Abstract
Importance
The value of robotically assisted surgery for mitral valve disease is questioned because the high cost of care associated with robotic technology may outweigh its clinical benefits.
Objective
To investigate conditions under which benefits of robotic surgery mitigate high technology costs.
Design
Clinical cohort study comparing costs of robotic vs. three contemporaneous conventional surgical approaches for degenerative mitral disease. Surgery was performed from 2006–2011, and comparisons were based on intent-to-treat, with propensity-matching used to reduce selection bias.
Setting
Large multi-specialty academic medical center.
Participants
1,290 patients aged 57±11 years, 27% women, underwent mitral repair for regurgitation from posterior leaflet prolapse. Robotic surgery was used in 473, complete sternotomy in 227, partial sternotomy in 349, and anterolateral thoracotomy in 241. Three propensity-matched groups were formed based on demographics, symptoms, cardiac and noncardiac comorbidities, valve pathophysiology, and echocardiographic measurements: robotic vs. sternotomy (n=198 pairs) vs. partial sternotomy (n=293 pairs) vs. thoracotomy (n=224 pairs).
Interventions
Mitral valve repair.
Main Outcome Measures
Cost of care, expressed as robotic capital investment, maintenance, and direct technical hospital cost, and benefit of care, based on differences in recovery time.
Results
Median cost of care for robotically assisted surgery exceeded the cost of alternative approaches by 27% (−5%, 68%), 32% (−6%, 70%), and 21% (−2%, 54%) (median [15th, 85th percentiles]) for complete sternotomy, partial sternotomy, and anterolateral thoracotomy, respectively. Higher operative costs were partially offset by lower postoperative costs and earlier return to work: median 35 days for robotic surgery, 49 for complete sternotomy, 56 for partial sternotomy, and 42 for anterolateral thoracotomy. Resulting net differences in cost of robotic surgery vs. the three alternatives were 16% (−15%, 55%), 16% (−19%, 51%), and 15% (−7%, 49%), respectively. Beyond a volume threshold of 55–100 robotic cases per year, confidence limits for the cost of robotic surgery broadly overlapped those of conventional approaches.
Conclusions
In exchange for higher procedural costs, robotically assisted mitral valve surgery offers the clinical benefit of least invasive surgery, lowest postoperative cost, and fastest return to work. The value of robotically assisted surgery comparable to conventional approaches can only be realized in high-volume centers.
INTRODUCTION
Robotically assisted surgery is the least invasive approach for treating myxomatous mitral valve disease. Benefits include less trauma, faster recovery leading to quicker return to work or normal activities, and superior cosmesis, with comparable success, safety, and effectiveness.1–5 However, the value of this emerging technology for mitral valve repair is questioned because associated incremental costs above those of conventional surgery—costs of capital investment, maintenance, and robotic-specific disposable instruments—may outweigh these clinical benefits. The purpose of this study was to investigate the value of robotically assisted mitral valve surgery by assessing whether benefits mitigate the additional cost.6 We compared its cost with those of conventional approaches, including offsets from shorter recovery and earlier return to work.
PATIENTS AND METHODS
Patients
From January 1, 2006, to January 1, 2011, 1,290 patients with degenerative mitral valve disease underwent first-time isolated posterior leaflet repair via complete sternotomy (n=227), partial sternotomy (n=349), anterolateral thoracotomy (n=241), or robotically assisted surgery (n=473) (Figure 1).1,4,7–14 The total number of intended robotic mitral valve operations during that time was 622; however, the study was limited to the 473 patients with myxomatous disease of the posterior leaflet to facilitate comparisons in a homogeneous patient population. This includes our robotic experience from the first case, performed by two surgeons with similar extensive prior experience with conventional and newer approaches for mitral valve repair. We excluded patients who underwent concomitant procedures, with the exception of suture closure of a patent foramen ovale or pulmonary vein isolation for atrial fibrillation. After propensity matching, 1,173 patients remained in the study.
Figure 1.

CONSORT diagram showing patient groups by approach: 91% of patients matched (87% of complete sternotomy, 84% of partial sternotomy, 93% of anterolateral thoracotomy, and 97% of robotic groups).
Average patient age was 57±11 years, 344 (27%) were women, and 605 (49%) were asymptomatic or minimally symptomatic with severe (n=1,027 [80%]) mitral valve regurgitation (Table 1). Preoperative and operative variables were retrieved from the Cleveland Clinic Cardiovascular Information Registry, an ongoing, prospective, concurrent registry of all cardiac operations, and by review of patients’ medical records.
Table 1.
Patient Characteristics and Surgical Details by Approach (n = 1,290)
| Variable | Robotic Approach (total n = 473)
|
Complete Sternotomy (total n = 227)
|
Partial Sternotomy (total n = 349)
|
Anterolateral Thoracotomy (total n = 241)
|
||||
|---|---|---|---|---|---|---|---|---|
| na | No. (%)or Mean ± SD | na | No. (%)or Mean ± SD | na | No. (%)or Mean ± SD | na | No. (%)or Mean ± SD | |
| Demography | ||||||||
| Female | 473 | 101 (21) | 227 | 66 (29) | 349 | 99 (28) | 241 | 78 (32) |
| Age (y) | 473 | 56 ± 10 | 227 | 62 ± 11 | 349 | 58 ± 12 | 241 | 54 ± 11 |
| Preoperative BSA (m2) | 472 | 2.0 ± 0.24 | 227 | 2.0 ± 0.29 | 347 | 2.0 ± 0.23 | 240 | 2.0 ± 0.25 |
| Symptoms | ||||||||
| NYHA functional class | 447 | 218 | 338 | 227 | ||||
| I | 244 (54) | 82 (38) | 160 (47) | 119 (52) | ||||
| II | 158 (35) | 94 (43) | 150 (44) | 92 (41) | ||||
| III | 44 (9.6) | 39 (18) | 26 (7.7) | 16 (7.0) | ||||
| IV | 2 (0.45) | 3 (1.4) | 2 (0.59) | 0 (0) | ||||
| Heart failure | 473 | 30 (6.3) | 227 | 38 (17) | 349 | 22 (6.3) | 241 | 7 (2.9) |
| Cardiac morbidity | ||||||||
| Mitral regurgitation grade | 473 | 227 | 349 | 241 | ||||
| 2+ | 6 (1.3) | 5 (2.2) | 7 (2.0) | 4 (1.7) | ||||
| 3+ | 73 (15) | 45 (20) | 57 (16) | 43 (18) | ||||
| 4+ | 386 (82) | 169 (74) | 282 (80) | 190 (79) | ||||
| Atrial fibrillation/flutter | 458 | 57 (12) | 198 | 63 (32) | 293 | 19 (6.5) | 224 | 22 (9.8) |
| Noncardiac comorbidity | ||||||||
| Stroke | 473 | 6 (1.3) | 227 | 5 (2.2) | 349 | 7 (2.0) | 241 | 2 (0.83) |
| Hypertension | 467 | 180 (38) | 226 | 110 (49) | 346 | 148 (43) | 237 | 92 (39) |
| Creatinine (mg•dL−1) | 473 | 0.98 ± 0.19 | 227 | 0.97 ± 0.21 | 349 | 0.99 ± 0.67 | 241 | 0.96 ± 0.20 |
| Smoking | 467 | 153 (33) | 226 | 85 (38) | 344 | 104 (30) | 235 | 71 (30) |
| COPD | 473 | 15 (3.2) | 227 | 19 (8.4) | 349 | 13 (3.7) | 241 | 8 (3.3) |
| Morphology | ||||||||
| Preoperative LA systolic area (cm2) | 448 | 28 ± 7.7 | 201 | 30 ± 7.9 | 332 | 28 ± 6.6 | 227 | 27 ± 6.3 |
| Preoperative calculated LV end-diastolic volume (cm3) | 462 | 148 ± 41 | 213 | 138 ± 43 | 342 | 146 ± 40 | 235 | 143 ± 39 |
| Preoperative calculated LV end-systolic volume (cm3) | 462 | 47 ± 21 | 212 | 47 ± 23 | 341 | 46 ± 20 | 234 | 46 ± 19 |
| Preoperative LV end-diastolic volume (BSA) (cm3) | 461 | 73 ± 19 | 213 | 70 ± 21 | 340 | 74 ± 20 | 234 | 72 ± 19 |
| Functional status | ||||||||
| LV ejection fraction (%) | 462 | 60 ± 4.5 | 216 | 59 ± 5.4 | 345 | 60 ± 4.5 | 237 | 59 ± 4.4 |
Patients with data available.
All patients were considered on an intent-to-treat basis to have mitral valve repair, but several were converted intraoperatively to mitral valve replacement.
Key: ASD, atrial septal defect; BSA, body surface area; COPD, chronic obstructive pulmonary disease; LA, left atrial; LV, left ventricular; NYHA, New York Heart Association; PFO, patent foramen ovale; SD, standard deviation.
Endpoints
Endpoints were 1) cost of care, expressed as robotic capital investment, maintenance of equipment, and direct technical hospital costs, and 2) benefit of care, expressed as recovery time translated into income difference.
Cost of care
Robotic costs included capital investment and fixed yearly maintenance costs for a Da Vinci Surgical System™ (Intuitive Surgical, Sunnyvale, CA). Direct technical hospital costs were obtained from Cleveland Clinic’s financial database. They were categorized into operative and postoperative costs (see eBox 1 for cost categories). Costs of robotic-specific instruments and procedure-specific disposables, such as double-lumen endotracheal tubes, external defibrillator patches, and special cannulae for cardiopulmonary bypass, were included in operative costs. Cost of any reoperation within the initial hospitalization was included as a postoperative cost. Indirect costs, including institutional indirect costs and costs of capital equipment used for all operations, could not be estimated on a per-patient basis and are not included, but they are assumed to be distributed uniformly across groups.
Benefit of care
Recovery time was assessed using a return-to-work survey (see eAppendix 1), which incorporated questions found in earlier studies to be predictive of return to work.15–23 For this study, we focused on self-reported work status, time to return to work after surgery, hours worked per week, and work-related income category before and after surgery. The survey was mailed to all 1,173 patients, from whom we received 918 responses (78%): complete sternotomy, 139 (70%); partial sternotomy, 219 (75%); anterolateral thoracotomy, 164 (73%); and robotic surgery, 396 (86%). Recovery time was translated into difference in income earned based on time to return to work. Specifically, patients were stratified by the questionnaire’s income levels: $0, $1 to $24,999, $25,000 to $49,999, $50,000 to $74,999, $75,000 to $99,999, and greater than $100,000. We queried the U.S. Internal Revenue Service database of 140 million tax returns from 2009 (most recent data available: http://www.irs.gov/pub/irs-soi/09in11si.xls, http://www.irs.gov/uac/SOI-Tax-Stats---Individual-Statistical-Tables-by-Size-of-Adjusted-Gross-Income) to estimate average income within each income level (using the $100,000 to $2 million category for the over $100,000 group, excluding only 0.04% of the U.S. population), applied these to our patients, and took the median to estimate the weekly income: $1,660. This estimated income was multiplied by the difference taken to return to work between the robotic group and the comparison groups.
Data Analysis
Analysis strategy
Patients were analyzed on an intent-to-treat basis. In the partial sternotomy group, 8 were converted to complete sternotomy; in the anterolateral thoracotomy group, 3 were converted to partial sternotomy and 3 to complete sternotomy; and in the robotic group, 19 were converted to anterolateral thoracotomy, 21 to partial sternotomy, and 13 to complete sternotomy. In the robotic group, 24 of the total of 53 patients were converted because of either small vessel size inappropriate for peripheral cannulation or difficulties in peripheral catheter placement, and 29 were converted after chest incision. A low threshold for conversion was maintained for all approaches so as not to compromise either patient safety or repair effectiveness. Patients remained in their intended procedure group for all analyses.
Propensity matching and comparisons
Characteristics of patients undergoing robotically assisted surgery differed from those of patients whose mitral valve surgery was via more conventional approaches (eTable 1). To reduce selection bias, propensity matching was used.24–26 Because our focus was on patients who underwent robotically assisted surgery, we created three propensity models: robotic surgery vs. 1) full sternotomy, 2) partial sternotomy, and 3) anterolateral thoracotomy.
We used preoperative variables (eAppendix 2) and multivariable logistic regression analysis to identify statistically significant factors associated with robotic surgery in each of the three comparisons (parsimonious models). We then added other variables representing groups of patient factors that might be related to unrecorded selection factors to create semi-saturated propensity models (see footnotes in eAppendix 2). Three propensity scores were calculated for each patient by solving the propensity models for the probability of receiving robotically assisted surgery vs. conventional approaches. Using the propensity score only, robotic cases were matched to non-robotic cases using a greedy matching strategy (eFigures 1 and 2).27 Robotic cases with propensity scores deviating more than 0.1 from those of non-robotic cases were considered unmatched.
Cost analysis
ROBOTIC COSTS
Amortization for the robot was made on a yearly case-volume basis, where fixed costs included the initial capital cost of the robot divided by an expected lifespan of 7 years, plus the yearly fixed maintenance costs. To obtain an average per-patient robotic cost, amortized robotic cost was divided by 167, the average yearly volume of robotic procedures of all types performed in cardiac surgery at Cleveland Clinic during the study time frame.
HOSPITAL DIRECT TECHNICAL COSTS
We examined three categories of cost: operative direct technical costs, postoperative direct technical costs, and the sum of these: total hospital direct technical cost. The cost ratio for each robotic/non-robotic propensity-matched pair was computed.
INCOME RELATED TO RETURN TO WORK
Although differences in income would ideally be calculated between propensity-matched pairs, response to the survey was incomplete (as previously described). In addition, some patients were retired and out of the workforce: 54 (39%) in the complete sternotomy group, 73 (33%) in the partial sternotomy group, 38 (23%) in the anterolateral thoracotomy group, and 99 (26%) in the robotic surgery group. Therefore, in subsequent calculations we used the median ratio based on income of the workforce in each group rather than the median of paired ratios. Within each matched group, the distribution of ratios for all possible pairs was also assessed to provide a measure of variability.
NET COST
Net cost was the sum of hospital direct technical costs, amortized per-patient robotic costs, and difference in median income due to return-to-work status.
CASE VOLUME–COST SIMULATION
To illustrate the relationship of per-patient net cost of robotic vs. conventional approaches to yearly case volume, we mathematically constructed curves showing how patient volume affects the net cost of robotically assisted surgery. For this, only amortized cost of the robot was varied as a function of yearly case volume, with all other components of net cost set at their median value.
Amortization of robotic costs was divided by yearly patient volume ranging from 1 to 300, rather than the 167 specific to this study. The resulting mathematically derived yearly amortization cost per patient was added to the median cost ratio of direct hospital cost and income related to return to work. Confidence limits were calculated using corresponding 15th and 85th percentile values in place of the median.
Statistical analysis
Continuous variables are summarized as mean ± standard deviation or as 15th, 50th, and 85th percentiles when data are skewed. Pairwise comparisons were made using the Wilcoxon rank-sum test, and for comparisons of more than two groups, the Kruskal-Wallis test. Categorical data are summarized by frequencies and percentages. Comparisons were made using the chi-squared or Fisher’s exact test when frequency was less than 5. All analyses were performed using SAS® statistical software (SAS version 9.1; SAS, Inc., Cary, NC). Median cost ratios are accompanied by 15th and 85th percentiles within parentheses.
RESULTS
Costs
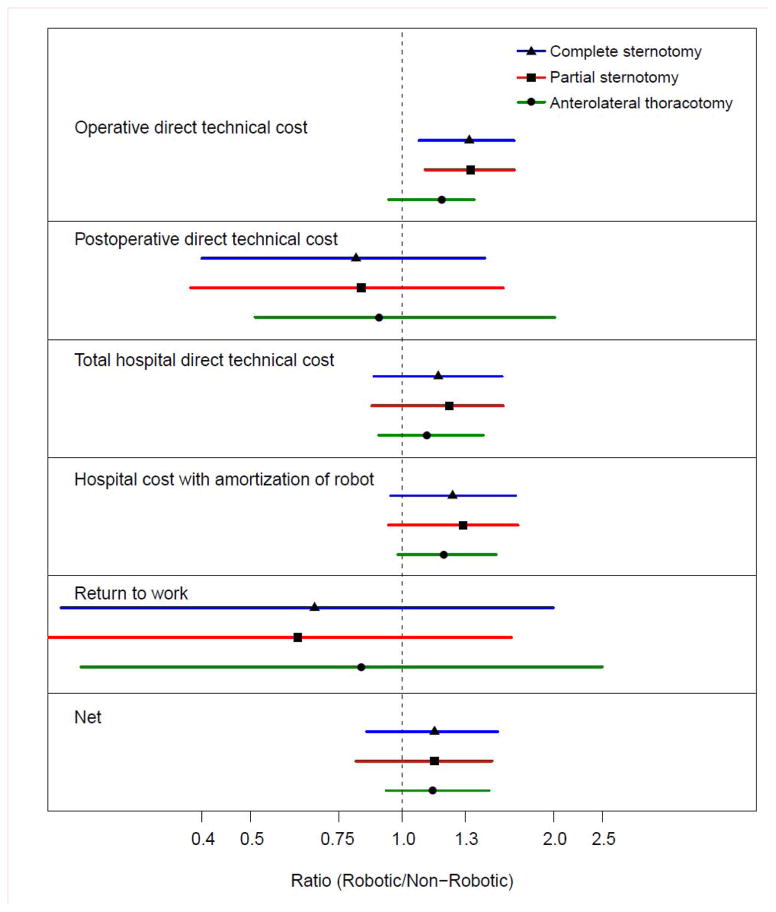
Direct technical operative costs, including specialized disposable robotic instruments, were 37%, 37%, and 20% higher for robotic mitral valve surgery than for operations performed through a complete sternotomy, partial sternotomy, or anterolateral thoracotomy, respectively (Figure 2).
Figure 2.
Forest plot of cost ratios. All cost ratios are presented as robotic/non-robotic. Each symbol denotes median cost ratio, with lines extending from 15th to 85th percentiles. Note that horizontal axis is logarithmic.
Direct technical postoperative costs were 16%, 17%, and 10% lower for robotic surgery than for these three alternatives, resulting in total direct hospital technical costs that were 18%, 24%, and 12% higher, respectively.
Total cost of care, including total hospital direct technical costs plus amortization of the capital investment and maintenance costs of the robot, were 27%, 32%, and 21% higher than for the three conventional approaches, respectively (see Figure 2).
Cost of recovery from surgery, expressed as median time from discharge to return to work (with 15th and 85th percentiles) was 35 days (19, 63) for patients undergoing robotic surgery, 49 days (21, 109) after a complete sternotomy, 56 days (30, 119) after partial sternotomy, and 42 days (18, 90) after anterolateral thoracotomy. Thus, return to work was 29%, 38%, and 17% earlier after robotic surgery than after the alternative approaches, respectively (see Figure 2).
Overall net cost of care, including operative and postoperative direct technical costs and amortized robotic costs, partially offset by difference in estimated income due to earlier return to work, was 16%, 16%, and 15% higher for robotic surgery than for the three alternatives, respectively (see Figure 2).
Case Volume–Cost Simulation
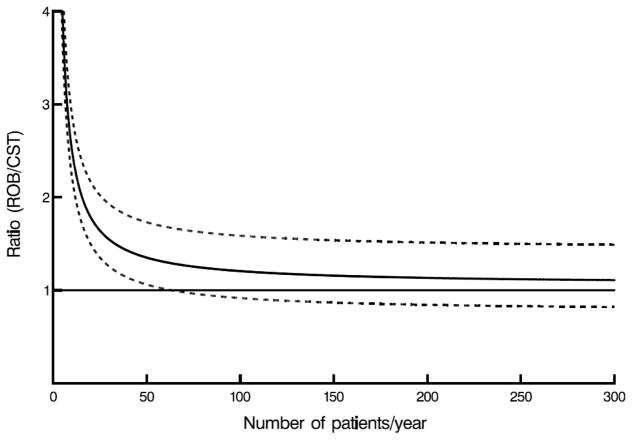
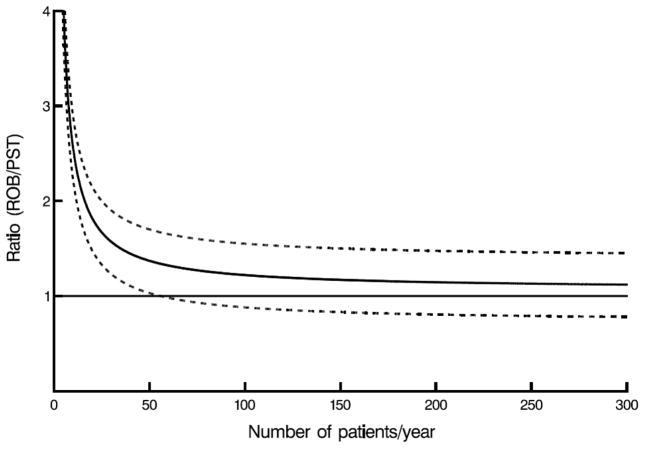
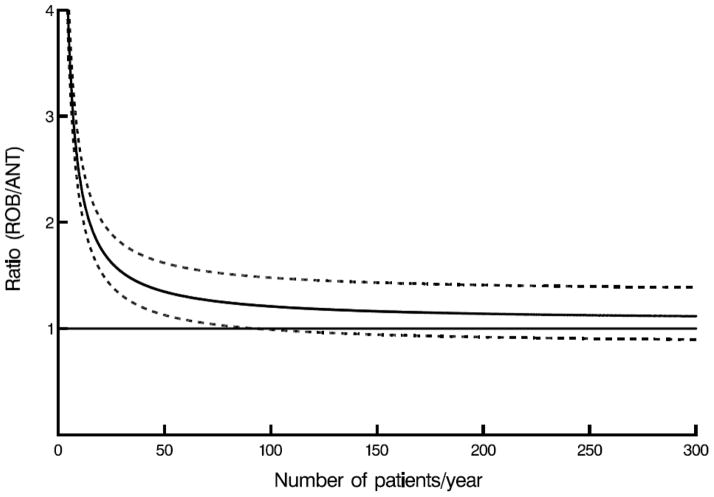
Mathematically, higher yearly case volume leads to a linear decrease in amortized costs of robotic technology. However, because other costs remain, an increase in case volume causes an exponentially decreasing cost per case, almost reaching an asymptotic yearly case volume between 55 and 100, at which point confidence limits for robotically assisted surgery for mitral valve disease overlap those of conventional approaches (Figure 3, A–C).
Figure 3.
Case volume–cost relationship. Horizontal line represents cost ratio of 1 (meaning costs are equal), solid curved line represents net cost ratio, including amortization for robotic capital investment and maintenance, direct technical hospital costs, and benefit of care translated into income difference, depending on annual number of patients for whom robotic procedures were performed. Dashed lines are confidence limits equivalent to ±1 standard error. A, Robotic (ROB) versus complete (CST) sternotomy. B, Robotic (ROB) versus partial sternotomy (PST). C, Robotic (ROB) versus anterolateral thoracotomy (ANT).
COMMENT
The principal finding of this study is that the cost of care associated with robotically assisted mitral valve repair is substantially higher than that of conventional approaches, but this cost is mitigated in large part by benefits of lesser invasiveness resulting in lower postoperative costs, shorter hospital stay, and earlier return to work. However, the value of robotically assisted mitral valve repair that is comparable to conventional approaches can only be realized in high-volume centers.
Three unique aspects of our study paint a more comprehensive picture of the introduction of new technology than generally has been the case. First, this was an intent-to-treat study design. Although such a design may seem to dilute the benefits of robotic surgery, it faithfully portrays a real-world robotic program as well as mimicks a randomized trial design. Second, it includes capital investment and maintenance costs of the robot. Few prior studies of robotically assisted mitral valve surgery have emphasized initial capital investment, which substantially increases the total cost of care for robotic procedures.28,29 Instead, studies dealing with new technologies focus on clinical results and hospital outcomes, neglecting potential downstream cost savings. Third, it considers what might be termed the social aspect of healthcare: rapidity of recovery from surgery. With increasing healthcare costs, it is important to review the introduction of new technologies from such a broad public health perspective to assess their value (benefit/cost).30 Our study takes a holistic view of patient healthcare cost by addressing the entire value proposition of a robotically assisted surgery program.
Apart from technology investment, other potential reasons behind the high operative costs we experienced include costs of additional personnel we believed were necessary for safe conduct of the procedure during its initial implementation, longer operative times, and more conversions from robotic to conventional surgery to maintain patient safety, all associated with inclusion of cases from the inception of our robotic experience. We must emphasize that this study is limited by technology that is still evolving away from large and expensive instruments. As with most advances in technology, in the future these will become smaller, more flexible, and more affordable. As surgical team efficiency and safety of the procedure improve, personnel costs, operative times, and conversions should decrease.
Strengths and Limitations
This is a single-institution study that includes only patients undergoing posterior mitral valve leaflet repair. Despite the problem of generalizing results from a single institution, this enabled a high level of detail in our analysis. In addition, in our environment, we use a surgical robot dedicated to cardiac surgery. Cost calculations could be different in a setting where the surgical robot is a shared resource for cardiac, thoracic, general, urologic, and gynecologic surgery. To compensate for selection bias in this nonrandomized observational study, we used the intent-to-treat principle and propensity scores to match patients with similar demographic and comorbidity profiles and similar extent of mitral valve disease to procedures performed contemporaneously by experienced surgeons in our institution.
A strength of our study is use of actual direct technical cost data rather than charges; however, we were unable to estimate indirect costs per patient for bricks and mortar, custodial services, security, and the like. Had these been added uniformly, the incremental relative cost of robotic surgery would be less (more favorable).
Incorporating information about recovery from surgery and its monetary transformation as we have done has several limitations. First, we used return to work as a surrogate for length of recovery in the same spirit as used in studies of the introduction of new therapies for ischemic heart disease in the 1970s. Second, it must be acknowledged that patients having a sternotomy are instructed to resume normal activities and return to work after about 6 weeks to ensure union of the sternum, whereas those undergoing operations that do not involve cutting through bone (anterolateral thoracotomy and robotic surgery) are told to resume these activities when they feel well enough to do so. Although this may introduce psychologic incentives, it also reflects real differences in healing time. Third, it translates differences in return to work into financial terms. We agree with the work of, and complied by, Murphy and Topol, showing that advances in healthcare contribute positively in often unacknowledged ways to the gross domestic product, and faster recovery and return to productive work have this social benefit apart from a clinical benefit. Fourth, we were able to obtain only ranges of individual-patient income data from our back-to-work questionnaire. Thus, we used reported income ranges and census data to approximate median income.
Clinical Implications
The major determinant of invasiveness in cardiac surgery is often not the size of the incision needed to perform the intracardiac procedure, but the size needed to expose the heart. Another determinant of incision size, and thus invasiveness, is the space needed to use the instruments for the procedure. Robotic instrumentation is a positive disruptive technology that minimizes invasiveness by affecting both these determinants. The often superior quality of visualization with robotic assistance is delivered through sophisticated three-dimensional technology. Small-caliber articulated instruments allow conduct of highly complex operations while being inserted through small port-like incisions. The overall result is uncompromised quality, accuracy, and precision of surgical manipulation. We envision that further refinement of robotic technology will lead to its wider adoption in cardiac surgery.
Evaluation of the value of new technology during its early phase of introduction into medical practice must consider not only its current value, but also future applications that inevitably follow technical accomplishments that increase its value. There are numerous examples of new technology implementation in surgery that encountered early economic obstacles, but then followed a path to wider adoption and application. Laparoscopic procedures in general surgery and gynecology, and thorascopic procedures in thoracic surgery, are now used more commonly than open approaches; however, each had to prove its value prior to wider implementation. Consider laparoscopic cholecystectomy, now performed in more than 90% of cases. Early literature suggested higher costs due to longer operative times, the learning curve, and the cost of disposable instruments,31 yet later literature established its higher value.32–35 Introduction of video-assisted thoracic surgery followed a similar path.36–39 We anticipate that implementation of robotic technology in cardiac surgery will progress in the same fashion, with a transition to newer and technologically more advanced procedures.
Conclusion
Robotically assisted mitral valve surgery offers the clinical benefit of the least invasive surgery, lowest postoperative cost, and fastest return to work in exchange for higher procedural costs. However, the maximum value of robotic mitral valve surgery can only be realized in high-volume centers.
Supplementary Material
Acknowledgments
Funding Support
This study was funded in part by the Cleveland Clinic Department of Thoracic and Cardiovascular Surgery and the Kenneth Gee and Paula Shaw, PhD, Chair in Heart Research (Dr. Blackstone); the Donna and Ken Lewis Chair in Cardiothoracic Surgery, and the Peter Boyle Research Fund (Dr. Mihaljevic).
Role of the Sponsors
The study sponsors had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the study.
Footnotes
public responsibility for part of the content
public responsibility for the whole of the content
conception and design of the study
acquisition of data
analysis and interpretation of data
drafting of manuscript
critical revision of manuscript for important intellectual content
statistical analysis
obtaining funding
administrative, technical, or material support
supervision
no additional contributions
Institutional Review Board Approval
Preoperative and operative variables were retrieved from the Cleveland Clinic Cardiovascular Information Registry, an ongoing, prospective, concurrent registry of all cardiac operations maintained concurrently with patient care, review of medical records, and back-to-work questionnaire. All data used in this study have been approved for use in research by the Institutional Review Board (IRB), with patient consent waived.
Disclosures
Dr. Gillinov has served as a consultant to Intuitive Surgical, Edwards Lifesciences, St. Jude Medical, Medtronic, Abbott, and On-X. None of the other authors has a conflict of interest regarding the material reported in this study.
Data Access and Responsibility
Sarah J. Williams and Eugene H. Blackstone had full access to all the data in the study and take responsibility for integrity of the data and accuracy of the data analysis.
Additional Contributions
Dr. A. Oberman at the University of Alabama at Birmingham helped develop the return-to-work survey.
Penny Houghtaling, MS, performed parts of the statistical analysis and verified all numeric data in the manuscript.
References
- 1.Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg. 2011 Jan;141(1):72–80. e71–74. doi: 10.1016/j.jtcvs.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Suri RM, Burkhart HM, Rehfeldt KH, et al. Robotic mitral valve repair for all categories of leaflet prolapse: improving patient appeal and advancing standard of care. Mayo Clin Proc. 2011 Sep;86(9):838–844. doi: 10.4065/mcp.2010.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy DA, Miller JS, Langford DA, Snyder AB. Endoscopic robotic mitral valve surgery. J Thorac Cardiovasc Surg. 2006 Oct;132(4):776–781. doi: 10.1016/j.jtcvs.2006.04.052. [DOI] [PubMed] [Google Scholar]
- 4.Nifong LW, Chitwood WR, Pappas PS, et al. Robotic mitral valve surgery: a United States multicenter trial. J Thorac Cardiovasc Surg. 2005 Jun;129(6):1395–1404. doi: 10.1016/j.jtcvs.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 5.Nifong LW, Rodriguez E, Chitwood WR., Jr 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann Thorac Surg. 2012 Jul;94(1):38–42. doi: 10.1016/j.athoracsur.2011.11.036. discussion 43. [DOI] [PubMed] [Google Scholar]
- 6.Porter ME. What is value in health care? N Engl J Med. 2010 Dec 23;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 7.Navia JL, Cosgrove DM., 3rd Minimally invasive mitral valve operations. Ann Thorac Surg. 1996 Nov;62(5):1542–1544. doi: 10.1016/0003-4975(96)00779-5. [DOI] [PubMed] [Google Scholar]
- 8.Cosgrove DM, 3rd, Sabik JF, Navia JL. Minimally invasive valve operations. Ann Thorac Surg. 1998 Jun;65(6):1535–1538. doi: 10.1016/s0003-4975(98)00300-2. discussion 1538–1539. [DOI] [PubMed] [Google Scholar]
- 9.Konertz W, Waldenberger F, Schmutzler M, Ritter J, Liu J. Minimal access valve surgery through superior partial sternotomy: a preliminary study. J Heart Valve Dis. 1996 Nov;5(6):638–640. [PubMed] [Google Scholar]
- 10.Carpentier A, Loulmet D, Le Bret E, Haugades B, Dassier P, Guibourt P. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. C R Acad Sci III. 1996 Mar;319(3):219–223. [PubMed] [Google Scholar]
- 11.Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg. 1997 Oct;226(4):421–426. doi: 10.1097/00000658-199710000-00003. discussion 427–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer FC, Galloway AC, Grossi EA, et al. Recent developments and evolving techniques of mitral valve reconstruction. Ann Thorac Surg. 1998 Feb;65(2):307–313. [PubMed] [Google Scholar]
- 13.Carpentier A, Loulmet D, Aupecle B, et al. Computer assisted open heart surgery. First case operated on with success. C R Acad Sci III. 1998 May;321(5):437–442. doi: 10.1016/s0764-4469(98)80309-0. [DOI] [PubMed] [Google Scholar]
- 14.Chitwood WR, Jr, Nifong LW, Elbeery JE, et al. Robotic mitral valve repair: trapezoidal resection and prosthetic annuloplasty with the da vinci surgical system. J Thorac Cardiovasc Surg. 2000 Dec;120(6):1171–1172. doi: 10.1067/mtc.2000.110177. [DOI] [PubMed] [Google Scholar]
- 15.Barnes GK, Ray MJ, Oberman A, Kouchoukos NT. Changes in working status of patients following coronary bypass surgery. Jama. 1977 Sep 19;238(12):1259–1262. [PubMed] [Google Scholar]
- 16.Anderson AJ, Barboriak JJ, Hoffmann RG, Mullen DC. Retention or resumption of employment after aortocoronary bypass operations. Jama. 1980 Feb 8;243(6):543–545. [PubMed] [Google Scholar]
- 17.Boulay FM, David PP, Bourassa MG. Strategies for improving the work status of patients after coronary artery bypass surgery. Circulation. 1982 Nov;66(5 Pt 2):III43–49. [PubMed] [Google Scholar]
- 18.Oberman A, Wayne JB, Kouchoukos NT, Charles ED, Russell RO, Jr, Rogers WJ. Employment status after coronary artery bypass surgery. Circulation. 1982 Jun;65(7 Pt 2):115–119. doi: 10.1161/01.cir.65.7.115. [DOI] [PubMed] [Google Scholar]
- 19.Coronary artery surgery study (CASS): a randomized trial of coronary artery bypass surgery. Quality of life in patients randomly assigned to treatment groups. Circulation. 1983 Nov;68(5):951–960. doi: 10.1161/01.cir.68.5.951. [DOI] [PubMed] [Google Scholar]
- 20.Varnauskas E. Survival, myocardial infarction, and employment status in a prospective randomized study of coronary bypass surgery. Circulation. 1985 Dec;72(6 Pt 2):V90–101. [PubMed] [Google Scholar]
- 21.Booth DC, Deupree RH, Hultgren HN, DeMaria AN, Scott SM, Luchi RJ. Quality of life after bypass surgery for unstable angina. 5-year follow-up results of a Veterans Affairs Cooperative Study. Circulation. 1991 Jan;83(1):87–95. doi: 10.1161/01.cir.83.1.87. [DOI] [PubMed] [Google Scholar]
- 22.Bradshaw PJ, Jamrozik K, Gilfillan IS, Thompson PL. Return to work after coronary artery bypass surgery in a population of long-term survivors. Heart Lung Circ. 2005 Sep;14(3):191–196. doi: 10.1016/j.hlc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Ellis JJ, Eagle KA, Kline-Rogers EM, Erickson SR. Perceived work performance of patients who experienced an acute coronary syndrome event. Cardiology. 2005;104(3):120–126. doi: 10.1159/000087410. [DOI] [PubMed] [Google Scholar]
- 24.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med. 2007 Jan 15;26(1):20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 26.Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg. 2002 Jan;123(1):8–15. doi: 10.1067/mtc.2002.120329. [DOI] [PubMed] [Google Scholar]
- 27.Bergstralh EJ, Konsanke JL. Technical report No 56. Department of Health Science Research; Rochester, MN: Mayo Clinic; 1995. Computerized matching of cases to controls. [Google Scholar]
- 28.Morgan JA, Thornton BA, Peacock JC, et al. Does robotic technology make minimally invasive cardiac surgery too expensive? A hospital cost analysis of robotic and conventional techniques. J Card Surg. 2005 May-Jun;20(3):246–251. doi: 10.1111/j.1540-8191.2005.200385.x. [DOI] [PubMed] [Google Scholar]
- 29.Kam JK, Cooray SD, Smith JA, Almeida AA. A cost-analysis study of robotic versus conventional mitral valve repair. Heart Lung Circ. 2010 Jul;19(7):413–418. doi: 10.1016/j.hlc.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Murphy KM, Topel RH. Measuring the gains from medical research: an economic approach. Chicago: University of Chicago Press; 2003. [Google Scholar]
- 31.Stoker ME, Vose J, O’Mara P, Maini BS. Laparoscopic cholecystectomy. A clinical and financial analysis of 280 operations. Arch Surg. 1992 May;127(5):589–594. doi: 10.1001/archsurg.1992.01420050117015. discussion 594–585. [DOI] [PubMed] [Google Scholar]
- 32.Fisher KS, Reddick EJ, Olsen DO. Laparoscopic cholecystectomy: cost analysis. Surg Laparosc Endosc. 1991 Jun;1(2):77–81. [PubMed] [Google Scholar]
- 33.Fajardo R, Valenzuela JI, Olaya SC, et al. Cost-effectiveness of laparoscopic versus open cholecystectomy. Biomedica. 2011 Oct-Dec;31(4):514–524. doi: 10.1590/S0120-41572011000400006. [DOI] [PubMed] [Google Scholar]
- 34.Zacks SL, Sandler RS, Rutledge R, Brown RS., Jr A population-based cohort study comparing laparoscopic cholecystectomy and open cholecystectomy. Am J Gastroenterol. 2002 Feb;97(2):334–340. doi: 10.1111/j.1572-0241.2002.05466.x. [DOI] [PubMed] [Google Scholar]
- 35.McIntyre RC, Jr, Zoeter MA, Weil KC, Cohen MM. A comparison of outcome and cost of open vs. laparoscopic cholecystectomy. J Laparoendosc Surg. 1992 Jun;2(3):143–148. doi: 10.1089/lps.1992.2.143. discussion 149. [DOI] [PubMed] [Google Scholar]
- 36.Miller JI., Jr The present role and future considerations of video-assisted thoracoscopy in general thoracic surgery. Ann Thorac Surg. 1993 Sep;56(3):804–806. doi: 10.1016/0003-4975(93)90986-r. [DOI] [PubMed] [Google Scholar]
- 37.Bhatnagar NK. The impact of video assisted thoracoscopic surgery (VATS) Respir Med. 1994 Jul;88(6):403–406. doi: 10.1016/s0954-6111(05)80041-1. [DOI] [PubMed] [Google Scholar]
- 38.Molin LJ, Steinberg JB, Lanza LA. VATS increases costs in patients undergoing lung biopsy for interstitial lung disease. Ann Thorac Surg. 1994 Dec;58(6):1595–1598. doi: 10.1016/0003-4975(94)91638-1. [DOI] [PubMed] [Google Scholar]
- 39.Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg. 2012 Apr;93(4):1027–1032. doi: 10.1016/j.athoracsur.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Breiman L. Bagging predictors. Machine Learning. 1996;24:123–140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.