Abstract
Background
Several clinical risk factors for death and heart transplantation have been identified in patients with Fontan circulation. It is unknown whether cardiac magnetic resonance (CMR) measurements of ventricular size and function are independently associated with these outcomes and further improve risk stratification.
Methods and Results
Data on Fontan patients who had a CMR study from 1/2002 to 1/2011 were retrospectively reviewed. The endpoint was time to death or listing for heart transplantation after the CMR study. The median age of the 215 patients was 18.3 years [25th, 75th percentiles: 14, 26] with a median age at Fontan of 3.6 years [2.3, 7.1]. Over a median post-CMR follow-up period of 4.1 years [2.6, 6.2], 24 patients (11%) reached the endpoint: 20 deaths, 3 transplants, and 1 transplant listing. In a multivariable Cox regression model with clinical parameters only, protein losing enteropathy (PLE) was associated with transplant-free survival. A multivariable model including clinical and CMR parameters showed that in addition to PLE, ventricular end-diastolic volume (EDVi) >125 mL/BSA1.3 was associated with transplant-free survival. A likelihood-ratio test comparing the 2 models showed that the addition of EDVi resulted in a significantly improved endpoint prediction (P<0.001) — C-index increased from 0.63 to 0.79.
Conclusions
CMR-derived ventricular EDVi is an independent predictor of transplant-free survival late after the Fontan operation and adds incremental value over clinical symptoms alone for risk stratification.
Keywords: Fontan procedure, heart defects, congenital, magnetic resonance imaging
Patients with functional single ventricle (FSV) congenital heart disease comprise a complex and heterogeneous population that is usually palliated with the Fontan procedure. Despite important improvements in both mortality and morbidity among young patients,1 adverse outcomes become increasingly frequent as patients approach adulthood.2, 3 Several risk factors such as protein-losing enteropathy (PLE) and decreased exercise parameters are associated with death and heart transplantation in this population.4, 5 Ventricular size and function are also thought to be clinically important; however, there are few studies that support their association with important outcomes.2, 3, 6 Indeed, most publications on FSV size and function in this population have used surrogate outcomes.1, 7–10 Because of the technical limitations of echocardiography in these patients, CMR remains the preferred modality for the assessment of ventricular size and function.11 Accordingly, the goal of this study was to determine whether CMR measurements of ventricular size and function improve risk stratification for transplant-free survival in patients late after the Fontan operation.
Methods
Patients
A database search identified all patients who underwent a Fontan operation, had a postoperative CMR study at Boston Children’s Hospital between January 2002 and January 2011, and had a minimum of 1 year of follow-up or reached the defined endpoint after CMR. Exclusion criteria were inability to measure ventricular size and function due to technically inadequate data and lack of clinical follow-up information. The Boston Children’s Hospital Committee on Clinical Investigation approved this retrospective study and waived the requirement for informed consent.
Clinical Parameters
Demographic and clinical data, including underlying diagnoses and type of FSV based on ventricular dominance, were abstracted from the medical records. The type of surgical palliation was classified as lateral tunnel, right atrium-to-pulmonary artery anastomosis, right atrium-to-right ventricle connection, or extracardiac conduit. Additional parameters included age at Fontan, time from Fontan to CMR, length of post-CMR follow-up, history of Fontan revision, and number and type of surgical and catheterization interventions prior to CMR, including age at volume unloading surgery (e.g., bidirectional Glenn). Arrhythmia history was compiled by review of Holter monitors, electrocardiograms, electrophysiology catheterizations, and clinic notes. Episodes of atrial ectopy, atrial fibrillation, atrial flutter, supraventricular tachycardia, ventricular ectopy, nonsustained ventricular tachycardia (3 or more beats lasting less than 30 seconds), sustained ventricular tachycardia (lasting ≥ 30 seconds), and arrhythmia-related cardiac arrest were recorded, along with their temporal relation to the CMR study. Other relevant clinical variables included history of congestive heart failure (CHF) (defined as New York Heart Association class II or greater), PLE, stroke, thrombus, seizures, liver disease, or pacemaker or defibrillator placement.
Clinical Endpoints
The primary endpoint was time to all-cause mortality, listing for cardiac transplantation, or receiving a heart transplant. Dates of listing for cardiac transplantation, transplantation, or death were confirmed against the New England Organ Bank and the Social Security Death Index databases. For survival analyses, follow-up was measured from date of CMR to either first occurrence of the endpoint or last known follow-up date with documented transplant-free survival.
CMR Techniques
CMR studies were performed with 1.5 Tesla scanners (GE Medical Systems, Milwaukee, Wisconsin, and Philips Healthcare, Best, the Netherlands). The details of the CMR protocols used in our laboratory for assessment of patients after the Fontan operation have been published.12–14 Briefly, ventricular assessment was performed by an electrocardiographically-gated, steady-state free precession cine CMR in vertical and horizontal ventricular long-axis planes, and a stack of slices in a ventricular short-axis plane encompassing the atrioventricular junction through the cardiac apex. Assessment for the presence of late gadolinium enhancement utilized a protocol with an inversion-recovery prepared, phase sensitive, ECG-triggered, breath-hold segmented fast gradient echo pulse sequence in the ventricular long- and short-axis planes. Imaging was performed approximately 15 minutes after injection of 0.2 mmol/kg gadopentetate dimeglumine (Magnevist, Bayer HealthCare, Tarrytown, New York).14
CMR Data Analysis
If a patient had multiple CMR studies, the most recent study was used for analysis. Ventricular volumes and function were measured by manual tracing of endocardial and epicardial borders on each short-axis steady-state free precession cine slice at end-diastole (maximal area) and end-systole (minimal area) as previously described.12 Analysis was performed using commercially available software (QMass, Medis Medical Imaging Systems, Leiden, the Netherlands). Simpson’s method was applied to calculate end-diastolic volume (EDV), end-systolic volume (ESV), ejection fraction, stroke volume (SV), ventricular mass, and mass-to-volume ratio. Ventricular morphology was classified as left ventricular, right ventricular, or biventricular (e.g., unbalanced atrioventricular canal), according to previously published criteria.1 Ventricular type was classified as biventricular if both ventricles had an end-diastolic volume z-score >-4. Published normative data from Alfakih et al. was used for ventricular z-score calculations.15 Regardless of the dominant ventricular morphology type, when 2 ventricles contributed to the systemic circulation, ventricular volumes and mass were summated to allow for calculation of total functional EDV, ESV, ejection fraction, SV, mass, and mass-to-volume ratio. In order to better account for differences in body size, EDV, ESV, SV, and ventricular mass were indexed to body surface area (BSA) raised to the 1.3 power.16 This method was selected based on studies showing that volumetric parameters are best adjusted to BSA raised to the 1.3–1.4 power.16–18 These studies have demonstrated a nonlinear relationship between ventricular volumes and BSA, a relationship that is primarily influenced by a change in heart rate as a function of age. Although commonly used in clinical studies in adult patients, indexing ventricular volumes to BSA alone fails to account for the variance across a population that includes pediatric patients.16–18 Analyses were repeated indexing to BSA alone to ensure the indexing strategy did not substantially change results. To facilitate comparison with other studies, results are also reported as indexed to BSA. For patients who had the myocardial delayed enhancement imaging technique performed, studies were reviewed for the presence of late gadolinium enhancement as previously described.14 Assessment of atrioventricular and semilunar valvar regurgitation by phase contrast imaging was performed using QFlow (Medis Medical Imaging Systems, Leiden, the Netherlands).19
Exercise Testing
Metabolic exercise testing data were included if the study occurred within 1 year of the CMR and if the patient reached maximal aerobic effort. Maximal aerobic effort was defined as a respiratory exchange ratio of ≥1.09 or achieving ≥ 75% of predicted heart rate for age and gender.20 Studies with submaximal aerobic effort were excluded from the analysis to eliminate bias from non-cardiac factors. The following parameters were recorded: work rate, relative oxygen consumption, oxygen consumption at ventilatory anaerobic threshold, oxygen pulse, heart rate, respiratory rate, blood pressure, ventilatory exchange ratio, and breathing reserve.
Statistical Analysis
Categorical data were summarized using frequencies and percentages, and compared between those who reached the endpoint and those who did not using a Fisher’s exact test. Continuous variables were summarized as median [25th, 75th percentiles] or mean ± SD, as appropriate, and were compared using a Mann-Whitney U test. A multivariable Cox proportional hazards survival model with forward stepwise selection was used to identify independent risk factors for the endpoint. The analysis included the entire study group. Bootstrap resampling with a sample size of 215 and 20,000 repetitions was used to estimate standard errors for hazard ratios and P values. Candidate predictor variables were initially considered for inclusion if P<0.2 in univariable analysis; P<0.05 was required for retention in the final model. Likelihood ratio statistics were used to compare models with clinical history variables only and those with clinical history and CMR variables. Harrell’s C-index was used to quantify how well each model discriminated between patients who experienced the endpoint and those who did not. If continuous variables were independently associated with the primary endpoint, cut points were calculated that allowed maximal discrimination by the C-index in order to improve interpretation of the data. Cumulative survival functions were constructed with Kaplan-Meier estimates for categorical variables included in the final Cox regression model, and event times were compared by the log-rank test.
All statistical tests were 2-sided and results were considered statistically significant if P<0.05. Data analysis was performed using SPSS version 21.0 (SPSS Inc, Chicago, IL).
Statement of Responsibility
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Patient Characteristics
During the study period, 261 patients who had undergone a Fontan operation had a total of 437 CMR examinations. Of these, 39 (15%) patients did not have quantitative assessment of ventricular size and function predominately because of metallic artifacts from stainless steel coils or other ferromagnetic implanted devices. An additional 7 patients were unable to complete the examination due to anxiety. The remaining 215 patients comprised the study cohort, and their most recent CMR examination was used for analysis. The demographic, clinical, surgical, and outcomes of the excluded patients were similar to those of the study group, except that the included patients had a longer time since their Fontan (14.6 [9.5, 19.3] vs. 11.2 [7.7, 16.1] years, P=0.004), and more commonly had right ventricular morphology (56% vs. 31%, P=0.006) and heterotaxy syndrome (22% vs. 9%, P=0.02).
Table 1 describes the demographic and clinical characteristics of the study cohort. Most notably, the patients had a median age of 18.3 [14, 26] years at the time of CMR and a median interval between Fontan surgery and CMR of 14.6 [10, 19] years. The lateral tunnel was the most common type of Fontan operation (70%).
Table 1.
Demographic and Clinical Characteristics
| All Patients (n=215) | Transplant Free Survival (n=191) | Death or Transplant (n=24) | P Value | |
|---|---|---|---|---|
| Gender (male) | 136 (63%) | 119 (62%) | 17 (71%) | 0.50 |
| Age at Fontan (years) | 3.6 [2.3, 7.1] | 3.5 [2.3, 6.6] | 4.2 [2.3, 13.0] | 0.31 |
| Age at CMR (years) | 18.3 [14, 26] | 18.3 [14, 26] | 18.1 [8, 33] | 0.98 |
| Time from Fontan to CMR (years) | 14.6 [10, 19] | 14.7 [10, 19] | 13.4 [5, 21] | 0.65 |
| Follow-up time after CMR (years) | 4.1 [2.6, 6.2] | 4.3 [2.7, 6.4] | 2.5 [1.1, 4.5] | <0.001 |
| Body surface area at CMR (m2) | 1.6 [1.4, 1.9] | 1.6 [1.4, 1.9] | 1.7 [1.0, 2.0] | 0.93 |
| Cardiac Diagnosis | 0.27 | |||
| Tricuspid atresia | 52 (24%) | 45 (24%) | 7 (29%) | |
| Double-inlet left ventricle | 36 (17%) | 35 (18%) | 1 (4%) | |
| HLHS | 34 (16%) | 26 (14%) | 8 (34%) | |
| Unbalanced AV canal | 21 (10%) | 18 (9%) | 3 (13%) | |
| Double-outlet right ventricle | 20 (9%) | 19 (10%) | 1 (4%) | |
| Complex 2 ventricle | 17 (8%) | 16 (8%) | 1 (4%) | |
| Hypoplastic TV/RV | 16 (7%) | 15 (8%) | 1 (4%) | |
| Pulmonary atresia/IVS | 10 (5%) | 9 (5%) | 1 (4%) | |
| Mitral atresia | 9 (4%) | 8 (4%) | 1 (4%) | |
| Heterotaxy | 20 (9%) | 17 (9%) | 3 (13%) | 0.47 |
| Ventricular morphology | 0.10 | |||
| Left ventricle | 96 (45%) | 90 (47%) | 6 (25%) | |
| Right ventricle | 66 (30%) | 55 (29%) | 11 (46%) | |
| Biventricular | 53 (25%) | 46 (24%) | 7 (29%) | |
| Surgical history | ||||
| Neonatal surgery | 108 (50%) | 99 (52%) | 9 (38%) | 0.20 |
| Bidirectional Glenn | 112 (52%) | 97 (51%) | 15 (63%) | 0.39 |
| Fontan revision (post CMR) | 19 (9%) | 15 (8%) | 4 (17%) | 0.24 |
| Fontan Type | 0.001 | |||
| Lateral tunnel | 151 (70%) | 140 (73%) | 11 (46%) | |
| RA-Pulmonary artery | 44 (21%) | 38 (20%) | 6 (25%) | |
| Extracardiac | 11 (5%) | 8 (4%) | 3 (12%) | |
| RA-RV | 9 (4%) | 5 (3%) | 4 (17%) | |
| Morbidity | ||||
| Liver disease | 34 (16%) | 25 (13%) | 9 (38%) | 0.005 |
| Congestive heart failure | 60 (28%) | 41 (22%) | 19 (79%) | <0.001 |
| Thrombus | 32 (15%) | 24 (13%) | 8 (33%) | 0.01 |
| Seizures | 22 (10%) | 19 (10%) | 3 (13%) | 0.72 |
| Stroke | 41 (19%) | 35 (18%) | 6 (25%) | 0.42 |
| Protein losing enteropathy | 14 (7%) | 6 (3%) | 8 (33%) | <0.001 |
| Atrial flutter/fibrillation | 65 (30%) | 53 (28%) | 12 (50%) | 0.03 |
| Nonsustained VT | 35 (16%) | 28 (15%) | 7 (29%) | 0.08 |
| Sustained VT | 8 (4%) | 5 (3%) | 3 (13%) | 0.05 |
| Resuscitated cardiac arrest | 2 (1%) | 1 (1%) | 1 (4%) | 0.21 |
| Defibrillator (post CMR) | 4 (2%) | 3 (2%) | 1 (4%) | 0.38 |
Values are expressed as n (%) or median [25th, 75th percentile].
Abbreviations: CMR = cardiac magnetic resonance; HLHS = hypoplastic left heart syndrome; IVS = intact ventricular septum; RA = right atrium; RV = right ventricle; TV = tricuspid valve; VT = ventricular tachycardia.
Death and Transplantation
Over a median follow-up period of 4.1 years [2.6, 6.2] after CMR, 24 of the 215 patients (11%) reached an endpoint: death (n=20), heart transplant (n=3), and heart transplant listing (n=1). Causes of death were heart failure (n=10), presumed arrhythmogenic cardiac arrest (n=5), complications immediately following surgical procedures (n=3), and complications after heart transplantation (n=2). For the entire cohort, cumulative freedom from the endpoint was 97% at 1 year and 87% at 5 years.
CMR Data
Table 2 compares the CMR parameters between patients with and without the endpoint. LGE data was available in 132 patients (61%), and of those, 43 (33%) had positive LGE within the ventricular myocardium. Positive LGE was not associated with the endpoint by univariate analysis (P=0.18) but was associated with a higher EDVi (104 [88,144] vs. 91 [73,108] mL/BSA1.3, P=0.001), ESVi (53 [40, 75] vs. 39 [30, 48] mL/BSA1.3, P<0.001), and massi (63 [47, 87] vs. 50 [40, 62] g/BSA1.3, P<0.001), and a lower EF (51% [41, 55] vs. 55% [50, 61], P=0.001). Similarly, heart rate at CMR was not associated with the endpoint by univariate analysis (P=0.25).
Table 2.
Comparison of CMR Parameters between Patients With and Without the Endpoint
| All Patients (n=215) | Transplant Free Survival (n=191) | Death or Transplant (n=24) | P Value | |
|---|---|---|---|---|
| Heart rate at CMR | 79 [66, 91] | 77 [65, 91] | 83 [66, 96] | 0.25 |
| EDVi (mL/BSA1.3) | 94 [76, 115] | 93 [75, 113] | 131 [90, 166] | 0.001 |
| EDVi (mL/BSA) | 107 [87, 130] | 104 [86, 127] | 128 [106, 182] | 0.001 |
| ESVi (mL/BSA1.3) | 42 [32, 57] | 41 [31, 54] | 55 [36, 94] | 0.007 |
| ESVi (mL/BSA) | 48 [36, 64] | 46 [36, 62] | 64 [39, 104] | 0.01 |
| SVi (mL/BSA1.3) | 50 [42, 60] | 50 [42, 57] | 63 [47, 81] | 0.008 |
| SVi (mL/BSA) | 57 [48, 67] | 56 [48, 65] | 65 [57, 85] | 0.007 |
| Ejection fraction (%) | 55 [47, 61] | 55 [48, 60] | 53 [38, 63] | 0.26 |
| Ejection fraction < 40% | 24 (11%) | 16 (8%) | 8 (33%) | 0.002 |
| Massi (g/BSA1.3) | 55 [43, 68] | 52 [43, 65] | 75 [56, 103] | 0.001 |
| Massi (g/BSA) | 60 [48, 81] | 58 [48, 77] | 84 [60, 104] | 0.002 |
| Mass/volume ratio (g/mL) | 0.57 [0.48, 0.70] | 0.57 [0.48, 0.70] | 0.55 [0.46, 0.73] | 0.62 |
| ≥ Moderate AVVR | 25 (12%) | 21 (11%) | 4 (17%) | 0.50 |
| ≥ Moderate SLVR | 10 (5%) | 8 (4%) | 2 (8%) | 0.31 |
| Positive LGE* | 43 (33%) | 35 (30%) | 8 (47%) | 0.18 |
Values are expressed as median [25th, 75th percentile] or n (%).
Subgroup analysis with 132 patients.
Abbreviations: AVVR = atrioventricular valve regurgitation; BSA = body surface area; EDV = end-diastolic volume; ESV = end-systolic volume; LGE = late gadolinium enhancement; SLVR = semilunar valve regurgitation; SV = stroke volume.
Exercise Testing
Of the 215 study patients, 103 (48%) had a metabolic exercise test within 1 year of their CMR study in which they achieved maximal aerobic capacity. Their exercise test results are shown in Table 3. In this group, 10 patients (10%) reached the endpoint. The data of all patients and a comparison between those with and without the endpoint are shown in Table 3. Lower peak oxygen consumption, relative oxygen consumption at ventilatory anaerobic threshold, and peak work rate were associated with the endpoint. The small number of endpoint events precluded multivariable analysis.
Table 3.
Exercise Testing Data
| Characteristic | All Patients (n=103) | Transplant Free Survival (n=93) | Death or Transplant (n=10) | P Value |
|---|---|---|---|---|
| Peak VO2 (ml/kg/min) | 23 [17, 26] | 23 [18, 28] | 15 [14, 18] | 0.001 |
| % Predicted Peak VO2 | 60 [49, 68] | 62 [52, 70] | 39 [36, 51] | <0.001 |
| VO2 at VAT (ml/kg/min) | 13 [10, 16] | 13 [10, 17] | 8 [7, 11] | <0.001 |
| % Predicted VO2 at VAT | 34 [30, 41] | 35 [32, 42] | 26 [19, 30] | <0.001 |
| Peak work rate (W) | 112 [84, 148] | 116 [89, 150] | 76 [59, 105] | 0.07 |
| % Predicted work rate | 53 [65, 74] | 65 [54, 74] | 42 [30, 66] | 0.001 |
| Peak O2 pulse index (ml O2/beat/BSA) | 9 [7, 11] | 9 [7, 11] | 10 [8, 10] | 0.87 |
| % Predicted peak O2 pulse | 74 [64, 86] | 75 [65, 87] | 67 [54, 85] | 0.21 |
Values are expressed as median [25th, 75th percentile].
Abbreviations: % Predicted = percent of predicted for age and gender, BSA = body surface index, VAT = ventilatory anaerobic threshold, VO2 = oxygen consumption.
Predictors of Death or Transplant
Table 1 compares the patient characteristics and clinical variables between those with and without the endpoint. Ventricular morphology, Fontan type, history of liver disease, CHF, thrombus, PLE, atrial fibrillation or flutter, and sustained ventricular tachycardia were all associated with the endpoint by univariate analysis. Table 2 compares CMR parameters between those with and without the endpoint. Patients with the endpoint had higher EDVi, ESVi, SVi, and massi by univariate analysis. Ventricular ejection fraction was not associated with the endpoint when analyzed as a continuous variable; however, an ejection fraction < 40% was associated with the endpoint (P=0.002).
Table 4 shows several multivariable Cox proportional hazard models with bootstrapping resampling for predicting the endpoint. The first model (C-index 0.75) was restricted to CMR parameters that were significantly associated with the endpoint in univariate analysis. The second model (C-index 0.63) was restricted to clinical variables that were significantly associated with the endpoint by univariate analysis. A third model (C-index 0.80) considered all significant clinical and CMR parameters, and showed that PLE and higher EDVi independently predicted death or transplant. The addition of CMR-measured EDVi, to a model with clinical parameters increased the model C-index from 0.63 to 0.80, with a statistically significant improvement in the model (P<0.001). To facilitate interpretation of the model and its practical use, an EDVi cutoff value of >125 mL/BSA1.3 was found to have the highest discrimination, with a C-index to 0.79. Comparison of the multivariable Cox proportional hazard model including clinical and CMR parameters with a dichotomized EDVi also showed a significant improvement in the model (P<0.001).
Table 4.
Independent Risk Factors for Death or Need for Transplant*
| Predictor | Hazard Ratio | 95% CI | P Value | C-index* |
|---|---|---|---|---|
| CMR variables only | 0.75 | |||
| EDVi (per 10 mL/BSA1.3) | 1.10 | 1.04 to 1.17 | 0.002 | |
| Clinical history variables only† ‡ | 0.63 | |||
| PLE | 3.5 | 1.5 to 8.5 | 0.005 | |
| Clinical history and CMR variables† | 0.80 | |||
| PLE | 8.5 | 1.9 to 38.2 | 0.005 | |
| EDVi (per 10 mL/BSA1.3 increase) | 1.12 | 1.05 to 1.19 | 0.001 | |
| Clinical history and CMR variables‡ | 0.79 | |||
| PLE | 7.8 | 1.1 to 57.1 | 0.04 | |
| EDVi > 125 mL/BSA1.3 | 7.7 | 2.8 to 21.1 | <0.001 |
Multivariable Cox proportional hazards survival model (n=215, total number of endpoints=24);
Comparison of the clinical history variable model vs. clinical history and CMR variable model: P=0.005.
Comparison of the clinical history variable model vs. clinical history and CMR variable model (with dichotomized EDVi): P<0.001.
Abbreviations: BSA = body surface area; EDVi = indexed end diastolic volume; PLE = protein losing enteropathy.
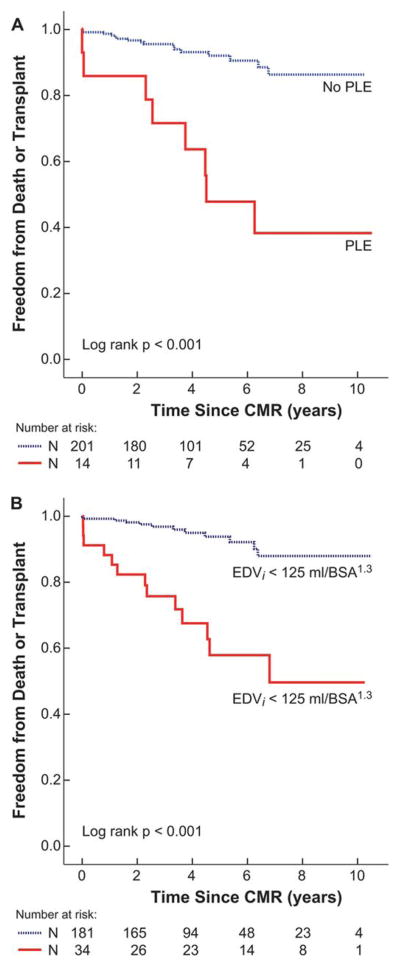
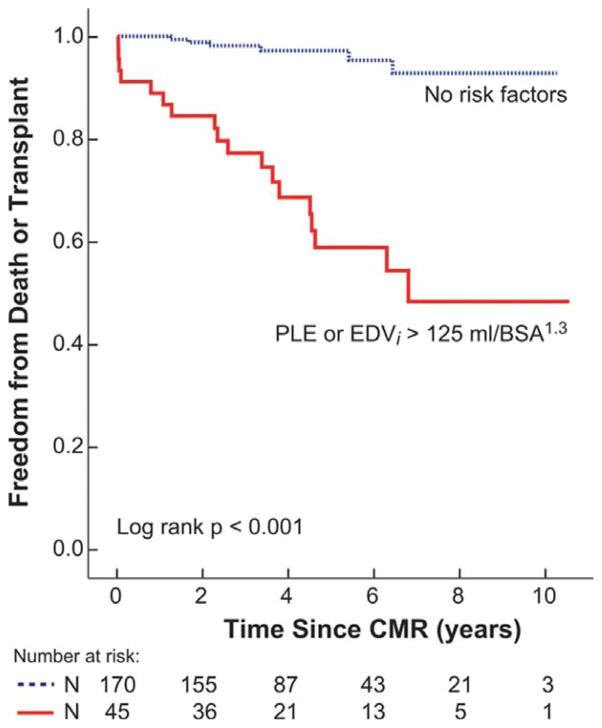
Kaplan-Meir plots showing freedom from death or transplant stratified by PLE status and EDVi >125 mL/BSA1.3, are shown in Figure 1. Kaplan-Meir plots of freedom from death or transplant comparing patients with no risk factors and clinical history risk factors plus CMR-measured EDVi are shown in Figure 2. The additive discriminating impact of EDVi >125 mL/BSA1.3 on freedom from the endpoint was significant (P<0.001).
Figure 1.
Freedom from Death or Transplant by Individual Risk Factors.
Kaplan-Meier plot of cumulative freedom from the endpoint (death or transplant) stratified by: A) protein losing enteropathy (P<0.001), and B) indexed end-diastolic volume (EDVi) > 125 mL/BSA1.3 (P<0.001).
Figure 2.
Freedom from Death or Transplant by Cumulative Risk Factors.
Kaplan-Meier plot of cumulative freedom from the endpoint (death or transplant) stratified by no risk factors and protein losing enteropathy or EDVi > 125 mL/BSA1.3 (P<0.001).
Discussion
This study provides the largest analysis of CMR-derived ventricular size and function measurements and their association with adverse clinical outcomes late after the Fontan operation. In this cohort, PLE and ventricular dilation were independently predictive of death or heart transplantation. Importantly, we found that CMR-derived ventricular EDVi significantly improved risk stratification for transplant-free survival in a relatively large cohort of patients with Fontan circulation.
Previous Studies
Khairy et al.2 analyzed a similar sized cohort (n=261) for determinants of long-term outcomes after the Fontan operation. In their multivariable model, PLE, hypoplastic left heart syndrome, elevated right atrial pressure, and need for diuretic therapy were predictors of death and transplantation. Our data support their findings of the importance of PLE. In contrast to our study, assessment of ventricular size and function by any modality was not part of their study.
The Pediatric Heart Network (PHN) has published several reports from the Fontan Cross-sectional Study, where CMR data was available in 161 patients.1 Compared to our study, patients in the PHN cohort had a smaller FSV EDVi (85 ± 25 vs. 102 ± 44 ml/BSA1.3, P<0.001). This cohort was also younger at the time of their evaluation (11.9 ± 3.4 vs. 20.5 ± 10.2 years; P<0.001), more likely to have a bidirectional Glenn operation (75% vs. 52%, P<0.001), and less likely to have a right atrium-to-pulmonary artery Fontan (13% vs. 21%, P=0.01). Longitudinal follow-up of the PHN cohort may shed light on changes in FSV size and function as the cohort reaches adulthood. Relevant to our study, analysis of the PHN data demonstrated good reproducibility of CMR-measured FSV size and function, which was superior to 2-dimensional echocardiographic measurements.11
Clinical Implications
The results of our study support the clinical value of measuring ventricular size by CMR in patients with a FSV. This study does not explain why ventricular dilation is such an important risk factor in this population. It is conceivable that ventricular dilation is the final common pathway of several pathophysiologic processes, including chronic volume overload from valvar regurgitation, aortopulmonary collateral burden,13 systolic dysfunction, delayed or lack of volume unloading surgery (e.g., bidirectional Glenn), or sinus node dysfunction. Given the association between higher EDVi and death or transplant, evaluating therapies aimed at inhibiting dilatation of the FSV is warranted. Such interventions may include diuretic and afterload reducing medications, transcatheter occlusion of aortopulmonary collaterals, or volume unloading surgery.
Although FSV EDVi was the strongest parameter associated with the endpoint, several other CMR parameters were significant by univariate analysis, including higher massi, ESVi, and stroke volumei, and an ejection fraction <40%. These observations highlight the importance of CMR-derived ventricular size and function parameters with regard to assessment of risk for death and heart transplant. As compared with 2-dimensional echocardiography, CMR offers improved reproducibility and is typically regarded as the optimal modality for the assessment of ventricular size and function parameters.11 Although positive late gadolinium enhancement analyzed as a binary parameter (present vs. absent) was not associated with the endpoint, further studies should evaluate the utility of quantitative analysis of degree of LGE. Indeed, in a smaller cohort, we have previously shown that quantitative analysis of LGE in Fontan patients is associated with ventricular dilatation and dysfunction.14 Future studies should investigate the clinical utility of recently described CMR parameters such as ventricular synchrony,21 quantitative measurements of ventricular LGE burden,14 and quantification of the myocardial extracellular volume fraction, an indicator of diffuse fibrosis.22 Finally, indices of diastolic function may also shed light on FSV mechanics and may be important for risk stratification in this population.
Limitations
The single-center, CMR centric study design limits the generalizability of this study across all Fontan patients. In particular, CMR evaluation in patients with pacemakers and defibrillators currently remains a strong relative contraindication.23 These devices were present in 13% of the PHN Fontan cross-sectional study patients.1 This selection bias may result in under-representation of arrhythmia-related events that could result in poor outcomes. Subgroup analyses of the PHN cohort showed that a pacemaker was associated with a poorer functional status and slight decrease in ventricular ejection fraction.24 Symptomatic and sicker patients could also be overrepresented in this study cohort as these patients often get heighten surveillance by CMR imaging. To facilitate comparison between patient characteristics and outcomes in our study and those of other recent studies, Table 1S (supplemental online) compares the current cohort with those published by the PHN1 and by Khairy et al.2 Measures of renal and liver function as calculated by the MELD-XI score have recently been shown to be associated with death and need for transplantation in this population.25 In our study, however, contemporaneous serologic data was not available for calculation of the MELD-XI score in most patients. Finally, from a methodological perspective, it would have been desirable to split the cohort into two, building a predictive model in one group of patients and validating the model in the other group. Unfortunately, the relatively small number of endpoint events precludes this type of analysis. Therefore, a prospective multicenter study evaluating all of these clinical, imaging, and serologic parameters in a larger cohort with more patients reaching the endpoint would likely improve our ability to stratify risk in patients late after the Fontan operation.
Conclusions
In patients late after the Fontan operation, ventricular dilation and presence of PLE are independent predictors of death and heart transplantation. This study is the first to demonstrate the predictive value of ventricular evaluation by CMR in a large cohort of patients. CMR derived parameters of ventricular size add incremental value for risk stratification than compared to clinical variables alone. These data may aid in the design of trials aimed at evaluating therapies that target these risk factors.
Supplementary Material
Acknowledgments
The authors thank Drs. Dionne Graham for her guidance regarding statistical methods, and Dr. Barbara Schaetzle for her help with data collection.
Funding Sources
This study was supported by the Eytan Singh Family and by the Higgins Family Noninvasive Cardiac Imaging Research Funds.
This work was supported by the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
None
References
- 1.Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, McCrindle BW, Paridon S, Schwartz M, Stylianou M, Williams RV, Clark BJ., 3rd Contemporary outcomes after the Fontan procedure: A pediatric heart network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khairy P, Fernandes SM, Mayer JE, Jr, Triedman JK, Walsh EP, Lock JE, Landzberg MJ. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 3.Piran S, Veldtman G, Siu S, Webb GD, Liu PP. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation. 2002;105:1189–1194. doi: 10.1161/hc1002.105182. [DOI] [PubMed] [Google Scholar]
- 4.Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein-losing enteropathy after the Fontan operation: An international multicenter study. Ple study group. J Thorac Cardiovasc Surg. 1998;115:1063–1073. doi: 10.1016/s0022-5223(98)70406-4. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes SM, Alexander ME, Graham DA, Khairy P, Clair M, Rodriguez E, Pearson DD, Landzberg MJ, Rhodes J. Exercise testing identifies patients at increased risk for morbidity and mortality following Fontan surgery. Congenit Heart Dis. 2011;6:294–303. doi: 10.1111/j.1747-0803.2011.00500.x. [DOI] [PubMed] [Google Scholar]
- 6.Sluysmans T, Sanders SP, van der Velde M, Matitiau A, Parness IA, Spevak PJ, Mayer JE, Jr, Colan SD. Natural history and patterns of recovery of contractile function in single left ventricle after Fontan operation. Circulation. 1992;86:1753–1761. doi: 10.1161/01.cir.86.6.1753. [DOI] [PubMed] [Google Scholar]
- 7.McCrindle BW, Zak V, Sleeper LA, Paridon SM, Colan SD, Geva T, Mahony L, Li JS, Breitbart RE, Margossian R, Williams RV, Gersony WM, Atz AM. Laboratory measures of exercise capacity and ventricular characteristics and function are weakly associated with functional health status after Fontan procedure. Circulation. 2010;121:34–42. doi: 10.1161/CIRCULATIONAHA.109.869396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moiduddin N, Texter KM, Zaidi AN, Hershenson JA, Stefaniak C, Hayes J, Cua CL. Two-dimensional speckle strain and dyssynchrony in single left ventricles vs. Normal left ventricles. Congenit Heart Dis. 2010;5:579–586. doi: 10.1111/j.1747-0803.2010.00460.x. [DOI] [PubMed] [Google Scholar]
- 9.Gokhale J, Husain N, Nicholson L, Texter KM, Zaidi AN, Cua CL. Qrs duration and mechanical dyssynchrony correlations with right ventricular function after fontan procedure. J Am Soc Echocardiogr. 2013;26:154–159. doi: 10.1016/j.echo.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes J, Margossian R, Sleeper LA, Barker P, Bradley TJ, Lu M, Fogel M, Harris MA, Lai WW, Powell AJ, Puchalski MD, Shirali G, Colan SD Pediatric Heart Network I. Non-geometric echocardiographic indices of ventricular function in patients with a Fontan circulation. J Am Soc Echocardiogr. 2011;24:1213–1219. doi: 10.1016/j.echo.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margossian R, Schwartz ML, Prakash A, Wruck L, Colan SD, Atz AM, Bradley TJ, Fogel MA, Hurwitz LM, Marcus E, Powell AJ, Printz BF, Puchalski MD, Rychik J, Shirali G, Williams R, Yoo SJ, Geva T. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the pediatric heart network Fontan cross-sectional study) Am J Cardiol. 2009;104:419–428. doi: 10.1016/j.amjcard.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg R, Powell AJ, Sena L, Marshall AC, Geva T. Effects of metallic implants on magnetic resonance imaging evaluation of Fontan palliation. Am J Cardiol. 2005;95:688–691. doi: 10.1016/j.amjcard.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 13.Prakash A, Rathod RH, Powell AJ, McElhinney DB, Banka P, Geva T. Relation of systemic-to-pulmonary artery collateral flow in single ventricle physiology to palliative stage and clinical status. Am J Cardiol. 2012;109:1038–1045. doi: 10.1016/j.amjcard.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rathod RH, Prakash A, Powell AJ, Geva T. Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after Fontan operation. J Am Coll Cardiol. 2010;55:1721–1728. doi: 10.1016/j.jacc.2009.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for mri as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–329. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 16.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 17.Gutgesell HP, Rembold CM. Growth of the human heart relative to body surface area. Am J Cardiol. 1990;65:662–668. doi: 10.1016/0002-9149(90)91048-b. [DOI] [PubMed] [Google Scholar]
- 18.Cantinotti M, Scalese M, Molinaro S, Murzi B, Passino C. Limitations of current echocardiographic nomograms for left ventricular, valvular, and arterial dimensions in children: A critical review. J Am Soc Echocardiogr. 2012;25:142–152. doi: 10.1016/j.echo.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Powell AJ, Geva T. Blood flow measurement by magnetic resonance imaging in congenital heart disease. Pediatr Cardiol. 2000;21:47–58. doi: 10.1007/s002469910007. [DOI] [PubMed] [Google Scholar]
- 20.Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 21.Valenti V, Zia MI, Shubayev L, Edelstein S, Supariwala A, Uretsky S, Fantozzi LM, Volpe M, Sciarretta S, Wolff SD. Cardiac magnetic resonance evaluation of the impact of interventricular and intraventricular dyssynchrony on cardiac ventricular systolic and diastolic function in patients with isolated left bundle branch block. Am J Cardiol. 2012;110:1651–1656. doi: 10.1016/j.amjcard.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 22.Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S, Ray SG, Yonan N, Williams SG, Flett AS, Moon JC, Greiser A, Parker GJ, Schmitt M. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013;6:373–383. doi: 10.1161/CIRCIMAGING.112.000192. [DOI] [PubMed] [Google Scholar]
- 23.Levine GN, Gomes AS, Arai AE, Bluemke DA, Flamm SD, Kanal E, Manning WJ, Martin ET, Smith JM, Wilke N, Shellock FS. Safety of magnetic resonance imaging in patients with cardiovascular devices: An american heart association scientific statement from the committee on diagnostic and interventional cardiac catheterization, council on clinical cardiology, and the council on cardiovascular radiology and intervention: Endorsed by the american college of cardiology foundation, the north american society for cardiac imaging, and the society for cardiovascular magnetic resonance. Circulation. 2007;116:2878–2891. doi: 10.1161/CIRCULATIONAHA.107.187256. [DOI] [PubMed] [Google Scholar]
- 24.Williams RV, Travison T, Kaltman JR, Cecchin F, Colan SD, Idriss SF, Lu M, Margossian R, Reed JH, Silver ES, Stephenson EA, Vetter VL Pediatric Heart Network I. Comparison of Fontan survivors with and without pacemakers: A report from the pediatric heart network Fontan cross-sectional study. Congenit Heart Dis. 2013;8:32–39. doi: 10.1111/j.1747-0803.2012.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assenza GE, Graham DA, Landzberg MJ, Valente AM, Singh MN, Bashir A, Fernandes S, Mortele KJ, Ukomadu C, Volpe M, Wu F. Meld-xi score and cardiac mortality or transplantation in patients after Fontan surgery. Heart. 2013;99:491–496. doi: 10.1136/heartjnl-2012-303347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.