar]
- 87.Nissen SE, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–80. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 88.Nissen SE, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. Jama. 2006;295:1556–65. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 89.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 90.Wiviott SD, et al. Can low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy: a PROVE IT-TIMI 22 substudy. J Am Coll Cardiol. 2005;46:1411–6. doi: 10.1016/j.jacc.2005.04.064. [DOI] [PubMed] [Google Scholar]
- 91.Nissen SE, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. Jama. 2003;290(17):2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 92.Tardif JC, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. Jama. 2007;297(15):1675–82. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 93.Shaw JA, et al. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103(10):1084–91. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 94.Robins SJ, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. Jama. 2001;285(12):1585–91. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 95.Taylor AJ, et al. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002;106(16):2055–60. doi: 10.1161/01.cir.0000034508.55617.65. [DOI] [PubMed] [Google Scholar]
- 96.Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin. 2006;22(11):2243–50. doi: 10.1185/030079906x148508. [DOI] [PubMed] [Google Scholar]
- 97.Taylor AJ, et al. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110(23):3512–7. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 98.Taylor AJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361(22):2113–22. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 99.Yu BL, Zhao SP. Anti-inflammatory effect is an important property of niacin on atherosclerosis beyond its lipid-altering effects. Med Hypotheses. 2007;69(1):90–4. doi: 10.1016/j.mehy.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 100.McKenney JM, et al. Effect of niacin and atorvastatin on lipoprotein subclasses in patients with atherogenic dyslipidemia. Am J Cardiol. 2001;88(3):270–4. doi: 10.1016/s0002-9149(01)01639-3. [DOI] [PubMed] [Google Scholar]
- 101.Superko HR, Krauss RM. Differential effects of nicotinic acid in subjects with different LDL subclass patterns. Atherosclerosis. 1992;95(1):69–76. doi: 10.1016/0021-9150(92)90177-i. [DOI] [PubMed] [Google Scholar]
- 102.Brown BG, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583–92. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 103.Boden WE, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 104.Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med. 2012;79(1):38–43. doi: 10.3949/ccjm.79a.11166. [DOI] [PubMed] [Google Scholar]
- 105.Barter PJ, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 106.Barter P. Lessons learned from the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Am J Cardiol. 2009;104(10 Suppl):10E–5E. doi: 10.1016/j.amjcard.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 107.Nicholls SJ, et al. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation) Circulation. 2008;118(24):2506–14. doi: 10.1161/CIRCULATIONAHA.108.790733. [DOI] [PubMed] [Google Scholar]
- 108.Yvan-Charvet L, et al. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler Thromb Vasc Biol. 2010;30(7):1430–8. doi: 10.1161/ATVBAHA.110.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yvan-Charvet L, et al. Inhibition of cholesteryl ester transfer protein by torcetrapib modestly increases macrophage cholesterol efflux to HDL. Arterioscler Thromb Vasc Biol. 2007;27(5):1132–8. doi: 10.1161/ATVBAHA.106.138347. [DOI] [PubMed] [Google Scholar]
- 110.Luscher TF, et al. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. Eur Heart J. 2012;33(7):857–65. doi: 10.1093/eurheartj/ehs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cannon CP, et al. Design of the DEFINE trial: determining the EFficacy and tolerability of CETP INhibition with AnacEtrapib. Am Heart J. 2009;158(4):513–519 e3. doi: 10.1016/j.ahj.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 112.Cannon CP, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363(25):2406–15. doi: 10.1056/NEJMoa1009744. [DOI] [PubMed] [Google Scholar]
- 113.Nicholls SJ, et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. Jama. 2011;306(19):2099–109. doi: 10.1001/jama.2011.1649. [DOI] [PubMed] [Google Scholar]
- 114.Hewing B, Fisher EA. Rationale for cholesteryl ester transfer protein inhibition. Curr Opin Lipidol. 2012;23(4):372–6. doi: 10.1097/MOL.0b013e328353ef1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miyamoto Y, et al. Plaque characteristics of thin-cap fibroatheroma evaluated by OCT and IVUS. JACC Cardiovasc Imaging. 2011;(6):638–46. doi: 10.1016/j.jcmg.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 116.Dixon SR, et al. Analysis of target lesion length before coronary artery stenting using angiography and near-infrared spectroscopy versus angiography alone. Am J Cadiol. 2012;109(1):60–6. doi: 10.1016/j.amjcard.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 117.Weinreb DB, et al. Non-invasive MRI of mouse models of atherosclerosis. NMR Biomed. 2007;20(3):256–64. doi: 10.1002/nbm.1148. [DOI] [PubMed] [Google Scholar]
- 118.Hyafil F, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13(5):636–41. doi: 10.1038/nm1571. [DOI] [PubMed] [Google Scholar]
- 119.Rosenbaum D, et al. Molecular imaging in atherosclerosis: FDG PET. Curr Atheroscler Rep. 2012;14(5):429–37. doi: 10.1007/s11883-012-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feig JE, et al. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS One. 2012;7(6):e39790. doi: 10.1371/journal.pone.0039790. [DOI] [PMC free article] [PubMed] [Google Scholar]