Abstract
Viruses that originate in bats may be the most notorious emerging zoonoses that spill over from wildlife into domestic animals and humans. Understanding how these infections filter through ecological systems to cause disease in humans is of profound importance to public health. Transmission of viruses from bats to humans requires a hierarchy of enabling conditions that connect the distribution of reservoir hosts, viral infection within these hosts, and exposure and susceptibility of recipient hosts. For many emerging bat viruses, spillover also requires viral shedding from bats, and survival of the virus in the environment. Focusing on Hendra virus, but also addressing Nipah virus, Ebola virus, Marburg virus and coronaviruses, we delineate this cross-species spillover dynamic from the within-host processes that drive virus excretion to land-use changes that increase interaction among species. We describe how land-use changes may affect co-occurrence and contact between bats and recipient hosts. Two hypotheses may explain temporal and spatial pulses of virus shedding in bat populations: episodic shedding from persistently infected bats or transient epidemics that occur as virus is transmitted among bat populations. Management of livestock also may affect the probability of exposure and disease. Interventions to decrease the probability of virus spillover can be implemented at multiple levels from targeting the reservoir host to managing recipient host exposure and susceptibility.
Keywords: emerging infectious diseases of bat origin, Hendra virus in flying-foxes, Nipah virus, severe acute respiratory syndrome coronavirus, Ebola virus, Marburg virus
1. Introduction
Bats are well-recognized reservoirs of zoonotic viruses. Agents that spill over from bats to humans—such as filoviruses (Ebola and Marburg virus), henipaviruses (Hendra and Nipah virus) and coronaviruses (including severe acute respiratory syndrome coronavirus [SARS-CoV] [1–3])—cause severe disease in recipient hosts and have pandemic potential. For each of these emerging zoonoses, spillover is predicated on ecological interactions between the infected bat, the pathogen and the recipient host species. Often the recipient is an intermediate host species frequently in contact with humans; the recipient then may infect humans. For example, humans were infected with SARS-CoV by civets, and in some outbreaks of Ebola and Nipah viruses by great apes and pigs, respectively [1–3]. In some cases, viruses are amplified by these intermediate hosts. The ecological events that drive interactions between source and recipient species are rarely understood, probably because the enabling conditions and drivers of cross-species transmission occur over many scales of time, space and ecological organization, from within-host pathogen evolution to spatially extensive processes such as land-use and climate change (figure 1). Such events, and how they lead to transmission of bat viruses to other species, are the focus of this review.
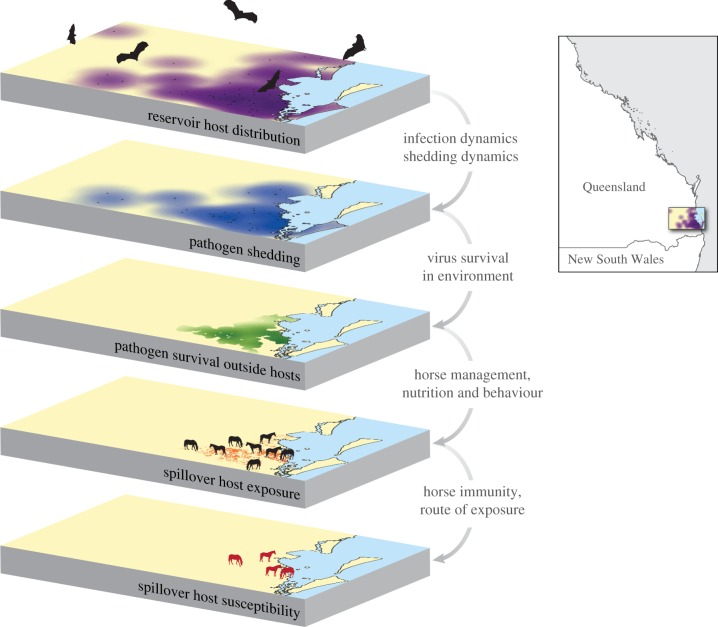
Figure 1.
Enabling conditions for Hendra virus spillover. A series of connected enabling conditions are necessary for spillover of emerging bat viruses. Bats must be present. Bats must be infected and in most cases shedding pathogen. Virus must survive outside of its reservoir host (if transmitted indirectly), with access to the recipient host. Recipient hosts must be exposed to the source of the virus in sufficient quantity for an infection to establish. Recipient hosts must be susceptible to the virus. The area depicted in the layers is southeastern Queensland, Australia (see inset). The purple areas over layer 1 correspond to 20 km foraging zones around known bat roost sites. Locations of the four horses on the bottom layer correspond to those of Hendra virus spillover events in 2011.
Although the role of bats as reservoir hosts of newly emerging pathogens has received considerable attention [4], that role may also have a deeper historical dimension. Common human and animal viruses, including the evolutionary progenitors of measles, mumps, parainfluenza, canine distemper and hepatitis C viruses, may have originated in bats [5,6]. Bats are unusual in the extent to which they host zoonotic viruses compared with ecologically similar taxonomic groups, such as rodents [7]. The reasons are not readily apparent, but one hypothesis is that chiropteran immune systems differ from those of most other mammals, perhaps as an indirect effect of evolutionary adaptations for sustained flight [8,9]. As a consequence, bats may be tolerant of infection and thus exceptionally hospitable reservoir hosts. Recent surveillance has discovered new bat viruses with zoonotic potential (e.g. [10,11]). These viruses may be spilling over undetected, particularly where disease surveillance is poor. Given the present limitations of global surveillance for zoonotic diseases, focusing on the spillover dynamics of bat diseases might be a wise use of the scarce resources for forecasting pandemics, as exemplified by the 2003 SARS-CoV pandemic and the 2014 Ebola epidemic in West Africa.
Hendra virus, the first and best understood of a recent series of high-profile emerging pathogens traced to bats, typifies the spillover process for many bat-borne zoonoses. Hendra virus, a negative sense, single-stranded RNA virus in the genus Henipavirus (family Paramyxoviridae) [12], is endemic in Australian Pteropus spp. (fruit bats or flying-foxes) (electronic supplementary material, appendix S1). Hendra virus spills over from bats into domestic animals, primarily horses, that amplify the virus and subsequently infect humans [13] (box 1).
Box 1. Within-host and among-host virus ecology in bats.
The persistence and propagation of viruses occur at multiple levels: cell of host, individual host, population of hosts, community of host species and landscape. Individual hosts are the habitat of viruses. To persist at the population level, viruses must replicate, exit from and be transmitted among hosts. A host's innate and adaptive immune responses work meanwhile, in opposition, to contain or eradicate virus. Bats and henipaviruses, and perhaps filoviruses and coronaviruses, share an evolutionary history with their hosts that may accommodate an interaction between virus and host cells that results in no apparent pathology or clinical disease [14–17] and in the case of henipaviruses, perhaps limited viral replication [14]. Yet such accommodation allows the viruses to survive and to be transmitted among host populations and metapopulations.
Various constraints have impeded research on the within-host ecology of emerging bat viruses. RNA from henipaviruses, filoviruses and coronaviruses is frequently detected in naturally infected bats, but virus is rarely recovered [16,18] (electronic supplementary material, appendix S2). Ebola virus and African bat henipaviruses are yet to be isolated from bat hosts [19,20]. All live-virus work with such agents requires maximum biocontainment, at biosafety level 4. Therefore, even when isolates exist, experiments are expensive, confined to certain laboratories and limited in sample size and duration. Additionally, henipavirus infections have been difficult to establish in captive bats. For example, when 20 bats were inoculated with high doses of Hendra virus, only one bat shed infectious virus [14].
The unusually low level of viral shedding from bats, and the difficulty of experimentally infecting bats, leads to hypotheses consistent with the distinct ecology of bats. Many bat species have dense, three-dimensional roost structures that facilitate indirect transmission through droplets or aerosols of viruses excreted in urine or faeces. Although the probability of developing infection from any given exposure to virus may be low, continuous exposure to a viral rain may lead to a high probability of infection (electronic supplementary material, appendix S2).
Whether bat viruses are patchily or evenly distributed among roosting sites depends on the viral infectious period in relation to movement rates of bats between roosts. Short infectious periods and low movement rates promote patchy viral dynamics across populations, whereas long infectious periods and high movement rates homogenize dynamics [21]. While infectious periods are unknown for emerging bat viruses, many bat species have high movement rates [22,23] with little spatial genetic structure (e.g. [20]). Consequently, antibodies (reflecting cumulative distribution of viruses) are often widely distributed across populations of bats and communities of bat species [20,24–26]. Viral shedding, in contrast, is often observed to occur in discrete pulses [24,27–29], suggesting short infectious periods with virus extinction and recolonization across roosts [30] or intermittent shedding from persistently infected individual hosts (electronic supplementary material, appendix S2).
We propose an integrative conceptual framework for assessing drivers of bat virus spillover from the cellular to the landscape level. We focus on Hendra virus, but also address emerging henipaviruses, filoviruses and coronaviruses where possible. Bat lyssaviruses have not been included because they are covered elsewhere [31]. Our approach describes data gaps and priorities for future research, and identifies potential interventions that may lead to prediction, control and mitigation of spillover events.
2. Enabling conditions for spillover of virus from bats
Spillover of the emerging bat viruses requires a series of hierarchical enabling conditions: reservoir hosts must be present; reservoir hosts must be infected; if transmission is indirect, reservoir hosts must be shedding pathogen and virus must survive outside of its reservoir host with access to the recipient host; recipient hosts must be exposed to the source of the virus in sufficient quantity for an infection to establish; and recipient hosts must be susceptible to the virus (figure 1).
Hendra virus (box 2) provides an ideal case study for developing insights into the dynamics of bat virus spillover. Hendra virus circulates in bat populations throughout their range [25,26,37] (electronic supplementary material, figure S3), yet spillover occurred in only a part of the overlapping distributions of fruit bats and horses [38], and affected only a small proportion of the horses in an outbreak area. We explored how the enabling conditions for Hendra virus spillover, and other bat viruses where possible, interact to explain spatio-temporal variation in spillover.
Box 2. Patterns of Hendra virus spillover events.
Fifty-two events of Hendra spillover have been detected, all of which became major public health concerns. The discovery of Hendra virus in 1994 was precipitated by a dramatic outbreak affecting 20 horses in a Thoroughbred-racing stable within the suburb of Hendra (Brisbane, Queensland; electronic supplementary material, appendix S2). Two people closely associated with the horses, the racehorse trainer and his assistant, became infected with Hendra virus. The trainer died from the virus, whereas his assistant recovered [13].
From 1995 through 2005, Hendra virus spillover was rare and sporadic. Since 2006, Hendra virus spillover into horses has been detected with increasing frequency and over an expanding geographical range. The scope of increase suggests an increasing spillover trend (figure 2; electronic supplementary material, figure S1) despite increased surveillance efforts, public reporting and detection. In 2011, there was an unprecedented cluster of 18 spillover events. The annual number of spillovers in 2012 (eight) and 2013 (eight) was also well above the pre-2011 average (electronic supplementary material, figure S2).
Figure 2.
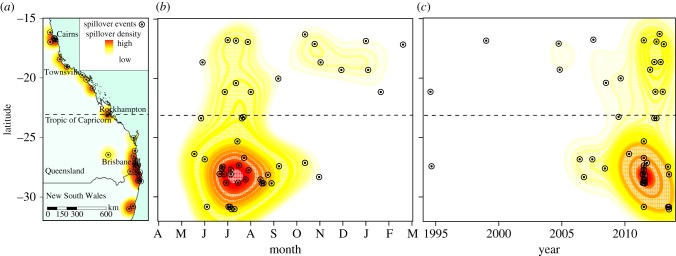
Hendra virus spillover events from 1 January 1994 to 1 December 2013 by latitude, month and date. (a) The distribution of Hendra virus spillover events across eastern Australia. The Tropic of Capricorn separates the northern tropics from the southern subtropics. (b) Hendra virus spillover events by latitude and date within month. Letters represent months from April through March. (c) Hendra virus spillover events by latitude and date within year. The colours represent the Gaussian kernel estimation of density of spillovers per unit area. Red represents areas and times with relatively high densities of spillover events.
The recent discovery of Hendra virus exposure in two dogs on infected horse properties suggests that horses can be intermediate hosts for infection of species other than humans [32,33]. Horse-to-horse transmission is usually limited to horses in close contact within paddocks or stables [34], and there is no evidence of human-to-human transmission. Nevertheless, repeated spillover events increase the likelihood of onward transmission of infectious agents in new hosts [35,36].
Hendra virus spillover events are clustered in time and space. All the subtropical spillovers have occurred in the cooler months of May through October, with the peak in July. No spillover events have occurred in the subtropics during summer (figure 2). In the northern tropics, spillover events have been detected throughout the year. The locations of clusters vary among years. In 2011, for example, most spillover events occurred within a 160 km coastal strip in southeast Queensland and northern New South Wales. In 2012, all eight spillover events were in the tropical north. The two spillover clusters in 2013 occurred in the subtropical south (electronic supplementary material, figure S2).
(a). Distribution and density of reservoir hosts
The overlapping distribution of reservoir and recipient hosts crudely delineates areas where recipient hosts are at risk of infection. In subtropical regions of Australia, shifting bat distributions, human population growth and changes in land use collectively increase the area and incidence of co-occurrence between bats and grazing horses (electronic supplementary material, appendix S3).
Hendra virus spillovers most often have occurred where urban and peri-urban areas are expanding and human population growth is high [30,38]. An increased presence of horses in these areas can be inferred [38]. Bats also have increased their use of these landscapes [39]. High-quality but ephemeral nectar resources in native flowering forests can support large, seasonally migratory or even nomadic bat populations [22,40]. However, when nectar flows are diminished due to seasonal conditions, habitat loss or climate change, bats seek alternative food sources in urban and peri-urban areas [39]. In some of these locations, the number of roost sites has increased fourfold since 1999 [41]. An increasing proportion of these urban and peri-urban bats forgo migration and switch to consistently available, but poorer quality, anthropogenic food sources [42,43], including asynchronously fruiting trees planted in horse paddocks [22,44]. These resident bats become particularly susceptible to winter and spring food shortages [42].
Coincident with these factors is another: the range of black flying-fox is expanding rapidly southward at rates faster than projected on the basis of climate change scenarios [40]. Black flying-fox have a stronger association with Hendra virus spillover events than other flying-fox species [45], and may be more likely to feed on the marginal foods that support resident populations in anthropogenic landscapes (K. Parry-Jones 2013, unpublished data). Thus, the range shift of black flying-fox may contribute to the increasing incidence and recent southern extension of Hendra virus spillover events in the subtropics.
(b). Pathogen shedding by reservoir hosts
Bat reservoir hosts must be infected and, in most cases, shedding virus for spillover to occur (although direct consumption of a bat, a bite from a bat or vector-borne transmission may circumvent the need for shedding [46]). New evidence suggests that virus excretion from bats may occur in spatial and temporal pulses that can drive spillover. For example, clusters of Nipah virus spillover in Bangladesh, Marburg virus spillover in Uganda and Hendra virus spillover in Australia have been associated with pulses of shedding from bats [28,29,32].
When shedding pulses are not occurring, many bat viruses are detected rarely or at low prevalence. For example, Hendra virus was rarely detected in bat populations [16,24] until 2011 when Hendra virus RNA was detected in up to two-thirds of pooled-urine samples from bats near cases in horses (G. Crameri 2011, unpublished data; H. Field 2011, unpublished data; electronic supplementary material, appendix S4). High prevalence in urine pools was sustained for two to three months [32]. Prevalence also was high in urine pools during the Hendra virus spillover clusters in 2013 (G. Crameri 2013, unpublished data; H. Field 2013, unpublished data). Similarly, Nipah virus prevalence in pooled bat urine was 22% following cases in humans in Bangladesh, but declined to 0% over two months [29], suggesting that detection may be more likely if sampling coincides with spillover. If outbreaks in humans are not documented, detection of virus in bats may be difficult. Delays between Ebola spillover, detection and sampling due to chains of infection in apes and humans may explain why spikes in bat seroprevalence [47], but not virus detection, have been linked with outbreaks in humans.
Two hypotheses may explain temporal and spatial pulses of virus shedding in bat populations: episodic shedding from persistently infected bats or transient epidemics passing as waves of bat-to-bat transmission between bat populations. These two processes have different drivers at the level of individuals, roosts and metapopulations.
(i). Episodic shedding hypothesis
There is a pervasive hypothesis that bats commonly host persistent infections that do not cause apparent pathology or disease [4,15,48], supported by the frequent isolation of viruses from healthy bats of different species [8,11]. If persistent infections are suppressed by the host's immune response [9,48], viral replication and episodic shedding could occur when intrinsic or extrinsic stressors weaken the immune response.
Bat populations excreting Hendra virus near the 2011 and 2013 spillover events experienced low food abundance and exhibited signs of nutritional stress (P. Eby 2011, 2013, unpublished data). Similarly, high antibody prevalence was observed in a population that was nutritionally compromised after a cyclone [25]. Given the susceptibility of urban and peri-urban bat populations to food shortages, the link between nutrition and Hendra virus shedding, including behavioural changes that may drive transmission, should be a research priority.
Pregnancy in bats has been noted to coincide with high seroprevalence and seasonal spillover of Hendra, Nipah and Ebola virus [25,26,30,47,49]. However, the relation between serological status (neutralizing or binding) and clearance or shedding of these viruses is unknown, and other factors (such as waning maternal antibody protection in pups) also coincide with spillover or shedding pulses [27,28,30,38]. Pups could contribute to shedding pulses if they develop productive infections during the acute phase of infection (when first infected), or because they provide a seasonal influx of susceptible individuals. However, before the effects of waning maternal immunity on infections in juveniles can be assessed, we must validate a protective effect of maternal antibodies.
More generally, experimental studies in which bats were held in presumably stressful conditions, but well fed, have not supported stress as a driver of shedding [14]. Nevertheless, different types of stress in captive and wild populations—for example, chronic, acute, nutritional and physiological—may have different effects on host–virus interactions. It is also plausible that physiological and environmental stressors discussed above, and co-infections—an increasingly recognized phenomenon in bats [10]—could increase the probability of individuals becoming shedders or even supershedders (electronic supplementary material, appendix S4).
The occurrence of relapsing (recrudescent) Hendra and Nipah virus encephalitis in humans has been used to support the theory of episodic shedding from bats [50]. However, relapsing Hendra and Nipah infections have been associated with defective forms of virus that were not infectious, and therefore do not contribute to transmission of disease (e.g. [51]). Furthermore, pathogenic mechanisms in novel recipient hosts do not provide evidence for related mechanisms in reservoir hosts; each host species is likely to have a different relationship with the pathogen. One study reported Nipah virus shedding in a captive bat as recrudescence [50]; however, there are alternative explanations (see the electronic supplementary material, appendix S4).
(ii). Transient epidemics hypothesis
Epidemics that travel as waves of infection among hosts could generate pulses of infection due to local virus extinction and recolonization across roosts [52]. The critical enabling factor for transient epidemics from a nonlethal virus is recovery from infection and subsequent immunity. Over time, waning population immunity (but not necessarily waning individual immunity) allows reinvasion of the virus. Halpin et al. [14] and Paweska et al. [53] provided experimental evidence for short periods of viral excretion for henipaviruses and Marburg virus, respectively. However, limited sample sizes and experiment durations restricted their ability to assess virus clearance (electronic supplementary material, appendix S4).
The gregariousness of bats, large group and population sizes, multiple host species and mixing over extensive areas could facilitate transient epidemics through extinction and recolonization metapopulation dynamics [30]. Under this scenario, decreases in migration observed in urban bat populations could disrupt transmission among host populations, reducing colony immunity and increasing the magnitude of epidemics when the virus is reintroduced [30]. The intensity of pulses of epidemic infection, and whether pulses fade out, reach a stable endemic state or recur, also depend on interactions among population size, transmission rate, infectious period, host replenishment rate, lifespan, rate of loss of immunity, environmental forcing, previous exposure and connectivity within and among subpopulations [30,52] (electronic supplementary material, appendix S4). Most of these parameters are unmeasured—with the exception of population size, which can, in many species of bats, vary rapidly from a few to hundreds of thousands of individuals through migration (e.g. [22]). If transmission increased with local population size (as with density-dependent transmission), population size could drive shedding pules [54]. There is no evidence that large populations of bats have been associated with periods of high prevalence or shedding of Hendra virus (electronic supplementary material, appendix S4).
(iii). Differentiating between hypotheses
Distinguishing episodic shedding from transient epidemics is challenging, and the two phenomena may not be mutually exclusive. For example, episodic shedding from an individual may generate waves of transmission through nearby susceptible individuals. Moreover, the pattern of shedding given persistent infection or transient epidemics may be indistinguishable, particularly when inferences are based on urine collected under roosts (detecting shedding but not infection status). One example may be the spatially extensive pulse of Hendra infection observed during 2011. Environmental conditions common among bat populations may have synchronized shedding by synchronizing stress [30], or may have synchronized transmission dynamics by creating similar density-dependent processes among populations [55].
Definitive evidence that bats are persistently infected with emerging viruses can only come from longitudinal studies of individual bats that are isolated from re-exposure—requiring experimental methods that establish patent infections in captive bats. Experiments, combined with field and modelling studies as well as viral phylogeny studies (when sequences become available), will ultimately decipher the complex relations that drive the dynamics of bat viruses.
(c). Survival of virus outside reservoir hosts and environmental load
Bats are volant, spending most of their time in trees in which they roost or feed, in caves or in transit. Bats spend little time on the ground. Therefore, transmission of virus from bats to non-volant species is most likely to occur indirectly via free virus particles shed from bats onto fomites or surfaces, or through virus-laden aerosolized urine or faeces (although note Ebola virus transmission linked to consumption of bats [46]). The stability of free virus in the environment determines the temporal window during which indirect cross-species transmission can occur.
Henipaviruses, filoviruses and coronoviruses are enveloped RNA viruses that are sensitive to increases in temperature, changes in pH, ultraviolet light and desiccation [56–58]. Under optimal laboratory conditions, henipaviruses may persist for several days, and filoviruses for several weeks [56–58]. Environmental conditions in nature may be less optimal for viral survival; temperature, humidity and microclimate under trees and in caves may influence viral decay rates and ultimately the likelihood of spillover. Hendra virus spillover has been associated with relatively cool months with conditions similar to those optimal for survival in the laboratory [57].
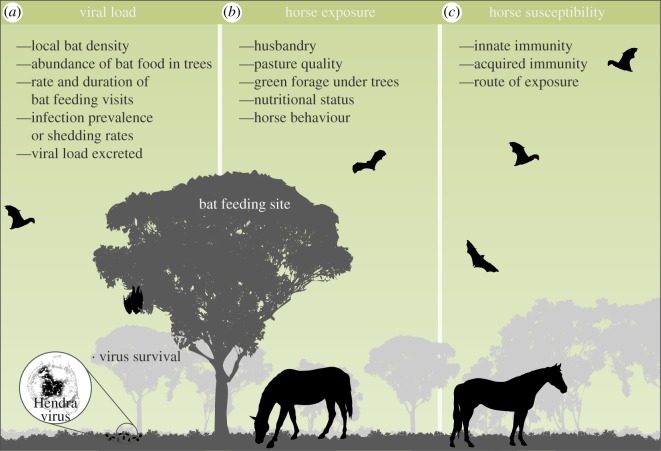
The interaction between virus survival and many other factors, including the amount of virus released into the environment and the time lag between virus shedding and recipient host exposure, affects how much virus is available to recipient hosts. The amount of virus shed from bats is determined by the number of bats present, the amount of time bats spend within the area, the shedding status of bats and the viral load excreted (figure 3).
Figure 3.
Risk factors for development of Hendra virus by horses. The concentration of virus in the environment is affected by the quantity of virus that bats are shedding and the probability of virus survival outside of the bat host, which in turn are affected by the factors in (a). Exposure of horses to virus is affected by the factors in (b). (c) The effectiveness of innate and acquired immunity determines whether horse exposure leads to fulminating infection.
(d). Recipient host exposure
During periods in which bats are shedding and contaminating the environment with virus, a small proportion of recipient hosts typically develop viral disease. For example, during a pulse of Nipah virus shedding in bats, an outbreak in humans was traced to two exposures to date palm sap [29]. Likewise, during the cluster of Hendra virus cases in horses in 2011, a small proportion of the horses within the area in which bats were shedding developed Hendra virus. Heterogeneity in exposure of recipient hosts to viruses—driven by interaction with the drip zone around trees—likely affects the probability of accumulating an infectious dose (figure 3).
Bats excrete urine, faeces and saliva (within partially eaten fruit) in a drip zone around trees where they feed or roost. Horses may be exposed to Hendra virus when consuming contaminated grass, fruit, feed or water; or when browsing or sniffing contaminated surfaces within this drip zone. Discarded fruit pulp is thought to be a route of transmission of Nipah virus to pigs in Malaysia [37] and Ebola virus to apes in Central Africa [3], while contaminated vessels used to collect date palm sap are a source of Nipah virus infection for humans in Bangladesh [59]. Exceptions to drip-zone transmission may occur when flying bats eliminate or drop partially eaten fruit or when virus is aerosolized in caves (reported for Marburg virus [60]).
Consumption rates within the drip zone may affect the accumulation of an infectious dose of a bat virus. For example, horses grazing on pastures with low nutritional quality, especially low fibre content, may eat bark, tree leaves and twigs for fibre, stomach fill and micronutrients [61], and new grass growth under trees [62], increasing exposure to Hendra virus. It is also conceivable that hungry horses are more likely to eat fruits partially consumed by bats, or even bat faeces, when other food is not available. The winter peak of Hendra virus spillover events in the subtropics coincides with the period of lowest pasture productivity [62] (electronic supplementary material, appendix S5). Ebola outbreaks in apes also occur during the dry season, when food is scarce and ape and bat populations compete for fruit [3,47].
(e). Susceptibility of recipient hosts
Henipaviruses and filoviruses have a broad species tropism that probably reflects their use of cell entry receptors that are highly conserved and widely distributed among vertebrates [12,63]. Within species, however, variation in susceptibility of recipient hosts to emerging bat viruses, and therefore the relation between cases in novel hosts and viral loads in the environment, is not known. For example, it is possible that environmental contamination and horse exposure to Hendra virus may be widespread during periods of shedding from bats, with susceptibility of individual horses determining their probability of infection (figure 3). Horses identified as spillover cases may be a small proportion of those exposed.
Some data suggest that fulminating infection may fall at one end of a spectrum of Hendra virus disease in horses (electronic supplementary material, appendix S2) [34]. Some horses may eliminate infection in the mucous membranes of the upper respiratory tract with rapid and effective innate immune responses. Others may seroconvert asymptomatically or seroconvert and recover after clinical disease. A similar spectrum of disease severity has been reported in humans infected with Ebola and Marburg virus [19].
Route of exposure probably affects susceptibility to Hendra virus infections. The likely primary routes of exposure for horses are nasal and oral [13,64]. However, behaviours such as sniffing the ground to avoid faeces and urine while foraging [65], along with the large surface area of nasal mucous membranes and large respiratory tidal volume, may increase exposure through inhalation. Perhaps this explains why cases have been observed in horses and not, for example, in cows, sheep, cats or other domestic animals.
Many additional factors affect the probability that an exposed recipient host will develop an infection. Genetics, general health and condition, secondary infections, previous exposures, climatic and nutritional conditions, and dose received can modulate the immune response and affect the outcome of exposure [66].
3. Summary
We suggest that the emergence of bat viruses in recipient hosts requires at least five hierarchical enabling conditions. The probability of occurrence of each is conditional on the occurrence of the preceding condition; removal of any condition should prevent spillover.
Interventions to decrease the probability of virus spillover can be implemented at each level. Interventions at the first level may include removal of the reservoir host. There has been public and political pressure in Queensland to manage Hendra virus by culling or dispersing fruit bat populations. We found no evidence that the prevalence of Hendra virus in bat populations was associated with population density, and therefore that decreases in host density would reduce virus prevalence. If increased levels of stress in bats facilitate virus shedding, or if culled populations compensate with higher birth rates or juvenile survival [67], disrupting colonies may increase the amplitude of viral shedding events.
At the level of virus shedding, conservation and restoration of critical bat feeding habitat should reduce the risk of nutritional stress and reduce urban colonization by bats. For Hendra virus in the subtropics, this would include forest habitats that are productive during winter and spring [42].
At the level of virus survival, delaying recipient hosts' interaction with bat excreta to allow viral decay should reduce exposure; for example, fencing horses away from trees at night should reduce exposure to Hendra virus. At the level of recipient host exposure, interventions can be targeted at the route of exposure. Barriers to collection pots for date palm sap can reduce exposure of humans to Nipah virus [68]. Horse exposure to Hendra virus can be reduced by watering and feeding horses away from trees, providing alternative shelter, and providing adequate dietary fibre and nutrient supplements (electronic supplementary material, appendix S5).
Vaccination is the standard intervention to modify host susceptibility. A recent vaccine for Hendra virus in horses initially had low uptake due to factors such as cost and lack of data in pregnant mares [69], highlighting the social challenge inherent when implementing interventions.
Although we have identified multiple, hierarchical enabling conditions for spillover, many conditions occur simultaneously and have common environmental drivers. Therefore, differentiating causal from correlational factors is a major challenge [70]. For example, winter in subtropical Australia is the peak of resource scarcity for both bats and horses. Bats move into human-dominated landscapes to find alternative food, increasing their co-occurrence with horses, their vulnerability to nutritional stress and possibly excretion of Hendra virus. Cool temperatures may maximize virus survival, increasing the cumulative dose available to horses. Low productivity of pastures leads to horse consumption of contaminated fruit or grass, as well as poor horse condition and higher susceptibility. Controlled experiments, in which some of these factors are manipulated and predictions compared with models, would be desirable. However, the difficulties and dangers of working with these viruses hinder such experimentation.
Tracking the dynamics of emerging diseases from the cell to the landscape will be necessary to assess the weight of evidence for potential causes and to elucidate how human activities affect one or more of the enabling conditions. Such a multiscale approach will move research into a realm that informs implementation of interventions and solutions.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank M. Rainey and R. Egloff for help producing figures, and E. Fleishman, J. Cawdell-Smith, C. Smith and D. Edson for comments on the manuscript.
Funding statement
This research was funded by the Commonwealth of Australia, the State of New South Wales and the State of Queensland under the National Hendra Virus Research Program, awarded through the Rural Industries Research and Development Corporation. R.K.P. was supported by the Cedar Tree Foundation, Morris Animal Foundation and P. Tye. R.K.P. and G.T. were supported by the Linnaeus Estate. P.J.H. was supported by the Research and Policy for Infectious Disease Dynamics program of the Science and Technology Directorate, Department of Homeland Security and Fogarty International Center, National Institutes of Health.
References
- 1.Wang L-F, Eaton B. 2007. Bats, civets and the emergence of SARS. In Wildlife and emerging zoonotic diseases: the biology, circumstances and consequences of cross-species transmission, pp. 325–344. New York, NY: Springer. [Google Scholar]
- 2.Chua K, et al. 2000. Nipah virus: a recently emergent deadly paramyxovirus. Science 288, 1432–1435. ( 10.1126/science.288.5470.1432) [DOI] [PubMed] [Google Scholar]
- 3.Leroy EM, et al. 2005. Fruit bats as reservoirs of Ebola virus. Nature 438, 575–576. ( 10.1038/438575a) [DOI] [PubMed] [Google Scholar]
- 4.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. 2006. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 19, 531–545. ( 10.1128/CMR.00017-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drexler JF, et al. 2012. Bats host major mammalian paramyxoviruses. Nat. Commun. 3, 796 ( 10.1038/ncomms1796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quan P-L, et al. 2013. Bats are a major natural reservoir for hepaciviruses and pegiviruses. Proc. Natl Acad. Sci. USA 110, 8194–8199. ( 10.1073/pnas.1303037110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luis AD, et al. 2013. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. R. Soc. B 280, 20122753 ( 10.1098/rspb.2012.2753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang GJ, et al. 2013. Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science 339, 456–460. ( 10.1126/science.1230835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Shea TJ, Cryan PM, Cunningham AA, Fooks AR, Hayman DTS, Luis AD, Peel AJ, Plowright RK, Wood JLN. 2014. Bat flight and zoonotic viruses. Emerg. Infect. Dis. 20, 741–745. ( 10.3201/eid2005.130539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh GA, et al. 2012. Cedar virus: a novel henipavirus isolated from Australian bats. PLoS Pathog. 8, e1002836 ( 10.1371/journal.ppat.1002836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker KS, et al. 2013. Novel, potentially zoonotic paramyxoviruses from the African straw-colored fruit bat Eidolon helvum. J. Virol. 87, 1348–1358. ( 10.1128/JVI.01202-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton BT, Broder CC, Middleton D, Wang LF. 2006. Hendra and Nipah viruses: different and dangerous. Nat. Rev. Microbiol. 4, 23–35. ( 10.1038/nrmicro1323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray K, et al. 1995. A morbillivirus that caused fatal disease in horses and humans. Science 268, 94–97. ( 10.1126/science.7701348) [DOI] [PubMed] [Google Scholar]
- 14.Halpin K, et al. 2011. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg. 85, 946–951. ( 10.4269/ajtmh.2011.10-0567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LF, Walker PJ, Poon LL. 2011. Mass extinctions, biodiversity and mitochondrial function: are bats ‘special’ as reservoirs for emerging viruses? Curr. Opin. Virol. 1, 649–657. ( 10.1016/j.coviro.2011.10.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith I, et al. 2011. Identifying Hendra virus diversity in pteropid bats. PLoS ONE 6, e25275 ( 10.1371/journal.pone.0025275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui J, et al. 2007. Evolutionary relationships between bat coronaviruses and their hosts. Emerg. Infect. Dis. 13, 1526 ( 10.3201/eid1310.070448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge X-Y, et al. 2013. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503, 535–538. ( 10.1038/nature12711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leroy E, Gonzalez JP, Baize S. 2011. Ebola and Marburg haemorrhagic fever viruses: major scientific advances, but a relatively minor public health threat for Africa. Clin. Microbiol. Infect. 17, 964–976. ( 10.1111/j.1469-0691.2011.03535.x) [DOI] [PubMed] [Google Scholar]
- 20.Peel AJ, et al. 2013. Continent-wide panmixia of an African fruit bat facilitates transmission of potentially zoonotic viruses. Nat. Commun. 4, 2770 ( 10.1038/ncomms3770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cross PC, Lloyd-Smith JO, Johnson PLF, Getz WM. 2005. Duelling timescales of host movement and disease recovery determine invasion of disease in structured populations. Ecol. Lett. 8, 587–595. ( 10.1111/j.1461-0248.2005.00760.x) [DOI] [Google Scholar]
- 22.Eby P. 1991. Seasonal movements of gray-headed flying-foxes, Pteropus poliocephalus (Chiroptera, Pteropodidae), from 2 maternity camps in Northern New-South-Wales. Wildl. Res. 18, 547–559. ( 10.1071/WR9910547) [DOI] [Google Scholar]
- 23.Roberts BJ, Catterall CP, Eby P, Kanowski J. 2012. Long-distance and frequent movements of the flying-fox Pteropus poliocephalus: implications for management. PLoS ONE 7, e42532 ( 10.1371/journal.pone.0042532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Field H, de Jong C, Melville D, Smith C, Smith I, Broos A, Kung YH, McLaughlin A, Zeddeman A. 2011. Hendra virus infection dynamics in Australian fruit bats. PLoS ONE 6, e28678 ( 10.1371/journal.pone.0028678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plowright RK, Field HE, Smith C, Divljan A, Palmer C, Tabor GM, Daszak P, Foley JE. 2008. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proc. R. Soc. B 275, 861–869. ( 10.1098/rspb.2007.1260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breed AC, Breed MF, Meers J, Field HE. 2011. Evidence of endemic Hendra virus infection in flying-foxes (Pteropus conspicillatus)--implications for disease risk management. PLoS ONE 6, e28816 ( 10.1371/journal.pone.0028816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drexler JF, Corman VM, Wegner T, Tateno AF, Zerbinati RM, Gloza-Rausch F, Seebens A, Müller MA, Drosten C. 2011. Amplification of emerging viruses in a bat colony. Emerg. Infect. Dis. 17, 449 ( 10.3201/eid1703.100526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amman BR, et al. 2012. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 8, e1002877 ( 10.1371/journal.ppat.1002877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan SU, et al. 2013. Nipah virus shedding among Pteropus bats in the context of a human outbreak in Bangladesh, 2012. In ASTMH 62nd Annual Meeting, 13–17 November, Washington, DC Deerfield, IL: American Society of Tropical Medicine and Hygiene. [Google Scholar]
- 30.Plowright RK, Foley P, Field HE, Dobson AP, Foley JE, Eby P, Daszak P. 2011. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. R. Soc. B 278, 3703–3712. ( 10.1098/rspb.2011.0522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banyard AC, Evans JS, Luo TR, Fooks AR. 2014. Lyssaviruses and bats: emergence and zoonotic threat. Viruses 6, 2974–2990. ( 10.3390/v6082974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Field H, Crameri G, NY K, Wang LF. 2012. Ecological aspects of hendra virus. Curr. Top. Microbiol. Immunol. 359, 11–23. ( 10.1007/82_2012_214) [DOI] [PubMed] [Google Scholar]
- 33.ProMED-mail. 2013. Hendra virus, equine—Australia (09): (New South Wales) dog affected. ProMED-mail 2013; 21 July; 20130721.1837123.
- 34.Field H, et al. 2010. Hendra virus outbreak with novel clinical features, Australia. Emerg. Infect. Dis. 16, 338 ( 10.3201/eid1602.090780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pulliam JR, et al. 2012. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J. R. Soc. Interface 9, 89–101. ( 10.1098/rsif.2011.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antia R, Regoes RR, Koella JC, Bergstrom CT. 2003. The role of evolution in the emergence of infectious diseases. Nature 426, 658–661. ( 10.1038/nature02104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Field H, Young P, Yob JM, Mills J, Hall L, Mackenzie J. 2001. The natural history of Hendra and Nipah viruses. Microb. Infect. 3, 307–314. ( 10.1016/S1286-4579(01)01384-3) [DOI] [PubMed] [Google Scholar]
- 38.McFarlane R, Becker N, Field H. 2011. Investigation of the climatic and environmental context of Hendra virus spillover events 1994–2010. PLoS ONE 6, e28374 ( 10.1371/journal.pone.0028374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eby P, Lunney D. 2002. Managing the grey-headed flying-fox as a threatened species in NSW. Sydney, Australia: Royal Zoological Society of New South Wales [Google Scholar]
- 40.Roberts BJ, Catterall CP, Eby P, Kanowski J. 2012. Latitudinal range shifts in Australian flying-foxes: a re-evaluation. Austral Ecol. 37, 12–22. ( 10.1111/j.1442-9993.2011.02243.x) [DOI] [Google Scholar]
- 41.Queensland Department of Environment and Heritage Protection. 2011. Flying-fox roost sites: south-eastern Queensland, 24 Aug 2011 edn Brisbane, Australia: Queensland Department of Environment and Heritage Protection. [Google Scholar]
- 42.Eby P, Richards G, Collins L, Parry-Jones K. 1999. The distribution, abundance and vulnerability to population reduction of a nomadic nectarivore, the grey-headed flying-fox Pteropus poliocephalus in New South Wales, during a period of resource concentration. Aust. Zool. 31, 240–253. ( 10.7882/AZ.1999.024) [DOI] [Google Scholar]
- 43.Markus N, Hall L. 2004. Foraging behaviour of the black flying-fox (Pteropus alecto) in the urban landscape of Brisbane, Queensland. Wildl. Res. 31, 345–355. ( 10.1071/WR01117) [DOI] [Google Scholar]
- 44.Parry-Jones K, Augee M. 2001. Factors affecting the occupation of a colony site in Sydney, New South Wales by the grey-headed flying-fox Pteropus poliocephalus (Pteropodidae). Austral Ecol. 26, 47–55. [Google Scholar]
- 45.Smith C, Skelly C, Kung N, Roberts B, Field H. 2014. Flying-fox species density-a spatial risk factor for Hendra virus infection in horses in Eastern Australia. PLoS ONE 9, e99965 ( 10.1371/journal.pone.0099965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leroy EM, Epelboin A, Mondonge V, Pourrut X, Gonzalez J-P, Muyembe-Tamfum J-J, Formenty P. 2009. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 9, 723–728. ( 10.1089/vbz.2008.0167) [DOI] [PubMed] [Google Scholar]
- 47.Pourrut X, Delicat A, Rollin P, Ksiazek T, Gonzalez J-P, Leroy E. 2007. Spatial and temporal patterns of Zaire ebolavirus antibody prevalence in the possible reservoir bat species. J. Infect. Dis. 196, S176–S183. ( 10.1086/520541) [DOI] [PubMed] [Google Scholar]
- 48.Baker ML, Schountz T, Wang LF. 2013. Antiviral immune responses of bats: a review. Zoonoses Public health 60, 104–116. ( 10.1111/j.1863-2378.2012.01528.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahman SA, et al. 2013. Risk factors for Nipah virus infection among pteropid bats, Peninsular Malaysia. Emerg. Infect. Dis. 19, 51–60. ( 10.3201/eid1901.120221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahman SA, et al. 2010. Characterization of Nipah virus from naturally infected Pteropus vampyrus bats, Malaysia. Emerg. Infect. Dis. 16, 1990–1993. ( 10.3201/eid1612.091790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan CT, et al. 2002. Relapsed and late-onset Nipah encephalitis. Ann. Neurol. 51, 703–708. ( 10.1002/ana.10212) [DOI] [PubMed] [Google Scholar]
- 52.Bolker B, Grenfell B. 1995. Space, persistence and dynamics of measles epidemics. Phil. Trans. R. Soc. Lond. B 348, 309–320. ( 10.1098/rstb.1995.0070) [DOI] [PubMed] [Google Scholar]
- 53.Paweska JT, van Vuren PJ, Masumu J, Leman PA, Grobbelaar AA, Birkhead M, Clift S, Swanepoel R, Kemp A. 2012. Virological and serological findings in Rousettus aegyptiacus experimentally inoculated with vero cells-adapted hogan strain of Marburg virus. PLoS ONE 7, e45479 ( 10.1371/journal.pone.0045479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCallum H, Barlow N, Hone J. 2001. How should pathogen transmission be modelled? Trends Ecol. Evol. 16, 295–300. ( 10.1016/S0169-5347(01)02144-9) [DOI] [PubMed] [Google Scholar]
- 55.Cattadori IM, Haydon DT, Hudson PJ. 2005. Parasites and climate synchronize red grouse populations. Nature 433, 737–741. ( 10.1038/nature03276) [DOI] [PubMed] [Google Scholar]
- 56.Sinclair R, Boone SA, Greenberg D, Keim P, Gerba CP. 2008. Persistence of category A select agents in the environment. Appl. Environ. Microbiol. 74, 555–563. ( 10.1128/AEM.02167-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fogarty R, Halpin K, Hyatt AD, Daszak P, Mungall BA. 2008. Henipavirus susceptibility to environmental variables. Virus Res. 132, 140–144. ( 10.1016/j.virusres.2007.11.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piercy T, Smither S, Steward J, Eastaugh L, Lever M. 2010. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J. Appl. Microbiol. 109, 1531–1539. [DOI] [PubMed] [Google Scholar]
- 59.Luby SP, et al. 2006. Foodborne transmission of Nipah virus, Bangladesh. Emerg. Infect. Dis. 12, 1888 ( 10.3201/eid1212.060732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adjemian J, et al. 2011. Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007. J. Infect. Dis. 204(Suppl. 3), S796–799. ( 10.1093/infdis/jir312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGreevy P. 2004. Equine behavior: a guide for veterinarians and equine scientists. London, UK: Elsevier. [Google Scholar]
- 62.Anderson DL, Bryden WL. 2012. Does pasture availability influence Hendra virus infection of grazing horses? In Proc. 4th Australasian Equine Science Symp., 13–15 June, Gold Coast, Australia, pp. 33–34. Gatton, Australia: Australasian Equine Science Symposium. See www.australasianequinescience.com/AESS2012.html. [Google Scholar]
- 63.Miller EH, et al. 2012. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 31, 1947–1960. ( 10.1038/emboj.2012.53) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marsh GA, et al. 2011. Experimental infection of horses with Hendra virus/Australia/horse/2008/Redlands. Emerg. Infect. Dis. 17, 2232–2238. ( 10.3201/eid1712.111162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ödberg F, Francis-Smith K. 1976. A study on eliminative and grazing behaviour—the use of the field by captive horses. Equine Vet. J. 8, 147–149. ( 10.1111/j.2042-3306.1976.tb03326.x) [DOI] [Google Scholar]
- 66.Moberg GP, Mench JA. 2000. The biology of animal stress: basic principles an implications for animal welfare. Wallingford, UK: CABI Publishing. [Google Scholar]
- 67.Choisy M, Rohani P. 2006. Harvesting can increase severity of wildlife disease epidemics. Proc. R. Soc. B 273, 2025–2034. ( 10.1098/rspb.2006.3554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan SU, Gurley ES, Hossain MJ, Nahar N, Sharker MY, Luby SP. 2012. A randomized controlled trial of interventions to impede date palm sap contamination by bats to prevent Nipah virus transmission in Bangladesh. PLoS ONE 7, e42689 ( 10.1371/journal.pone.0042689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Middleton D, et al. 2014. Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg. Infect. Dis. 20, 372 ( 10.3201/eid2003.131159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plowright RK, Sokolow SH, Gorman ME, Daszak P, Foley JE. 2008. Causal inference in disease ecology: investigating ecological drivers of disease emergence. Frontiers in Ecology and the Environment 6, 420–429. ( 10.1890/070086) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.