Abstract
Males that produce conspicuous mate attraction signals are often at high risk of predation from eavesdropping predators. Females of such species typically search for signalling males and their higher motility may also place them at risk. The relative predation risk faced by males and females in the context of mate-finding using long-distance signals has rarely been investigated. In this study, we show, using a combination of diet analysis and behavioural experiments, that katydid females, who do not produce acoustic signals, are at higher risk of predation from a major bat predator, Megaderma spasma, than calling males. Female katydids were represented in much higher numbers than males in the culled remains beneath roosts of M. spasma. Playback experiments using katydid calls revealed that male calls were approached in only about one-third of the trials overall, whereas tethered, flying katydids were always approached and attacked. Our results question the idea that necessary costs of mate-finding, including risk of predation, are higher in signalling males than in searching females.
Keywords: bushcrickets, palaeotropics, lesser false vampire, sex-biased predation, eavesdropping, bat predation
1. Introduction
Long-distance mate attraction signals (visual, acoustic or chemical) are deployed by a variety of animal taxa [1]. Typically, one sex signals from a stationary position and the other sex responds by moving towards the signaller, facilitating pair formation, a prerequisite for mating [1]. In addition to potential mates, these conspicuous mate attraction signals attract eavesdropping predators and parasitoids [2]. Acoustic signals are also energetically expensive [3], thus imposing a dual cost on signal producers. In acoustically signalling systems, such as those of frogs, crickets (Orthoptera: Gryllidae) and katydids (Orthoptera: Tettigoniidae), males tend to be the signalling sex, whereas females typically are silent and use male signals to locate them [4].
The costs of signalling imposed by predation and parasitism on males have been demonstrated in a number of studies. Notable examples include predation by bats on calling frogs, crickets and katydids [5–11] and parasitism by acoustically orienting flies on calling male crickets and katydids [12–15]. In the case of attack by acoustically orienting parasitoid flies on cricket and katydid hosts, males suffer much higher levels of parasitism than females [13,14]. This is also implied by the dramatically lower levels of parasitism found in mute Teleogryllus oceanicus (field cricket) males that show the ‘flatwing’ phenotype [16].
Signalling males are known to incur costs but females that have to move towards and locate these signalling males may also incur predation costs [17]. The relative predation costs of signalling by males versus mate-finding by females have however received little attention in empirical studies. A few studies that have examined relative risk of predation between the sexes have in fact found evidence to suggest that females are either under equal or, perhaps, even higher risk of predation than males [18,19]. Males and females of the katydid species Tettigonia viridissima were found to be preyed upon in almost equal numbers by the Scops owl, Otus scops [18]. In a comparative field study of two katydid species Poecilimon veluchianus and P. affinis, survival rates of males and females of P. veluchianus were found to be similar, whereas males of P. affinis had lower survival rates than females [19]. This study did not however specifically examine relative predation risk as survivorship (or rather, disappearance of individuals in the field) was used as a proxy for predation. There is thus still much to be learned about sex-specific predation risks in acoustically signalling species.
Bat predators in the Neotropics have been shown to approach the calls of katydid species and diet analyses have confirmed that these bat species prey heavily on katydids [8]. In addition, the observation that males of neotropical forest katydid species that are sympatric with these bat predators often produce calls at infrequent intervals (low duty cycles) and intersperse them with bouts of silent vibrational signalling [8,20,21] lends support to the idea that bat predation imposes a high cost as well as acts as a strong selection pressure, determining the structures of katydid acoustic signals and signalling behaviour [8]. The fact that the calls and calling behaviour of palaeotropical katydid species known to date [22–24] do not show these adaptations (low duty cycle calls or male vibrational signalling) is puzzling and has raised the question of the relevance of bat predation as a selection pressure on katydid acoustic signals, especially in the palaeotropics [22].
We investigated these questions using bat and katydid species of a palaeotropical forest community [23–25]. We investigated the relative predation risk posed to male and female katydids by a predatory bat species, Megaderma spasma, a gleaning insectivorous bat [26–29], using a combination of diet analyses and playback experiments. (i) We used diet analyses to confirm that M. spasma is a major predator of katydids and to examine the relative numbers of male and female katydids preyed upon by this bat species. We predicted that male katydids, which have stridulatory structures and call, would be preyed upon in higher numbers than females, which lack stridulatory structures and do not call. (ii) We also tested, in outdoor flight tents, the response of M. spasma to playback of the male calls of acoustically conspicuous, sympatric katydid species and to tethered, flying, female katydids. We predicted that if calling males were at higher risk of predation, then M. spasma should more frequently approach male calls than flying females. (iii) We also predicted that the bats would approach high duty-cycle and high-bandwidth calls more often than low duty-cycle, low-bandwidth calls as the former should be easier to locate [8].
2. Material and methods
(a). Animals and study site
The bat species M. spasma was used since it is a known katydid predator [26–28]. Our study was conducted in and around Kadari village in Karnataka, India (13°21′–75°08′). The diet study was conducted in 2007–2008 and the playback experiment was carried out over two dry seasons ( January to May, which is the breeding and therefore the calling season for the katydid species tested [23,24]) in 2010 and 2011. For the diet study, we located five roosts of M. spasma within a 10 km radius of Kadari village. For the playback experiments, adult bats were collected from the roosts two at a time using hand nets. They were released in separate flight rooms (2 × 2 × 2 m), provided with water ad libitum and fed three to four katydids every night. They were acclimated for at least 24 h before testing in playback trials but were not trained to approach either speakers or insect bait. Every bat was sexed and collared using custom-made rings (length × breadth × thickness: 5 cm × 5 mm × 1 mm) of soft aluminium covered with reflective tape (Tape India, Chennai, India) before testing. After the experiment, bats were returned unharmed to the roosts from which they had been captured. None of the bats was kept in captivity for more than 2 days.
(b). Diet analysis
Polythene sheets (5 × 8 m) were spread beneath each of the five bat roosts. The culled insect parts such as wings, partly eaten insects, legs, antennae or any other prey fragments were collected from the sheets and stored in polythene bags. We made collections every month during the start of the third week, from June 2007 until May 2008, spanning all the seasons. The insect fragments were taxonomically identified to the Order and, wherever possible, to the Family level. Results were expressed as percentage frequency (i.e. the percentage of the total number of fragments identified), which is a measure of prey taxa in the diet [30]. In addition, to examine the relative proportion of male versus female katydids consumed by M. spasma, we counted the total number of katydid forewings with typical male stridulatory structures (indicative of the number of males of katydid species capable of acoustic signal production) and compared them with the total number of katydid forewings without stridulatory structures (indicative of the number of females) in the culled remains. Calling katydid species typically have stridulatory structures on both forewings and the structures on the two wings are morphologically distinct [31]. As the numbers of left and right forewings found under a given roost were not always equal, we counted the total number of forewings with and without stridulatory structures. All forewing fragments with the proximal part (that typically contains the stridulatory structures) intact were considered for the analysis, which was carried out using a Leica Stereo Zoom Microscope (M165C, Leica Microsystems GmbH, Wetzlar, Germany).
(c). Behaviour experiments
Playback trials were conducted in an outdoor flight tent (5 × 5 × 2.5 m) made of nylon mesh (pore size 1 × 1 mm). Eighteen adult bats were tested, including nine males and nine females. Each bat was exposed to four playback stimuli and one live tethered katydid that was induced to fly, making a total of five trials per night. Bats were not retested across nights. The playback stimuli consisted of typical exemplars of calls of three abundant katydid species (Onomarchus uninotatus, Brochopeplus sp., Mecopoda ‘Two-Part’) whose male calls are acoustically conspicuous and which co-occur with M. spasma [23,24]. Calls were played back at natural calling rates and sound pressure levels (details in the electronic supplementary material). The fourth stimulus consisted of the previously recorded flight sounds of a Mecopoda sp. female in tethered flight played back at 76.5 dB SPL. The fifth treatment consisted of a tethered, live katydid (mostly a female of Mecopoda sp.) that was induced to fly. The order of presentation of the five treatments was randomized using a random number generator for each bat.
Each playback trial consisted of a 3 min silent period followed by 3 min of playback. For each trial, a silent speaker was also placed inside the arena in addition to the active one as a control. Both active and silent speaker were covered using identical iron mesh cages to prevent the bats from damaging the speakers. An immobilized katydid was kept on a leaf on top of each of the speaker cages as a reward. A minimum gap of 30 min was provided between trials. If the bat captured the insect reward placed on the active speaker and consumed it during a trial, a gap of 2 h was provided. If the bat did not pick up the reward in two consecutive trials, it was fed with one Mecopoda and a gap of 2 h was provided.
Each flight trial consisted of a 3 min silent period followed by 3 min of a flight session. For flight stimuli, live animals (mostly females of Mecopoda sp.) were tied around the pronotum using a soft cotton thread (approx. 20 cm) that was attached to a thin, wooden stick (25 cm) suspended from the top of the tent, such that the animal was about 10–12 inches from the ground. The stick served to limit the flying radius of the tethered insect. A freshly killed Mecopoda was also similarly suspended as a control in another randomly picked location in the test arena. During the flight session, the animal was prodded with a stick or blown upon through a long hollow tube introduced from outside the cage to induce it to initiate flight. During the flight trials, the insects typically flew in short bouts (of about 10 s each) rather than continuously and had to be stimulated multiple times. The occasions when the insects did not initiate flight in response to our stimulation served as additional controls, as they represented situations where all the other conditions were the same (being prodded or blown upon, movement of the tether or legs and antenna of the insect, vibration of the stick) except that the insect did not initiate flight.
An area of 2 × 1.2 m was chosen as the active experimental arena in the middle of the tent and a grid with 15 squares (each of size 40 × 40 cm) was drawn close to the ground using ropes. The positions of the active speaker/insect and the control silent speaker/insect for each trial were randomly chosen out of the 15 squares using a random number generator in MatLab v. 7 (v. 7, Mathworks, Natick, MA, USA). During randomization for flight trials, the experimenters ensured that the control and the flying animals were not in two adjacent squares of the grid. This was done to make sure that the insect had enough flight range and did not get entangled with the control insect during flight and to enable us to clearly distinguish between approach of the bat to the active and immobile insects.
Bat response was monitored and recorded using two infrared-sensitive video cameras (details in the electronic supplementary material). Responses of the bats were analysed by viewing the videos during the pre-playback silent periods and during playback. A response was scored as positive if the bat approached at least once during the trial to within 40 cm of the active speaker/insect, hovered above or close to it or attempted to land on the speaker/capture the insect.
(d). Statistical analysis
The proportions of males and females consumed by bats were compared using a χ2-test. Proportions of responders across treatments in the behavioural experiment were compared using Cochran's Q test and pairwise comparisons were made using McNemar's test with continuity correction. Proportions of bat responses between the two scenarios in the flight treatment (trial occasions when the insects did not initiate flight in response to our stimulation versus when the insects actually flew) were compared using the proportion test [32].
3. Results
(a). Diet analysis
Over 95% of the prey remains collected beneath the roosts of M. spasma belonged to the Class Insecta, establishing that this bat species is primarily insectivorous (figure 1). Vertebrate prey remains were rare (figure 1) and were only found on six sampling occasions (out of a total of 60): these included feathers of small birds such as sunbirds and sparrows and a single instance of the remains of a tree frog, Rhacophorus malabaricus. The insects identified from these fragments (n = 3714) (figure 1) included katydids and crickets (Order Orthoptera), beetles (Coleoptera), moths (Lepidoptera), stick insects (Phasmida), roaches (Dictyoptera), cicadas (Homoptera) and termites (Isoptera). The major insect groups consumed were Orthoptera (59.8%), Coleoptera (16%) and Lepidoptera (9.9%) (figure 1). Orthopteran fragments (n = 2221 in total) constituted about 60% of all culled insect remains pooled across roosts. The proportion of orthopteran fragments varied between 76.7 and 43.2% across the five roosts and dominated in all of the roosts except one (electronic supplementary material, figure S1), where lepidopteran remains were almost equally abundant.
Figure 1.
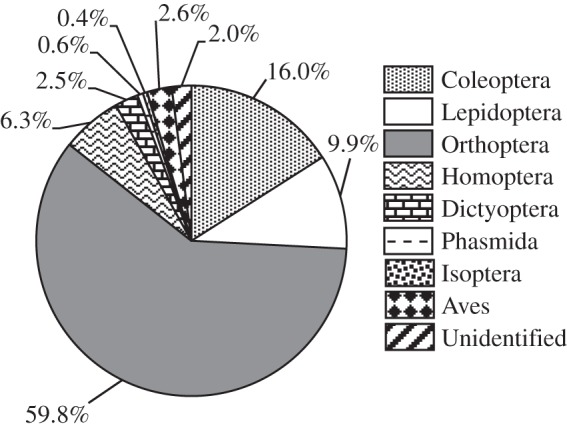
Diet analysis. Pie chart showing the relative proportions of different taxa consumed over a year by M. spasma revealed from culled remains under roosts. Data from five roosts were pooled.
Within the orthopteran fragments, across all roosts, katydids (Family Tettigoniidae) constituted the major proportion (98%), whereas crickets (Family Gryllidae) constituted a very small proportion (2%). Of the tettigoniid fragments, 27% belonged to the sub-family Pseudophyllinae, 63% to the Phaenopterinae and the remaining 10% to the Mecopodinae (Genus Mecopoda). Out of a total of 453 forewings examined, 294 contained no stridulatory structures (indicative of females), whereas 159 bore typical male stridulatory structures. The number of female forewings was thus 1.85 times the number of male forewings, which is significantly different from a male : female ratio of 1 : 1 (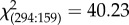 , d.f. = 1, p = 2.255 × 10−10). This indicates that female katydids, which do not call, are consumed in greater numbers by M. spasma than male katydids, which are capable of calling.
, d.f. = 1, p = 2.255 × 10−10). This indicates that female katydids, which do not call, are consumed in greater numbers by M. spasma than male katydids, which are capable of calling.
(b). Behaviour experiments
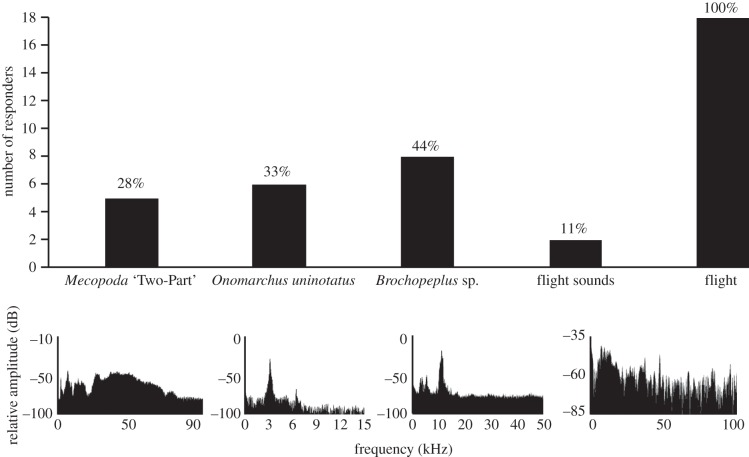
Bats showed significant differences in their responses to the different treatments (Q = 35.04, d.f. = 4, p = 4.55 × 10−7). Bats never approached the active speakers/insects during the silent, pre-playback periods in any of the trials and also did not approach the control silent speaker/immobile insect during playback/flight trials. The calls of Mecopoda ‘Two-Part’, O. uninotatus and Brochopeplus sp. were effective in eliciting responses in 28%, 33% and 44% of the trials, respectively (figure 2). The responses to the calls of the three katydid species were not significantly different from each other (Q = 1.55, d.f. = 2, p = 0.45). Response to playback of flight sounds was rare (two out of 18 trials: figure 2) and not significantly different from the silent control (χ2 = 0.5, d.f. = 1, p = 0.45). Flight was the most effective stimulus: all 18 bats (100%) approached tethered katydids that initiated flight. Within each of these flight trials, bats never approached these live, tethered insects when stationary or on occasions when they failed to initiate flight in response to our stimulation (which was the case in 193 out of 246, i.e. in 78% of our attempts to initiate flight). Across all trials, bats approached the insects in 70% (37 out of 53) of all flight bouts but never (0 out of 193) in cases of no flight initiation in response to our stimulation ( χ2 = 153.2, p = 2.2 × 10−26).
Figure 2.
Behavioural responses of M. spasma to playback of katydid calls, flight sounds and tethered flying katydids. The panels below the graph depict the power spectra of the four playback stimuli: note that Mecopoda ‘Two-Part’ has a high-bandwidth call, whereas O. uninotatus and Brochopeplus sp. have calls of narrow bandwidth.
4. Discussion
(a). Diet of Megaderma spasma
Our results are consistent with previous studies on the diet of M. spasma [26–28]. Davison & Zubaid [28] found that tettigoniids constituted 51.5% of the culled remains collected, but their study was carried out on only a single roost and sampling was carried out in an irregular fashion. We monitored five roosts in a systematic fashion through a whole year, thus taking into account seasonal fluctuations in insect abundance. Megaderma spasma is shown to be primarily insectivorous, with a very minor component (less than 5%) of its diet consisting of small vertebrates. This is in contrast to its larger congeneric species, Megaderma lyra, with which it is sympatric in our study site [25]. Megaderma lyra consumes a much higher proportion of larger vertebrate prey, although it will consume insects as well when they are abundant [33]. Katydids do not, however, appear to form part of the diet of M. lyra [33]. The two congeneric, sympatric species (M. spasma and M. lyra) thus show partitioning of their dietary niches.
In a previous study characterizing the species diversity of bats in the same area [25], we found a total of 20 species, of which four were frugivorous. Of the remaining 16, seven were small insectivorous species (Pipistrellus ceylonicus, P. mimus, P. affinis, P. coromandra, Murina cyclotis, Scotophilus kuhlii and Tylonycteris pachypus) that do not typically consume katydids [29]. The three rhinolophid and one hipposiderid species (Rhinolophus rouxii, R. lepidus, R. beddomei and Hipposideros galeritus) showed no trace of orthopteran remains beneath their roosts and M. lyra specializes mostly on vertebrate prey and does not consume katydids [33]. The remaining three species (Harpiocephalus harpia, Hesperoptenus tickelli and Myotis horsefieldii) were rare and their food habits are unknown. Thus, 16 of the other 19 species that we found in these forests do not prey upon katydids in significant numbers and M. spasma is likely to be the major bat predator of this katydid community.
(b). Relative predation risk of male and female katydids
Analysis of culled remains under M. spasma roosts indicated that female katydids were consumed in much higher numbers than males. This sex bias is unlikely to be explained by seasonal differences in sex ratios of available katydids, since we monitored and pooled data over an entire year. This is also unlikely to be simply a consequence of the activity patterns of male and female katydids and bats: for example, one may argue that culled remains at roosts represent a small sample of what a bat actually eats through the night and if these represent only the final meal in the early morning, when female katydids are possibly more active than males, then these do not give a reliable signature of the diet. We monitored bat activity at three roosts through the night (18.00 to 7.00) and found that bats do return to their day roosts at intervals throughout the night (also observed in [26]), sometimes with prey items, suggesting that the above explanation is unlikely.
The female-biased predation suggested by the diet analysis was corroborated by the results of the behavioural experiments. Our results suggest that a calling male katydid faces only one-third the probability of attack by the bat predator, M. spasma as a flying female. The scenario most closely replicated in our experiments is that of either a calling male or a flying female katydid present within a few metres of, and easily accessible to, an M. spasma predator: it suggests that a female that may fly in towards a calling male is at much higher risk of predation from a bat in the vicinity than the male himself. Belwood & Morris [8] also found that 50% of the culled remains recovered from the roosts of foliage-gleaning, insectivorous bats consisted of females, suggesting that katydid females have at least as high a risk of bat predation as males in the neotropics as well.
Both the behaviour and the diet data thus suggest that female katydids are likely to face higher predation risk from a major bat predator than calling males, especially since males often call from highly cluttered and relatively inaccessible sites. In fact, male Mecopoda sp. typically call from within dense brambles close to the ground, which may make them hard to access, and O. uninotatus calls from the leaf clutter in the canopy [34]. Females of Mecopoda sp. typically fly in towards calling males as do females of several phaneropterine species in the understorey (R. Balakrishnan 2005, personal observations), lending further support to the idea of female-biased predation in these katydid species. Future studies will examine sex-biased predation within single species of katydids, using both laboratory experiments and field observations.
(c). Implications of bat predation for katydid signal evolution
Megaderma spasma approached the calls of sympatric katydid species on an average of one-third of the playback trials, establishing that it does eavesdrop on these communication signals and can use them to find prey. Interestingly, there were no differences in response to the calls of the three species tested. This was unexpected as the three call structures differ greatly in bandwidth and dominant frequency, ranging from the low frequency, tonal call of O. uninotatus (3.2 kHz) to the exceptionally high-bandwidth (2–80 kHz), high duty cycle (98%) call of Mecopoda ‘Two-Part’. This is in contrast to previous studies with neotropical katydids [8]; however, none of our calls had the exceptionally low duty cycle characteristics (less than 1 call min−1) of the neotropical katydid species tested (Acanthodis curvidens [8]). It is also possible that the task of signal localization was much too easy in our set-up and that differences in locatability of signals will emerge when tested with more natural levels of clutter. Further experiments and observations are thus required to understand the role of bat predation in determining katydid signal structure and signalling behaviour, especially in the paleotropics.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Sudhakar Gowda and Sekar Chettigar for assistance in the field and Mahindran Valliyappan, Ranjana Jaiswara and Ashok Mallick for help with the playback experiments. We thank Yeshwant Buttur, Satish Buttur, Babu Nayak, Anantha Poojari and Hemanantha for their tolerance in allowing bats to roost in the attics of buildings on their property and for generously allowing us to collect culled remains and observe these bats. We thank Dennis Francis for allowing us to conduct playback experiments on his plantation. H.R. carried out the diet data collection and analysis, R.D. designed and set up the behaviour experiment; H.R., R.D. and D.N. carried out the behaviour experiment and analysed the results; R.B. designed the study and wrote the manuscript.
Ethics statement
This experimental protocol conforms to institutional and national guidelines for the ethical treatment of animals.
Data accessibility
Data files may be accessed from Dryad (doi:10.5061/dryad.4129d).
Funding statement
We thank the Ministry of Environment and Forests, Government of India for funding and the Karnataka State Forest Department for permits. H.R. was funded by a Research Associate fellowship and R.D. by a Senior Research Fellowship of the Council for Scientific and Industrial Research (CSIR), Government of India.
References
- 1.Bradbury JW, Vehrencamp SL. 1998. Principles of animal communication. Sunderland, UK: Sinauer Associates. [Google Scholar]
- 2.Zuk M, Kolluru GR. 1998. Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 73, 415–438. ( 10.1086/420412) [DOI] [Google Scholar]
- 3.Prestwich KN. 1994. The energetics of acoustic signaling in anurans and insects. Am. Zool. 34, 625–643. [Google Scholar]
- 4.Gerhardt HC, Huber F. 2002. Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago, IL: University of Chicago Press. [Google Scholar]
- 5.Tuttle MD, Ryan MJ. 1981. Bat predation and the evolution of frog vocalizations in the Neotropics. Science 214, 677–678. ( 10.1126/science.214.4521.677) [DOI] [PubMed] [Google Scholar]
- 6.Tuttle MD, Ryan MJ, Belwood JJ. 1985. Acoustical resource partitioning by two species of phyllostomid bats (Trachops cirrhosus and Tonatia sylvicola). Anim. Behav. 33, 1369–1371. ( 10.1016/S0003-3472(85)80204-9) [DOI] [Google Scholar]
- 7.Ryan MJ, Tuttle MD. 1987. The role of prey-generated sounds, vision, and echolocation in prey localization by the African bat Cardioderma cor (Megadermatidae). J. Comp. Physiol. A 161, 59–66. ( 10.1007/BF00609455) [DOI] [Google Scholar]
- 8.Belwood JJ, Morris GK. 1987. Bat predation and its influence on calling behavior in neotropical katydids. Science 238, 64–67. ( 10.1126/science.238.4823.64) [DOI] [PubMed] [Google Scholar]
- 9.Hosken DJ, Bailey WJ, Oshea JE, Roberts JD. 1994. Localization of insect calls by the bat Nyctophilus geoffroyi (Chiroptera, Vespertilionidae)—a laboratory study. Aust. J. Zool. 42, 177–184. ( 10.1071/ZO9940177) [DOI] [Google Scholar]
- 10.Bailey WJ, Haythornthwaite S. 1998. Risks of calling by the field cricket Teleogryllus oceanicus; potential predation by Australian long-eared bats. J. Zool. 244, 505–513. ( 10.1111/j.1469-7998.1998.tb00056.x) [DOI] [Google Scholar]
- 11.Jones PL, Page RA, Hartbauer M, Siemers BM. 2011. Behavioral evidence for eavesdropping on prey song in two Palearctic sibling bat species. Behav. Ecol. Sociobiol. 65, 333–340. ( 10.1007/s00265-010-1050-9) [DOI] [Google Scholar]
- 12.Cade W. 1975. Acoustically orienting parasitoids: fly phonotaxis to cricket song. Science 190, 1312–1313. ( 10.1126/science.190.4221.1312) [DOI] [Google Scholar]
- 13.Zuk M, Simmons LW, Cupp L. 1993. Calling characteristics of parasitized and unparasitized populations of the field cricket Teleogryllus oceanicus. Behav. Ecol. Sociobiol. 33, 339–343. [Google Scholar]
- 14.Lakes-Harlan R, Heller KG. 1992. Ultrasound-sensitive ears in a parasitoid fly. Naturwissenschaften 79, 224–226. ( 10.1007/BF01227133) [DOI] [Google Scholar]
- 15.Lehmann GUC, Heller K-G. 1998. Bushcricket song structure and predation by the acoustically orienting parasitoid fly Therobia leonidei (Diptera: Tachinidae: Ormiini). Behav. Ecol. Sociobiol. 43, 239–245. ( 10.1007/s002650050488) [DOI] [Google Scholar]
- 16.Zuk M, Rotenberry JT, Tinghitella RM. 2006. Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 2, 521–524. ( 10.1098/rsbl.2006.0539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaluk SK, Belwood JJ. 1984. Gecko phonotaxis to cricket calling song: a case of satellite predation. Anim. Behav. 32, 659–662. ( 10.1016/S0003-3472(84)80141-4) [DOI] [Google Scholar]
- 18.Heller KG, Arlettaz R. 1994. Is there a sex ratio bias in the bushcricket prey of the scops owl due to predation on calling males? J. Orthoptera Res. 2, 41–42. ( 10.2307/3503607) [DOI] [Google Scholar]
- 19.Heller KG. 1992. Risk shift between males and females in the pair-forming behavior of bushcrickets. Naturwissenschaften 79, 89–91. ( 10.1007/BF01131812) [DOI] [Google Scholar]
- 20.Morris GK, Mason AC, Wall P, Belwood JJ. 1994. High ultrasonic and tremulation signals in neotropical katydids (Orthoptera: Tettigoniidae). J. Zool. 233, 129–163. ( 10.1111/j.1469-7998.1994.tb05266.x) [DOI] [Google Scholar]
- 21.Römer H, Lang A, Hartbauer M. 2010. The Signaller's Dilemma: a cost–benefit analysis of public and private communication. PLoS ONE 5, e13325 ( 10.1371/journal.pone.0013325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heller KG. 1995. Acoustic signalling in palaeotropical bushcrickets (Orthoptera: Tettigonioidea: Pseudophyllidae): does predation pressure by eavesdropping enemies differ in the Palaeo- and Neotropics? J. Zool. 237, 469–485. ( 10.1111/j.1469-7998.1995.tb02775.x) [DOI] [Google Scholar]
- 23.Diwakar S, Balakrishnan R. 2007. The assemblage of acoustically communicating crickets of a tropical evergreen forest in Southern India: call diversity and diel calling patterns. Bioacoustics 16, 113–135. ( 10.1080/09524622.2007.9753571) [DOI] [Google Scholar]
- 24.Nityananda V, Balakrishnan R. 2006. A diversity of songs among morphologically indistinguishable katydids of the genus Mecopoda (Orthoptera: Tettigoniidae) from southern India. Bioacoustics 15, 223–250. ( 10.1080/09524622.2006.9753552) [DOI] [Google Scholar]
- 25.Raghuram H, Jain M, Balakrishnan R. 2014. Species and acoustic diversity of bats in a paleotropical wet evergreen forest in southern India. Curr. Sci. 107, 631–641. [Google Scholar]
- 26.Brosset A. 1962. The bats of central and western India. J. Bombay Nat. Hist. Soc. 59, 707–746. [Google Scholar]
- 27.Tyrell K. 1990. The ethology of the Malayan false vampire bat (Megaderma spasma), with special emphasis on auditory cues used in foraging. PhD dissertation, University of Illinois at Urbana–Champaign, Champaign, IL, USA. (http://hdl.handle.net/2142/23567) [Google Scholar]
- 28.Davison GWH, Zubaid A. 1992. Food habits of the lesser false vampire, Megaderma spasma, from Kuala Lompat, Peninsular Malaysia. Z. Säugetierkunde 57, 310–312. [Google Scholar]
- 29.Bates PJJ, Harrison DL. 2000. Bats of the Indian subcontinent. Amsterdam, The Netherlands: ETI, University of Amsterdam. [Google Scholar]
- 30.Whitaker JO, Jr, Kunz TH. 1988. Food habits analysis of insectivorous bats. In Ecological and behavioral methods for the study of bats (ed. Kunz TH.), pp. 171–189. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 31.Stärk AA. 1957. Untersuchungen am Lautorgan einiger Grillen-und Laubheuschrecken-Arten, zugleichein Beitrag zum Rechts-links problem. Zool. Jahrbucher Abteilung für Anatomie und Ontogenie der Tiere 77, 9–50. [Google Scholar]
- 32.Zar JH. 1999. Biostatistical analysis. Delhi, India: Pearson Education India. [Google Scholar]
- 33.Advani R. 1981. Seasonal fluctuations in the feeding ecology of the Indian false vampire, Megaderma llyra (Chiroptera: Megadermatidae) in Rajasthan. Z. Säugetierkunde 46, 90–93. [Google Scholar]
- 34.Jain M, Balakrishnan R. 2011. Microhabitat selection in an assemblage of crickets (Orthoptera: Ensifera) of a tropical evergreen forest in Southern India. Insect Conserv. Divers. 4, 152–158. ( 10.1111/j.1752-4598.2010.00118.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data files may be accessed from Dryad (doi:10.5061/dryad.4129d).