Abstract
Although bladder cancer (BC) is a significant health threat to the US population, integrated clinical and laboratory investigations of this disease lag behind those of other types of cancer. Advances in BC are especially challenged due in part to a general decreased level of funding over the past 5 years. It is ironic that despite the awareness that BC is the 5th most commonly diagnosed solid malignancy in the United States, and one of the most costly to treat, funding for this organ site lags far behind that of other less common malignancies. Moreover, BC offers several unique opportunities for translational research that make it an ideal candidate for investigation. One distinct advantage over other solid tumor sites is that urine and tissue are readily available for translational studies that can direct the development of novel therapy for this disease. The NCI sponsored “Novel Neoadjuvant Therapy for Bladder Cancer” forum held in brought leading clinical and laboratory-based scientists together with the advocacy community to lay the groundwork for collaborative discovery and translation. The goal of the meeting was to bridge the gaps in translational science and develop the concepts for two novel biomarker-driven clinical trials, one in the neoadjuvant presurgical setting and the other in the setting of bladder preservation with chemoradiation. The meeting provided a unique opportunity to launch a collective effort to establish molecular-based therapy for UC. Herein, we summarize the proceedings of this meeting, and the future plans resulting from this forum.
Introduction
Bladder cancer (BC) is a significant health threat to the US population, affecting approximately 70,000 individuals and resulting in an estimated 15,000 deaths in the US annually [1]. Moreover, the cost of clinical care to the nation is substantial, over 50% more than the care of patients with prostate cancer (Agency for Health Care Policy & Research, 1995 and 1999, U.S. Public Health Service (HHS). Limited progress has been made in BC therapy over the past several decades as a consequence of: 1) the lack of targeted agents and associated predictive biomarkers for clinical management; 2) the paucity of published data supporting the implementation of new and potentially effective therapeutics in BC; and 3) limited funding for integrated clinical and laboratory investigation of BC compared to other US cancers. To address these issues, the National Cancer Institute (NCI) sponsored the “Novel Neoadjuvant Therapy for Bladder Cancer” forum in September of 2011 in Bethesda Maryland, which brought together clinicians, scientists and patient advocates to establish meaningful collaborations to accelerate translational research and innovative clinical trial design in BC. A primary goal of the forum was to lay the groundwork for two prospective multi-disciplinary clinical trials for patients with muscle-invasive bladder cancer (MIBC); that will prospectively validate novel biomarkers that predicts benefit for patients receiving neoadjuvant chemotherapy (NAC) in the cystectomy setting and the other for patients managed with a combination of radiation and chemotherapy for bladder preservation.
Designing “smart trials” in bladder cancer
BC is a heterogeneous malignancy even when the disease is confined to the bladder. Despite laboratory discoveries identifying specific tumor subtypes which exhibit distinct genetic and molecular signatures [2], current clinical practice treats these molecularly diverse cancers similarly. Innovations in high-throughput array-based assays for both gene sequencing and expression analysis should bridge this gap and facilitate the development of ‘precision’ medicine for this disease [3,4]. In other diseases such as breast cancer, tumor-specific signatures are utilized to stratify patients according to disease aggressiveness and inform clinical decisions [3,4].
The focus of the NCI forum was to promote the application of biomarkers to conventional and investigational clinical trial design. Working groups focused on two specific aspects of biomarker development. One group reviewed emerging biomarkers and high-throughput technologies that may be relevant for predicting sensitivity or resistance of BC to therapeutic regimens, while the other discussed tissue-based applications for analyzing the proposed biomarker(s) and associated technologies using new standards in the field. The results from these discussions were ultimately incorporated into the design of the two clinical trials that emerged from the summit.
The workshop participants reached the consensus that the analysis of any proposed biomarker should be feasible using routine clinical materials (i.e., formalin- fixed, paraffin-embedded tissues; FFPE) with attention to new strategies for specimen collection, preservation, processing and analysis that minimize preanalytic confounders inherent to the processing of FFPE tissue [5–7]. These recommendations reflected several practical considerations including: 1) pre-therapy tumor is often sampled by transurethral resection (TUR) and tumor material may be limited and/or fully submitted for pathology diagnosis; 2) the volume of tumor remaining post-therapy may also be limited, especially if downstaging from prior treatment has occurred; and, 3) accurate biomarker comparisons require material to be available from patients both pre- and post-therapy which most commonly involves the use of FFPE material. This pragmatism contrasts with the strict tissue quality control standards that The Cancer Genome Atlas (TCGA) requires of the tumors that are being deeply annotated within their projects, where rapidly flash frozen materials are required and specimens must have minimal amounts of necrosis. While the practicality of using FFPE material for study was agreed upon, limitations of this approach were also noted. Specifically, whole genome DNA and RNA sequencing technologies are still not optimized for use with FFPE tissues and lack of these technologies may limit discovery of novel predictive biomarkers in the proposed BC trials. In lieu of these approaches, the working groups recommended that more focused methods of biomarker identification be applied, including DNA exome sequencing and measurements of copy number variations, immunohistochemistry, gene expression profiling and whole genome micro RNA analysis.
There was talk of attempting to link the presence of one of the more common MIBC mutations to response to a novel biological agent but the consensus was that an approach focused on predicting response to existing conventional therapies would have more near-term impact and potentially attract pharmaceutical interest and support. As current therapies are based on the use of DNA damaging agents[8], the group considered how tumor sensitivity or resistance might be linked to mutational status and/or relative expression of DNA repair enzymes. Examples of such putative biomarkers include ERCC1[8], BRCA1/2 [9,10], and/or PTEN11 for cisplatin-based therapy, MRE11 [12] for radiation-based therapy, and/or “p53-ness” [13,14]. Immunohistochemistry (IHC), expression profiling, and focused gene sequencing can be readily employed to assess these biomarkers.
There was considerable interest in isolating and characterizing cancer stem cells (CSCs) within the trial designs. One speaker introduced evidence implicating CSCs that lack MHC class I expression in chemoresistance and tumor relapse. Other speakers pointed to critical roles for “basal cell” markers, such as Np63 [15,16], miR- 205 [17], the 67kD laminin receptor[18] and cytokeratins 5 and 14 [19–21] in promoting bladder tumorigenesis and progression. These putative CSC markers could be analyzed by CSC-specific IHC and/or mRNA measurements of pre- and post-therapy specimens and correlated with de novo resistance to conventional therapy.
The use of proper methodologies is crucial to ensure that biomarker evaluation in FFPE is both reproducible and quantitative. It was determined that appropriate pathological assessment and designation of standard operating procedures (SOPs) for tissue collection and storage be defined prior to the initiation of a new clinical trial. Specific considerations in pathological assessment include: 1) recognition of prognostic histological features such as variant morphology, angiolymphatic invasion, and depth of invasion that may independently predict outcome; 2) characterization of tumor composition with emphasis on tumor heterogeneity; and, 3) identification of features that reflect suboptimal specimen handling and possible variability in biomarker analysis. Additionally, specification of tissue collection and storage protocols that optimize specimen preservation was detailed. Recent reports have shown that changes in protein/phosphoprotein levels occur during the slow (1 mm/hr) fixation time of formalin, rendering the molecular information suspect [22–25]. However, new types of rapid molecular fixatives [5] and tissue processing methods [6] have been recently developed specifically to preserve these labile analytes and these advances in generating FFPE- like histomorphology and immunohistochemical results may be incorporated into new trial designs [7]. When significant tumor heterogeneity is evident, in situ-based methods, such as laser capture microdissection (LCM), may be valuable for assessing biomarker expression and molecular profiles in different tumor regions, as well as in the associated stroma [26,27]. A recommendation was made to generate tissue microarrays (TMAs) from tissues collected in clinical trials, in order to reduce “batch” variability in IHC-based studies, and to use the SOPs developed by a recent National Institutes of Health (NIH) sponsored a working group (http://cdp.cancer.gov/diagnostics/templates.htm and http://ctep.cancer.gov/protocolDevelopment/default.htm#protocoldevelopmentwebsite link) when evaluating the results. In addition, index TMAs, which are composed of a mix of patient specimens and cell lines, can be used to standardize the reproducibility of antibody use from batch to batch, and between different laboratories. Immunohistochemistry can also be more quantitative with the use of emerging technology. Recent studies in breast cancer focused on estrogen receptor (ER) IHC [28] reported that a more sophisticated Analytical Quantitative Assessment (AQUA) approach improved the predictive power of immunostains [29] over standard light microscopy interpretation of IHC.
Phosphorylated protein assessment may be possible using new fixatives and emerging technologies such as the Reverse Phase Protein Microarrays (RPPA) [30,31] that allows the quantitative measurements of hundreds of phosphoproteins and proteins from microscopic-sized cellular inputs including fine-needle aspiration (FNA) and needle biopsy [32,33] and has recently been used to analyze biomarkers as part of the ISPY-2 breast cancer trial (http://ispy2.org). Finally, it was recommended that genetic and molecular testing including gene expression (Affymetrix and Illumina’s DASL platforms), mutational and copy number analysis, and whole genome micro RNA analysis using either quantitative real-time PCR or Nanostring technology be incorporated into biomarker analysis going forward.
The novel gene expression algorithm, Co-eXpression ExtrapolatioN (COXEN), which combines in vitro and in vivo molecular profiling of cancer cell lines and drug responsiveness [34], has recently been shown to be a useful tool for drug discovery[35] and one that can translate drug sensitivity of carcinoma cell lines into prediction of clinical responses of patients. The COXEN algorithm starts by deriving candidate biomarkers by comparing gene expression data between cell lines from the NCI-60 that are sensitive or resistant to drug X, a step which can be regarded as biomarker discovery. Next candidate biomarkers are triaged to ensure concordant expression between cell line and tumor (for the tumor of interest) gene expression data sets. The concordant biomarkers are then used to derive gene expression biomarkers (GEMs), predictive of sensitivity to individual drugs or combinations. Finally, such models are used to classify cell lines or tumors on the basis of their own gene expression data, and prediction scores are examined with respects to empirical (in vitro) or observed (clinical trial) response outcomes. In the case of drug discovery, GEMs can be derived for each of thousands of drugs tested against the NCI-60 panel, then drugs ranked by percentage predicted responders to prioritize them for preclinical evaluation. Additional details about the COXEN approach can be found in the following publication [34].
In retrospective studies, multigene biomarker panels developed using COXEN have been shown to effectively stratify clinical response in patients with a variety of tumor types including breast [36], ovarian [37], lung [38] and head and neck [39] treated with chemotherapy and/or radiation. In the case of BC, GEMs panels developed using COXEN have been shown to predict clinical responses of patients treated with GC and M-VAC [34] in several clinical settings including neoadjuvant use. If successful in predicting the benefit of neoadjuvant chemotherapy, this approach would be similar to the Oncotype DX testing paradigm developed for the adjuvant treatment of breast cancer. As the patient-based component of this work has been previously performed on FFPE material [40], it is likely that this approach could be readily used in assessing pre- therapy BC material from the newly designed trials.
Incorporation of biomarkers and biomarker-based endpoints into correlative studies needs to be done prospectively with appropriate statistical design and analysis plans. Different levels of biomarker validation must also be considered. Biomarkers intended for making treatment decisions (integral biomarkers) would require a higher level of assay validation (for example specificity, sensitivity and precision) than biomarkers not intended for making treatment decisions (integrated or exploratory biomarkers). A large, formal prospective validation study will be required to confirm the effectiveness of a biomarker to predict outcome or benefit of a specific therapy; a prerequisite for the successful completion of such a validation trial is robust prior (preliminary) data. In fact, the vast majority of trials involving biomarkers are conceived in order to obtain these preliminary data. Importantly, a clear association between the marker and the clinical outcome of interest is a necessary (but not sufficient) condition for a marker to be useful for prediction; an early study is needed to establish this association.
Clinical trial design using the cystectomy model
Despite level-one evidence supporting the benefit of NAC with methotrexate, doxorubicin, vinblastine and cisplatin (M-VAC) for MIBC [41–45], contemporary studies have shown dramatic underutilization of this approach, with less than 20% of patients receiving the recommended care [46]. There are several explanations for the lack of widespread acceptance of NAC. The overall benefit to an unselected population with MIBC is modest, revealed by the approximate 14% improvement in 5-year survival reported by SWOG 8710 [44]. Furthermore, only the 30–40% of those rendered pT0 or ≤ pT1 benefit [44,47], and it is still not possible to reliably identify these individuals prior to chemotherapy. And while peri-operative mortality from NAC is unaffected, the morbidity from chemotherapy in this older population of patients is significant; it is thus desirable to limit NAC to patients most likely to benefit. Many patients are likewise ineligible for cisplatin based chemotherapy on the basis of renal insufficiency and other co-morbidities [46]. To add to the impasse, not everyone requires NAC; patients with pathologically confirmed organ-confined MIBC have a durable disease-free survival approaching 85% [48]. The dilemma is furthered by the knowledge that current clinical staging paradigms are inadequate to accurately identify those with extravesical disease, such that more than 50% of patients at risk of harboring occult metastasis are clinically understaged [49]. We are left with the quandary of either offering NAC to every eligible patient regardless of their risk or likelihood of benefit, or the challenges of identifying those likely to progress despite local therapy, and developing biomarkers that identify those likely to respond to systemic therapy. The latter, more selective approach may ultimately result in a higher rate of NAC use among urologists and medical oncologists and serves as the basis for the NAC clinical trials discussion at this forum.
In moving the care of BC forward, a rational application of our past successes, most notably NAC must be made in the context of emerging knowledge of the molecular genetics underlying BC pathogenesis and the development of new biomarkers that prospectively identify BCs that are likely to respond to conventional and investigational therapies. Bladder cancer offers several unique opportunities for translational research that make it an ideal candidate for investigation. Pathologic response assessment to NAC is a reliable surrogate for patient survival and urine and tissue are readily available pre- and post-operatively for translational pharmacodynamic studies [34]. Innovations in high-throughput array-based assays for both gene sequencing and expression analysis have made possible the development of ‘precision’ medicine based on molecular profiling. These tumor-specific signatures can be utilized to stratify patients according to disease aggressiveness and inform clinical decisions. The awareness of tumor heterogeneity and differences in molecular profiles can be addressed in both pre- and post-treatment specimens. These opportunities make the neoadjuvant setting ideal for the development and assessment of novel therapeutics in BC to advance a “precision” approach to stratify patients according to disease aggressiveness and inform clinical decisions.
A neoadjuvant clinical trial to prospectively test the hypothesis that COXEN can be used to predict the efficacy of cisplatin-based chemotherapy has been proposed to the Southwest Oncology Group (SWOG). As many of the workshop participants were involved with the design of this trial, it was agreed upon that this trial should serve as the basis for an expanded trial that would incorporate additional relevant biomarkers, including DNA repair genes and CSC markers, as exploratory endpoints. The draft study design is found is Figure 1; in brief, patients with MIBC will be randomized to either gemcitabine and cisplatin (GC) or MVAC chemotherapy. Gene expression, microRNA expression and patient samples for TMA construction will be collected prior to chemotherapy. The COXEN algorithm and biomarker expression panel will be used to confirm whether it accurately predicts response to chemotherapy, defined as resolution of the tumor (pT0) in the cystectomy specimen. If successful, this predictive paradigm would provide a rational basis for the administration of NAC in patients defined as chemotherapy sensitive. Specifically, this model would be used to recommend immediate cystectomy in those patients unlikely to respond to NAC thus avoiding potentially detrimental delays and unnecessary toxicity from predicted ineffective chemotherapy. Furthermore, this model would also allow patients who are likely to respond to chemotherapy to be recommended for the optimal chemotherapeutic regimen based on their COXEN gene expression profile. Robust surgical quality control is mandated in the trial design to minimize the variability in surgical technique as a confounder in interpreting the impact of NAC on patient survival.
FIGURE 1.
SWOG COXEN-directed neoadjuvant chemotherapy trial: This Phase 2 Biomarker validation and discovery will randomize 184 eligible subjects between GC and MVAC chemotherapy, stratified by clinical stage (T2 vs. T3-T4a) and tumor grade. Cystectomy is recommended between 14 and 56 days from the last dose of neoadjuvant chemotherapy. Primary study objective is to characterize the relationship of MVAC-and GC-specific COXEN scores in terms of pT0 rate in patients treated with neoadjuvant chemotherapy. This will be done in three ways 1) assess whether either treatment-specific COXEN score is prognostic of pT0 rate or favorable downgrading in this patient population; 2) evaluate the correlation between the GC COXEN score and the MVAC COXEN score; 3) assess in a preliminary fashion whether either COXEN score is predictive of the respective treatment regimen’s effect on pT0 rate. This study will provide a rich tissue resource for future predictive and prognostic biomarker studies in bladder cancer, develop the intergroup infrastructure to complete neoadjuvant trials in bladder cancer and transform thinking about patient selection for neoadjuvant chemotherapy in TCC.
The trial, “A Randomized Phase II Study of CO-eXpression ExtrapolatioN (COXEN)-Directed Neoadjuvant Chemotherapy for Localized, Muscle-Invasive Bladder Cancer” has been evaluated and approved by the NCI Genitourinary Steering Committee. It has also undergone expedited approval by the Cancer Therapy and Evaluation Program (CTEP) and the final stages of the full protocol development are currently underway.
Bladder preservation and utility in novel clinical trial models for bladder cancer
Bladder preservation is an option for many patients with MIBC, notably those who are elderly or with significant co-morbidities. The safety and effectiveness of bladder conservation therapy for MIBC patients by trimodality therapy (TMT) using maximal TURBT and concurrent chemoradiation has been established by the Radiation Therapy Oncology Group (RTOG) with complete response (CR) rates in the order of 67–74%, and with cure of the primary tumor and bladder preservation with adequate bladder function in over 85% of those achieving a CR [50]. The need for an alternative therapy to radical cystectomy in the elderly is highlighted in data from American College of Surgeons Commission on Cancer accredited facilities, in which almost 30,000 MIBC patients were characterized by treatment patterns [51]. Significantly fewer patients with advanced age received potentially curative treatment (cystectomy or TMT or XRT alone); only 45% of those over age 70 and only 33% of those over age 80. This data indicates a significant unmet clinical need in physician education regarding appropriate selection of elderly patients for definitive therapy of MIBC. If the patient is not suitable for consideration of cystectomy, TMT certainly could bridge the gap and offer more patients cure from BC. Recent analysis of outcomes of patients entered on TMT protocols indicate the elderly have good outcomes with disease-specific survivals at 5 and 10 years equal to the younger patients [50,52]. However, radiosensitization with cisplatin may not be well tolerated by some older patients with MIBC, especially those with impaired renal function or poor performance status. The U.K. multicenter Bladder Cancer Phase III study randomized 360 patients with MIBC with a median age of 72 years to undergo radiotherapy alone or with synchronous chemotherapy, consisting of 5-FU (500 mg/m2 on days 1–5 and 22–26) and mitomycin C (12 mg/m2 given only on day 1) [53]. With a median follow-up of 70 months, the 2-year locoregional recurrence free survival was 67 %, invasive local relapse rate was 18 %, and 5-year OS rate was 48 % in the chemo-RT arm, with a statistically significant improved locoregional free survival hazard ratio (HR) of 0.68 (p=.03). Out of 360 patients, 51 (14 %) underwent cystectomy. Although this study did not compare 5-FU/mitomycin C with cisplatin- based chemoradiation, these data clearly established an alternative effective and tolerable radiosensitization regimen [54]. A 2008 United Kingdom retrospective analysis of radiotherapy or cystectomy as curative treatment for MIBC patients found similar and stable disease specific survival rates of 55% at from 5 to 9 years [55]. The Massachusetts General Hospital group also recently reported 59% and 57% disease specific survivals at 10- and 15- years for 348 MIBC patients [56]. These results were similar to the large retrospective cystectomy series for comparably clinically staged MIBC patients from the University of Southern California and Bern, Switzerland [57].
Unfortunately until recently there has been limited enthusiasm for this approach in North America and, as such, bladder preservation has not been widely endorsed by urologists. The identification of biomarkers that reliably identify patients with MIBC likely be cured by well tolerated chemo-radiation could dramatically enhance the acceptance and use of this approach. At the NCI bladder cancer workshop there was an active discussion of recent biomarker discoveries for radiation based approaches. While several biomarkers (normal HER-2, High Ki-67 and High XRCC1) have been linked to a favorable response, the most encouraging translational research data comes from Leeds, Manchester and Oxford in the United Kingdom. They, in several separate cohorts of MIBC patients, reported the pre-treatment tumor specimen evaluated by of IHC tumor staining for MRE 11 expressions (which is active in the cellular response to radiation) was predictive of bladder tumor eradication following radiation alone [12]. Patients with high expression of MRE 11 had significantly higher cancer specific survival after radiation alone (65% vs. 40% at 3 years, p< 0.001). In contrast higher MRE 11 staining levels were not associated with higher cancer specific survival following cystectomy.
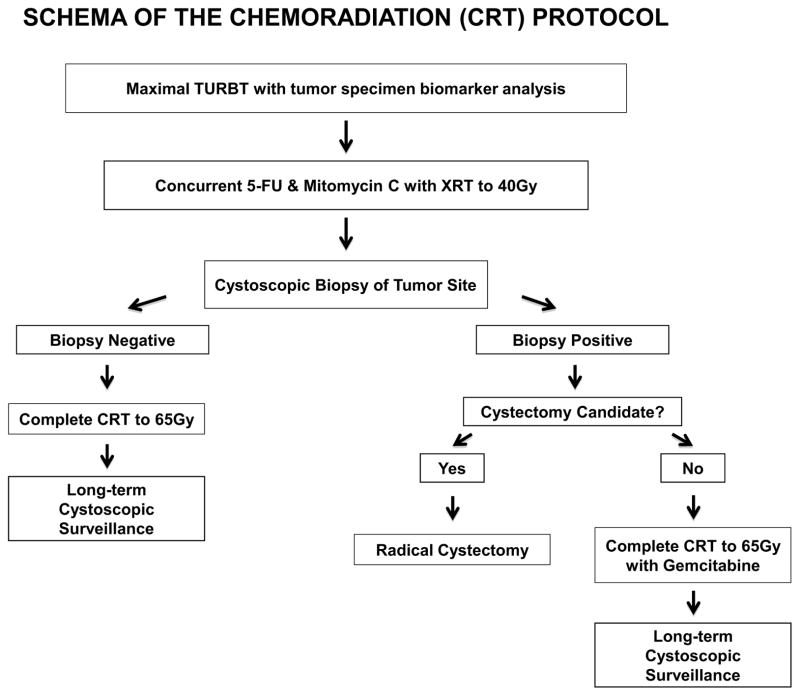
The bladder preservation trial that evolved from this NCI-sponsored forum will use a similar structure as the recent phase III Birmingham, UK trial that showed increased bladder tumor eradication without recurrence of an invasive tumor following concurrent 5-FU and Mitomycin C (FMC) chemotherapy with radiation as compared to radiation alone (82% vs. 68%, p < 0.001) without a significant increase in acute or late toxicity [53]. This chemo-radiation regimen is a more desirable, less toxic alternative to concurrent cisplatin chemotherapy with radiation for elderly MIBC patients because 40–50% of elderly patients have inadequate renal function to tolerate cisplatin. Secondary correlative studies will correlate the pretreatment of expression of MRE 11 by IHC with local CR and disease-specific survival and the tumor CR rate to induction chemoradiation. A draft of the study design which received overwhelming support is shown in Figure 2.
Figure 2.
Schema for the proposed NCI Bladder Task Force translational research trial to evaluate tumor biomarkers for predicting response to concurrent chemoradiation.
Future novel therapies for bladder cancer
The last element needed for a more customized treatment approach in BC is an increased availability of targeted agents for this disease; as of the current publication, no FDA approved targeted agents for BC are available. Future opportunities that link the presence of a common MIBC mutation to response to a novel biological agent, such as FGFR3 to an FGFR inhibitor, or PIK3CA, TSC-1, or PTEN to a PI3 kinase/AKT/mTOR inhibitor [58,59,60], hold unique promise in BC patients, where NAC regimens may be employed and pre- and post-therapy specimens are readily acquired. Ongoing large-scale efforts in BC, including the Cancer Genome Atlas Project (TCGA) sponsored by the NCI, are likely to yield new insights into MIBC. The dedication of the BC clinical and research community to discovery and application of these new findings is clear, given the commitment and enthusiasm of the participants at this forum.
Summary
The NCI-sponsored forum on Novel Neoadjuvant Therapy for Bladder Cancer was met with overwhelming enthusiasm and provided a unique opportunity to launch a collective effort to establish molecular-based therapy for BC. Leading basic scientists were engaged prospectively so that both trials were designed with a focus on hypothesis testing and high quality translational research as top priorities. There is now a unique opportunity to capitalize on this multi-disciplinary forum to conduct prospective clinical trials with the intent of establishing new paradigms for the treatment of BC cancer based on state-of –the-art scientific discoveries. Support from the oncology community will be instrumental in achieving this goal.
Acknowledgments
This Workshop was supported by resources from the National Cancer Institute of the National Institutes of Health. Dr. Colin Dinney wishes to acknowledge grant support from the SPORE in Genitourinary Cancer at The University of Texas MD Anderson Cancer Center (P50 CA91846). Dr. Dean Bajorin wishes to acknowledge support from the Clinical and Translational Science Center at the Weill Medical College of Cornell University (UL1-RR024996)
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jema A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Sjodahl G, Lauss M, Lovgren K, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012;18:3377–86. doi: 10.1158/1078-0432.CCR-12-0077-T. [DOI] [PubMed] [Google Scholar]
- 3.Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–49. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 5.Mueller C, Edmiston KH, Carpenter C, et al. One-step preservation of phosphoproteins and tissue morphology at room temperature for diagnostic and research specimens. PLoS One. 2011;6(8):e23780. doi: 10.1371/journal.pone.0023780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rountree CB, Van Kirk CA, You H, et al. Clinical application for the preservation of phospho-proteins through in-situ tissue stabilization. Proteome Sci. 2010;8:6. doi: 10.1186/1477-5956-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicks DG, Boyce BF. The challenge and importance of standardizing pre-analytical variables in surgical pathology specimens for clinical care and translational research. Biotech Histochem. 2012;87:14–17. doi: 10.3109/10520295.2011.591832. [DOI] [PubMed] [Google Scholar]
- 8.Sonpavde G, Sternberg CN. Treatment of metastatic urothelial cancer: opportunities for drug discovery and development. BJU Int. 2008;102:1354–60. doi: 10.1111/j.1464-410X.2008.07982.x. [DOI] [PubMed] [Google Scholar]
- 9.Bartz SR, Zhang Z, Burchard J, et al. Small interfering RNA screens reveal enhanced cisplatin cytotoxicity in tumor cells having both BRCA network and TP53 disruptions. Mol Cell Biol. 2006;26:9377–86. doi: 10.1128/MCB.01229-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narod SA. BRCA mutations in the management of breast cancer: the state of the art. Nat Rev Clin Oncol. 2010;7:702–707. doi: 10.1038/nrclinonc.2010.166. [DOI] [PubMed] [Google Scholar]
- 11.Mendes-Pereira AM, Martin SA, Brough R, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1:315–22. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhury A, Nelson LD, Teo MT, et al. MRE11 expression is predictive of cause- specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 2010;70:7017–26. doi: 10.1158/0008-5472.CAN-10-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esserman LJ, Berry DA, Cheang MC, et al. Chemotherapy response and recurrence- free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Breast Cancer Res Treat. 2012;132:1049–62. doi: 10.1007/s10549-011-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troester MA, Herschkowitz JI, Oh DS, et al. Gene expression patterns associated with p53 status in breast cancer. BMC Cancer. 2006;6:276. doi: 10.1186/1471-2407-6-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi W, Shah JB, Tran M, et al. p63 expression defines a lethal subset of muscle- invasive bladder cancers. PLoS One. 2012;7(1):e30206. doi: 10.1371/journal.pone.0030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karni-Schmidt O, Castillo-Martin M, Shen TH, et al. Distinct expression profiles of p63 variants during urothelial development and bladder cancer progression. Am J Pathol. 2011;178:1350–60. doi: 10.1016/j.ajpath.2010.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran MN, Choi W, Wszolek MF, et al. The p63 isoform DNp63a inhibits epithelial- mesenchymal transition in human bladder cancer cells: Role of miR-205. J Biol Chem. 2013;288 (5):3275–88. doi: 10.1074/jbc.M112.408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandt WD, Matsui W, Rosenberg JE, et al. Urothelial carcinoma: stem cells on the edge. Cancer Metastasis Rev. 2009;28:291–304. doi: 10.1007/s10555-009-9187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KS, Espinosa I, Chao M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:14016–21. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho PL, Kurtova A, Chan KS. Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat Rev Urol. 2012;9:583–94. doi: 10.1038/nrurol.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkmer JP, Sahoo D, Chin RK, et al. Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc Natl Acad Sci U S A. 2012;109:2078–83. doi: 10.1073/pnas.1120605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai Y, Tolles J, Cheng H, et al. Quantitative assessment shows loss of antigenic epitopes as a function of pre-analytic variables. Lab Invest. 2011;91:1253–61. doi: 10.1038/labinvest.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espina V, Edmiston KH, Heiby M, et al. A portrait of tissue phosphoprotein stability in the clinical tissue procurement process. Mol Cell Proteomics. 2008;7:1998–2018. doi: 10.1074/mcp.M700596-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espina V, Mueller C. Reduction of preanalytical variability in specimen procurement for molecular profiling. Methods Mol Biol. 2012;823:49–57. doi: 10.1007/978-1-60327-216-2_4. [DOI] [PubMed] [Google Scholar]
- 25.Holzer TR, Fulford AD, Arkins AM, et al. Ischemic time impacts biological integrity of phospho-proteins in PI3K/Akt, Erk/MAPK, and p38 MAPK signaling networks. Anticancer Res. 2011;31:2073–81. [PubMed] [Google Scholar]
- 26.Silvestri A, Colombatti A, Calvert VS, et al. Protein pathway biomarker analysis of human cancer reveals requirement for upfront cellular-enrichment processing. Lab Invest. 2010;90:787–96. doi: 10.1038/labinvest.2010.47. [DOI] [PubMed] [Google Scholar]
- 27.Wulfkuhle JD, Speer R, Pierobon M, et al. Multiplexed cell signaling analysis of human breast cancer applications for personalized therapy. J Proteome Res. 2008;7:1508–17. doi: 10.1021/pr7008127. [DOI] [PubMed] [Google Scholar]
- 28.Dimou A, Agarwal S, Anagnostou V, et al. Standardization of epidermal growth factor receptor (EGFR) measurement by quantitative immunofluorescence and impact on antibody-based mutation detection in non-small cell lung cancer. Am J Pathol. 2011;179:580–89. doi: 10.1016/j.ajpath.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welsh AW, Moeder CB, Kumar S, et al. Standardization of estrogen receptor measurement in breast cancer suggests false-negative results are a function of threshold intensity rather than percentage of positive cells. J Clin Oncol. 2011;29:2978–84. doi: 10.1200/JCO.2010.32.9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paweletz CP, Charboneau L, Bichsel VE, et al. Reverse phase protein microarrays which capture disease progression show activation of pro-survival pathways at the cancer invasion front. Oncogene. 2001;20:1981–89. doi: 10.1038/sj.onc.1204265. [DOI] [PubMed] [Google Scholar]
- 31.Wulfkuhle JD, Edmiston KH, Liotta LA, et al. Technology insight: pharmacoproteomics for cancer--promises of patient-tailored medicine using protein microarrays. Nat Clin Pract Oncol. 2006;3:256–68. doi: 10.1038/ncponc0485. [DOI] [PubMed] [Google Scholar]
- 32.Einspahr JG, Calvert V, Alberts DS, et al. Functional protein pathway activation mapping of the progression of normal skin to squamous cell carcinoma. Cancer Prev Res (Phila) 2012;5:403–13. doi: 10.1158/1940-6207.CAPR-11-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibarra-Drendall C, Troch MM, Barry WT, et al. Pilot and feasibility study: prospective proteomic profiling of mammary epithelial cells from high-risk women provides evidence of activation of pro-survival pathways. Breast Cancer Res Treat. 2012;132:487–98. doi: 10.1007/s10549-011-1609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JK, Havaleshko DM, Cho H, et al. A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proc Natl Acad Sci U S A. 2007 Aug 7;104(32):13086–91. doi: 10.1073/pnas.0610292104. Epub 2007 Jul 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith SC, Havaleshko DM, Moon K, et al. Use of yeast chemigenomics and COXEN informatics in preclinical evaluation of anticancer agents. Neoplasia. 2011;13(1):72–80. doi: 10.1593/neo.101214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen K, Song N, Kim Y, Tian C, et al. A systematic evaluation of multi-gene predictors for the pathological response of breast cancer patients to chemotherapy. PLoS One. 2012;7(11):e49529. doi: 10.1371/journal.pone.0049529. Epub 2012 Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferriss JS, Kim Y, Duska L, et al. Multi-gene expression predictors of single drug responses to adjuvant chemotherapy in ovarian carcinoma: predicting platinum resistance. PLoS One. 2012;7(2):e30550. doi: 10.1371/journal.pone.0030550. Epub 2012 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagji AS, Cho SH, Liu Y, et al. Multigene expression-based predictors for sensitivity to Vorinostat and Velcade in non-small cell lung cancer. Mol Cancer Ther. 2010;9(10):2834–43. doi: 10.1158/1535-7163.MCT-10-0327. Epub 2010 Aug 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams PD, Owens CR, Dziegielewski J, et al. Cyclophilin B expression is associated with in vitro radioresistance and clinical outcome after radiotherapy. Neoplasia. 2011;13(12):1122–31. doi: 10.1593/neo.111398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith SC, Baras AS, Dancik G, et al. A 20-gene model for molecular nodal staging of bladder cancer: development and prospective assessment. Lancet Oncol. 2011;12(2):137–43. doi: 10.1016/S1470-2045(10)70296-5. Epub 2011 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta- analysis. Lancet. 2003;361:1927–34. doi: 10.1016/s0140-6736(03)13580-5. [DOI] [PubMed] [Google Scholar]
- 42.Stenzl A, Cowan NC, De Santis M, et al. The updated EAU guidelines on muscle- invasive and metastatic bladder cancer. Eur Urol. 2009;55:815–25. doi: 10.1016/j.eururo.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Griffiths G, Hall R, Sylvester R, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–77. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 45.National Comprehensive Cancer Network. [Accessed 7/25/12];Bladder Cancer Guidelines Version 2.2012. http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- 46.Raj GV, Karavadia S, Schlomer B, et al. Contemporary use of perioperative cisplatin- based chemotherapy in patients with muscle-invasive bladder cancer. Cancer. 2011;117:276–82. doi: 10.1002/cncr.25429. [DOI] [PubMed] [Google Scholar]
- 47.Millikan R, Dinney C, Swanson D, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol. 2001;19:4005–13. doi: 10.1200/JCO.2001.19.20.4005. [DOI] [PubMed] [Google Scholar]
- 48.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 49.Svatek RS, Shariat SF, Novara G, et al. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int. 2011;107:898–04. doi: 10.1111/j.1464-410X.2010.09628.x. [DOI] [PubMed] [Google Scholar]
- 50.Mak RH, Hunt D, Shipley WU, Efstathiou JA, Yan Y, Tester WJ, Hagan MP, Kaufman DS, Heney NM, Zietman AL. Long-term outcomes after bladder preserving combined modality therapy for muscle-invasive bladder cancer: a pooled analysis of RTOG. 8802, 8903, 9506, 9706, 9906, and 0233. Int J Radiat Oncol Biol Phys. 2012;84(3):S121. [Google Scholar]
- 51.Gray PJ, Fedewa SA, Shipley WU, et al. use of potentially curative therapies for muscle-invasive bladder cancer in the united states: results from the National Cancer Data Base. Eur Urol. 2012;12:1346–52. doi: 10.1016/j.eururo.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 52.Clayman RH, Shipley WU, Galland-Girodet S, et al. Outcomes of selective bladder preservation in the elderly treated with conservative surgery and chemoradiation. Int J Radiat Oncol Biol Phys. 2013;87(2S):S202. [Google Scholar]
- 53.James ND, Hussain SA, Hall E, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366:1477–88. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 54.Efstathiou JA, Shipley WU. Re. Radiotherapy with or without chemotherapy in muscle-invasive Bladder Cancer. Eur Urol. 2013;63(1):181–2. doi: 10.1016/j.eururo.2012.10.032. doi:10.1056NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 55.Kotwal S, Choudhury A, Johnston C, et al. Similar treatment outcomes for radical cystectomy and radical radiotherapy in invasive bladder cancer treated at a United Kingdom specialist treatment center. Int J Radiat Oncol Biol Phys. 2008;70:456–63. doi: 10.1016/j.ijrobp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 56.Efstathiou JA, Spiegel DY, Shipley WU, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol. 2012;61:705–11. doi: 10.1016/j.eururo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Zehnder P, Studer UE, Skinner EC, et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: a comparative study. J Urol. 2011;186:1261–68. doi: 10.1016/j.juro.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Knowles MA, Platt FM, Ross RL, et al. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009;28:305–16. doi: 10.1007/s10555-009-9198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iyer G, Hanrahan AJ, Milowsky MI, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iyer G, Al-Ahmadie H, Schultz N, et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J Clin Oncol. 2013;31(25):3133–40. doi: 10.1200/JCO.2012.46.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]