Abstract
Individual animals frequently exhibit repeatable differences from other members of their population, differences now commonly referred to as ‘animal personality’. Personality differences can arise, for example, from differences in permanent environmental effects―including parental and epigenetic contributors―and the effect of additive genetic variation. Although several studies have evaluated the heritability of behaviour, less is known about general patterns of heritability and additive genetic variation in animal personality. As overall variation in behaviour includes both the among-individual differences that reflect different personalities and temporary environmental effects, it is possible for personality to be largely genetically influenced even when heritability of behaviour per se is quite low. The relative contribution of additive genetic variation to personality variation can be estimated whenever both repeatability and heritability are estimated for the same data. Using published estimates to address this issue, we found that approximately 52% of animal personality variation was attributable to additive genetic variation. Thus, while the heritability of behaviour is often moderate or low, the heritability of personality is much higher. Our results therefore (i) demonstrate that genetic differences are likely to be a major contributor to variation in animal personality and (ii) support the phenotypic gambit: that evolutionary inferences drawn from repeatability estimates may often be justified.
Keywords: personality, additive genetic variation, among-individual variation
1. Introduction
Personality differences among individuals, if genetically based, have potentially profound evolutionary implications [1–5]. However, while some studies have examined the heritability of animal personality (e.g. [6–8]) and others have looked at the specific actions of one or a few genes or gene products on specific behaviours (recent examples include [9,10]), whether or not there is broad support for a genetic basis of animal personality is not currently clear. Within this context, current efforts to define personality in terms of repeatable variation have the potential to reveal the genetic basis of personality, and thus their evolutionary importance.
Personality variation has been defined as consistent individual differences in behaviours [11,12] that can be operationally defined mathematically in terms of repeatable differences among individuals [13–15]. Specifically, among-individual variation (henceforth Vind)—the numerator in repeatability—corresponds to personality variation [13,14]. Defining personality mathematically in terms of repeatability has several advantages. First, it allows application of well-developed statistical tools (i.e. mixed-effects models) to address questions about personality variation [13]. Second, and more biologically important, repeatability has long been of interest in evolutionary ecology and is closely connected to quantitative genetics and evolutionary theory [16]. This connection thus bridges personality research with evolutionary biology and its tools, increasing the scope of questions that can be asked and allowing well-developed questions in evolutionary theory to be asked with regard to personality (see also [17]). This definition also allows the general question of the degree to which animal personality variation is genetically determined to be asked.
The connection between repeatability and quantitative genetics becomes apparent when the former is defined based on the factors that contribute to phenotypic variation. Phenotypic variation (VP) is composed of multiple components:
| 1.1 |
where VA represents the effects of additive genetic variation on VP, VD represents dominance genetic effects, VPE represents permanent environmental effects, and VTE represents temporary environmental effects with only transitory effects on the phenotype and can include effects like temporary variation in state (e.g. motivation, energy reserves etc.). VPE includes parental effects (both maternal and paternal effects), epigenetic effects and other contributors that have long-term impacts on phenotypes (e.g. nutritional state during development). From this list of contributors, we can define repeatability (τ) as follows:
| 1.2 |
which also represents the variability among individuals relative to total phenotypic variability [14,16]. By defining animal personality as among-individual variation (Vind), variation in personality is equal to the numerator of τ [14]:
| 1.3 |
This definition of personality variation explicitly distinguishes personality variation from behavioural variation at large. Specifically, as seen from comparing equation (1.1) with equation (1.3), personality variation does not include the influence of temporary environmental variation on behaviour.
Narrow-sense heritability (h2) is formally defined from the same list of contributors as are repeatability and personality variation:
| 1.4 |
One unanswered question in personality research is the relative contribution of genetic variation to personality variation. The relationships expressed in equations (1.2)–(1.4) allow this question to be addressed. Specifically, if we want to know the contribution of additive genetic variation to personality variation, we can divide heritability by repeatability,
| 1.5 |
which simplifies to
| 1.6 |
Put another way, equation (1.6) can be considered the heritability of personality, and the relative contribution of additive genetic differences to personality differences can be directly estimated whenever both τ and h2 are estimated from the same data. Heritability of personality differs from the conventional definition of either narrow- or broad-sense heritability primarily in that VTE is missing from the denominator. Further, because VTE is excluded from the denominator of equation (1.6) but not equation (1.4), the heritability of personality will necessarily be greater than that of behaviours generally.
Whereas (narrow-sense) heritability estimates the proportion of total phenotypic variation attributable to additive genetic variance, heritability of personality refers strictly to the proportion of personality variation attributable to additive genetic variance. Distinguishing heritability of personality from heritability in general is important for a variety of reasons, most notably because doing so allows the explicit consideration of how additive genetic and non-genetic factors might influence the evolution of personality, a topic of great interest. While others have reviewed estimates of the heritability of behaviour (e.g. [18–20]), general patterns regarding the heritability of personality specifically have not been similarly reviewed.
Understanding the relationship between heritability and repeatability is also of general interest to behavioural ecology and, more broadly, evolutionary ecology, because these fields typically make the ‘phenotypic gambit’ [21]. The phenotypic gambit—that evolutionary inferences can be reliably drawn from phenotypic observations—is a common but often unstated assumption of evolutionary ecology, and is often made despite ignorance about proximate mechanisms and underlying genetics. Importantly, whether the phenotypic gambit is appropriate rests on whether observed phenotypes correspond to underlying genotypes, or at least behave as though their distributions do [21]. The gambit—and therefore many of the inferences evolutionary ecologists draw—thus depends on the degree to which observed phenotypic variation corresponds to underlying additive genetic variation. Unfortunately, the degree to which the phenotypic gambit holds for behaviours and other traits is often unclear, but can, again, be assessed when both τ and h2 are estimated from the same data. Finally, although our discussion of the heritability of personality is strictly focused on behaviour, the relationship of VA/Vind is similarly important to evolutionary ecologists as a whole. As is the case for behaviour, this relationship likewise demonstrates the relative contribution of additive genetic variation to among-individual variation for other types of traits.
Here, using meta-analysis, we tested (i) whether the phenotypic gambit is supported for behaviours and (ii) the degree to which personality variation can be attributed to additive genetic variation (i.e. the heritability of personality).
2. Material and methods
(a). Dataset
To test the contribution of additive genetic variance to personality variation, we obtained estimates of τ and h2 from the literature in two ways. First, we used data sources previously collected by Stirling et al. [19] in their review of heritabilities of behaviour. This previous search reviewed the behavioural literature to the end of the year 2000 and yielded 70 articles. Second, we conducted a search of 12 leading behavioural ecology, behavioural genetics and evolutionary ecology journals. The journals we included in our search were The American Naturalist, Evolution, Ecology, Behavioral Ecology, Animal Behaviour, Behavior Genetics, Heredity, Behaviour, Ethology, Journal of Evolutionary Biology, Journal of Animal Ecology and Proceedings of the Royal Society B. For behavioural journals, we used the keywords ‘heritability’ and ‘heritab*’, while for evolutionary ecology journals we used the keywords ‘heritab* AND behav*’ for all articles published in these journals between January 2000 to September 2012. This yielded an additional 236 articles. Of these 306 total articles, only 12 reported both heritability and repeatability of at least one behaviour. The other 294 articles may have reported one parameter or the other, or simply discussed both heritability and repeatability. From these 12 studies―which included 121 pairs of estimates―we extracted all reported estimates of τ and h2, species names, and traits measured. We only included non-human animals in the dataset—thereby excluding one study and 13 pairs of estimates. We also excluded h2 or τ estimates greater than 1 or less than 0 [22], which removed 14 pairs of estimates and one article entirely. From the remaining 10 articles and 94 estimates, we excluded all cases in which h2 was estimated as greater than τ. While h2 can be greater than τ under special circumstances [23], a review of available estimates did not suggest these circumstances were met and suggested that these instances were instead a product of estimation error. This screening reduced the dataset to 71 estimates. We removed an additional pair of estimates (i.e. one record in the dataset) as they showed up twice in the dataset, once via mid-parent : son and once as mid-parent : mid-offspring (we retained the mid-offspring estimate). These searches and inclusion criteria resulted in a dataset of 70 instances from 10 studies in which h2 and τ were jointly estimated for the same behaviour with the same data (electronic supplementary material, table S1).
(b). Data analysis
To assess support for both the phenotypic gambit as it pertains to behaviours and the degree to which personality variation can be attributed to additive genetic variation, we calculated the ratio of heritability to repeatability for each of the 70 estimates from 10 studies. This ratio, as demonstrated in equations (1.5) and (1.6), is key to both questions. First, as this ratio increases, the phenotypic gambit can be made more reliably. Second, this ratio explicitly estimates the relative contribution of additive genetic variation to personality variation.
To estimate this ratio, we used a linear random-effects model with the study from which estimates were drawn included as a random effect. This model was fitted using restricted estimate maximum likelihood. The intercept of this model provides an estimate of equations (1.5) and (1.6) after controlling for non-independence of studies. We also estimated the 95% confidence interval (CoI) around this estimate.
Finally, we qualitatively compared differences in the relative contribution of additive genetic variation to personality variation based on the types of behaviours assayed.
3. Results
Across the 70 estimates, h2 had an average of 0.14 and τ had an average of 0.29. The ratio of h2 to τ was estimated as 0.52 (CoI = 0.33 : 0.70), indicating that 52% of personality variation present across the included studies was attributable to additive genetic variation (figure 1). Put another way, the heritability of personality was estimated as 0.52.
Figure 1.
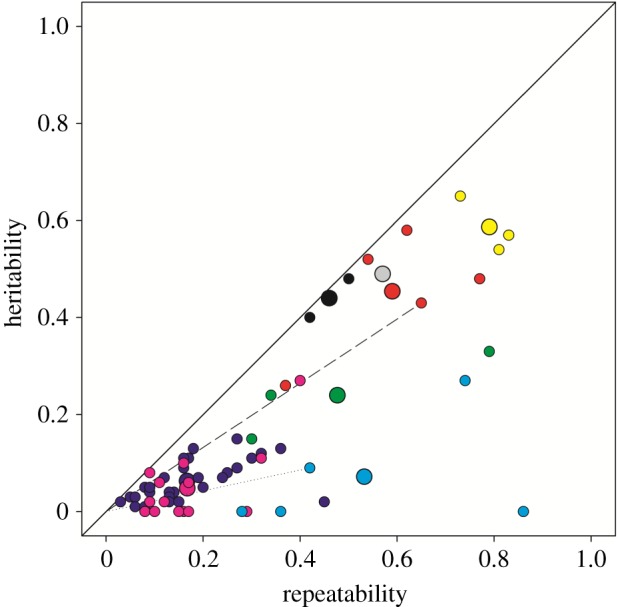
Heritability relative to repeatability. The solid line represents a 1 : 1 relationship between the heritability and repeatability. Large circles are study-level means for heritability and repeatability. Smaller circles are individual estimates from each study. Individual and mean estimates share the same colour by study. A point that falls directly on the solid line would represent one in which all personality (i.e. repeatable) variation was attributable to additive genetic variation. The slope of the relationship between any particular point and the origin (0,0) estimates the proportion of personality variation for that behavioural measure attributable to additive genetic variation. For example, the dashed and dotted lines correspond, respectively, to behavioural responses where 66% and 21% of observed personality variation was attributable to additive genetic effects.
We did not have sufficient estimates to statistically determine how different behaviours might differ in the contribution of additive genetic variation to personality variation. However, qualitatively it appears that personality variation in aggression and antipredator behaviour may have a stronger genetic component than for other types of behaviours included in our dataset (figure 2). These behaviours also tended towards having higher repeatabilities and higher heritabilities (figure 2).
Figure 2.
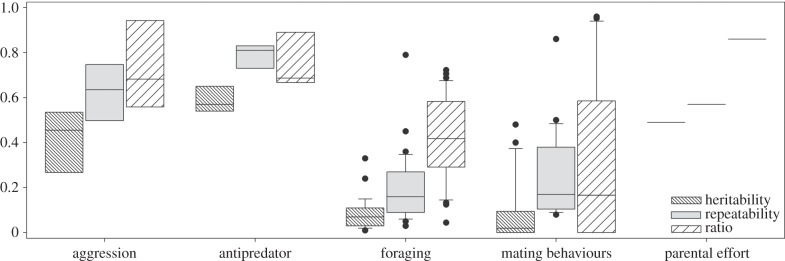
Boxplots for estimates of heritability (narrowly hatched), repeatability (grey fill) and the ratio between the two (widely hatched) by general behavioural classification. Horizontal lines within box correspond to behavioural medians, box boundaries correspond to first and third quartiles. When present, whiskers correspond to 10th and 90th percentiles, and points correspond to outliers. For parental effort, only a single estimate was available.
4. Discussion
The ratio of heritability to repeatability (0.52) suggests that evolutionary inferences based on repeatabilities could be appropriate. More specifically, this result suggests that the repeatable component of behavioural responses has a substantive genetic component, a prerequisite for the phenotypic gambit. However, the broad CoI around this estimate suggests that further verification is needed and that in the interim such a conclusion should be considered provisional. This general support for the phenotypic gambit, as it pertains to personality variation, is also in agreement with analyses conducted elsewhere that behavioural phenotypic correlations are generally consistent with genetic correlations (e.g. [22,24]), although this is not necessarily the case for correlations among other types of traits [25,26]. Thus, while estimation of genetic variances and covariances remains key for understanding animal personalities [14,27], some evolutionary inferences can be drawn from phenotypic estimates. Importantly, this finding should not be taken to suggest that repeatability estimates can be used as straightforward proxies for heritability as the ratio between the two ranged from 0 to 0.96 (see also [16]). Further, details such as G × E interactions will not necessarily be properly revealed with phenotypic estimates alone.
Our finding that the ratio of heritability to repeatability is 0.52 is particularly interesting for the field of personality research. As personality can be defined as repeatable variation in behaviour [12–14], this result means that 52% of personality variation is at the additive genetic level (i.e. that the heritability of personality is 0.52). That personality variation represents standing genetic variation is assumed in many empirical studies, but has not been broadly tested previously. Our findings provide some support for this assumption. Elsewhere it has been demonstrated that phenotypic correlations might closely correspond to genetic correlations [22]; that finding, along with our results here, bolsters recent suggestions that personality and behavioural syndromes might substantially constrain evolutionary responses [2].
As an aside, our discussion of repeatability and heritability variance components (e.g. equations (1.3)–(1.6)) has excluded mention of measurement error as a source, of variation. Measurement error will be present in all studies, but will typically be conflated with VTE, leading to underestimations of repeatability, heritability and the heritability of personality. However, sources of error might occasionally be conflated with Vind, for example when different recording methods or different observers are used on a particular subset of study subjects.
Our estimate that the mean heritability of personality is 0.52—although the heritability of behaviour for the same data was 0.14—is interesting in additional ways. First, this difference emphasizes that while behavioural heritabilities might be quite low, the heritability of personality might still be high. This difference is determined by the contribution of temporary environmental effects (VTE; equation (1.1)) to the expression of a behaviour, which includes short-term plastic responses (and measurement error). Other published estimates of behavioural heritabilities provide average estimates that range from 0.26 [20], via approximately 0.3 [18,19], to approximately 0.5 [28]. Our results suggest that the contribution of additive genetic variation to personality would be underestimated if based on these estimates.
A second implication of our estimate of heritability of personality as 0.52 is that it also provides clues as to how much and to what degree other types of factors might contribute to personality differences. Specifically, an average of 48% of personality variation is necessarily due to variation in permanent environmental effects and genetic dominance (equation (1.6)). Genetic dominance is often assumed to play only a minor role in quantitative traits [29] (but see [30]), although this may not be the case with sex-linked traits. If we assume a small role for genetic dominance, permanent environmental effects explain around half of standing personality variation. As discussed above, permanent environmental effects include maternal and paternal effects, epigenetics and environmental effects that have long-term effects on phenotypes (relative to the time span of measurements). In the personality literature, an exciting possibility that is getting attention involves permanent environmental effects due to positive feedback loops between an individual's personality and its choice of environments. For example, individuals might exhibit personality-dependent social niche specializations, where different individuals are consistently more aggressive, fearful or cooperative depending on their social niche (rank or role), which favours them maintaining both that personality and that social niche over the long term [31,32]. Thus, while additive genetic variation is a primary contributor to personality, considerable variation remains to be explained. Importantly, this interpretation has broader applicability and can likewise be applied to, for example, physiology and life-history traits.
While available data did not allow statistical comparison of how the contribution of additive genetic variation to personality varied across behavioural types, some qualitative observations can be drawn. As stated earlier, aggression and antipredator personality variation seem to be more heavily influenced by additive genetic variation, and tend to have higher heritabilities, than other behaviours. This result is somewhat surprising as elsewhere [20] aggressive behaviours have been found to exhibit relatively low heritabilities. This result is further complicated by the fact that repeatability of aggressive behaviours can be complicated by indirect genetic effects based on behavioural variation among opponents [33]. Our result may also be atypical in that it was restricted only to studies reporting both heritabilities and repeatabilities, although why this would impart a directional bias is unclear.
In contrast to empirical research, theoretical research into the evolutionary causes of personality variation has not necessarily assumed a genetic basis [34,35]. Our results suggest that this assumption may be neither necessary nor appropriate. Moreover, the finding that a considerable amount of personality variation corresponds to additive genetic variation reinforces a point made in the numerous reviews of behavioural ecological research regarding personality. Specifically, classical and current evolutionary theory regarding the maintenance of genetic variance can be put towards questions regarding personality variation [27]. For example, it is generally expected that variation will be depleted under selection [18]. However, when adjusting for scaling issues, traits more closely connected to fitness typically harbour greater amounts of genetic variation [36]. Likewise, although small populations are typically assumed to harbour less fitness affecting genetic variation than large populations, this is not typically actually the case [37]. What patterns might occur for behaviours is unclear; however, our results suggest that qualitative and quantitative predictions for this and other questions may be available within the broader body of evolutionary research.
Supplementary Material
Data accessibility
Data used for these analyses are included as the electronic supplementary material.
Funding statement
N.A.D. would like to thank the ND-EPSCoR programme for funding support, and A.S. would like to thank support from NSF IOS 0952132.
References
- 1.Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW. 2012. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198. ( 10.1111/j.1461-0248.2012.01846.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dochtermann NA, Dingemanse NJ. 2013. Behavioral syndromes as evolutionary constraints. Behav. Ecol. 24, 806–811. ( 10.1093/beheco/art002) [DOI] [Google Scholar]
- 3.Sih A, Bell AM, Johnson JC, Ziemba RE. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277. ( 10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 4.Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. ( 10.1111/j.1461-0248.2011.01731.x) [DOI] [PubMed] [Google Scholar]
- 5.Wolf M, van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. ( 10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 6.Bell AM. 2005. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 18, 464–473. ( 10.1111/j.1420-9101.2004.00817.x) [DOI] [PubMed] [Google Scholar]
- 7.Dingemanse NJ, Barber I, Wright J, Brommer JE. 2012. Quantitative genetics of behavioural reaction norms: genetic correlations between personality and behavioural plasticity vary across stickleback populations. J. Evol. Biol. 25, 485–496. ( 10.1111/j.1420-9101.2011.02439.x) [DOI] [PubMed] [Google Scholar]
- 8.Taylor RW, Boon AK, Dantzer B, Reale D, Humphries MM, Boutin S, Gorrell JC, Coltman DW, McAdam AG. 2012. Low heritabilities, but genetic and maternal correlations between red squirrel behaviours. J. Evol. Biol. 25, 614–624. ( 10.1111/j.1420-9101.2012.02456.x) [DOI] [PubMed] [Google Scholar]
- 9.Mueller JC, Edelaar P, Carrete M, Serrano D, Potti J, Blas J, Dingemanse NJ, Kempenaers B, Tella JL. 2014. Behaviour-related DRD4 polymorphisms in invasive bird populations. Mol. Ecol. 23, 2876–2885. ( 10.1111/mec.12763) [DOI] [PubMed] [Google Scholar]
- 10.Sanogo YO, Hankison S, Band M, Obregon A, Bell AM. 2011. Brain transcriptomic response of threespine sticklebacks to cues of a predator. Brain Behav. Evol. 77, 270–285. ( 10.1159/000328221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell AM, Hankson SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. ( 10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 13.Dingemanse NJ, Dochtermann NA. 2013. Quantifying individual variation in behaviour: mixed-effect modelling approaches. J. Anim. Ecol. 82, 39–54. ( 10.1111/1365-2656.12013) [DOI] [PubMed] [Google Scholar]
- 14.Dingemanse NJ, Dochtermann NA. 2014. Individual behaviour: behavioural ecology meets quantitative genetics. In Quantitative genetics in the wild (eds Charmantier A, Garant D, Kruuk LEB.), pp. 54–67. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Dingemanse NJ, Kazem AJN, Réale D, Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. ( 10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 16.Boake CRB. 1989. Repeatability: its role in evolutionary studies of mating-behavior. Evol. Ecol. 3, 173–182. ( 10.1007/BF02270919) [DOI] [Google Scholar]
- 17.Penke L, Denissen JJA, Miller GF. 2007. The evolutionary genetics of personality. Eur. J. Pers. 21, 549–587. ( 10.1002/per.629) [DOI] [Google Scholar]
- 18.Mousseau TA, Roff DA. 1987. Natural selection and the heritability of fitness components. Heredity 59, 181–197. ( 10.1038/hdy.1987.113) [DOI] [PubMed] [Google Scholar]
- 19.Stirling DG, Reale D, Roff DA. 2002. Selection, structure and the heritability of behaviour. J. Evol. Biol. 15, 277–289. ( 10.1046/j.1420-9101.2002.00389.x) [DOI] [Google Scholar]
- 20.van Oers K, Sinn DL. 2013. Quantitative and molecular genetics of animal personality. In Animal personalities: behavior, physiology, and evolution (eds Carere C, Maestripieri D.), pp. 159–200. Chicago, IL: University of Chicago Press. [Google Scholar]
- 21.Grafen A. 1984. Natural selection, kin selection and group selection. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB.), 2nd edn, pp. 62–84. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 22.Dochtermann NA. 2011. Testing Cheverud's conjecture for behavioral correlations and behavioral syndromes. Evolution 65, 1814–1820. ( 10.1111/j.1558-5646.2011.01264.x) [DOI] [PubMed] [Google Scholar]
- 23.Dohm MR. 2002. Repeatability estimates do not always set an upper limit to heritability. Funct. Ecol. 16, 273–280. ( 10.1046/j.1365-2435.2002.00621.x) [DOI] [Google Scholar]
- 24.Brommer JE, Kluen E. 2012. Exploring the genetics of nestling personality traits in a wild passerine bird: testing the phenotypic gambit. Ecol. Evol. 2, 3032–3044. ( 10.1002/ece3.412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadfield JD, Nutall A, Osorio D, Owens IPF. 2007. Testing the phenotypic gambit: phenotypic, genetic and environmental correlations of colour. J. Evol. Biol. 20, 549–557. ( 10.1111/j.1420-9101.2006.01262.x) [DOI] [PubMed] [Google Scholar]
- 26.Kruuk LEB, Slate J, Wilson AJ. 2008. New answers for old questions: the evolutionary quantitative genetics of wild animal populations. Annu. Rev. Ecol. Evol. Syst. 39, 525–548. ( 10.1146/annurev.ecolsys.39.110707.173542) [DOI] [Google Scholar]
- 27.Dochtermann NA, Roff DA. 2010. Applying a quantitative genetics framework to behavioural syndrome research. Phil. Trans. R. Soc. B 365, 4013–4020. ( 10.1098/rstb.2010.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potsma E. 2014. Four decades of estimating heritabilities in wild vertebrate populations: improved methods, more data, better estimates? In Quantitative genetics in the wild (eds Charmantier A, Garant D, Kruuk LEB.), pp. 16–33. Oxford, UK: Oxford University Press. [Google Scholar]
- 29.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 30.Meffert LM, Hicks SK, Regan JL. 2002. Nonadditive genetic effects in animal behavior. Am. Nat. 160, S198–S213. ( 10.1086/342896) [DOI] [PubMed] [Google Scholar]
- 31.Bergmuller R, Taborsky M. 2010. Animal personality due to social niche specialisation. Trends Ecol. Evol. 25, 504–511. ( 10.1016/j.tree.2010.06.012) [DOI] [PubMed] [Google Scholar]
- 32.Montiglio PO, Ferrari C, Reale D. 2013. Social niche specialization under constraints: personality, social interactions and environmental heterogeneity. Phil. Trans. R. Soc. B 368, 20120343 ( 10.1098/rstb.2012.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson AJ, Gelin U, Perron MC, Reale D. 2009. Indirect genetic effects and the evolution of aggression in a vertebrate system. Proc. R. Soc. B 276, 533–541. ( 10.1098/rspb.2008.1193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dingemanse NJ, Wolf M. 2010. Recent models for adaptive personality differences: a review. Phil. Trans. R. Soc. B 365, 3947–3958. ( 10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf M, Weissing FJ. 2010. An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959–3968. ( 10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houle D. 1992. Comparing evolvability and variability of quantitative traits. Genetics 130, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson T, Barton N. 2005. Theoretical models of selection and mutation on quantitative traits. Phil. Trans. R. Soc. B 360, 1411–1425. ( 10.1098/rstb.2005.1667) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for these analyses are included as the electronic supplementary material.


