Abstract
Background & Aims
Chronic hepatitis B (CHB) infection is associated with reduced bone mineral density, but its association with fractures is unknown. Our objectives were to determine whether untreated or treated CHB-infected persons are at increased risk for hip fracture compared to uninfected persons.
Methods
We conducted a cohort study among 18,796 untreated CHB-infected, 7,777 treated CHB-infected, and 979,751 randomly sampled uninfected persons within the U.S. Medicaid populations of California, Florida, New York, Ohio, and Pennsylvania (1999 – 2007). CHB infection was defined by two CHB diagnoses recorded >6 months apart and was classified as treated if a diagnosis was recorded and antiviral therapy was dispensed. After propensity score matching of CHB-infected and uninfected persons, Cox regression was used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) of incident hip fracture in: 1) untreated CHB-infected versus uninfected, and 2) treated CHB-infected versus uninfected patients.
Results
Untreated CHB-infected patients of black race had a higher rate of hip fracture than uninfected black persons (HR, 2.55 [95% CI, 1.42 – 4.58]). Compared to uninfected persons, relative hazards of hip fracture were increased for untreated white (HR, 1.26 [95% CI, 0.98 – 1.62]) and Hispanic (HR, 1.36 [95% CI, 0.77 – 2.40]) CHB-infected patients, and treated black (HR, 3.09 [95% CI, 0.59 – 16.22) and white (HR, 1.90 [95% CI, 0.81 – 4.47]) CHB-infected patients, but these associations were not statistically significant.
Conclusions
Among U.S. Medicaid enrollees, untreated CHB-infected patients of black race had a higher risk of hip fracture than uninfected black persons.
Keywords: Hepatitis B, fracture, bone
INTRODUCTION
Chronic hepatitis B (CHB) infection is a major global health problem, with an estimated 365 million persons affected worldwide (6% of the world population) [1–3]. Long-term sequelae of CHB infection, which include cirrhosis, hepatic decompensation, and hepatocellular carcinoma, affect approximately one million persons annually [4, 5]. CHB infection can also affect organ systems outside of the liver, particularly the skeletal system [6–8].
Several cross-sectional studies in CHB-infected patients alone [9, 10] and among non-cirrhotic CHB- and chronic hepatitis C-infected patients [11–14] have demonstrated that persons with CHB have reduced bone mineral density compared to uninfected individuals. A number of factors related to CHB infection have been hypothesized to contribute to low bone mineral density. Prior studies in animals and humans have suggested that CHB-associated inflammation could inhibit bone formation and increase bone resorption, leading to a decrease in bone mineral density [15, 16]. Moreover, CHB-induced hepatic decompensation could further contribute to a decrease in bone mineral density by impairing production of factors (e.g., 25-hydroxyvitamin D, insulin-like growth factor-1) that promote bone formation [7, 8].
Although CHB infection is associated with reduced bone mineral density [9–14], no longitudinal study has been performed to evaluate incidence rates of fractures. Evaluating the risk of fracture associated with CHB is important because this chronic infection is prevalent [1], and because fractures, particularly those at the hip, adversely affect survival, with an effect on mortality similar to that of cardiovascular disease [17]. Further, hip fractures cause significant pain and disability and typically require an emergency department visit, hospitalization, surgery, and rehabilitation stay, resulting in substantial health care costs.
The objective of this study was to determine the risk of hip fracture associated with CHB infection. We hypothesized that the risk of hip fracture is higher among patients with CHB infection than uninfected individuals. We separately evaluated hip fracture rates in persons with untreated CHB infection and in those who received antiviral therapy compared to uninfected individuals. Since CHB-associated inflammation likely contributes to decreased bone mineral density and therefore might increase fracture risk [15, 16], we hypothesized that rates of hip fracture might be the highest among patients requiring antiviral therapy for CHB infection, given that treated persons typically have elevated hepatitis B DNA levels and significant hepatic inflammation. Further, since rates of fractures differ according to age, sex, and race/ethnicity [18–21], we examined these factors as effect modifiers.
PATIENTS AND METHODS
Study Design and Data Source
We performed a retrospective cohort study using data from the U.S. Medicaid programs of California, Florida, New York, Ohio, and Pennsylvania between January 1, 1999 and December 31, 2007. The Medicaid program consists of state-run programs with joint federal and state funding for hospital, medical, and outpatient care and drug benefits for low-income and special-needs individuals [22]. The states included in this study were selected because they represent five of the largest Medicaid programs in the U.S., comprising approximately 45 million active enrollees, or almost 38% of the U.S. Medicaid population [23, 24]. Medicaid claims report demographic information, inpatient and outpatient medical diagnoses (recorded using International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis codes), and dispensed medications. Laboratory tests that were ordered can be identified, but the results of these tests are not recorded in the Medicaid database. Death dates were ascertained using Centers for Medicare and Medicaid Services data supplemented with mortality information from the Social Security Administration Death Master File. Since 17% of Medicaid beneficiaries are co-enrolled in the U.S. Medicare program, we obtained Medicare data on dually-eligible persons to ensure no diagnoses were missed [25]. Prior analyses of the linked Medicaid and Medicare claims indicate that the data are of high quality [26]. The study was approved by the University of Pennsylvania Institutional Review Board, and a data use agreement was obtained from the Centers for Medicare and Medicaid Services.
Study Patients
Patients aged 18 years or older with at least 180 days of Medicaid enrollment were eligible for inclusion. Patients were identified as CHB-infected if they had two ICD-9-CM diagnoses of CHB infection (Supplementary Table 1) recorded >6 months apart. A prior study validated this definition, with 96% of diagnoses confirmed by medical records [27]. We required untreated CHB-infected patients to have two CHB diagnoses recorded >6 months apart because a clinical diagnosis of CHB requires evidence of persistence of infection for >6 months [28]. Treated CHB-infected patients had at least one CHB diagnosis plus a prescription claim for an anti-CHB nucleos(t)ide analogue (i.e., adefovir, emtricitabine, entecavir, lamivudine, telbivudine, or tenofovir) or interferon alfa (standard or pegylated). Uninfected persons had no CHB diagnoses and no claims for anti-CHB antiviral medications.
Patients were excluded if they had: 1) diagnosis of hip fracture prior to the start of follow-up (defined below), 2) diagnosis of hepatitis C virus infection (to isolate the effect of CHB and since chronic hepatitis C is associated with an increased risk of hip fracture [29]), 3) diagnosis of human immunodeficiency virus (HIV) infection or claims for antiretroviral therapy (since HIV is associated with an increased hip fracture risk [30, 31]), 4) acute hepatitis B diagnosis only, or 5) no claims after becoming eligible for the study. ICD-9-CM diagnoses used to identify hepatitis C, HIV, and acute hepatitis B are listed in Supplementary Table 1.
All eligible CHB-infected patients were included. Because the prevalence of CHB infection was substantially higher in persons of Asian/Pacific Islander race [27], we selected a 20% systematic random sample of CHB-uninfected Asians/Pacific Islanders and 5% systematic random sample of CHB-uninfected patients of other races, stratified on age, sex, and state, to reduce to workable proportions the number of uninfected individuals for subsequent matching and analysis and to ensure that this sample represented the uninfected population [32].
Because of the high likelihood that Medicaid patients with hepatitis B developed chronic infection prior to their first-recorded CHB diagnosis [27], follow-up for untreated CHB-infected patients began 180 days after their first Medicaid claim of any type. Follow-up for treated CHB-infected patients began on the date an anti-CHB antiviral medication was dispensed or 180 days after their first Medicaid claim of any type, whichever was later, so that baseline variables could be defined. Follow-up for uninfected patients began 180 days after their first claim. The 6 months prior to the start of follow-up represented the baseline period, during which baseline comorbidities and therapies were identified. Follow-up for all cohorts continued until a hip fracture, death, or last claim before December 31, 2007. For untreated CHB-infected patients, follow-up was also censored if there was initiation of CHB therapy. When a patient was initiated on CHB treatment, that patient began contributing follow-up time to the treated CHB-infected cohort.
Main Study Outcome
The primary outcome was incident fracture of the proximal femur (hip). Hip fracture diagnoses (Supplementary Table 1) from Centers for Medicare and Medicaid Services claims are highly valid, with 94% of cases confirmed via medical records [33].
Data Collection
Demographic data collected included: age, sex, race/ethnicity, and state. Baseline diagnoses associated with osteoporosis or risk of falling (Table 1; Supplementary Table 2) were also collected. Baseline hepatic decompensation was defined by a diagnosis of ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy [34]. We also examined baseline use of medications that impact bone metabolism (Table 1; Supplementary Table 2). Patients were considered exposed to a medication if a drug claim was recorded during the baseline period.
Table 1.
Baseline characteristics of all eligible untreated chronic hepatitis B (CHB) patients, all eligible CHB patients who were treated with anti-CHB therapy, and a sample of uninfected persons among the U.S. Medicaid populations of California, Florida, New York, Ohio, and Pennsylvania.
| Characteristic | CHB-Infected | CHB-Uninfected Sample† (n=979,751) |
|
|---|---|---|---|
| Untreated (n=18,796) |
Treated* (n=7,777) |
||
|
| |||
| Mean age (years, SD) | 49 (16) | 46 (16) | 43 (21) |
|
| |||
| Female sex (n, %) | 9,369 (49.8%) | 3,257 (41.9%) | 688,938 (70.3%) |
|
| |||
| Race/ethnicity (n, %) | |||
| Asian/Pacific Islander | 13,444 (71.5%) | 5,724 (73.6%) | 232,957 (23.8%) |
| White | 2,336 (12.5%) | 759 (9.8%) | 317,008 (32.4%) |
| Black | 973 (5.2%) | 384 (4.9%) | 124,182 (12.7%) |
| Hispanic/Multiracial/American Indian | 1,133 (6.0%) | 569 (7.3%) | 270,527 (27.6%) |
| Unknown | 910 (4.8%) | 341 (4.4%) | 35,077 (3.5%) |
|
| |||
| State (n, %) | |||
| California | 8,405 (44.7%) | 2,458 (31.6%) | 505,701 (51.6%) |
| Florida | 448 (2.4%) | 220 (2.9%) | 101,363 (10.4%) |
| New York | 9,186 (48.9%) | 4,916 (63.2%) | 232,665 (23.7%) |
| Ohio | 414 (2.2%) | 86 (1.1%) | 66,436 (6.8%) |
| Pennsylvania | 343 (1.8%) | 97 (1.2%) | 73,586 (7.5%) |
|
| |||
| Mean follow-up (years, SD) | 4.9 (2.8) | 2.1 (1.8) | 3.5 (2.9) |
|
| |||
| Total follow-up time (person-years) | 92,725 | 16,598 | 3,448,002 |
|
| |||
| Baseline health condition (n, %) ‡ | |||
| Asthma/chronic obstructive pulmonary disease | 1,243 (6.6%) | 440 (5.7%) | 60,897 (6.2%) |
| Cancer | 660 (3.5%) | 664 (8.5%) | 29,769 (3.0%) |
| Chronic kidney disease | 1,439 (7.7%) | 829 (10.7%) | 35,318 (3.6%) |
| Congestive heart failure | 498 (2.6%) | 215 (2.8%) | 37,054 (3.8%) |
| Coronary artery disease/myocardial infarction | 1,184 (6.3%) | 416 (5.3%) | 59,937 (6.1%) |
| Dementia | 169 (0.9%) | 67 (0.9%) | 23,409 (2.4%) |
| Depression/bipolar disorder | 777 (4.1%) | 250 (3.2%) | 43,311 (4.4%) |
| Diabetes mellitus | 1,785 (9.5%) | 975 (12.5%) | 81,742 (8.3%) |
| Hepatic decompensation | 246 (1.3%) | 455 (5.9%) | 2,631 (0.3%) |
| Peptic ulcer disease | 736 (3.9%) | 286 (3.7%) | 12,951 (1.3%) |
| Peripheral vascular disease | 336 (1.8%) | 137 (1.8%) | 26,633 (2.7%) |
| Rheumatoid arthritis/non-specific inflammatory arthropathy | 629 (3.3%) | 244 (3.1%) | 26,438 (2.7%) |
| Seizure disorder | 392 (2.1%) | 85 (1.1%) | 15,221 (4.4%) |
| Stroke | 417 (2.2%) | 176 (2.3%) | 34,815 (3.6%) |
|
| |||
| Baseline medication use (n, %) § | |||
| Anxiolytic | 1,536 (8.2%) | 647 (8.3%) | 59,067 (6.0%) |
| Antidepressant | 1,511 (8.0%) | 651 (8.4%) | 78,786 (8.0%) |
| Anticonvulsant/gabapentin | 882 (4.7%) | 437 (5.6%) | 47,385 (4.8%) |
| Antiparkinsonian | 708 (3.8%) | 236 (3.0%) | 23,696 (2.4%) |
| Antipsychotic | 909 (4.8%) | 424 (5.5%) | 38,739 (4.0%) |
| Bisphosphonate | 498 (2.6%) | 417 (5.4%) | 10,011 (1.0%) |
| Calcium supplementation | 986 (5.2%) | 331 (4.3%) | 16,712 (1.7%) |
| Corticosteroid (inhaled) | 394 (2.1%) | 217 (2.8%) | 17,315 (1.8%) |
| Corticosteroids (oral) | 430 (2.3%) | 409 (5.3%) | 24,101 (2.5%) |
| Hormone therapy (estrogen) | 502 (2.7%) | 86 (1.1%) | 16,856 (1.7%) |
| Nonsteroidal anti-inflammatory drug/aspirin | 3,336 (17.7%) | 1,556 (20.0%) | 129,882 (13.3%) |
| Proton pump inhibitor | 1,716 (9.1%) | 1,816 (23.4%) | 46,659 (4.8%) |
| Statin | 1,211 (6.4%) | 646 (8.3%) | 51,058 (5.2%) |
| Thiazide diuretic | 639 (3.4%) | 634 (8.2%) | 45,875 (4.7%) |
| Thyroxine | 344 (1.8%) | 149 (1.9%) | 21,347 (2.2%) |
| Vitamin D supplementation | 685 (3.6%) | 343 (4.4%) | 5,690 (0.6%) |
Abbreviations: CHB, chronic hepatitis B; SD, standard deviation
Untreated CHB-infected patients who subsequently initiated CHB treatment were separately classified within the treated CHB-infected cohort.
Represents a 20% systematic random sample of chronic hepatitis B-uninfected Asians/Pacific Islanders and 5% systematic random sample of chronic hepatitis B-uninfected patients of other races.
Cohorts were also evaluated for alcoholism, hyperparathyroidism, inflammatory bowel disease, obesity, osteomalacia, schizophrenia, and systemic lupus erythematosus, but the prevalence of these conditions was <2% within each group. Please see Supplementary Table 2 for the prevalence of these additional health conditions within each cohort.
Cohorts were also evaluated for use of calcitonin, cholestyramine, and testosterone, but the prevalence of these medications was <2% within each group. Please see Supplementary Table 2 for the prevalence of baseline use of these medications within each cohort.
Statistical Analysis
We first determined unadjusted incidence rates of hip fractures (in events/1,000 person-years) with 95% confidence intervals (CIs) for the untreated CHB, treated CHB, and uninfected cohorts. Because the prevalence of CHB infection and incidence of hip fracture varied substantially by race/ethnicity, we stratified these results by race/ethnicity.
The primary analysis compared the time to incident hip fracture in: 1) untreated CHB-infected versus uninfected persons, and 2) treated CHB-infected versus uninfected persons. Differences in baseline comorbidities and usage of medications associated with osteoporosis were observed among the three cohorts (Table 1; Supplementary Table 2). Because of the many potential confounding variables relative to the number of hip fractures, we developed propensity scores to control for these confounders [35, 36]. Since uninfected persons were far more numerous than CHB-infected patients, we elected to match uninfected persons to CHB-infected persons on propensity scores. This matching allowed us to create a comparison group of uninfected persons whose baseline characteristics resembled those of the CHB-infected patients. Due to the differences in the prevalence of CHB infection by race/ethnicity and because of disparities across states in patient demographics, we first stratified the three cohorts before matching on race/ethnicity within U.S. state. We then developed propensity scores using separate logistic regression models for each racial/ethnic group within California, New York, and for Florida, Ohio, and Pennsylvania combined (due to the small numbers of CHB-infected patients within each of these three states). All variables in Table 1 and Supplementary Table 2 were included within propensity score models except for follow-up time. We also excluded age, sex, and race/ethnicity (to allow us to evaluate these variables as effect modifiers) and hepatic decompensation (since this condition might be in the causal pathway to fracture) from propensity score models. Within the two CHB-infected cohorts (treated and untreated), each infected patient was matched with up to four uninfected persons within strata formed by race/ethnicity and state using nearest-neighbor matching and a caliper of one-fourth of the standard deviation of the propensity score on the log odds scale [37].
Because of the variable number of matches for each CHB-infected patient, we used a weighted analysis, with weights of infected patients equaling 1.0 and weights of the matched uninfected patients equaling the inverse of the number of uninfected in the matched set. This form of weighting ensured that the weighted sum of each matched uninfected cohort would equal the sample size of each infected cohort. It also ensured that uninfected patients were similar in characteristics to the infected patients [37].
Weighted Cox proportional hazards regression analyses were then used to estimate hazard ratios (HRs) of hip fracture. To compensate for effects of weights on variances, we used robust (empirical) variance estimates to calculate 95% CIs [38]. In an initial model among the untreated patients, we fit main effects and all two-, three-, and four-way interactions for CHB infection status, age, sex, and race/ethnicity. We removed all non-significant (p>0.05) interactions. The final model included main effects for CHB status, age, sex, race/ethnicity, and interactions for CHB status*race/ethnicity, age*sex, and age*race/ethnicity. Because there was a statistically significant CHB status*race/ethnicity interaction, analyses are presented stratified by race/ethnicity. To be consistent, in a subsequent model among the treated patients, we included the same main effects and three two-way interactions.
To evaluate the risk of hip fracture associated with CHB infection in the absence of advanced liver disease, we repeated the above matched analysis, excluding CHB-infected and uninfected patients with baseline hepatic decompensation diagnoses. If decompensation was recorded at baseline in a CHB-infected patient, we dropped not only this patient but also all uninfected patients in that matched set. If decompensation was recorded in an uninfected patient, we preserved the matched set but re-weighted the remaining uninfected patients in that set to reflect the loss of one or more uninfected patients. In this analysis, follow-up was additionally censored at any decompensation-related diagnosis.
Finally, to evaluate the potential impact of hepatic decompensation on fracture outcomes, we compared the hip fracture rate among CHB-infected patients after an initial decompensation diagnosis with that of CHB-infected patients without decompensation, who were followed until they either were censored or developed decompensation. For this analysis, we combined both treated and untreated CHB-infected patients.
We estimated that 1,841 CHB-infected patients would provide 90% power to detect a relative hazard of hip fracture of 2.0 between CHB-infected and uninfected persons, assuming a two-tailed alpha of 0.05, 4:1 ratio of unexposed:exposed, and a 1% rate of hip fracture among the uninfected cohort [29]. Data were analyzed using SAS 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
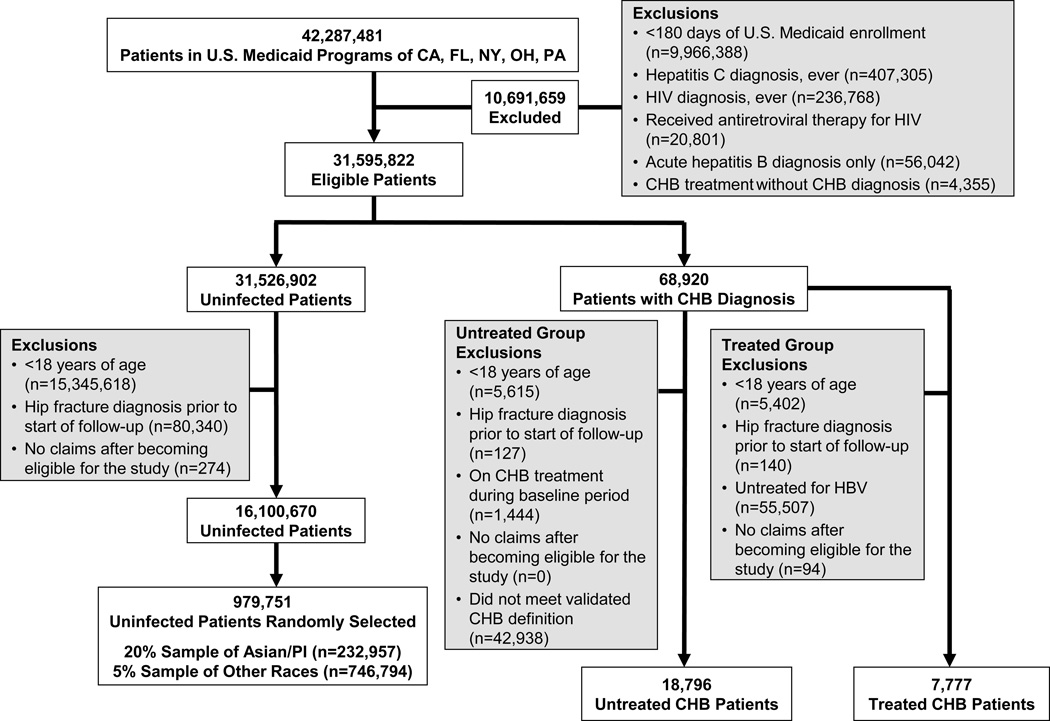
Among 42,287,481 persons enrolled in the U.S. Medicaid programs within the five states between 1999 and 2007 (Figure 1), we identified 18,796 (0.04%) patients with untreated CHB infection and 7,777 (0.02%) CHB-infected persons who received antiviral treatment. After exclusions (Figure 1), 16,100,670 eligible uninfected persons were identified. From this group, the 20% systematic random sample of Asian/Pacific Islanders (n=232,957) and 5% random sample of other races (n=746,794) yielded a total of 979,751 CHB-uninfected individuals.
Fig. 1. Selection of chronic hepatitis B (CHB)-infected and uninfected patients in the study.
CHB treatment was defined by a prescription claim for adefovir, emtricitabine, entecavir, lamivudine, telbivudine, tenofovir, or interferon alfa (standard or pegylated).
Table 1 and Supplementary Table 2 summarize the characteristics of the untreated CHB, treated CHB, and uninfected cohorts. Untreated and treated CHB-infected patients were older, more frequently female, and much more commonly of Asian race than uninfected patients. Both cohorts of CHB-infected patients more commonly had diabetes mellitus, chronic kidney disease, hyperparathyroidism, and rheumatoid arthritis. Diagnoses of cancer and hepatic decompensation were more frequent among treated CHB-infected patients. Further, both CHB-infected cohorts more commonly received calcium and vitamin D supplements, bisphosphonates, anti-Parkinson drugs, and proton pump inhibitors. The mean follow-up time was 4.9 years for untreated CHB-infected patients, 2.1 years for treated CHB-infected patients, and 3.5 years for uninfected persons. A higher proportion of uninfected persons died during follow-up (60,714 [6.2%]) compared to either untreated (706 [3.8%]) or treated (325 [4.2%]) CHB-infected patients.
Among the 7,777 treated CHB-infected patients, 2,888 (37.1%) were prescribed adefovir, 37 (0.5%) emtricitabine, 853 (11.0%) entecavir, 3,942 (50.7%) lamivudine, 143 (1.8%) telbivudine, 36 (0.5%) tenofovir, and 3,922 (50.4%) standard or pegylated interferon alfa during the baseline period or at the start of follow-up.
Hip Fracture Rates in Untreated CHB-Infected Versus Uninfected Persons
Among both untreated CHB patients and uninfected persons, unadjusted incidence rates of hip fracture varied significantly by race/ethnicity (p-value for interaction=0.002). Unadjusted incidence rates and relative hazards of hip fracture for untreated CHB-infected patients compared to uninfected persons are therefore shown in Table 2 stratified by race/ethnicity.
Table 2.
Estimated unadjusted incidence rates and relative hazards of hip fracture (with 95% confidence intervals) for untreated chronic hepatitis B (CHB)-infected patients compared to uninfected persons, overall and by race/ethnicity, among the U.S. Medicaid populations of California, Florida, New York, Ohio, and Pennsylvania.
| Untreated CHB Infection | CHB-Uninfected | Propensity-Score Matched and Adjusted Hazard Ratio of Hip Fracture, Untreated CHB vs. Uninfected (95% CI)† |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Patients (% Total) |
No.
(%) Hip Fractures |
Person- Years |
Raw Hip Fracture Incidence Rate, Events/1,000 person-years (95% CI) |
No. Patients (% Total) |
No.
(%) Hip Fractures |
Person- Years |
Raw Hip Fracture Incidence Rate, Events/1,000 person-years (95% CI) |
||
|
| |||||||||
| Total * | 18,796 (100%) |
227 (1.2%) |
92,725 | 2.4 (2.1 – 2.8) |
979,751 (100%) |
13,668 (1.4%) |
3,448,002 | 4.0 (3.9 – 4.1) |
1.08 (0.93 – 1.26) |
|
| |||||||||
| Asian/PI | 13,444 (71.5%) |
92 (0.7%) |
63,927 | 1.4 (1.2 – 1.8) |
232,957 (23.8%) |
2,088 (0.9%) |
866,499 | 2.4 (2.3 – 2.5) |
0.85 (0.68 – 1.06) |
|
| |||||||||
| White | 2,336 (12.4%) |
86 (3.7%) |
13,920 | 6.2 (4.9 – 7.6) |
317,008 (32.4%) |
8,550 (2.7%) |
1,123,939 | 7.6 (7.4 – 7.8) |
1.26 (0.98 – 1.62) |
|
| |||||||||
| Black | 973 (5.2%) |
22 (2.3%) |
4,982 | 4.4 (2.8 – 6.7) |
124,182 (12.7%) |
1,019 (0.8%) |
489,963 | 2.1 (2.0 – 2.2) |
2.55 (1.42 – 4.58) |
|
| |||||||||
| Hispanic | 1,133 (6.0%) |
16 (1.4%) |
5,166 | 3.1 (1.8 – 5.0) |
270,527 (27.6%) |
1,142 (0.4%) |
852,265 | 1.3 (1.3 – 1.4) |
1.36 (0.77 – 2.40) |
Abbreviations: CHB, chronic hepatitis B; CI, confidence interval; PI, Pacific Islander
Persons of unknown race are included in all totals except for hazard ratios. Persons of unknown race are not displayed separately.
Hazard ratios of hip fracture in CHB-infected versus uninfected persons are based on propensity score-matched Cox regression analyses with further adjustment for age, sex, race, and interactions for age*race and age*sex. Persons of unknown race are not included in hazard ratios.
After propensity score matching (Supplementary Table 3), untreated CHB-infected patients of black race had a higher relative hazard of hip fracture compared to uninfected black persons (adjusted HR, 2.55; 95% CI, 1.42 – 4.58). Hazard ratios of hip fracture were also increased in untreated white and Hispanic CHB-infected patients compared to uninfected persons of these racial/ethnic groups, but these associations did not achieve statistical significance. No association between untreated CHB infection and hip fracture was observed among Asians/Pacific Islanders. Results were similar when analyses were repeated among patients without hepatic decompensation diagnoses (Supplementary Table 4). Hazard ratios of hip fracture remained higher in untreated CHB-infected black patients (adjusted HR, 2.52; 95% CI, 1.34 – 4.75) but were also significantly higher in CHB-infected white patients (adjusted HR, 1.31; 95% CI, 1.01 – 1.70) in this analysis.
Hip Fracture Rates in Treated CHB-Infected Versus Uninfected Persons
Among treated CHB-infected and uninfected persons, unadjusted incidence rates of hip fracture again varied by race/ethnicity. Table 3 presents the unadjusted incidence rates and relative hazards of hip fracture for treated CHB-infected compared to uninfected persons, stratified by race/ethnicity. No hip fractures were observed in treated CHB patients of Hispanic ethnicity. As a result, relative hazards of hip fracture were not determined for this group.
Table 3.
Estimated unadjusted incidence rates and relative hazards of hip fracture (with 95% confidence intervals) for treated chronic hepatitis B (CHB)-infected patients compared to uninfected persons, overall and by race/ethnicity, among the U.S. Medicaid populations of California, Florida, New York, Ohio, and Pennsylvania.
| Treated CHB Infection | CHB-Uninfected | Propensity-Score Matched and Adjusted Hazard Ratio of Hip Fracture, Treated CHB vs. Uninfected (95% CI)† |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Patients (% Total) |
No.
(%) Hip Fractures |
Person- Years |
Raw Hip Fracture Incidence Rate, Events/1,000 person-years (95% CI) |
No. Patients (% Total) |
No.
(%) Hip Fractures |
Person- Years |
Raw Hip Fracture Incidence Rate, Events/1,000 person-years (95% CI) |
||
|
| |||||||||
| Total * | 7,777 (100%) |
27 (0.3%) |
16,598 | 1.6 (1.1 – 2.4) |
979,751 (100%) |
13,668 (1.4%) |
3,448,002 | 4.0 (3.9 – 4.1) |
1.17 (0.76 – 1.78) |
|
| |||||||||
| Asian/PI | 5,724 (73.6%) |
16 (0.3%) |
12,142 | 1.3 (0.8 – 2.1) |
232,957 (23.8%) |
2,088 (0.9%) |
866,499 | 2.4 (2.3 – 2.5) |
0.98 (0.59 – 1.64) |
|
| |||||||||
| White | 759 (9.8%) |
7 (0.9%) |
1,675 | 4.2 (1.7 – 8.6) |
317,008 (32.4%) |
8,550 (2.7%) |
1,123,939 | 7.6 (7.4 – 7.8) |
2.19 (0.94 – 5.09) |
|
| |||||||||
| Black | 384 (4.9%) |
2 (0.5%) |
869 | 2.3 (0.3 – 8.3) |
124,182 (12.7%) |
1,019 (0.8%) |
489,963 | 2.1 (2.0 – 2.2) |
3.39 (0.61 – 18.80) |
|
| |||||||||
| Hispanic | 569 (7.3%) |
0 (0.0%) |
1,123 | 0.0 (0.0 – 2.7) |
270,527 (27.6%) |
1,142 (0.4%) |
852,265 | 1.3 (1.3 – 1.4) |
–‡ |
Abbreviations: CHB, chronic hepatitis B; CI, confidence interval; PI, Pacific Islander
Persons of unknown race are included in all totals except for hazard ratios. Persons of unknown race are not displayed separately.
Hazard ratios of hip fracture in CHB-infected versus uninfected persons are based on propensity score-matched Cox regression analyses with further adjustment for age, sex, race, and interactions for age*race and age*sex. Persons of unknown race are not included in hazard ratios.
Relative hazards of hip fracture were not determined because no fracture events occurred in this group.
After propensity score matching (Supplementary Table 5), treated CHB-infected patients of black and white races had higher relative hazards of hip fracture compared to uninfected persons of these races (Table 3), but these associations were not statistically significant. No association between treated CHB infection and hip fracture was observed among Asians/Pacific Islanders. Similar results were observed when analyses were repeated among patients without hepatic decompensation diagnoses (Supplementary Table 6).
Hip Fracture Rates in CHB-Associated Hepatic Decompensation
CHB-infected patients with hepatic decompensation had a higher incidence rate of hip fracture than CHB-infected patients without decompensation (Table 4). CHB-infected patients with decompensated cirrhosis who were of black and white race had higher hip fracture rates than non-decompensated CHB-infected persons of these races.
Table 4.
Estimated unadjusted incidence rates (with 95% confidence intervals) for chronic hepatitis B (CHB)-infected patients with hepatic decompensation compared to CHB-infected persons without decompensation, overall and by race/ethnicity, among the U.S. Medicaid populations of California, Florida, New York, Ohio, and Pennsylvania.
| CHB-Infected (Untreated +
Treated) With Hepatic Decompensation* |
CHB-Infected (Untreated +
Treated) Without Hepatic Decompensation* |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Patients (% Total) |
No. (%) Hip Fractures |
Person- Years† |
Raw Hip Fracture Incidence Rate, Events/1,000 person-years (95% CI) |
No. Patients (% Total) |
No. (%) Hip Fractures |
Person- Years |
Raw Hip Fracture Incidence Rate, Events/1,000 person-years (95% CI) |
||
|
| |||||||||
| Total ‡ | 2,382 (100%) |
24 (1.0%) |
6,487 | 3.7 (2.4 – 5.5) | 21,481 (100%) |
230 (1.1%) |
102,836 | 2.2 (2.0 – 2.5) | |
|
| |||||||||
| Asian/PI | 1,384 (58.1%) |
6 (0.4%) |
3,793 | 1.6 (0.6 – 3.4) | 15,349 (71.5%) |
102 (0.7%) |
72,275 | 1.4 (1.2 – 1.7) | |
|
| |||||||||
| White | 467 (19.6%) |
10 (2.1%) |
1,368 | 7.3 (3.5 – 13.4) | 2,603 (12.1%) |
83 (3.2%) |
14,227 | 5.8 (4.6 – 7.2) | |
|
| |||||||||
| Black | 184 (7.7%) |
6 (3.3%) |
513 | 11.7 (4.3 – 25.4) | 1,126 (5.2%) |
18 (1.6%) |
5,337 | 3.4 (2.0 – 5.3) | |
|
| |||||||||
| Hispanic | 173 (7.3%) |
1 (0.6%) |
411 | 2.4 (0.1 – 13.6) | 1,385 (6.4%) |
15 (1.1%) |
5,878 | 2.6 (1.4 – 4.2) | |
Abbreviations: CHB, chronic hepatitis B; CI, confidence interval; PI, Pacific Islander
Some CHB-infected patients with decompensation contributed follow-up time to the non-decompensation group prior to observing a decompensation diagnosis.
If a diagnosis indicative of hepatic decompensation (i.e., ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) was recorded prior to an untreated or treated CHB-infected patient’s start of follow-up, then that patient contributed all of their follow-up time to the hepatic decompensation group. Otherwise, follow-up for CHB-infected patients with hepatic decompensation began on the date a decompensation diagnosis was first recorded.
Persons of unknown race are included in the total but are not displayed separately.
DISCUSSION
Our study of U.S. Medicaid enrollees found that hip fracture rates associated with both untreated and treated CHB infection varied by race/ethnicity. Untreated CHB-infected patients of black race had a higher rate of hip fracture compared to uninfected black persons. Relative hazards of hip fracture were also increased in untreated white and Hispanic CHB patients but were not statistically significant. Black and white treated CHB-infected patients had higher relative hazards of hip fracture compared to uninfected persons of these races, but associations did not reach conventional levels of statistical significance. No associations with hip fracture were observed with untreated or treated Asian/Pacific Islander CHB-infected patients. Results were similar when all patients with hepatic decompensation were excluded from comparisons, with white CHB-infected patients also observed to have a significantly higher hazard ratio of hip fracture. These results suggest that CHB infection contributes to hip fracture risk even in the absence of advanced liver disease. CHB-infected patients with hepatic decompensation had a higher incidence rate of hip fracture than CHB-infected patients without decompensation.
The mechanisms for the association between CHB infection and hip fracture are unknown. Inflammatory cytokines associated with CHB infection (e.g., tumor necrosis factor-alpha, interleukin-1, interleukin-6) could increase receptor activator of nuclear factor kappa-B ligand (RANKL), which can stimulate osteoclastogenesis and bone resorption [15, 16]. Tumor necrosis factor-alpha can also promote osteoblast apoptosis and inhibit osteoblast differentiation [15]. The combination of these effects could lead to uncoupling of bone formation and resorption, leading to reduced bone mineral density, a decrease in bone strength over time, and increased fracture risk. In addition, concomitant non-alcoholic fatty liver disease can induce oxidative stress, which could further reduce bone formation through reductions in beta-catenin-mediated osteogenesis [39, 40]. Further, CHB-associated hepatic decompensation could increase the risk of hypogonadism [41], reduce hepatic hydroxylation of vitamin D [6], and impair hepatic production of insulin-like growth factor-1, which promotes bone formation [8]. Decompensated cirrhosis can also result in metabolic acidosis, which can induce calcium efflux from bone, further leading to reduced bone mass and strength [42]. Our finding that CHB-infected patients with hepatic decompensation had a higher hip fracture rate than CHB-infected patients without decompensation suggests that liver synthetic dysfunction also contributes to the mechanism of CHB-associated bone disease. Finally, alcohol abuse, illicit drug use, poor nutrition, and fragility among CHB-infected patients might further contribute to increased fracture risk from trauma, irrespective of the impact of CHB infection on bone density. Future studies should evaluate the mechanisms by which CHB affects bone density.
We observed a significantly increased risk of hip fracture among untreated black CHB-infected patients. Hazard ratios of hip fracture associated with treated CHB infection in blacks were also the highest among all races evaluated. Additionally, black CHB-infected patients with hepatic decompensation had substantially higher incidence rates of hip fracture than CHB-infected decompensated cirrhotic patients of all other races. Certain factors contributing to risk of fracture might be more prevalent in black CHB-infected patients. Future research should evaluate risk factors for decreased bone mineral density within different racial groups infected with CHB.
We observed no associations between untreated or treated CHB infection and hip fracture among Asians/Pacific Islanders. This finding may have been because a large proportion of these patients had acquired their infection at birth or during their youth. Consequently, many of these patients could have been in the low replicative (or inactive) carrier phase of CHB infection, during which HBV DNA levels are low or undetectable and hepatic inflammation may be absent [43]. Given the potential role of CHB-associated inflammation on bone deficits [15, 16], the low levels of inflammation in the low replicative carrier phase of CHB infection might not be sufficient to contribute to fracture risk. The U.S. Medicaid database does not include laboratory results, so we were unable to determine the prevalence of the low replicative carrier phase among the CHB-infected cohorts. However, no significant differences in rates of hip fracture were also observed between uninfected persons and treated Asian/Pacific Islander CHB-infected patients, a group that should have high levels of inflammation that could contribute to fracture risk. Thus, there may be other factors mitigating the hip fracture risk associated with CHB infection in this racial group. Additionally, the Asian/Pacific Islander group is quite heterogeneous, and subgroups within this population (e.g., Hawaiian, recent immigrants, American-born Asians) might have different hip fracture rates.
For all racial/ethnic subgroups, hazard ratios of hip fracture were the highest among those treated for CHB infection. CHB-infected patients requiring antiviral therapy typically have more active disease, with high hepatitis B DNA levels before treatment and evidence of hepatic inflammation [28]. Systemic inflammation, induced by such active CHB infection [44], could contribute to an increased risk of fracture, regardless of race.
Prior epidemiologic studies have demonstrated that fracture rates vary by race/ethnicity [18–21]. These studies reported higher fracture rates among whites and lower rates for blacks, Hispanic, and Asians, as was observed in this analysis. The variation in fracture rates among the racial/ethnic groups is thought to be due to differences in risk factors for fractures, particularly bone mineral density, bone geometry, body mass index, physical activity, tobacco and alcohol use, and concomitant medication use [45].
Our study had several limitations. First, we lacked laboratory data to confirm CHB infection and did not have radiographic determination of fracture diagnoses. There is potential for misclassification of CHB status (e.g., low-replicative carrier phase) among uninfected persons (especially Asians/Pacific Islanders), which might have biased results towards a null association. However, diagnoses of CHB infection [27] and hip fracture [33] were identified using previously validated definitions. Second, we observed major differences in the prevalence of comorbidities and drugs associated with osteoporosis between the cohorts. To address this issue, we developed separate propensity score models for the two comparisons of interest in order to balance, or control for, potential confounding variables across the comparison groups. Third, our analyses accounted only for baseline use of pharmacologic therapies for osteoporosis but not use during follow-up. Fourth, residual confounding by unmeasured factors is possible in observational studies. We did not have information on body mass index, smoking, alcohol, illicit drug use, and duration of CHB infection. The absence of laboratory data on hepatitis B e antigen and antibody status, hepatitis B DNA, and extent of hepatic fibrosis did not allow us to determine the stage of CHB infection for patients in this analysis. Since vitamin D is available without a prescription, we likely had incomplete capture of this variable. We also could not assess whether fractures were specifically trauma-related. Fifth, the study sample consisted of U.S. Medicaid enrollees and might not be generalizable to other settings. However, Medicaid is a large source of care for patients with CHB infection [27], and the study cohorts were representative of U.S. CHB populations [46–48].
Our study had several strengths. It evaluated the association between both untreated and treated CHB infection and hip fracture in a large population. Analyses among CHB-infected patients without hepatic decompensation were conducted to explore if associations with hip fracture remained in the absence of advanced liver disease. Additionally, incidence rates of hip fracture in CHB-infected patients with hepatic decompensation were compared to infected patients without end-stage liver disease to explore the impact of decompensation on fracture outcomes. Further, matching based on propensity scores enabled us to adjust for many potential confounding variables that have not been considered in prior fracture studies.
In conclusion, we found that relative rates of hip fracture associated with CHB infection varied by race/ethnicity. Untreated CHB-infected patients of black race had a particularly high rate of hip fracture. Future studies should confirm these findings and evaluate the potential mechanisms that might contribute to fracture incidence in this group.
ACKNOWLEDGEMENTS
Financial support was provided by an investigator-initiated research grant (to the University of Pennsylvania) from Gilead Sciences, Inc.
Conflict of Interest:
Dr. Jay R. Kostman has served as a consultant to Merck and Vertex. Dr. Vincent Lo Re III has received research grant support (to the University of Pennsylvania) from AstraZeneca, Bristol-Myers Squibb, and Merck.
List of Abbreviations in the order of appearance
- CHB
Chronic hepatitis B virus
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- HIV
Human immunodeficiency virus
- CI
Confidence interval
- HR
Hazard ratio
- SD
Standard deviation
- PI
Pacific Islander
REFERENCES
- 1.Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol. 2004;38:S158–S168. doi: 10.1097/00004836-200411003-00008. [DOI] [PubMed] [Google Scholar]
- 2.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 3.McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25(Suppl 1):3–8. doi: 10.1055/s-2005-915644. [DOI] [PubMed] [Google Scholar]
- 4.Pungpapong S, Kim WR, Poterucha JJ. Natural history of hepatitis B virus infection: an update for clinicians. Mayo Clin Proc. 2007;82:967–975. doi: 10.4065/82.8.967. [DOI] [PubMed] [Google Scholar]
- 5.Pungpapong S, Kim WR. In the eye of the B-holder: natural history of chronic hepatitis B. Hepatology. 2013;58:6–8. doi: 10.1002/hep.26356. [DOI] [PubMed] [Google Scholar]
- 6.Hay JE. Bone disease in cholestatic liver disease. Gastroenterology. 1995;108:276–283. doi: 10.1016/0016-5085(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 7.Leslie WD, Bernstein CN, Leboff MS. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology. 2003;125:941–966. doi: 10.1016/s0016-5085(03)01062-x. [DOI] [PubMed] [Google Scholar]
- 8.Rouillard S, Lane NE. Hepatic osteodystrophy. Hepatology. 2001;33:301–307. doi: 10.1053/jhep.2001.20533. [DOI] [PubMed] [Google Scholar]
- 9.Fung S, Fabri M, Wong F, Heathcote J, Gurel S, Kwan WCP, et al. Reduced bone mineral density derived from dual x-ray absorptiometry (DEXA) assessments in patients with chronic hepatitis B (CHB); 46th Annual Meeting of the European Association for the Study of the Liver; March 30 – April 3, 2011; Berlin, Germany. [Abstract 2382]. [Google Scholar]
- 10.Gill US, Al-Shamma S, Burke K, Ross V, Marley R, Kooner P, et al. Factors determining bone mineral density loss in chronic hepatitis B patients: Is tenofovir disoproxil fumarate the main culprit? J Hepatol. 2011;54(Supp 1):S286. [Google Scholar]
- 11.Olsson R, Johansson C, Lindstedt G, Mellstrom D. Risk factors for bone loss in chronic active hepatitis and primary biliary cirrhosis. Scand J Gastroenterol. 1994;29:753–756. doi: 10.3109/00365529409092505. [DOI] [PubMed] [Google Scholar]
- 12.Schiefke I, Fach A, Wiedmann M, Aretin AV, Schenker E, Borte G, et al. Reduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World J Gastroenterol. 2005;11:1843–1847. doi: 10.3748/wjg.v11.i12.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo Re V, 3rd, Guaraldi G, Leonard MB, Localio AR, Lin J, Orlando G, et al. Viral hepatitis is associated with reduced bone mineral density in HIV-infected women but not men. AIDS. 2009;23:2191–2198. doi: 10.1097/QAD.0b013e32832ec258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson-Ayayi S, Cazanave C, Kpozehouen A, Barthe N, Mehsen N, Hessamfar M, et al. Chronic viral hepatitis is associated with low bone mineral density in HIV-infected patients, ANRS CO 3 Aquitaine Cohort. J Acquir Immune Defic Syndr. 2013;62:430–435. doi: 10.1097/QAI.0b013e3182845d88. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Calvin JL, Gallego-Rojo F, Fernandez-Perez R, Casado-Caballero F, Ruiz-Escolano E, Olivares EG. Osteoporosis, mineral metabolism, and serum soluble tumor necrosis factor receptor p55 in viral cirrhosis. J Clin Endocrinol Metab. 2004;89:4325–4330. doi: 10.1210/jc.2004-0077. [DOI] [PubMed] [Google Scholar]
- 17.Mittalhenkle A, Gillen DL, Stehman-Breen CO. Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis. 2004;44:672–679. [PubMed] [Google Scholar]
- 18.Baron JA, Barrett J, Malenka D, Fisher E, Kniffin W, Bubolz T, et al. Racial differences in fracture risk. Epidemiology. 1994;5:42–47. doi: 10.1097/00001648-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Fang J, Freeman R, Jeganathan R, Alderman MH. Variations in hip fracture hospitalization rates among different race/ethnicity groups in New York City. Ethn Dis. 2004;14:280–284. [PubMed] [Google Scholar]
- 20.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573–1579. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright NC, Saag KG, Curtis JR, Smith WK, Kilgore ML, Morrisey MA, et al. Recent trends in hip fracture rates by race/ethnicity among older US adults. J Bone Miner Res. 2012;27:2325–2332. doi: 10.1002/jbmr.1684. [DOI] [PubMed] [Google Scholar]
- 22.Hennessy S, Bilker WB, Weber A, Strom BL. Descriptive analyses of the integrity of a US Medicaid claims database. Pharmacoepidemiol Drug Saf. 2003;12:103–111. doi: 10.1002/pds.765. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Medicare & Medicaid Services. Medicaid Statistical Information System (MSIS) Tables. [Accessed December 22, 2013]; http://www.cms.gov/MedicaidDataSourcesGenInfo/MSIS/list.asp.
- 24.Centers for Medicare & Medicaid Services. Medicaid Analytic eXtract (MAX) Validation Reports. [Accessed December 22, 2013]; http://www.cms.gov/MedicaidDataSourcesGenInfo/MVR/list.asp.
- 25.Medicare Payment Advisory Commission. Report to the Congress: New Approaches in Medicare. 2004 [Google Scholar]
- 26.Hennessy S, Leonard CE, Palumbo CM, Newcomb C, Bilker WB. Quality of Medicaid and Medicare data obtained through Centers for Medicare and Medicaid Services (CMS) Med Care. 2007;45:1216–1220. doi: 10.1097/MLR.0b013e318148435a. [DOI] [PubMed] [Google Scholar]
- 27.Byrne DD, Newcomb CW, Carbonari DM, Nezamzadeh MS, Leidl KBF, Herlim M, et al. Prevalence of diagnosed chronic hepatitis B infection among U.S. Medicaid enrollees, 2000 – 2007. Ann Epidemiol. 2014 doi: 10.1016/j.annepidem.2014.02.013. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 29.Lo Re V, 3rd, Volk J, Newcomb CW, Yang YX, Freeman CP, Hennessy S, et al. Risk of hip fracture associated with hepatitis C virus infection and hepatitis C/human immunodeficiency virus coinfection. Hepatology. 2012;56:1688–1698. doi: 10.1002/hep.25866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young B, Dao CN, Buchacz K, Baker R, Brooks JT. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis. 2011;52:1061–1068. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
- 31.Womack JA, Goulet JL, Gibert C, Brandt C, Chang CC, Gulanski B, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One. 2011;6:e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kish L. Survey Sampling. New York, NY: John Wiley & Sons, Inc; 1965. [Google Scholar]
- 33.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45:703–714. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg D, Lewis J, Halpern S, Weiner M, Lo Re V., 3rd Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21:765–769. doi: 10.1002/pds.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137:693–695. doi: 10.7326/0003-4819-137-8-200210150-00015. [DOI] [PubMed] [Google Scholar]
- 36.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 37.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collett D. Modeling survival data in medical research. New York: Chapman and Hall; 2003. [Google Scholar]
- 39.Chen Y, Whetstone HC, Youn A, Nadesan P, Chow EC, Lin AC, et al. Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J Biol Chem. 2007;282:526–533. doi: 10.1074/jbc.M602700200. [DOI] [PubMed] [Google Scholar]
- 40.Yilmaz Y. Review article: non-alcoholic fatty liver disease and osteoporosis--clinical and molecular crosstalk. Aliment Pharmacol Ther. 2012;36:345–352. doi: 10.1111/j.1365-2036.2012.05196.x. [DOI] [PubMed] [Google Scholar]
- 41.Pignata S, Daniele B, Galati MG, Esposito G, Vallone P, Fiore F, et al. Oestradiol and testosterone blood levels in patients with viral cirrhosis and hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 1997;9:283–286. doi: 10.1097/00042737-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Krieger NS, Frick KK, Bushinsky DA. Mechanism of acid-induced bone resorption. Curr Opin Nephrol Hypertens. 2004;13:423–436. doi: 10.1097/01.mnh.0000133975.32559.6b. [DOI] [PubMed] [Google Scholar]
- 43.Tong MJ, Trieu J. Hepatitis B inactive carriers: clinical course and outcomes. J Dig Dis. 2013;14:311–317. doi: 10.1111/1751-2980.12051. [DOI] [PubMed] [Google Scholar]
- 44.Yu Y, Gong R, Mu Y, Chen Y, Zhu C, Sun Z, et al. Hepatitis B virus induces a novel inflammation network involving three inflammatory factors, IL-29, IL-8, and cyclooxygenase-2. J Immunol. 2011;187:4844–4860. doi: 10.4049/jimmunol.1100998. [DOI] [PubMed] [Google Scholar]
- 45.Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469:1891–1899. doi: 10.1007/s11999-011-1863-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ioannou GN. Hepatitis B virus in the United States: infection, exposure, and immunity rates in a nationally representative survey. Ann Intern Med. 2011;154:319–328. doi: 10.7326/0003-4819-154-5-201103010-00006. [DOI] [PubMed] [Google Scholar]
- 47.McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89:14–18. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wasley A, Kruszon-Moran D, Kuhnert W, Simard EP, Finelli L, McQuillan G, et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192–201. doi: 10.1086/653622. [DOI] [PubMed] [Google Scholar]