Abstract
Objectives
We contrast risk profiles and compare outcomes of patients with severe aortic stenosis (AS) and coronary artery disease (CAD) who underwent aortic valve replacement (AVR) and coronary artery bypass grafting (AS+CABG) with those of patients with isolated AS who underwent AVR alone.
Background
In patients with severe AS, CAD is often an incidental finding with underappreciated survival implications.
Methods
From 10/1991–7/2010, 2,286 patients underwent AVR+CABG and 1,637 AVR alone. A propensity score was developed and used for matched comparisons of outcomes (1,082 patient pairs). Analyses of long-term mortality were performed for each group, then combined to identify common and unique risk factors.
Results
Patients with AS+CAD vs. isolated AS were older, more symptomatic, more likely to be hypertensive, had lower ejection fraction and greater arteriosclerotic burden, but less severe AS. Hospital morbidity and long-term survival were poorer (43% vs. 59% at 10 years). Both groups shared many mortality risk factors; however, early risk among AS+CAD patients reflected effects of CAD; late risk reflected diastolic left ventricular dysfunction expressed as ventricular hypertrophy and left atrial enlargement. Patients with isolated AS and few comorbidities had the best outcome, those with CAD without myocardial damage had intermediate outcome equivalent to propensity-matched isolated AS patients, and those with CAD, myocardial damage, and advanced comorbidities had the worst outcome.
Conclusions
Cardiovascular risk factors and comorbidities must be considered in managing patients with severe AS. Patients with severe AS and CAD risk factors should undergo early diagnostics and AVR+CABG before ischemic myocardial damage occurs.
Keywords: coronary artery disease, aortic valve replacement, aortic stenosis, outcomes
INTRODUCTION
Patients treated for severe aortic stenosis (AS) constitute a heterogeneous population ranging from young patients with isolated bicuspid valve disease to elderly patients with degenerative disease complicated by comorbidities. The most common comorbidity importantly influencing outcomes after aortic valve replacement (AVR), affecting a third of patients and half of those above age 70 years, is coronary artery disease (CAD) (1–3). Current guidelines recommend bypass of all significant stenoses at the time of AVR, with evidence level C (4); however, addition of coronary artery bypass grafting (CABG) to AVR is associated with elevated short- and long-term mortality (5–10). This association may be causal (e.g., by increasing myocardial ischemic time (11)) or simply a marker for a high-risk patient profile. Clarifying this may lead to more targeted diagnostics, therapy, and chronic disease management.
To provide insight into severe AS with and without CAD and current treatment and patient outcomes, we contrasted risk profiles, compared outcomes, and identified risk factors for mortality. To accomplish these objectives, we studied a large group of patients who had routine preoperative coronary angiography before AVR and received the most common AVR device implanted at our institution, with or without CABG. We sought to identify risk factors both unique to AS and AS+CAD patients and ones in common.
For fair comparison of outcomes in comparable patients, we used propensity-score–based matching. Characteristics of AS or AS+CAD patients who could not be propensity matched provided insight into the profile of a subgroup of patients expected to have excellent long-term survival after operation, and another with substantially poorer survival. We believe these investigations provide both support for current guidelines as well as information useful for amplifying and refining them.
PATIENTS AND METHODS
Patients
From 10/1991 to 7/2010, 4,372 patients at Cleveland Clinic underwent primary AVR with a single type of bovine pericardial prosthesis (Carpentier-Edwards PERIMOUNT, Edwards Lifesciences Corporation, Irvine, CA, USA) for severe AS (aortic valve area <1 cm2 on transthoracic echocardiography [TTE]), with or without CABG. During this period, it was our policy to perform coronary angiography on all patients considered for AVR. Presence of CAD was defined as at least one epicardial artery (left main trunk [LMT], left anterior descending coronary artery [LAD], left circumflex coronary artery [LCx], and right coronary artery [RCA]) with at least 50% stenosis or history of percutaneous coronary intervention. Groups were defined based on the presence of CAD and CABG. Thus, we excluded 22 patients with less than 50% stenosis in all coronary vessels who underwent AVR and CABG, as well as 427 patients with 50% or greater coronary stenosis who underwent AVR alone. Remaining study groups consisted of 1,637 patients with severe AS without CAD who underwent AVR alone (Isolated AS group) and 2,286 patients with severe AS and CAD who underwent AVR and CABG (AS+CAD group) (eFigure 1). Patients with missing coronary stenosis data, prior cardiac surgery, infective endocarditis, rheumatic valve disease, indications for AVR other than AS, and those who underwent thoracic aorta or valvar operations other than mitral or tricuspid procedures for functional or ischemic regurgitation were excluded. Patient characteristics are presented in Table 1.
Table 1.
Patient Characteristics and Operative Details in Those with Severe Aortic Stenosis Alone (Isolated AS) vs. Aortic Stenosis and Coronary Artery Disease (AS+CAD)
| Characteristic | Overall
|
Propensity Matched
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Isolated AS (n=1,637)
|
AS+CAD (n=2,286)
|
P | Isolated AS (n=1,082)
|
AS+CAD (n=1,082)
|
P | |||||
| na | No. (%) or Mean ± SD | na | No. (%) or Mean ± SD | na | No. (%) or Mean ± SD | na | No. (%) or Mean ± SD | |||
| Demography | ||||||||||
| Female | 1,637 | 769 (47) | 2,286 | 774 (34) | <.0001 | 1,082 | 450 (42) | 1,082 | 458 (42) | .7 |
| Age (y) | 1,637 | 70 ± 11 | 2,286 | 75 ± 8.1 | <.0001 | 1,082 | 73 ± 9.4 | 1,082 | 73 ± 8.6 | .5 |
| BSA (m2) | 1,607 | 2.0 ± 0.27 | 2,263 | 2.0 ± 0.25 | .03 | 1,059 | 2.0 ± 0.26 | 1,066 | 2.0 ± 0.26 | .04 |
| Symptoms | ||||||||||
| Preoperative NYHA functional class | 1,607 | 2,263 | <.0001 | 1,059 | 1,066 | >.9 | ||||
| I | 395 (25) | 346 (15) | 222 (21) | 227 (21) | ||||||
| II | 835 (52) | 1,180 (52) | 558 (53) | 559 (52) | ||||||
| III | 325 (20) | 573 (25) | 233 (22) | 239 (22) | ||||||
| IV | 52 (3.2) | 164 (7.2) | 46 (4.3) | 41 (3.8) | ||||||
| Canadian Angina class | 1,231 | 1,946 | <.0001 | 833 | 882 | <.0001 | ||||
| 0 | 537 (44) | 624 (32) | 366 (44) | 307 (35) | ||||||
| I | 391 (32) | 469 (24) | 275 (33) | 230 (26) | ||||||
| II | 260 (21) | 656 (34) | 163 (20) | 286 (32) | ||||||
| III | 39 (3.2) | 162 (8.3) | 26 (3.1) | 53 (6.01) | ||||||
| IV | 4 (0.32) | 35 (1.8) | 3 (0.36) | 6 (0.68) | ||||||
| Syncope | 1,271 | 176 (14) | 1,892 | 271 (14) | .7 | 860 | 120 (14) | 860 | 130 (15) | .5 |
| Dyspnea on exertion | 1,170 | 613 (52) | 1,771 | 897 (51) | .4 | 789 | 418 (53) | 800 | 410 (51) | .5 |
| Shortness of breath | 1,169 | 687 (59) | 1,772 | 976 (55) | .05 | 788 | 451 (57) | 801 | 463 (58) | .8 |
| Paroxysmal nocturnal dyspnea | 1,168 | 84 (7.2) | 1,771 | 171 (9.7) | .02 | 787 | 61 (7.8) | 800 | 69 (8.6) | .5 |
| Orthopnea | 1,168 | 150 (13) | 1,772 | 231 (13) | .9 | 787 | 105 (13) | 801 | 111 (14) | .8 |
| Valve pathology | ||||||||||
| Pure aortic stenosis | 1,603 | 1,203 (75) | 2,219 | 1,682 (76) | .6 | 1,054 | 778 (74) | 1,056 | 794 (75) | .5 |
| Mixed aortic stenosis/regurgitation | 1,603 | 332 (21) | 2,219 | 468 (21) | .8 | 1,054 | 223 (21) | 1,056 | 225 (21) | >.9 |
| Bicuspid aortic valve | 1,637 | 659 (40) | 2,286 | 411 (18) | <.0001 | 1,082 | 298 (28) | 1,082 | 296 (27) | >.9 |
| Mitral valve regurgitation severity | 1,509 | 2,096 | <.0001 | 982 | 977 | >.9 | ||||
| None | 811 (54) | 923 (44) | 478 (49) | 490 (50) | >9 | |||||
| Mild | 419 (28) | 637 (30) | 294 (30) | 287 (29) | ||||||
| Moderate | 218 (14) | 372 (18) | 158 (16) | 150 (15) | ||||||
| Moderately severe | 50 (3.3) | 133 (6.3) | 43 (4.4) | 41 (4.2) | ||||||
| Severe | 11 (0.73) | 31 (1.5) | 9 (0.92) | 9 (0.92) | ||||||
| Tricuspid valve regurgitation severity | 1,518 | 2,095 | .02 | 990 | 980 | .4 | ||||
| None | 1,020 (67) | 1,317 (63) | 643 (65) | 624 (64) | ||||||
| Mild | 318 (21) | 462 (22) | 211 (21) | 219 (22) | ||||||
| Moderate | 120 (7.9) | 220 (11) | 91 (9.2) | 87 (8.9) | ||||||
| Moderately severe | 42 (2.8) | 74 (3.5) | 31 (3.1) | 42 (4.3) | ||||||
| Severe | 18 (1.2) | 22 (1.1) | 14 (1.4) | 8 (0.82) | ||||||
| Coronary artery disease | ||||||||||
| Number of systems diseased ≥ 50% | 1,629 | 2,284 | <.0001 | 1,076 | 1,080 | <.0001 | ||||
| 0 | 1,629 (100) | 105 (4.6) | 1,076 (100) | 73 (6.8) | ||||||
| 1 | 0 (0) | 803 (35) | 0 (0) | 473 (44) | ||||||
| 2 | 0 (0) | 745 (33) | 0 (0) | 322 (30) | ||||||
| 3 | 0 (0) | 631 (28) | 0 (0) | 212 (20) | ||||||
| LMT disease ≥50% | 1,533 | 0 (0) | 2,032 | 296 (15) | <.0001 | 1,001 | 0 (0) | 973 | 107 (11) | <.0001 |
| LAD system disease ≥50% | 1,595 | 0 (0) | 2,249 | 1,624 (72) | <.0001 | 1,049 | 0 (0) | 1,062 | 709 (68) | <.0001 |
| LCx system disease ≥50% | 1,561 | 0 (0) | 2,189 | 1,134 (52) | <.0001 | 1,027 | 0 (0) | 1,036 | 455 (44) | <.0001 |
| RCA system disease ≥50% | 1,637 | 0 (0) | 2,286 | 1,428 (62) | <.0001 | 1,082 | 0 (0) | 1,082 | 589 (54) | <.0001 |
| Cardiac comorbidity | ||||||||||
| Previous myocardial infarction | 1,637 | 126 (7.7) | 2,286 | 652 (29) | <.0001 | 1,082 | 117 (11) | 1,082 | 112 (10) | .7 |
| Atrial fibrillation/flutter | 1,458 | 114 (7.8) | 2,009 | 204 (10) | .02 | 957 | 90 (9.4) | 927 | 79 (8.5) | .5 |
| Complete heart block/pacer | 1,452 | 62 (4.3) | 2,002 | 115 (5.7) | .05 | 951 | 55 (5.8) | 926 | 51 (5.5) | .8 |
| Ventricular arrhythmia | 1,492 | 167 (11) | 2,074 | 274 (13) | .07 | 982 | 121 (12) | 967 | 130 (13) | .5 |
| Heart failure | 1,637 | 330 (20) | 2,286 | 691 (30) | <.0001 | 1,082 | 257 (24) | 1,082 | 248 (23) | .6 |
| Noncardiac comorbidity | ||||||||||
| Peripheral arterial disease | 1,637 | 65 (4.0) | 2,286 | 278 (12) | <.0001 | 1,082 | 57 (5.3) | 1,082 | 65 (6.0) | .5 |
| Carotid disease | 1,637 | 500 (31) | 2,286 | 1,215 (53) | <.0001 | 1,082 | 424 (39) | 1,082 | 430 (40) | .8 |
| Stroke | 1,637 | 102 (6.2) | 2,286 | 221 (9.7) | .0001 | 1,082 | 85 (7.9) | 1,082 | 78 (7.2) | .6 |
| Hypertension | 1,637 | 1,063 (65) | 2,286 | 1,811 (79) | <.0001 | 1,082 | 782 (72) | 1,082 | 798 (74) | .4 |
| Insulin-treated diabetes | 1,600 | 61 (3.8) | 2,213 | 212 (9.6) | <.0001 | 1,055 | 55 (5.2) | 1,056 | 53 (5.0) | .8 |
| Pharmacologically treated diabetes | 1,602 | 243 (15) | 2,219 | 582 (26) | <.0001 | 1,055 | 199 (19) | 1,059 | 201 (19) | >.9 |
| BUN (mg·dL−1) | 1,606 | 20 ± 8.3 | 2,249 | 23 ± 11 | <.0001 | 1,057 | 21 ± 8.7 | 1,062 | 21 ± 9.6 | .6 |
| Creatinine (mg·dL−1) | 1,606 | 1.03 ± 0.45 | 2,235 | 1.2 ± 0.59 | <.0001 | 1,058 | 1.08 ± 0.47 | 1,053 | 1.08 ± 0.51 | .6 |
| Preoperative renal dialysis | 1,377 | 12 (0.87) | 1,929 | 34 (1.8) | .03 | 898 | 9 (1.0) | 908 | 14 (1.5) | .3 |
| COPD | 1,637 | 201 (12) | 2,286 | 327 (14) | .07 | 1,082 | 132 (12) | 1,082 | 149 (14) | .3 |
| Smoking | 1,630 | 803 (49) | 2,266 | 1,247 (55) | .0004 | 1,077 | 536 (50) | 1,077 | 529 (49) | .8 |
| Cholesterol (mg·dL−1) | 1,266 | 187 ± 45 | 1,683 | 181 ± 46 | .0002 | 813 | 183 ± 44 | 834 | 186 ± 45 | .2 |
| Triglycerides (mg·dL−1) | 1,262 | 132 ± 75 | 1,668 | 135 ± 80 | .2 | 810 | 132 ± 74 | 829 | 134 ± 77 | .4 |
| HDL cholesterol (mg·dL−1) | 1,261 | 54 ± 18 | 1,672 | 48 ± 15 | <.0001 | 808 | 51 ± 16 | 830 | 51 ± 15 | .6 |
| LDL cholesterol (mg·dL−1) | 1,260 | 108 ± 39 | 1,666 | 106 ± 41 | .2 | 807 | 106 ± 38 | 830 | 109 ± 40 | .15 |
| Bilirubin (mg·dL−1) | 1,529 | 0.67 ± 0.46 | 2,102 | 0.68 ± 0.54 | >.9 | 1,000 | 0.69 ± 0.52 | 1,000 | 0.69 ± 0.67 | .6 |
| Hematocrit (%) | 1,551 | 39 ± 5.4 | 2,124 | 38 ± 5.6 | <.0001 | 1,017 | 38 ± 5.5 | 1,010 | 38 ± 5.5 | .6 |
| Concomitant procedures | ||||||||||
| Mitral valve repair | 1,637 | 22 (1.3) | 2,286 | 77 (3.4) | <.0001 | 1,082 | 17 (1.6) | 1,082 | 13 (1.2) | .5 |
| Tricuspid valve repair | 1,637 | 33 (2.0) | 2,286 | 53 (2.3) | .5 | 1,082 | 26 (2.4) | 1,082 | 27 (2.5) | .9 |
| Aortic endarterectomy | 1,637 | 49 (3.0) | 2,286 | 125 (5.5) | .0002 | 1,082 | 37 (3.4) | 1,082 | 40 (3.7) | .7 |
| Any atrial fibrillation procedure | 1,637 | 97 (5.9) | 2,286 | 143 (6.3) | .7 | 1,082 | 74 (6.8) | 1,082 | 71 (6.6) | .8 |
| Septal myectomy | 1,637 | 30 (1.8) | 2,286 | 34 (1.5) | .4 | 1,082 | 21 (1.9) | 1,082 | 26 (2.4) | .5 |
| ASD/PFO suture closure | 1,637 | 27 (1.6) | 2,286 | 37 (1.6) | .9 | 1,082 | 16 (1.5) | 1,082 | 18 (1.7) | .7 |
| Aortic valve prosthesis | ||||||||||
| Valve size (mm) | 1,637 | 2,286 | .5 | 1,082 | 1,082 | .4 | ||||
| 19 | 240 (15) | 350 (15) | 163 (15) | 190 (18) | ||||||
| 21 | 483 (30) | 666 (29) | 312 (29) | 319 (29) | ||||||
| 23 | 532 (32) | 786 (34) | 353 (33) | 316 (29) | ||||||
| 25 | 303 (18) | 394 (17) | 200 (18) | 207 (19) | ||||||
| 27 | 68 (4.2) | 79 (35) | 50 (4.6) | 44 (4.1) | ||||||
| 29 | 11 (0.67) | 11 (0.48) | 4 (0.37) | 6 (0.55) | ||||||
| Standardized size (Z-value) | 1,605 | −0.42 ± 0.99 | 2,262 | −0.41 ± 0.95 | .8 | 1,059 | −0.42 ± 0.99 | 1,066 | −1.42 ± 1.01 | .9 |
Patients with data available.
Key: AS, aortic stenosis, ASD/PFO, atrial septal defect/patent foramen ovale, BSA, body surface area; BUN, blood urea nitrogen; CAD, coronary artery disease, COPD, chronic obstructive pulmonary disease; HDL, high-density lipoprotein; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; LDL, low-density lipoprotein; LMT, left main trunk; NYHA, New York Heart Association; RCA, right coronary artery; SD, standard deviation.
Preoperative, operative, and postoperative variables, including echocardiographic variables, were retrieved from prospective databases approved for use in research by the Institutional Review Board, with patient consent waived.
Preoperative Echocardiography
Preoperative measurements were retrieved from the TTE performed nearest to, but preceding, the operation. Median interval between TTE and surgery was 7 days, and 3,020 patients (81%) underwent AVR within 30 days. Left ventricular (LV) mass was calculated using the formula validated by Devereux and colleagues (12), indexed to body surface area. Peak instantaneous aortic valve gradients were calculated from Doppler velocity. Preoperative TTE measurements are summarized in Table 2.
Table 2.
Preoperative Echocardiographic Measurements
| Measurement | Overall
|
Propensity Matched
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Isolated AS (n=1,637)
|
AS+CAD (n=2,286)
|
P | Isolated AS (n=1,082)
|
AS+CAD (n=1,082)
|
P | |||||
| na | Mean ± SD | na | Mean ± SD | na | Mean ± SD | na | Mean ± SD | |||
| Aortic valve hemodynamics | ||||||||||
| Area (cm2) | 1,426 | 0.65 ± 0.14 | 1,992 | 0.67 ± 0.14 | <.0001 | 942 | 0.65 ± 0.14 | 932 | 0.66 ± 0.14 | .5 |
| Mean gradient (mmHg) | 1,468 | 52 ± 16 | 2,042 | 45 ± 16 | <.0001 | 969 | 50 ± 16 | 957 | 49 ± 16 | .4 |
| Peak gradient (mmHg) | 1,474 | 87 ± 26 | 2,042 | 76 ± 25 | <.0001 | 975 | 84 ± 25 | 956 | 84 ± 24 | >.9 |
| Left ventricle | ||||||||||
| Morphology | ||||||||||
| Posterior wall thickness (cm) | 1,430 | 1.3 ± 0.24 | 1,894 | 1.3 ± 0.23 | .5 | 932 | 1.3 ± 0.24 | 905 | 1.3 ± 0.22 | .8 |
| Septal thickness (cm) | 1,440 | 1.5 ± 0.29 | 1,915 | 1.5 ± 0.31 | .6 | 939 | 1.5 ± 0.28 | 915 | 1.5 ± 0.29 | .9 |
| Mass index (g·m−2) | 1,398 | 128 ± 42 | 1,863 | 134 ± 41 | <.0001 | 908 | 132 ± 43 | 886 | 131 ± 42 | .6 |
| End-diastolic diameter (cm) | 1,446 | 4.6 ± 0.79 | 1,934 | 4.7 ± 0.81 | <.0001 | 945 | 4.6 ± 0.82 | 927 | 4.6 ± 0.82 | .2 |
| End-systolic diameter (cm) | 1,432 | 3.0 ± 0.84 | 1,911 | 3.1 ± 0.90 | <.0001 | 940 | 3.0 ± 0.86 | 920 | 3.0 ± 0.85 | .2 |
| Function | ||||||||||
| Ejection fraction (%) | 1,509 | 55 ± 11 | 2,134 | 51 ± 13 | <.0001 | 987 | 54 ± 11 | 1,006 | 54 ± 11 | .4 |
| Left atrium | ||||||||||
| Diameter (cm) | 1,352 | 4.1 ± 0.76 | 1,836 | 4.3 ± 0.75 | <.0001 | 887 | 4.2 ± 0.75 | 857 | 4.2 ± 0.75 | .4 |
Patients with data available.
Key: AS, aortic stenosis, CAD, coronary artery disease; SD, standard deviation.
Endpoints
Our primary endpoint was all-cause mortality from date of operation. Patients were systematically followed at 2 years, then every 5 years by telephone or mailed questionnaire. This active follow-up was supplemented with passive Social Security Death Master File data. Follow-up information was unavailable for 60 patients (1.5%). Median follow-up was 4.7 years, 25% of patients were followed more than 8 years and 10% more than 11 years; 21,005 patient-years of data were available for analysis.
Survival was estimated nonparametrically by the Kaplan-Meier method and parametrically by a multiphase hazard model (13). Parametric modeling was used to resolve the number of phases of instantaneous risk of death (hazard function) and to estimate shaping parameters. These were modeled separately for each group. As is characteristic of cardiac surgery procedures, there was an initial phase of high risk immediately after surgery that merged with a phase of lower risk (14). The temporal decomposition of this phenomenon is illustrated in eFigure 2, which shows what we term an early, rapidly declining hazard phase starting immediately after surgery, and a late rising hazard phase, which cross at about 7–12 months. Factors modulating each phase are expected to be quite different (nonproportional hazards), which is the motivation behind the approach. Because the temporal decomposition produces hazard phases with little overlap, modulating factors are processed simultaneously for all hazard phases (two in this case). For additional details, see http://www.clevelandclinic.org/heartcenter/hazard.
Reference population survival estimates were generated from equations for the U.S. life tables for each patient according to age, race, and sex (http://www.cdc.gov/nchs/products/life_tables.htm). These were averaged overall and within subgroups of patients.
Secondary endpoints were in-hospital morbidities defined by the Society of Thoracic Surgeons National Database (http://www.ctsnet.org/file/rptDataSpecifications252_1_ForVendorsPGS.pdf).
Data Analysis
Patient characteristics
Simple comparisons were made using Wilcoxon rank-sum nonparametric tests. When the frequency was less than five, comparisons were made using chi-squared and Fisher’s exact tests.
Differences in preoperative patient and echocardiographic measures between isolated AS vs. AS+CAD patients were analyzed by multivariable logistic regression using variables listed in eAppendix 1. CAD- and CABG-related variables defined the AS+CAD group, as did history of myocardial infarction and coronary artery stenosis variables; thus, we did not include them in the modeling. Variable selection, with a P value of .05 for retention of variables, utilized bagging (15,16). Briefly, automated stepwise variable selection was performed on 250 bootstrap samples, and frequency of occurrence of variables related to procedure performed was ascertained by the median rule (15). In doing this, it became apparent that a number of continuous variables demonstrated a nonlinear relationship to group membership. Therefore, to demonstrate the shape of these relationships, we performed a Random Forests classification analysis, using all variables considered in the analysis, to produce nonparametric partial dependency risk-adjusted graphs of the probability of being in the AS+CAD group as a function of these variables (see eAppendix 2 for details).
Unique risk factors
To identify risk factors that may be unique to isolated AS and AS+CAD, separate parsimonious risk factor models were developed using variables listed in eAppendix 1. Risk factors were then combined from the two parsimonious models (eTables 1a and b) to create semi-saturated models (eTable 1c) for each group, with all factors identified in both analyses included. Based on these, an overall model was constructed in which group-specific risk factors were incorporated as interaction effects.
Survival analysis
Due to differences in underlying patient characteristics, propensity matching of isolated AS with AS+CAD patients was employed (17). Multivariable logistic regression using preoperative and procedure variables was used to identify factors associated with isolated AS vs. AS+CAD, as described under “Patient Characteristics.” After developing that parsimonious model, additional variables representing patient factors that might relate to unrecorded selection factors were added (semi-saturated model; see Appendix 1). A propensity score was calculated for each patient by solving the saturated model for the probability of being in the AS+CAD group. Using only propensity scores, 1,082 isolated AS patients (66%) were matched to AS+CAD patients using a greedy matching strategy (Tables 1 and 2, eFigure 3) (18,19). Isolated AS patients whose propensity scores deviated more than 0.10 in probability scale from those of AS+CAD patients were considered unmatched.
Survival was compared for propensity-matched patients using the log-rank test and contrasted with that of unmatched isolated AS patients who did not fit the comorbidity profile of AS+CAD patients, and with unmatched AS+CAD patients whose comorbidity profile did not fit that of isolated AS patients (19).
Missing Data
To account for missing values for some variables, five-fold multiple imputation was performed (17) using a Markov Chain Monte Carlo technique (SAS PROC MI in v9.1, SAS, Inc., Cary, NC). Only covariables were imputed, not outcomes. Bootstrap bagging for variable selection, as described earlier, used one imputed dataset. Regression coefficients and their variance–covariance matrix for the final models were subsequently estimated for each imputed dataset and combined using the method of Rubin and colleagues to produce the final estimates (17).
Presentation
Continuous variables are summarized as mean±standard deviation and as equivalent 15th, 50th (median), and 85th percentiles when values are skewed. Categorical data are summarized using frequencies and percentages. All analyses were performed using SAS statistical software (SAS v9.1;). Parametric survival estimates are accompanied by asymmetric 68% confidence limits, comparable to ±1 standard error.
RESULTS
Unadjusted Outcome Comparisons
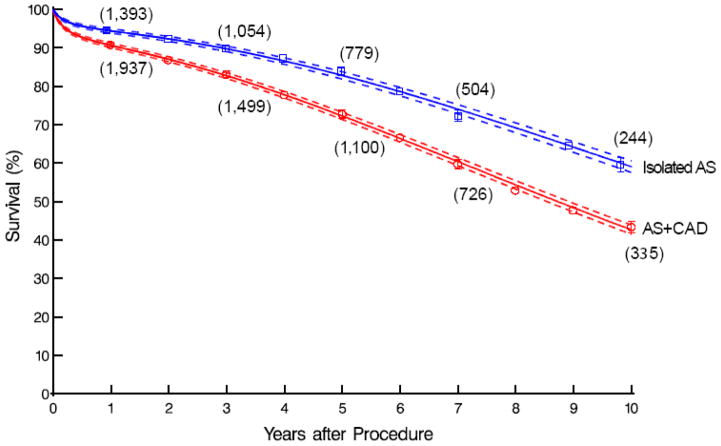
Compared with isolated AS patients, AS+CAD patients had worse early and late unadjusted survival (97.6% vs. 98.7%, 91% vs. 94%, 83% vs. 90%, and 43% vs. and 59% at 30 days, 1, 5, and 10 years, respectively; Figure 1). They also experienced greater postoperative morbidity with significantly more septicemia (2.8% vs. 1.6%), renal failure (6.4% vs. 3.5%), prolonged ventilation (14% vs. 6.4%), and atrial fibrillation (36% vs. 32%) (eTable 2).
Figure 1.
Unadjusted survival among patients after surgery for isolated aortic stenosis (blue; Isolated AS) vs. aortic stenosis with coronary artery disease (red; AS+CAD). Symbols represent Kaplan-Meier nonparametric estimates at yearly intervals, accompanied by vertical bars representing confidence limits equivalent to ±1 standard error. Solid lines enclosed within dashed confidence bands equivalent to ±1 standard error represent parametric estimates.
Patient Characteristics
Compared with isolated AS patients, AS+CAD patients tended to be older males with more severe symptoms; they were more likely to have LV dysfunction and comorbidities, including vasculopathies, systolic hypertension, diabetes, and anemia (Table 3). In contrast, isolated AS patients were more likely young and overweight with more severe AS, bicuspid valves, diastolic hypertension, less LV hypertrophy, and smaller left atria compared with AS+CAD patients.
Table 3.
Patient Variables Associated with Severe Aortic Stenosis Alone (Isolated AS) vs. Aortic Stenosis and Coronary Artery Disease (AS+CAD)
| Variablea | Coefficient ± SE | Odds Ratio (68% CI) | P | Reliability (%)b |
|---|---|---|---|---|
| Higher likelihood of isolated AS | ||||
| Larger BMIc | 0.34 ± 0.094 | —a | .0003 | 82 |
| Shortness of breath | 0.29 ± 0.10 | 1.3 (1.2, 1.5) | .006 | 97 |
| COPD | 0.22 ± 0.11 | 1.2 (1.1, 1.4) | .05 | 41 |
| Higher aortic valve peak gradientd | −0.85 ± 0.098 | —a | <.0001 | 100 |
| Bicuspid aortic valve | 0.56 ± 0.092 | 1.8 (1.6, 1.9) | <.0001 | 100 |
| Tricuspid valve regurgitation 3+/4+ | 0.45 ± 0.20 | 1.6 (1.3, 1.9) | .02 | 64 |
| Higher diastolic blood pressuree | −0.20 ± 0.085 | —a | .02 | 74 |
| Higher likelihood of AS+CAD | ||||
| Older agef | 2.2 ± 0.32 | —a | <.0001 | 89 |
| Male | 0.78 ± 0.087 | 2.2 (2.0, 2.4) | <.0001 | 98 |
| Higher NYHA functional class | 0.25 ± 0.053 | 1.3 (1.2, 1.4) | <.0001 | 99 |
| Lower ejection fractiong | −0.42 ± 0.12 | —a | .0005 | 98 |
| Peripheral arterial disease | 0.58 ± 0.16 | 1.8 (1.5, 2.1) | .0002 | 82 |
| Carotid disease | 0.63 ± 0.078 | 1.9 (1.7, 2.03) | <.0001 | 100 |
| Hypertension | 0.54 ± 0.088 | 1.7 (1.6, 1.9) | <.0001 | 100 |
| Higher systolic blood pressureh | 0.33 ± 0.15 | —a | .03 | 74 |
| Diabetes | 0.47 ± 0.099 | 1.6 (1.4, 1.8) | <.0001 | 100 |
| Lower hematocriti | −0.90 ± 0.28 | —a | .001 | 81 |
| Lower HDLj | −0.60 ± 0.14 | —a | <.0001 | 100 |
| Aorta procedure | 0.43 ± 0.19 | 1.5 (1.3, 1.9) | .02 | 81 |
See eFigure 4 for an illustration of the nature of the relationship of continuous variables to likelihood of having coronary artery disease.
Percent of times variables appeared in 250 bootstrap models.
(BMI/30)2, squared transformation.
(80/AV peak gradient), inverse transformation.
(75/diastolic blood pressure)2, inverse squared transformation.
Log(age), logarithmic transformation.
(Ejection fraction/55)2, squared transformation.
(Systolic blood pressure/135)2, squared transformation.
Log(hematocrit), logarithmic transformation.
Log(HDL), logarithmic transformation.
Key: AS, aortic stenosis; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association; HDL, high-density lipoprotein; SE, standard error.
Unique Risk Factors
In both groups, patients who were elderly and more symptomatic had increased risk of death early after surgery (Table 4). Late risk was associated with smoking and LV dysfunction. Some of these common factors had statistically significant different strengths between groups, including higher creatinine levels in the early hazard phase and older age and higher blood urea nitrogen in the late hazard phase.
Table 4.
Overall Model for Incremental Risk Factors for Death after Procedure
| Risk Factor | Common
|
Isolated AS
|
AS+CAD
|
|||
|---|---|---|---|---|---|---|
| Coefficient ± SE | P | Coefficient ± SE | P | Coefficient ± SE | P | |
| Early hazard phase | ||||||
| Isolated AS | 3.7 ± 0.89 | <.0001 | ||||
| AS+CAD | ||||||
| LMT stenosis ≥70% | 0.76 ± 0.25 | .002 | ||||
| LAD stenosis ≥70% | 0.34 ± 0.16 | .03 | ||||
| RCA stenosis ≥50% | 0.404 ± 0.18 | .02 | ||||
| LCx stenosis >0% | 0.62 ± 0.27 | .02 | ||||
| Older agea | 1.9 ± 0.34 | < .0001 | ||||
| Larger height/weight ratio | 0.55 ± 0.19 | .004 | ||||
| NYHA functional class III/IV | 0.66 ± 0.13 | <.0001 | ||||
| Heart failure | 0.34 ± 0.16 | .04 | ||||
| Aortic valve regurgitation 3+/4+ | 0.64 ± 0.27 | .02 | ||||
| Tricuspid valve regurgitation 4+ | 1.02 ± 0.38 | .007 | ||||
| Lower LV diastolic volumeb | 0.097 ± 0.028 | .0004 | ||||
| Lower ejection fraction | 0.061 ± 0.022 | .006 | ||||
| Lower HDLc | 0.11 ± 0.034 | .002 | ||||
| Carotid disease (less risk) | −0.35 ± 0.15 | .02 | ||||
| COPD | 0.51 ± 0.18 | .005 | ||||
| Treated diabetes | 0.52 ± 0.17 | .002 | ||||
| Higher creatinined | −1.3 ± 0.43 | .003 | −0.64 ± 0.25 | .01 | ||
| Higher bilirubin | 0.24 ± 0.12 | .05 | ||||
| Lower hematocrite | −1.6 ± 0.51 | .002 | −0.60 ± 0.34 | .08 | ||
| Smaller AV prosthesis (Z-value) | −0.25 ± 0.086 | .004 | ||||
| Earlier date of operationf | 1.2 ± 0.38 | .001 | ||||
| Late hazard phase | ||||||
| AS alone | −3.2 ± 0.76 | <.0001 | ||||
| AS+CAD | ||||||
| ITA graft used (less risk) | −0.24 ± 0.079 | .002 | ||||
| Older agea | 3.7 ± 0.43 | <.0001 | 2.3 ± 0.24 | <.0001 | ||
| Myocardial infarction | 0.24 ± 0.082 | .004 | ||||
| Larger BMIg | 0.41 ± 0.11 | .0002 | ||||
| Syncope | 0.34 ± 0.14 | .02 | ||||
| Lower ejection fractionh | −0.52 ± 0.15 | .001 | ||||
| Larger LA diameteri | 0.86 ± 0.35 | .01 | 0.34 ± 0.44 | .4 | ||
| LV mass indexj | 0.73 ± 0.18 | <.0001 | −0.35 ± 0.202 | .09 | ||
| Interaction: LA diameter • LV mass index | 0.42 ± 0.19 | .03 | ||||
| Interaction: age • LV mass indexl | −0.65 ± 0.21 | .002 | ||||
| Insulin-treated diabetes | 0.72 ± 0.13 | <.0001 | ||||
| Smoking | 0.25 ± 0.065 | <.0001 | ||||
| COPD | 0.59 ± 0.16 | .0002 | ||||
| Dialysis | 1.4 ± 0.303 | <.0001 | ||||
| Higher BUNm | 0.14 ± 0.036 | .0002 | 0.062 ± 0.015 | <.0001 | ||
| Lower hematocrite | −0.60 ± 0.18 | .001 | ||||
| Any atrial fibrillation procedure | 0.64 ± 0.20 | .001 | ||||
| ASD/PFO suture closure | 0.83 ± 0.26 | .002 | ||||
(Age/75)2, squared transformation.
(100/LV diastolic volume)2, inverse squared transformation.
(50/HDL)2, inverse squared transformation.
(1/creatinine), inverse transformation.
(Hematocrit/40)2, squared transformation.
(1/[interval to date of operation from 1/1/91]), inverse transformation.
(BMI/30)2, squared transformation.
(Ejection fraction/55), scaled variable.
(LA diameter/4.5), scaled variable.
(LV mass index/125)2, squared transformation.
Interaction: (LA diameter/4.5) • (LV mass index/125)2.
Interaction: (age/75)2 • (LV mass index/125)2.
(BUN/20)2, squared transformation.
Key: AS, aortic stenosis; ASD/PFO, atrial septal defect/patent foramen ovale; BMI, body mass index; BUN, blood urea nitrogen; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; HDL, high-density lipoprotein; ITA, internal thoracic artery; LA, left atrial; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; LMT, left main trunk; LV, left ventricle; NYHA, New York Heart Association; RCA, right coronary artery; SE, standard error.
Unique to isolated AS patients was early mortality associated with smaller LV diastolic volume and other systemic illnesses, including anemia and liver dysfunction (see Table 4). The status of the LV at time of surgery, notably presence of severe LV hypertrophy and LA dilatation, was associated with increased late risk of death, with a more pronounced effect in younger patients (eFigure 5).
Unique to AS+CAD patients was early mortality associated with more severe LV systolic dysfunction and aortic or tricuspid valve regurgitation (see Table 4). In contrast to the isolated AS group, prior myocardial infarction, insulin-dependent diabetes, dialysis, and anemia were associated with increased risk of late death. Addition of an atrial fibrillation procedure or patent foramen ovale suture closure was associated with increased risk of death. Internal thoracic artery grafts used for CABG were associated with decreased mortality.
Matched Outcome Comparisons
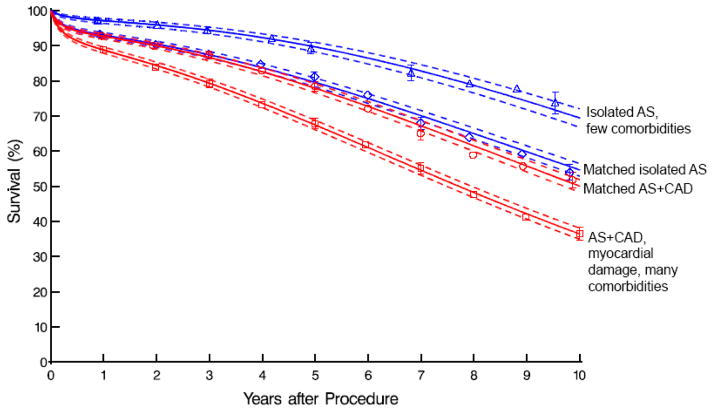
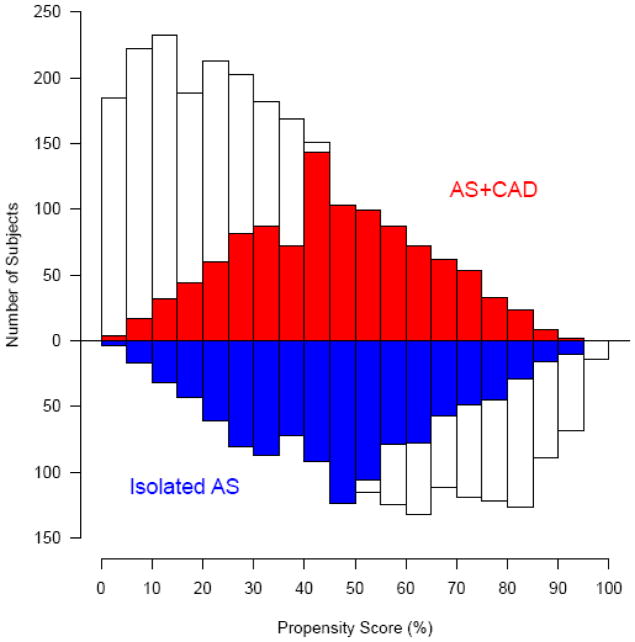
Survival was similar among propensity-matched isolated AS and AS+CAD patients (93% vs. 93%, 80% vs. 80%, and 55% vs. 50% at 1, 5, and 10 years, respectively; Figure 2). Within the isolated AS group, matched patients had a significantly lower survival than unmatched patients with few comorbidities (93% vs. 97%, 80% vs. 90%, and 55% vs. 70% at 1, 5, and 10 years, respectively; eTables 3a and 3b and Figure 2). Conversely, AS+CAD matched patients had significantly higher survival than unmatched patients with myocardial damage and many comorbidities (93% vs. 89%, 78% vs. 67%, and 50% vs. 36% at 1, 5, and 10 years, respectively; eTables 4a and 4b and Figure 2). Differences in survival relate to the disparate patient profiles that resulted in propensity matching intermediate profile groups (Figure 3; see Table 1).
Figure 2.
Survival of patients with isolated aortic stenosis (AS) with few comorbidities (blue triangles) compared with isolated AS and noncoronary artery disease (CAD) comorbidities matching those of patients with both AS (blue diamonds) and AS+CAD (red circles). These are all contrasted with patients having AS and CAD with myocardial damage and a comorbidity profile that is completely unlike patients with isolated AS (red squares). Format is as in Figure 1.
Figure 3.
Mirrored histogram of distribution of propensity scores for patients with isolated aortic stenosis (blue; Isolated AS) vs. aortic stenosis and coronary artery disease (red; AS+CAD). Red and blue areas represent 1,082 matched patient pairs.
Postoperative in-hospital morbidity was similar among matched patients, except for more prolonged ventilation in AS+CAD patients (see eTable 2).
Matched and Unmatched Patient Characteristics
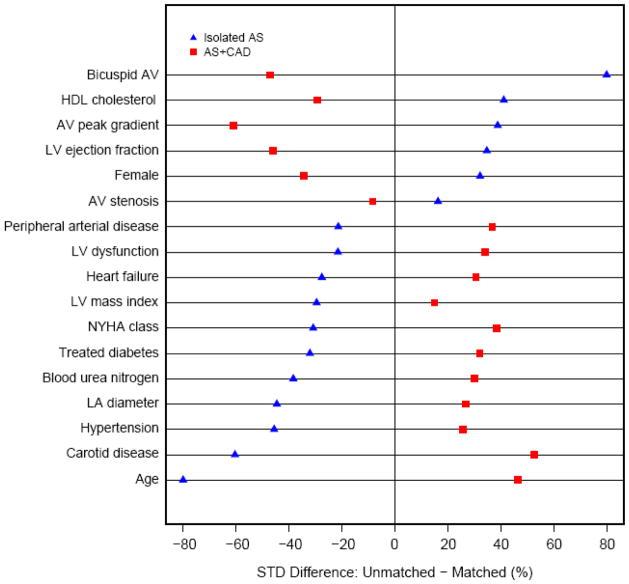
Unmatched patients within the isolated AS group, on average, represent younger persons with mild symptoms and few to no comorbidities (Figure 4 and see eTables 3a and 3b). However, they had the most hemodynamically severe form of AS among the groups, with high aortic valve gradients and a predominance of bicuspid valves (65% matched vs. 28% unmatched). They tended to have isolated AS, with few having concomitant mitral or tricuspid valve disease. This stands in contrast to matched isolated AS patients who had hemodynamically less severe AS, but significantly higher prevalence of comorbid conditions, including vasculopathies, stroke, hypertension, diabetes, and renal disease. These matched patients also had worse LV systolic function, more heart failure, complete heart block, atrial fibrillation, and greater LV hypertrophy and LA dilatation compared with unmatched isolated AS patients.
Figure 4.
Standardized differences between unmatched patients with isolated aortic stenosis (blue triangles; Isolated AS) group and aortic stenosis with coronary artery disease (red squares; AS+CAD) group (1). Key: AV, aortic valve; HDL, high- density lipoprotein; LA, left atrial; LV, left ventricular; NYHA, New York Heart Association.
In contrast, unmatched AS+CAD patients represent the opposite spectrum of disease severity. They tended to be older males with the most severe symptoms and extensive comorbidities (see Figure 4 and eTables 4a and 4b). Unmatched AS+CAD patients had the least severe AS; however, they had the highest prevalence of CAD risk factors among the groups, including vasculopathies, smoking, hypertension, diabetes, and renal disease. They also had more heart failure and LV dysfunction, the most severe LV hypertrophy and dilatation, and more ischemic mitral disease and atrial fibrillation.
DISCUSSION
Principal Findings
Overall, AS+CAD patients had poorer short- and long-term prognosis than those with isolated AS and more postoperative morbidity. Increased mortality of AS+CAD patients was associated with effects of preexisting ischemic myocardial damage and comorbidities. In contrast, prognosis for isolated AS patients was adversely influenced by the negative influence of LV hypertrophy and diastolic dysfunction at operation. But further analyses using propensity matching clearly identified distinct patient subgroups with differing prognosis. The first comprised patients with isolated AS who had the best outcome and whose survival was adversely affected by LV hypertrophy and diastolic dysfunction. The second comprised patients with CAD without evidence of ischemic myocardial damage. These patients had outcome similar to those with isolated AS and similar non-CAD comorbidities. The third group comprised patients with severe AS+CAD and ischemic damage and multiple comorbidities, unlike patients with isolated severe AS. They had the poorest survival, despite the least degree of AS.
Patient Profiles
That patients with AS+CAD were older and more symptomatic with less severe AS, but more risk factors for CAD than patients with isolated AS, has been documented by others (3). Onset of symptoms likely results from the combined effects of CAD and AS, resulting in catheterization and earlier intervention for their valvar and coronary diseases. This stands in contrast to milder, but slowly developing symptoms more characteristic of patients with isolated AS.
Unique Risk Factors
Patients with AS+CAD display risk profiles reflecting CAD (20–22). Survival is dominated by comorbidities, particularly vasculopathy. Many of their risk factors have been described by others (5,23,24), including older age, more severe symptoms, vasculopathy, previous stroke, diabetes, previous myocardial infarction, and renal disease.
In contrast, survival of patients with isolated AS is dominated by secondary effects of AS on myocardium, notably LV hypertrophy and diastolic dysfunction (25). Severe LV hypertrophy only partly regresses after AVR (26,27), is associated with decreased long-term survival (28), and results in decreased ventricular compliance and ischemia-induced myocardial fibrosis, contributing to LV diastolic dysfunction (29,30). Although LV mass index and LA diameter were smaller in the isolated AS group, these factors, particularly LA diameter (31), are strongly associated with increased late mortality not seen in AS+CAD patients despite their having larger left heart measures. This suggests that structural heart changes in the two groups are different. Given the extensive comorbidity profile of the AS+CAD group, secondary effects of more prominent disease processes, such as hypertension (32), may be responsible. Nonetheless, long-term survival of patients with isolated AS appears to be more sensitive to the presence of LV hypertrophy and diastolic dysfunction.
Survival Comparison
In general, patients with severe AS and coexisting CAD have worse survival than those with isolated AS. However, the heterogeneity of patient characteristics makes simple survival comparisons between the two groups inaccurate (19). Propensity matching provides an opportunity to compare outcomes of patients with isolated AS with those of patients with AS+CAD and otherwise similar non-CAD comorbidity profiles. Matched survival was similar, consistent with the propensity-matched comparison by Roberts and colleagues (3), suggesting that surgical revascularization at the time of AVR neutralizes the adverse effects of CAD, provided that ischemic myocardial damage has not occurred. However, the distribution of propensity scores (see Figure 3) demonstrates that this matched population is an intermediate risk group, different from both average isolated AS and AS+CAD patients. This is reflected in survival of the unmatched groups. Unmatched isolated AS patients had better, and AS+CAD patients worse, survival than their respective matched group. Given the age discrepancies of patients, it is helpful to reference survival curves to expected U.S. age-sex-race–matched survival. Isolated AS patients, both matched and unmatched, had better than expected survival, as did the matched AS+CAD patients (eFigure 6). However, unmatched AS+CAD patients have poor survival, falling below that of the U.S. life table by 8 to 10 years postoperatively.
Patient Profile: A Determinant of Outcomes in Severe AS
Patients’ comorbidity profiles affected survival more than the procedure they underwent. Even in the absence of CAD, patients who underwent AVR, but had comorbidities including smoking, vasculopathies, hypertension, diabetes, and renal disease, had survival equal to matched patients with CAD who underwent AVR+CABG. Unmatched AS+CAD patients had poor survival with a high-risk comorbidity profile and signs of irreversible ischemic heart damage. Even if these patients survive surgery, they have poor survival relative to other patients undergoing the same procedures. They require chronic disease management for their comorbidities, as these will ultimately determine their outcomes. It may be argued whether surgery should be performed on these patients. The answer may have implications for future therapies. For example, late results of transcatheter AVR+percutaneous coronary intervention may also be determined by patients’ systemic diseases. Therefore, because of their patient profile, results may not be as good as those for patients receiving transcatheter AVR alone (33).
Strengths and Limitations
This is a large, contemporary, single-institution observational study comparing patients who received either AVR alone for isolated severe AS, or AVR+CABG for severe AS and CAD. All patients were evaluated by heart catheterization to define coronary artery stenosis and underwent primary heart surgery with a single prosthesis type for isolated degenerative AS. Prior analyses of these groups evaluated patient risk factors for survival, which, as both this and other studies noted, are limited based on the distinct profiles of the patients undergoing these procedures (6). The application of propensity analysis enabled direct comparison of a subset of these patients. Given their similar profiles after matching, patients undergoing surgery for isolated AS and AS+CAD had equal survival and thus no effect of CAD after intervention.
Diagnosis of CAD was based on catheterization with at least one vessel with stenosis ≥50%. This definition was based on clinical practice within our institution for treating of CAD, guided by current American Heart Association and American College of Cardiology recommendations (4). This is reflected in the relatively small number of patients (n=427) who underwent AVR alone, but had CAD meeting these criteria; these patients were excluded from this analysis. Others have applied stricter criteria, such as 70%–75% stenosis (34). Only 22 patients underwent AVR+CABG without meeting our criteria for CAD, but 20 of these procedures were for ostial occlusion by the prosthesis. Thus, our definition reflects current clinical practice.
Implications
Patients with severe AS range from young patients with isolated bicuspid valve disease and excellent expected survival to elderly patients with extensive comorbidities resulting in poor functional status and survival. Therefore, for many patients, AS is not a simple mechanical disease process, but represents one of many concurrent comorbidities. Our analysis distinguishes between groups based on comorbidities and risk factors. These factors should be used for accurate interpretation of results for current and future therapies for AS, including percutaneous procedures. Although patients with isolated AS have excellent survival, their outcomes are more sensitive to the consequences of long-standing pressure overload leading to LV hypertrophy and diastolic dysfunction, as reflected by left atrial size (31,35–37). These results add to evidence that these patients may need AVR before symptom development and irreversible left heart remodeling. In contrast, those with the highest risk profile have poor survival because of advanced comorbidities that are not reversed by AVR.
Complete assessment of patient characteristics should be incorporated into decision-making. In patients with severe AS and LV hypertrophy, early surgery may be indicated before symptoms develop. Although current guidelines for treating valvar heart disease recognize that risk factors for CAD and AS frequently coexist, there are no recommendations for early evaluation or diagnosis of CAD in these patients. Our study demonstrates the devastating effects on survival of ischemic damage in patients with AS; this indicates the need for early diagnosis of CAD in these patients.
The common practice of advocating delay of surgery for patients with AS in order to avoid anticoagulation associated with mechanical prostheses can adversely affect long-term survival because of the potential for myocardial ischemic damage. The results of our study strongly suggest changing practice and modifying guidelines to include early evaluation of CAD in asymptomatic patients with severe AS and risk factors for CAD so that timely AVR+CABG is performed before ischemic myocardial damage occurs. Generally these patients will have reached an age for which contemporary bioprostheses have lifetime durability in the great majority (38) and transcatheter valve-in-valve procedures may obviate need for reoperation in the future (39–41). Elderly patients with AS and risk factors for CAD should be considered for active investigation of CAD before evaluation for AVR. In contrast, patients with poor functional status and advanced comorbidities may be best served with medical management alone.
Supplementary Material
Acknowledgments
This work was supported by awards from the American Heart Association (Dr. Beach). It was also supported in part by the Donna and Ken Lewis Chair in Cardiothoracic Surgery (Dr. Mihaljevic) and the Kenneth Gee and Paula Shaw, PhD, Chair in Heart Research (Dr. Blackstone).
ABBREVIATIONS USED IN TEXT
- AS
Aortic stenosis
- AVR
Aortic valve replacement
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- LA
Left atrial
- LAD
Left anterior descending coronary artery
- LCx
Left circumflex artery
- LMT
Left main trunk
- LV
Left ventricle
- RCA
Right coronary artery
- TTE
Transthoracic echocardiography
References
- 1.de Waard GA, Jansen EK, de Mulder M, Vonk AB, Umans VA. Long-term outcomes of isolated aortic valve replacement and concomitant AVR and coronary artery bypass grafting. Neth Heart J. 2012;20:110–117. doi: 10.1007/s12471-011-0238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsoufi B, Karamlou T, Slater M, Shen I, Ungerleider R, Ravichandran P. Results of concomitant aortic valve replacement and coronary artery bypass grafting in the VA population. J Heart Valve Dis. 2006;15:12–18. discussion 18–19. [PubMed] [Google Scholar]
- 3.Roberts WC, Roberts CC, Vowels TJ, Ko JM, Filardo G, Hamman BL, et al. Effect of coronary bypass and valve structure on outcome in isolated valve replacement for aortic stenosis. Am J Cardiol. 2012;109:1334–1340. doi: 10.1016/j.amjcard.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–142. doi: 10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Edwards FH, Peterson ED, Coombs LP, DeLong ER, Jamieson WR, Shroyer ALW, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol. 2001;37:885–892. doi: 10.1016/s0735-1097(00)01202-x. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi KJ, Williams JA, Nwakanma LU, Weiss ES, Gott VL, Baumgartner WA, et al. EuroSCORE predicts short- and mid-term mortality in combined aortic valve replacement and coronary artery bypass patients. J Card Surg. 2009;24:637–643. doi: 10.1111/j.1540-8191.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- 7.Donzeau-Gouge P, Blondeau P, Enriquez O, Benomar M, Nottin R, Chauvaud S, et al. Calcified aortic stenosis and coronary disease. Apropos of 115 surgically-treated cases. Arch Mal Coeur Vaiss. 1984;77:856–864. [PubMed] [Google Scholar]
- 8.Craver JM, Weintraub WS, Jones EL, Guyton RA, Hatcher CR., Jr Predictors of mortality, complications, and length of stay in aortic valve replacement for aortic stenosis. Circulation. 1988;78:I85–90. [PubMed] [Google Scholar]
- 9.Stahle E, Bergstrom R, Nystrom SO, Hansson HE. Early results of aortic valve replacement with or without concomitant coronary artery bypass grafting. Scand J Thorac Cardiovasc Surg. 1991;25:29–35. doi: 10.3109/14017439109098080. [DOI] [PubMed] [Google Scholar]
- 10.Aranki SF, Rizzo RJ, Couper GS, Adams DH, Collins JJ, Jr, Gildea JS, et al. Aortic valve replacement in the elderly. Effect of gender and coronary artery disease on operative mortality. Circulation. 1993;88:II17–23. [PubMed] [Google Scholar]
- 11.Mattila S, Harjula A, Jarvinen A, Kyllonen KE, Tala P. Combined valve replacement and myocardial revascularization. Factors influencing early and late results. Scand J Thorac Cardiovasc Surg. 1984;18:49–52. doi: 10.3109/14017438409099383. [DOI] [PubMed] [Google Scholar]
- 12.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 13.Blackstone EH, Naftel DC, Turner ME., Jr The decomposition of time-varying hazard into phases, each incorporating a separate stream of concomitant information. J Am Stat Assoc. 1986;81:615–624. [Google Scholar]
- 14.Osswald BR, Blackstone EH, Tochtermann U, Thomas G, Vahl CF, Hagl S. The meaning of early mortality after CABG. Eur J Cardiothorac Surg. 1999;15:401–407. doi: 10.1016/s1010-7940(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 15.Breiman L. Bagging predictors. Machine Learning. 1996;24:123–140. [Google Scholar]
- 16.Blackstone EH. Breaking down barriers: helpful breakthrough statistical methods you need to understand better. J Thorac Cardiovasc Surg. 2001;122:430–439. doi: 10.1067/mtc.2001.117536. [DOI] [PubMed] [Google Scholar]
- 17.Rubin DB. Multiple imputation for non-response in surveys. New York: Wiley; 1987. [Google Scholar]
- 18.Bergstralh EJ, Konsanke JL. Technical report No 56. Department of Health Science Research; Rochester, MN: Mayo Clinic; 1995. Computerized matching of cases to controls. [Google Scholar]
- 19.Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg. 2002;123:8–15. doi: 10.1067/mtc.2002.120329. [DOI] [PubMed] [Google Scholar]
- 20.Taylor HA, Jr, Clark BL, Garrison RJ, Andrew ME, Han H, Fox ER, et al. Relation of aortic valve sclerosis to risk of coronary heart disease in African-Americans. Am J Cardiol. 2005;95:401–404. doi: 10.1016/j.amjcard.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 21.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 22.Olsen MH, Wachtell K, Bella JN, Gerdts E, Palmieri V, Nieminen MS, et al. Aortic valve sclerosis relates to cardiovascular events in patients with hypertension (a LIFE substudy) Am J Cardiol. 2005;95:132–136. doi: 10.1016/j.amjcard.2004.08.080. [DOI] [PubMed] [Google Scholar]
- 23.Gardner SC, Grunwald GK, Rumsfeld JS, Cleveland JC, Jr, Schooley LM, Gao D, et al. Comparison of short-term mortality risk factors for valve replacement versus coronary artery bypass graft surgery. Ann Thorac Surg. 2004;77:549–556. doi: 10.1016/S0003-4975(03)01585-6. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of The Society of Thoracic Surgeons. Ann Thorac Surg. 1999;67:943–951. doi: 10.1016/s0003-4975(99)00175-7. [DOI] [PubMed] [Google Scholar]
- 25.Casaclang-Verzosa G, Malouf JF, Scott CG, Juracan EM, Nishimura RA, Pellikka PA. Does left atrial size predict mortality in asymptomatic patients with severe aortic stenosis? Echocardiography (Mount Kisco, NY. 2010;27:105–109. doi: 10.1111/j.1540-8175.2009.01002.x. [DOI] [PubMed] [Google Scholar]
- 26.Lund O, Erlandsen M, Dorup I, Emmertsen K, Flo C, Jensen FT. Predictable changes in left ventricular mass and function during ten years after valve replacement for aortic stenosis. J Heart Valve Dis. 2004;13:357–368. [PubMed] [Google Scholar]
- 27.Lim E, Ali A, Theodorou P, Sousa I, Ashrafian H, Chamageorgakis T, et al. Longitudinal study of the profile and predictors of left ventricular mass regression after stentless aortic valve replacement. Ann Thorac Surg. 2008;85:2026–2029. doi: 10.1016/j.athoracsur.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Mihaljevic T, Nowicki ER, Rajeswaran J, Blackstone EH, Lagazzi L, Thomas J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg. 2008;135:1270–1278. doi: 10.1016/j.jtcvs.2007.12.042. discussion 1278–1279. [DOI] [PubMed] [Google Scholar]
- 29.Triposkiadis F, Pitsavos C, Boudoulas H, Trikas A, Kallikazaros I, Stefanadis C, et al. Left atrial volume and function in valvular aortic stenosis. J Heart Valve Dis. 1993;2:104–113. [PubMed] [Google Scholar]
- 30.Dalsgaard M, Egstrup K, Wachtell K, Gerdts E, Cramariuc D, Kjaergaard J, et al. Left atrial volume in patients with asymptomatic aortic valve stenosis (the Simvastatin and Ezetimibe in Aortic Stenosis study) Am J Cardiol. 2008;101:1030–1034. doi: 10.1016/j.amjcard.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 31.Beach JM, Mihaljevic T, Rajeswaran J, Marwick T, Nowicki ER, Thomas J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.12.016. Submitted. [DOI] [PubMed] [Google Scholar]
- 32.Tjang YS, van Hees Y, Korfer R, Grobbee DE, van der Heijden GJ. Predictors of mortality after aortic valve replacement. Eur J Cardiothorac Surg. 2007;32:469–474. doi: 10.1016/j.ejcts.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 34.Richardson JV, Kouchoukos NT, Wright JO, 3rd, Karp RB. Combined aortic valve replacement and myocardial revascularization: results in 220 patients. Circulation. 1979;59:75–81. doi: 10.1161/01.cir.59.1.75. [DOI] [PubMed] [Google Scholar]
- 35.Gjertsson P, Caidahl K, Bech-Hanssen O. Left ventricular diastolic dysfunction late after aortic valve replacement in patients with aortic stenosis. Am J Cardiol. 2005;96:722–727. doi: 10.1016/j.amjcard.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 36.De Castro S, Caselli S, Di Angelantonio E, Del Colle S, Mirabelli F, Marcantonio A, et al. Relation of left atrial maximal volume measured by real-time 3D echocardiography to demographic, clinical, and Doppler variables. Am J Cardiol. 2008;101:1347–1352. doi: 10.1016/j.amjcard.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Gjertsson P, Caidahl K, Farasati M, Oden A, Bech-Hanssen O. Preoperative moderate to severe diastolic dysfunction: a novel Doppler echocardiographic long-term prognostic factor in patients with severe aortic stenosis. J Thorac Cardiovasc Surg. 2005;129:890–896. doi: 10.1016/j.jtcvs.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Smedira NG, Blackstone EH, Roselli EE, Laffey CC, Cosgrove DM. Are allografts the biologic valve of choice for aortic valve replacement in nonelderly patients? Comparison of explantation for structural valve deterioration of allograft and pericardial prostheses. J Thorac Cardiovasc Surg. 2006;131:558–564. e554. doi: 10.1016/j.jtcvs.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Gotzmann M, Mugge A, Bojara W. Transcatheter aortic valve implantation for treatment of patients with degenerated aortic bioprostheses--valve-in-valve technique. Catheter Cardiovasc Interv. 2010;76:1000–1006. doi: 10.1002/ccd.22738. [DOI] [PubMed] [Google Scholar]
- 40.Dworakowski R, Maccarthy P. Where should transcatheter aortic valve implantation go beyond 2012? J Cardiovasc Med (Hagerstown) 2012;13:516–523. doi: 10.2459/JCM.0b013e328354cdac. [DOI] [PubMed] [Google Scholar]
- 41.Greif M, Lange P, Mair H, Becker C, Schmitz C, Steinbeck G, et al. Transcatheter Edwards Sapien XT valve in valve implantation in degenerated aortic bioprostheses via transfemoral access. Clin Res Cardiol. 2012 doi: 10.1007/s00392-012-0488-3. [DOI] [PubMed] [Google Scholar]
- 42.Breiman L. Random forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 43.Liaw A, Wiener M. Classification and regression by random. Forest Rnews. 2002;2/3:18–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.