Abstract
The goal of the present study was to examine relationships between individual differences in resting state functional connectivity as ascertained by fMRI (rs-fcMRI) and performance on tasks of executive function (EF), broadly defined as the ability to regulate thoughts and actions. Unlike most previous research that focused on the relationship between rs-fcMRI and a single behavioral measure of EF, in the current study we examined the relationship of rs-fcMRI with individual differences in subcomponents of EF. Ninety-one adults completed a resting state fMRI scan and three separate EF tasks outside the magnet: inhibition of prepotent responses, task set shifting, and working memory updating. From these three measures, we derived estimates of common aspects of EF, as well as abilities specific to working memory updating and task shifting. Using Independent Components Analysis (ICA), we identified across the group of participants several networks of regions (Resting State Networks, RSNs) with temporally correlated time courses. We then used dual regression to explore how these RSNs covaried with individual differences in EF. Dual regression revealed that increased higher common EF was associated with connectivity of a) frontal pole with an attentional RSN, and b) Crus I and II of the cerebellum with the right frontoparietal RSN. Moreover, higher shifting-specific abilities were associated with increased connectivity of angular gyrus with a ventral attention RSN. The results of the current study suggest that the organization of the brain at rest may have important implications for individual differences in EF, and that individuals higher in EF may have expanded resting state networks as compared to individuals with lower EF.
Keywords: executive control, rs-fcMRI, individual differences, behavior, ICA
1 Introduction
When individuals are not engaged in an experimentally-directed task (i.e., are in a “resting state”), distinct networks of widely separated brain regions can be identified as sharing similar temporal patterns of functional activity (Fox & Raichle, 2007) – a phenomenon often referred to as “resting state functional connectivity MRI” (rs-fcMRI). These “resting state networks” [RSNs] show strong correspondence with regions that tend to co-activate during performance of a class of tasks (e.g., language processing tasks; Smith et al., 2009). Moreover, the organization of such networks has been found to have behavioral and clinical relevance. A large body of literature indicates that RSNs are altered across a plethora of neurological and clinical populations, including Alzheimer’s disease, schizophrenia, depression, attention deficit hyperactivity disorder, and others (for reviews see Greicius, 2008; Zhang & Raichle, 2010).
More recently, research has focused on how individual differences in abilities among neurologically normal individuals are related to the organization and extent of networks identified by rs-fcMRI. For example, patterns of rs-MRI are associated with fluid intelligence (Cole et al., 2012), attentional vigilance (Thompson et al., 2012), performance on the trail making test (Seeley et al., 2007), working memory (Hampson et al., 2006; Gordon et al., 2012), and the ability to maintain attentional control in the face of distracting information (Kelly et al., 2008). In general, however, there is a paucity of studies that examine the relationship between rs-fcMRI and individual differences in executive function (EF), the ability to engage in and guide goal-oriented behavior. Because EF is a broad umbrella term that encompasses a wide variety of specific functions and component processes (Miyake et al., 2000), our approach in the current study is to examine the relationship between RSNs and individual differences in both general and specific subcomponents of EF in a large sample of participants. Moreover, we take a novel approach of investigating this issue by embedding our research within the framework of a prominent and well-grounded theoretical model of EF, known as the unity and diversity model (for a review, see Miyake & Friedman, 2012). This model, based on intercorrelated patterns of performance across individuals on multiple measures of EF, suggests that many important aspects of EF can be reduced into at least three latent factors. The first is a common EF factor, representing the unity aspect of the model, on which all measured EF tasks load. This factor is thought to represent the general capacity to maintain a task goal, or “attentional set,” and is thought to be a common feature of all EF tasks. The second two orthogonal factors represent the diversity aspect of the model and are more specific processes above and beyond common EF. Statistically speaking, these factors are residuals of the EF abilities once common EF has been taken into account. One factor, the shifting-specific factor, captures processes relating to flexibly shifting between different task or mental sets, while the other factor, the updating-specific factor, indexes the process of rapidly adding or deleting information from the contents of working memory.
Theoretical considerations, computational modeling, and empirical research by our group and others suggest that these three EF factors are likely to be supported by overlapping yet somewhat distinct brain systems (Miyake & Friedman, 2012; Herd et al., in press). The ability to stably maintain a task goal is thought to rely on areas of lateral prefrontal cortex extending from BA 10 through mid-dorsolateral prefrontal cortex (Banich, 2009; Braver, 2012; Herd, Banich, & O’Reilly, 2006; Sakai, 2008), potentially including the anterior cingulate and frontal operculum as well (Dosenbach et al., 2008). Set shifting involves changes in the focus of attention and may engage more posterior regions of dorsolateral prefrontal cortex (e.g., inferior frontal junction) as well as parietal regions (e.g., intraparietal sulcus; Wager, Jonides, & Reading, 2004; Derrfuss et al., 2005). Working memory updating has been suggested to involve fronto-striatal connections and require input from the basal ganglia (Braver et al., 1997; O’Reilly & Frank, 2006; McNab & Klingberg, 2008). Using task-related fMRI across multiple EF tasks, Collette and colleagues (2005) found that regions commonly activated across EF tasks include the left superior parietal gyrus and the right intraparietal sulcus, and to a lesser degree, mid- and inferior prefrontal regions. Moreover, left frontopolar cortex (BA 10) activity was specifically associated with updating-specific EF, while activity of the left intraparietal sulcus was associated with shifting-specific EF.
Given the relatively limited scope of prior research on rs-fcMRI and EF, the current study had a number of major objectives. First, we wanted to determine whether patterns of rs-fcMRI are associated with individual differences in both common and specific factors underlying EF. Second, given the research suggesting that these three EF factors may engage somewhat different brain regions, we wanted to ascertain whether different aspects of rs-fcMRI predicted individual differences for each of the three EF factors investigated (i.e., common EF, updating-specific EF, shifting-specific EF). Third, we wanted to disentangle whether individual differences in these three aspects of EF are associated with activity in RSNs that are composed of regions commonly activated across individuals when performing EF tasks (e.g., the fronto-parietal network), and/or whether they are influenced by activity in RSNs outside those traditionally thought to be engaged in EF (e.g., medial frontal/limbic network). Finally, we wanted to investigate how individual differences in EF might predict alterations in either the degree to which specific subregions coactivate as part of a particular RSNs (e.g., more intense connectivity of DLPFC within the fronto-parietal network) or the composition of particular RSNs (e.g., a greater spatial extent of the fronto-parietal network). Our hypothesis was that rs-fcMRI would be associated with individual differences in these three aspects of EF. However, based on the paucity of prior research, our investigation was more exploratory with regards to how exactly such individual differences would manifest. To investigate these questions, we utilized dual regression to extract subject-specific versions of classic RSNs and then performed statistical tests to determine how individual variation in these RSNs predicted EF as characterized by the unity and diversity model.
2 Material and Methods
2.1 Participants
One hundred individuals aged 18 to 34 years (M = 22.3, SD = 9.92) from the University of Colorado Boulder participated for payment over two sessions. Participants were paid $25.00 per hour for the fMRI session and $10.00 per hour for the behavioral session. Session one involved the administration of behavioral tasks that measured EF ability. Session two involved the acquisition of anatomical and functional brain data via magnetic resonance imaging. The two sessions occurred within an average of 31.6 days of each other. Functional brain data from six participants were discarded due to excessive levels of movement during the scanning session (greater than 3mm in a single dimension). Additionally, data from three participants were discarded due to failure to comply with rules on one of more of the behavioral tasks. All presented results are from analyses of data from the remaining 91 participants (48 females).
2.2 Procedures
In session one, three behavioral tasks were administered from the battery of nine tasks typically used in studies that have provided evidence for the unity and diversity model of EF (see Miyake et al., 2000; updated in Miyake & Friedman, 2012): antisaccade, category switching, and keep track. These three tasks were chosen because they load most highly on common EF, switching-specific, and updating-specific factors, respectively, in a prior large scale study in which the full battery of EF tasks was administered (Friedman et al., 2012). A variety of self-report questionnaires (e.g., emotion regulation style, trait rumination, worry, distractibility) and genetic data were acquired during session 1. Analyses of questionnaire data are outside the scope of the current study. Analyses of genetic data were not performed due to lack of a replication sample.
In session two, participants were scanned in a Siemens Tim Trio 3T scanner. During a 5.5 minute resting state scan, participants were instructed to relax and close their eyes.
2.3 Session 1: Behavioral Tasks
Antisaccade task
(adapted from Roberts, Hager, & Heron, 1994). This task measures a person’s ability to inhibit an automatic process (an eye movement). Participants were instructed to focus on a centrally located fixation cross (lasting 1.5–3.5 sec). When the fixation cross disappeared, an initial box cue flashed 10 cm either to the right or to the left of fixation. The cue disappeared after a fixed interval (233, 200, or 183 ms), after which the target (a digit, 1 through 9) appeared for 150ms before being masked with gray cross-hatching. Participants named the number they saw aloud and the experimenter typed in their response, triggering the next trial to begin. For some trials, the cue was helpful in that it indicated the location at which the target appeared (prosaccade trials). In other trials – antisaccade trials – the cue appeared on the opposite side of the screen as the target. The task began with a block of 18 prosaccade trials in which the cue disappeared after 183 ms to establish that participants could perform the easy prosaccade trials within the most stringent time demands. Participants were then given three blocks of 36 antisaccade trials (with 233, 200, or 183 ms cue durations, respectively). Participants typically vary in their ability to identify the target on antisaccade trials because it is difficult to inhibit the automatic tendency to look towards an object, in this case the cue. The dependent measure was average accuracy for the three blocks of antisaccade trials.
Category Switch task
(adapted from Mayr & Kliegl, 2000). This task measures a person’s ability to quickly and accurately switch between different modes of categorization. Participants were asked to categorize words (e.g., alligator, knob, coat, lion) either with regards to animacy (living/non-living) or size (smaller/larger than a soccer ball) depending on a cue that appeared above the word (heart or crossed arrows). After two pure blocks of 32 trials each that involved categorizing items along a single dimensions (e.g., just on animacy), participants completed two blocks of 64 trials each that contained a mixture of trials in which some trials required judgments regarding animacy and others required judgments regarding size. The trials in these blocks were presented in a fixed pseudorandom order such that the subtasks occurred equally often, and 50% of the trials involved a switch from one subtask to the other. Participants were given unlimited time to respond on each trial, but were instructed to respond as swiftly and accurately as possible. The dependent measure was the switch cost: the difference between average reaction time for correct switch trials and correct repeat trials during the mixed blocks for each subject. Trials following errors were eliminated because it was not clear that the correct set was achieved (precluding categorization of whether the subsequent trial was either a switch or repeat trial). Reaction times identified as within-subject outliers by the Wilcox-Kessleman trimming procedure (Wilcox & Keselman, 2003) were also removed before averaging.
Keep Track task
(adapted from Yntema, 1963). This task measures the ability to update working memory. A stream of words is presented, one at a time. The words belong to six categories: relatives, countries, colors, animals, metals, and distances, with six words in each category. Participants were asked to keep track of the most recently presented words from two to five given categories and report them verbally at the end of the trial. Sixteen trials were administered, with each trial containing a stream of 15–25 words. After two practice trials with two categories to remember, there were four blocks, each with one two-, three-, four-, and five-category trial, for a total of 16 trials. The order of the trials within each block was fixed in a pseudorandom order. Each trial began with the list of categories, which remained at the bottom of the screen until the final recall. Each word appeared for 2000ms, followed by the next word. The dependent measure was each participant’s accuracy in recalling the target words.
EF scores
We extracted three factors – common EF, shifting-specific, and updating-specific – in accordance with prior research. The lack of an inhibition-specific factor reflects a recent update to the unity and diversity framework that highlighted the complete overlap of common EF and inhibition-specific variance in behavioral tasks in several samples of adults and adolescents (Miyake & Friedman, 2012). Common EF was calculated by taking the average of each subject's three tasks converted to a Z-value (across the group of 91 participants). Shifting-specific was the residual variance in the category switch task, regressing out common EF. Updating-specific was the residual variance in the keep track task, regressing out common EF. This procedure left shifting- and updating-specific orthogonal to Common EF; however, the shifting- and updating-specific residuals were significantly negatively correlated (r = −.61, p < 0.05). This method of calculating EF component scores is similar to that performed in a recent related study from our laboratory demonstrating that individual differences in these EF component scores predict individual differences in grey matter volume and gyrification index of prefrontal regions as well as fractional anisotropy of specific neural tracts that connect prefrontal regions with posterior brain areas (Smolker, Depue, et al., 2014). Shapiro-Wilks tests confirmed that all three EF measures were normally distributed. Higher scores on three composite measures correspond to greater ability in that construct (i.e. - maintain a goal, shift between task/mental sets, or update working memory).
2.4 Session 2: Brain Imaging
Neuroanatomical data were acquired with T1-weighted MP RAGE sequence (acquisition parameters: repetition time (TR) = 2,530ms, echo time (TE) = 1.64, matrix size = 256 × 256 × 192, flip angle (FA) = 7 deg., slice thickness = 1mm). Resting state data was acquired with a T2*-weighted echo-planar functional scan (acquisition parameters: number of volumes = 165, TR = 2,000ms, TE = 29ms, matrix size = 64 × 64 × 33, FA = 75 deg., slice thickness = 3.5mm, field of view (FOV) = 240mm).
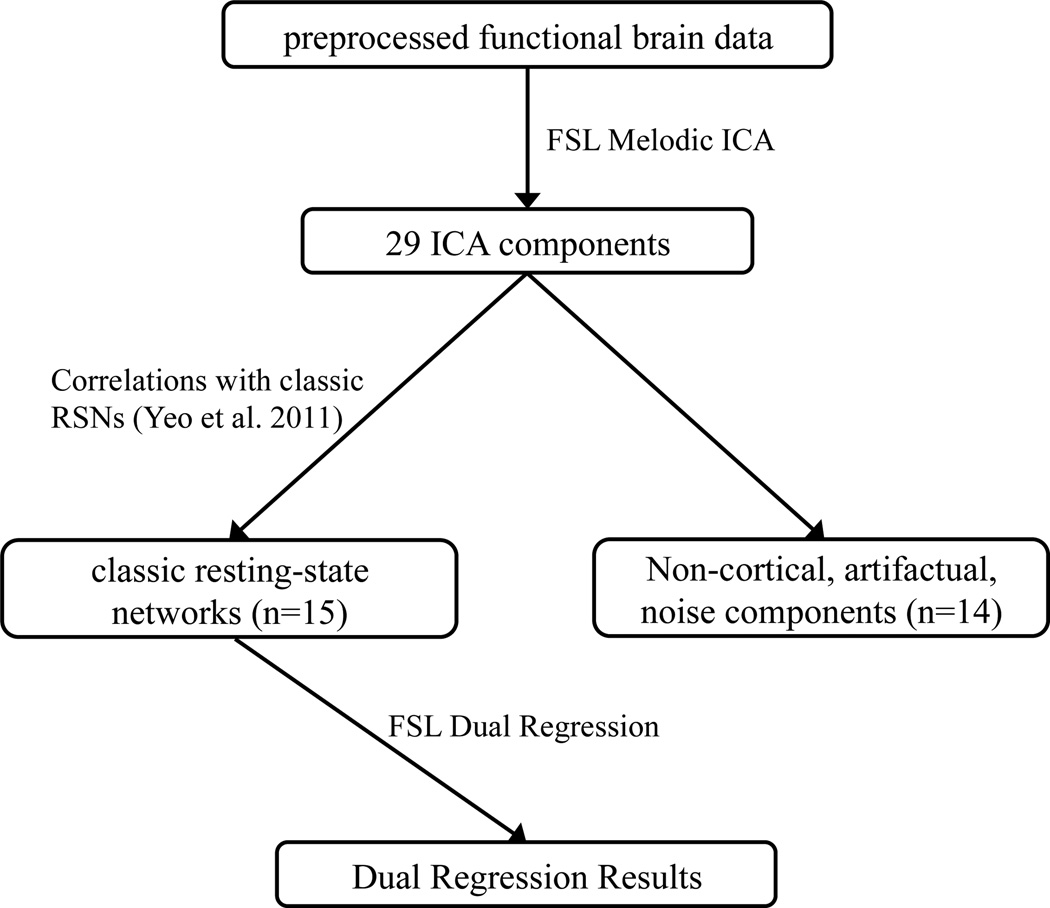
Analysis of brain data was performed via a multi-step process (see Figure 1 for summary). First, Independent Components Analysis (ICA) was used to identify networks of brain regions whose activity was correlated across the group of participants. From these so-identified ICA components, we selected those that were significantly correlated with those of a reference study with a larger number of individuals (Yeo et al., 2011) and discarded those that are were irrelevant to the current investigation (e.g., atypical RSNs and artifactual components; see below for procedure). For the relevant networks, dual regression was used to derive subject-specific maps of the group-identified RSNs. Finally, statistical analyses were performed to identify differences in the subject-specific RSN maps that predicted EF ability.
Figure 1.
The data processing pipeline for resting state data in the current study.
2.5 Preprocessing
All processing of brain data was performed in a standard install of FSL build 5.06 (Jenkinson et al., 2012). To account for signal stabilization, the first four volumes of each individual functional scan were removed, yielding 161 volumes per subject for additional analysis. The functional scans were corrected for head motion using MCFLIRT, FSL’s motion correction tool. Brain extraction (BET) was used to remove signal associated with non-brain material (e.g., skull, sinuses, etc.). FSL’s FLIRT utility was used to perform a boundary-based registration of each participant’s functional scan to his or her anatomical volume and a 6 degree of freedom affine registration to MNI152 standard space. Finally, the scans were converted to 4mm voxel size, smoothed (5mm FWHM), and high-pass filtered (.01 Hz threshold).
2.6 Independent Components Analysis
To decompose the functional brain data into various independent spatiotemporal components, Independent Components Analysis (ICA) was performed on the preprocessed functional scans using Melodic ICA version 3.14 (Beckmann & Smith, 2004). A dimensionality estimation using the Laplace approximation to the Bayesian evidence of the model order was performed (Beckman & Smith, 2004). This procedure yielded 29 spatiotemporal components. While one common approach for identifying “classical” resting-state networks from a pool of ICA components is to have an expert subjectively label ICA components as signal (e.g., right frontoparietal network, default network, etc.) or noise (edge effects, movement, etc.), we opted to use a different RSN identification procedure to select RSNs for further analysis. We statistically compared the spatial map of each ICA component to a set of 7 popular RSNs from analysis of resting-state data from approximately 1000 participants (Yeo et al., 2011). We used FLS’s “fslcc” tool to calculate Pearson’s r for each pairwise relationship and kept only those ICA components that yielded a significant spatial correlation (Pearson’s r > .207) with one of the RSNs from Yeo et al. (2011). This procedure identified and helped label 15 RSNs, and identified 14 ICA components that did not significantly correlate with a reference network. ICA components that did not significantly correlate with a reference network were eliminated from further analysis. Further inspection confirmed that the eliminated components were likely artifactual (e.g., edge effects) or were predominantly high frequency signal according to a power frequency distribution curve (i.e., physiological noise such as heartbeat-induced movement).
2.7 Dual Regression
Dual regression is a method that uses unthresholded group-level independent component maps to generate both subject-specific component time courses and subject-specific spatial maps as output (Beckmann et al., 2009). Here we focus on subject-specific spatial maps to examine how EF influences the composition of the networks. Dual regression can be broken down into two steps: First, for each subject, the group-average set of spatial maps is regressed (as spatial regressors in a multiple regression) on the subject’s 4D spatio-temporal dataset (i.e., brain volumes across time). This process results in a set of subject-specific time series, one per group-level component. Next, those time series are regressed (as temporal regressors, again in a multiple regression) into the same 4D dataset, resulting in a set of subject-specific components, one per group-level component. Subject-specific components are whole brain images. Some subjects express a given RSN that is very similar to the group level RSN while others have variations of the group level RSN (e.g., have an expanded RSN or high connectivity of a particular regions of a given RSN). Statistical analyses (discussed below) are performed on these whole brain subject-specific RSNs to determine areas covary with behavioral covariates of interest, in our case, level of EF.
In the present study, the associations between EF measures and subject-specific RSNs were analyzed using Randomise, FSL’s nonparametric permutation testing tool (Jenkinson et al., 2012), with 5000 permutations and threshold free cluster enhancement (TFCE) to correct for multiple comparisons. Permutation testing was performed while controlling for between-subject differences in transient movement throughout the scanning session in accordance with Van Dijk, Sabuncu, & Buckner (2012). Two summary motion regressors were created for each subject and entered into Randomise as control variables: average motion in the x, y, and z planes (mean translation) and average roll, pitch, and yaw (mean rotation) across the resting state run. The permutation testing procedure was run for each set of subject-specific RSNs (one for each group-level RSN of interest), thus the resulting statistical images reveal how variation in RSNs predict differences in EF. For example, the permutation testing procedure could reveal that individuals with an expanded RSN (i.e., expanded to areas outside the areas included in the group-level RSN) have greater EF.
To account for the possibility that performance of EF tasks in session one could affect resting-state functional connectivity in session two, we performed an additional series of dual regression analyses adding time between session one and two as a covariate in addition to EF and motion variables. We found no difference in the observed effects of EF after the addition of this covariate and no main effects of time between sessions. For the sake of simplicity, only results from models that included EF, mean translation, and mean rotation are reported below.
3 Results
3.1 Behavioral Data
We performed a check to ensure the data was suitable for the proposed analyses. As anticipated, there were no floor or ceiling effects and scores varied considerably across the group of participants. Average results on measures of interest were slightly higher than performance in a large (n = 735+) population sample of young adults (mean age of 22.8 compared to 20.8 in the current study; Friedman et al., in preparation) : The mean antisaccade accuracy was 75.5% (SD = 31.4), the mean category-switch switch cost was 174.3 ms (SD = 123.9 ms), and the mean keep-track accuracy was 76.1% (SD = 8.33).
3.2 Independent Components Analysis
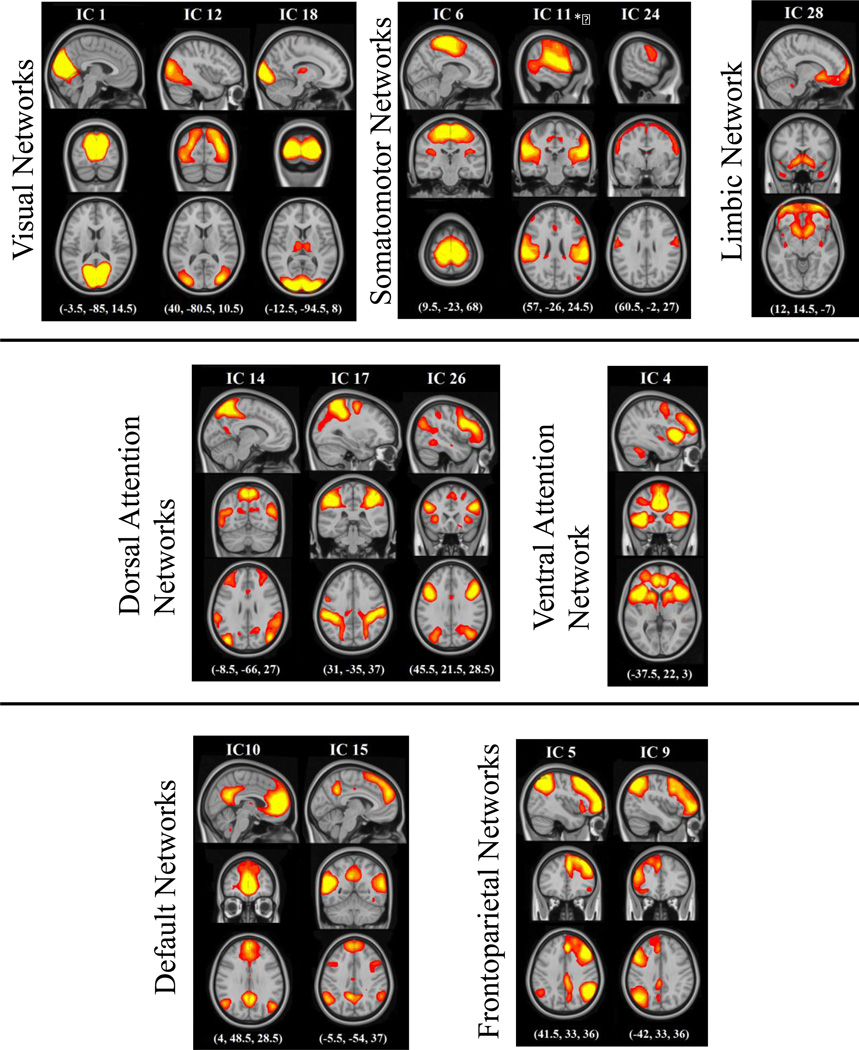
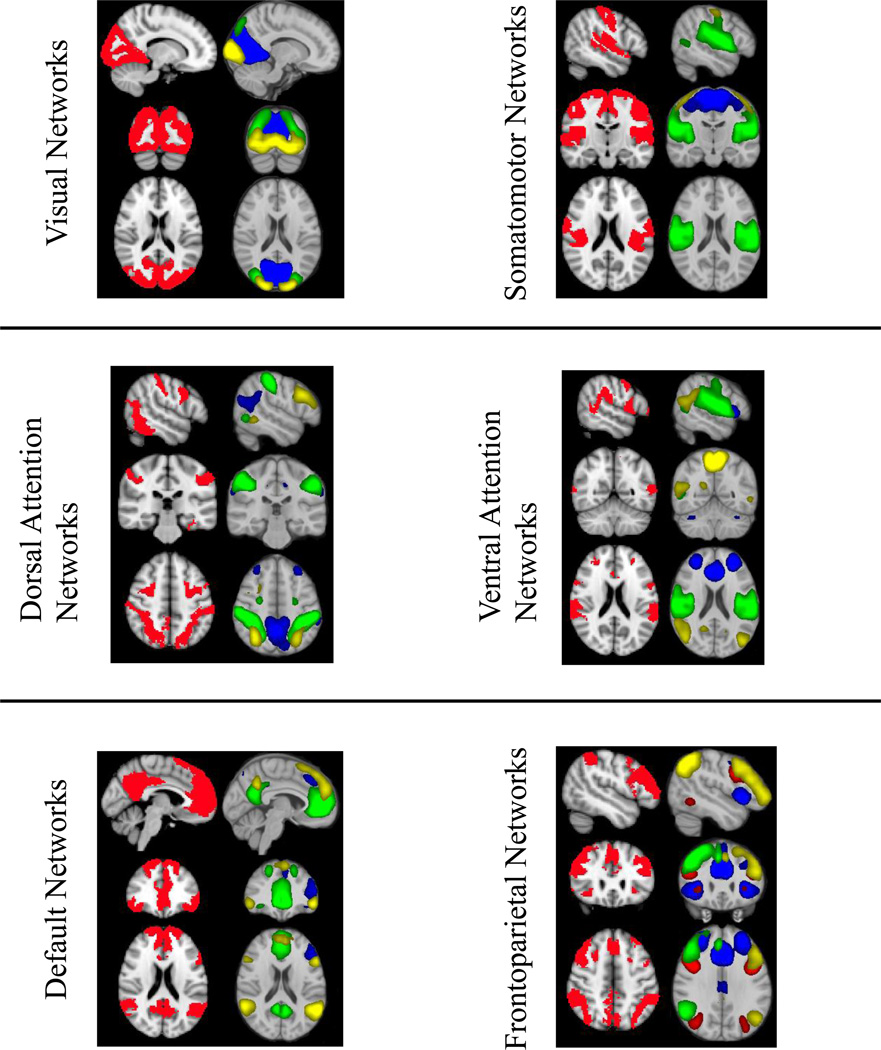
The 15 RSNs of interest in the present study were, with one exception, subsets of the large seven major RSNs identified by Yeo et al. (2011): visual, somatomotor, dorsal attention, limbic, ventral attention, default, and frontoparietal networks (see Figure 2). For all but Yeo’s ventral attention and limbic network, there was more than a single RSN that correlated significantly with the masks from Yeo et al. (2011). Additionally, five of the 15 RSNs from the current study significantly correlated with more than one network (e.g., to both the ventral attention network and the frontoparietal network of Yeo et al. (2011). In Figure 2, we show the independent component (IC) numbers from our Melodic output under the label of the reference networks from Yeo et al. (2011). In Figure 2, our independent components are thresholded at a level consistent with previous research (z = 5, compared to 3 < z < 9 (Rytty et al., 2013), 4 standard deviations above the mean (Allen et al, 2013), and p (signal > noise) > .5 (FSL default)). This threshold was also used for determining the parent network of any region later identified in the Dual Regression analyses. Any IC that significantly correlated with more than one template network is grouped in Figure 2 with the template network with which it is most strongly correlated. The three networks that correlated with Yeo’s visual network were composed of regions extending from occipital pole through cuneal cortex and lingual gyrus (ICs 1, 12, and 18). The three networks that correlated with Yeo’s somatomotor network were composed of the superior pre- and post-central gyrus (IC 6), primary auditory cortex and superior temporal gyrus (IC 11), and post-central gyrus (IC 24). There was a single network that extended through orbitofrontal and ventromedial prefrontal cortex that correlated with Yeo’s limbic network (IC 28). The three networks that correlated with the Yeo’s dorsal attention network were composed of dorsolateral frontal, parietal, and occipital regions (ICs 14, 17 and 26). The single networks that correlated with Yeo’s ventral attention network was composed of a conglomerate of medial and lateral frontal regions (IC 4). The two networks that correlated with Yeo’s default network were composed of medial prefrontal cortex, posterior cingulate cortex, and precuneus (ICs 10 and 15). Finally, there were two networks that correlated with Yeo’s frontoparietal networks: classic right and left frontoparietal networks (ICs 5 and 9, respectively). In Figure 3, we show ICs from the current study (plotted in multiple colors) next to template networks of Yeo et al. (2011) (plotted in red).
Figure 2.
Resting-state networks from the current study thresholded at z > 5. The RSNs are grouped into seven categories based on relation to reference networks – visual, somatomotor, limbic, dorsal attention, ventral attention, default, and frontoparietal. Independent components that significantly overlapped with more than one template are grouped with the template they correlate most strongly. In addition to the pictured groupings, independent components 11 and 14 significantly overlapped with the ventral attention template network; independent component 9 also significantly overlapped with the default template network; and independent components 4 and 26 significantly overlapped with the frontoparietal template network.
Figure 3.
Resting state networks from the current study compared to reference networks (Yeo et al., 2011). Reference networks are plotted on the left in red. RSNs from the current study were spatially combined into a single image and plotted in contrasting colors.
3.3 Dual Regression
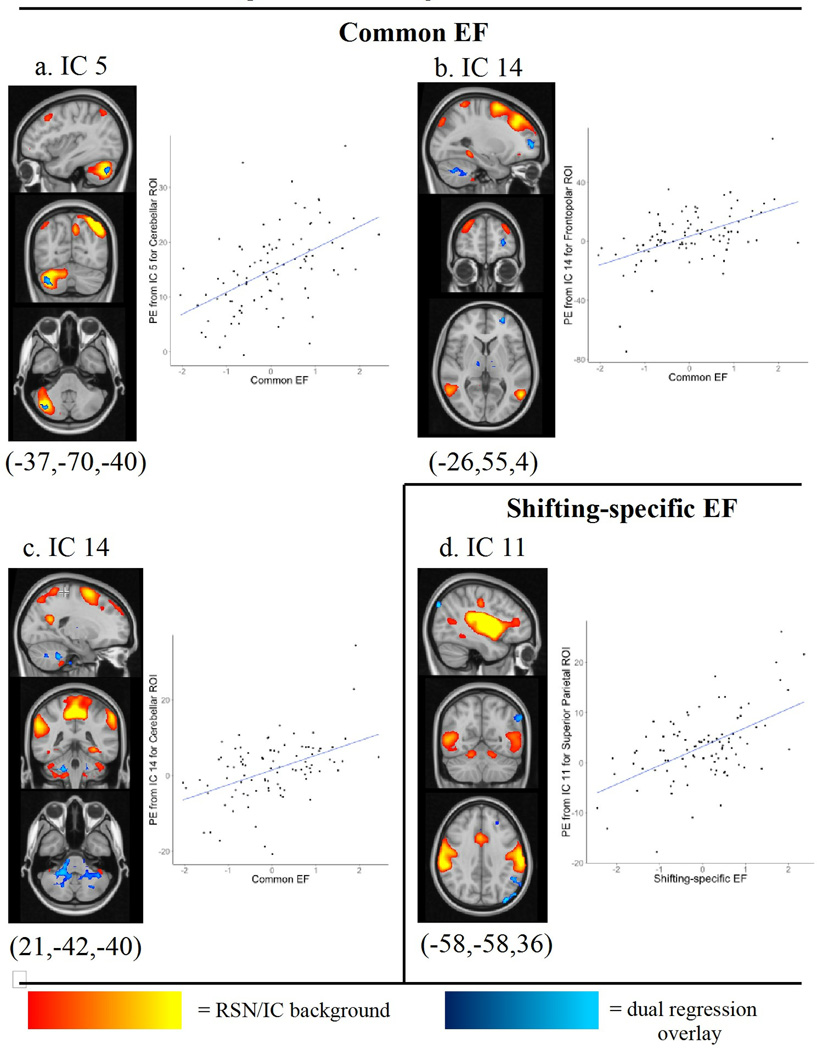
Common EF
Individual differences in common EF were associated with variation of two RSNs. First, we observed that increased connectivity of Crus I and II of the cerebellum within the right frontoparietal network (IC 5) was associated with greater common EF ability (Figure 4a; p(corrected) = 0.037). Second, we observed that expansion of IC14, a dorsal attentional RSN, to two regions – the left frontopolar cortex (Figure 4b; p(corrected) = 0.028) and right cerebellar regions (Figure 4c; p(corrected) = 0.035) – was associated with greater common EF. The frontopolar region is part of ICs 4 and 9.
Figure 4.
Dual regression analyses reveal RSNs vary with common and switching-specific components of EF. The input RSN for dual regression is plotted in warm colors as background image. The dual regression result is plotted as an overlay in blue. Blue regions are regions that covary with individual differences in EF. For all results within the input RSN (4a), increased intensity of the highlighted region (thresholded at p(corrected) < .05) corresponds to higher EF construct scores. For outside-group-network results (4b, c), expansion of the input RSN to the highlighted region corresponds to higher EF construct scores. Next to each dual regression result is a scatterplot of parameter estimates extracted from the voxels identified in the dual regression analysis for each participant’s independent component plotted against each participant’s EF score. For size of significant clusters identified in the dual regression analysis, see Table 1.
Shifting-specific
Greater shifting-specific EF was associated with increased coupling of the left angular gyrus with IC 11, a network that correlated significantly with both the somatomotor and ventral attention template networks. (Figure 4d; p(corrected) = 0.007). Left angular gyrus is not part of IC 11 from the group level ICA analysis.
4 Discussion
Our findings indicate that aspects of the functional architecture of the resting brain are associated with variation amongst individuals in different aspects of EF. While an abundance of studies have identified sets of regions that consistently coactivate together during periods of rest, we have shown that the composition of these networks vary based on individual differences in EF. More specifically, higher common EF is associated with intensity differences within the right frontoparietal RSN, and the expansion of an attention RSN to frontopolar cortex and cerebellum. Better shifting-specific ability was associated with expansion of a somatomotor/attention network to angular gyrus. No relationships were observed between rs-fcMRI and updating-specific ability.
4.1 Common EF
Higher common EF was associated with differences in the right frontoparietal RSN. Specifically, increased coupling between the right frontoparietal RSN and Crus I and II of the cerebellum predicted higher common EF. Such a result is consistent with task-based research showing that frontal and parietal regions emerge as important in both region-of-interest (Alvarez & Emory, 2006; Buckner, 2013) and network-based analyses (Zhang & Li, 2012) of executive function tasks. This result is also in line with work that shows a strong connection between Crus I/II and frontoparietal regions both functionally (Buckner et al., 2011) and anatomically (Kelly & Strick, 2003). Additionally, Crus I and II have been shown to activate in task-based fMRI studies of working memory and EF, and greater activation and functional connectivity of these regions is associated with better performance (Bernard et al., 2013; Salmi et al., 2010; Stoodley & Schmahmann, 2009). This result supplements these task-based findings by demonstrating that the degree to which Crus I and II couples with the frontoparietal network at rest predicts differences in EF. The exact role of cerebellar regions in the service of EF is still debated. Two prominent theories stemming from the motor control literature suggest that cerebellum is crucial for timing of cognitive events and/or provides a means for predicting the outcome of implemented plans (Ivry & Keele, 1989; Ramnani, 2006). While our study cannot speak directly to these theories, we have shown that a key difference between individuals with high versus low Common EF may be the degree to which cerebellum is involved in a network of regions broadly implicated in higher-order cognitive operations.
Additionally, Common EF was associated with variation in a network supporting attention to the external environment (IC 14). Individuals with greater common EF showed increased coupling of frontopolar region with this attentional RSN, which is composed of lateral frontal and superior parietal/occipital regions. An attention network similar to IC 14 has previously been linked to performance on the antisaccade task (Schaeffer et al., 2013), which is a task that loads strongly on the Common EF construct (Miyake & Friedman, 2012). Notably though, the frontopolar region identified in the dual regression analysis has not been characterized as part of the dorsal attention network and is not contained within the dorsal attention RSN that was identified at the group level of the current study. Rather, this frontopolar region is part of two frontoparietal RSNs – ICs 4 and 9. Importantly, this frontopolar region has previously been linked to maintenance of goals and abstract task sets (Ramnani & Owen, 2004; Vincent et al., 2008; Christoff et al., 2009; Dosenbach et al., 2008; Orr & Banich, 2014). As such, co-activation of regions implicated in the maintenance of abstract representation and/or goals with the attentional machinery to implement those goals, characterizes individuals with high levels of common EF.
In summary, alterations in resting state networks associated with high common EF can be characterized as expanded compared to those with low common EF, encompassing both higher-order areas involved in setting abstract goals (i.e., frontopolar cortex) and lower-level regions that could aid in implement those goals more automatically (i.e., cerebellum).
4.2 Shifting-specific
Higher Shifting-specific EF was associated with variation in a somatomotor/attentional RSN (IC 11), suggesting it is involved in sensory aspects of spatial processing. Specifically, individuals with higher EF had greater recruitment of the angular gyrus, which lies outside IC. Lateral parietal regions are frequently implicated in executive processes, and are especially important for shifting-specific aspects of EF (Collette et al., 2005; Esterman et al., 2009), perhaps due to their ability to integrate multimodal information. Additionally, this particular region of the angular gyrus is characterized by a distinct pattern of anatomical connectivity to a variety of regions implicated in higher order cognitive process, such as ventrolateral prefrontal cortex, among others (Uddin et al., 2010). Shifting-specific tasks depends not only on active abilities to move from one task set to the other, but also the passive linking between lower order centers of control and appropriate targets (e.g., motor regions). It may be that individuals with higher shifting-specific EF effectively utilize parietal regions to control the stimulus-response mappings that are required to perform different tasks.
4.3 Updating-specific
One unexpected aspect of our findings was a lack of a relationship between individual differences in updating-specific ability and any rs-fcMRI network or region. On the basis of both theoretical and empirical findings regarding the neural substrates of updating (Frank, Loughry, & O’Reilly, 2001), one might have predicted a priori that connectivity between the DLPFC and basal ganglia might influence individual differences in updating ability. However, we did not find such an effect. Our null results may arise because of the qualitative differences between processes supporting updating-specific EF as compared to common EF and shifting-specific EF. Updating requires the contents of working memory to be manipulated, and as such may rely more on a more circumscribed brain region, notably DLPFC, without connectivity to other regions playing as much of a role. In contrast, when switching between tasks, new task sets and stimulus-response mappings need to be loaded to perform the task, and hence switching may rely on a more distributed network (e.g., frontopolar regions for selecting the task set, parietal regions for stimulus-response mappings, etc.). Of course one must be circumspect when discussing potential reasons for a null result.
4.4 General Discussion
One notable aspect of our results was that different RSNs were associated with different aspects of EF. As such, our findings support the idea that EF represents a family of abilities. More importantly, however, it suggests that these different abilities may preferentially recruit distinct neural substrates, at least with respect to individual differences in EF.
Although our findings clearly demonstrate a relationship between individual differences in EF and rs-fcMRI, our study cannot speak directly to the source of that relationship. On the one hand, a dominant theme emerging from prior literature is that patterns of rs-fcMRI reflect the intrinsic functional organization of the brain (Fox & Raichle, 2007), sculpted by a history of coherent neuronal firing and anatomical wiring between distributed brain regions (Wig, Schlaggar, & Petersen, 2011). Indeed, patterns of rs-fcMRI are stable across time within individuals (Guo et al., 2012; Shehzad et al., 2009), relate to a variety of genetic factors (Glahn et al., 2010), and persist to some degree under various stages of consciousness and anesthesia (Boly et al., 2008; Greicius et al., 2008). From this perspective, the findings of the present study may potentially reflect an individual’s biological heritage, such as genetic influences on EF that might influence the structural and/or functional connectivity of the brain (see Friedman et al., 2008 for evidence of genetic influences on EF). However, this does not preclude environmental influences also working to sculpt brain activity, such as the amount of training/schooling during childhood with regards to activities or tasks that require EF (Diamond, 2012), SES (Hackman & Farah, 2009), or other such factors. The relationship between behavior-related RSN variability and specific individual traits, whether they have genetic and/or experiential causes, is an important direction for future work.
It is also possible that aspects of an individual’s state at the time of scanning may influence the patterns we observed. Several recent findings suggest that patterns of functional connectivity may be partially influenced by the participant’s mental/task state (Andrews-Hanna et al., 2010; Doucet et al., 2012; Shirer et al., 2012), and can be modified on a rapid time scale (Lewis et al., 2009; Tambini, Ketz, & Davachi, 2010; Stevens, Buckner, & Schacter, 2010). Future research should also explore the possibility that behavior-related RSN variability is caused by differences in the cognitive processes of high versus low EF individuals during resting-state scans (e.g., high EF individuals plan their day, while low EF individuals daydream).
Although we believe our findings establish a strong groundwork for further exploration of neuropsychological correlates of executive function as assessed during resting state, it will be important for future studies to replicate our findings and examine their possible dysfunction in psychiatric and neurological disorders. In addition to replication and extension, future studies should consider genetic and behavioral variation that could account for differences between high and low EF individuals’ resting-state functional connectivity.
4.5 Conclusion
In a large group of individuals, we demonstrate that the resting state architecture of the brain is associated with individual differences in different aspects of a theoretically motivated framework of EF – the unity and diversity model. The results are notable for providing a fine-grained picture of the relationships with specific regions within and outside of well-known RSNs. In the case of common EF, individuals higher in EF had greater recruitment of cerebellar regions within a group-identified frontoparietal RSN, and increased coupling of a frontopolar region to an attention RSN. Those individuals higher in shifting-specific EF had increased coupling of angular gyrus and a somatomotor/attention network. The current study significantly expands our knowledge of neural influences on EF, showing that variability in EF across individuals man be sculpted by patterns of resting-state functional connectivity within and between large-scale cognitive brain networks.
Table 1. Dual Regression results.
summarizes each dual regression result. Center of mass is provided for each cluster for the purposes of anatomical localization.
| Executive Function | IC | Center of Mass | p-value TFCE-corrected |
cluster size 4mm3 (2mm3) |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Common EF | 5 | −37 | −70 | −40 | 0.037 | 3 (24) |
| Common EF | 14 | −26 | 55 | 4 | 0.028 | 3 (24) |
| Common EF | 14 | 21 | −42 | −40 | 0.035 | 3 (24) |
| Shifting-specific EF | 11 | −58 | −58 | 36 | 0.007 | 31 (248) |
EF = executive function. IC = independent component. TFCE = threshold-free cluster enhancement.
Acknowledgments
This work was supported by an NIMH-funded Interdisciplinary Behavioral Science Center grant (P50 MH079485). The authors thank Harry Smolker and Amy Turner for assistance with data collection, Tim Curran and Randy O’Reilly for their discussion and feedback on an early draft of the manuscript, and Jessica Bernard for valuable discussion related to this project.
References
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychology Review. 2006;16(1):17–142. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network’s role in spontaneous cognition. Journal of Neurophysiology. 2010;104(1):322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18(2):89–94. [Google Scholar]
- Beckmann CF, Mackay CE, Filippini N, Smith SM. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. OHBM. 2009 [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic Independent Component Analysis for Functional Magnetic Resonance Imaging. IEEE Trans. Med. Imaging. 2004;1(23):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bernard Ja, Peltier SJ, Wiggins JL, Jaeggi SM, Buschkuehl M, Fling BW, Seidler RD. Disrupted cortico-cerebellar connectivity in older adults. NeuroImage. 2013;83:103–119. doi: 10.1016/j.neuroimage.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang-Vu TT, Laureys S. Intrinsic brain activity in altered states of consciousness: How conscious is the default mode of brain function? Annals of the New York Academy of Sciences. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Sciences. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80(3):807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Keramatian K, Gordon AM, Smith R, Mädler B. Prefrontal organization of cognitive control according to levels of abstraction. Brain Research. 2009;1286:94–105. doi: 10.1016/j.brainres.2009.05.096. [DOI] [PubMed] [Google Scholar]
- Cole MW, Yarkoni T, Repovs G, Anticevic A, Braver TS. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(26):8988–8999. doi: 10.1523/JNEUROSCI.0536-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, Salmon E. Exploring the unity and diversity of the neural substrates of executive functioning. Human Brain Mapping. 2005;25(4):409–423. doi: 10.1002/hbm.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Human Brain Mapping. 2005;25(1):22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Activities and Programs That Improve Children’s Executive Functions. Current Directions in Psychological Science. 2012;21(5):335–341. doi: 10.1177/0963721412453722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G, Naveau M, Petit L, Zago L, Crivello F, Jobard G, Joliot M. Patterns of hemodynamic low-frequency oscillations in the brain are modulated by the nature of free thought during rest. NeuroImage. 2012;59(4):3194–3200. doi: 10.1016/j.neuroimage.2011.11.059. [DOI] [PubMed] [Google Scholar]
- Esterman M, Chiu Y-C, Tamber-Rosenau BJ, Yantis S. Decoding cognitive control in human parietal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(42):17974–17979. doi: 10.1073/pnas.0903593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews. Neuroscience. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cognitive, Affective & Behavioral Neuroscience. 2001;1(2):137–160. doi: 10.3758/cabn.1.2.137. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12467110. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Altamirano LJ, Miyake A, Young SE, Corley RP, Hewitt JK. Etiology of stability and change in executive functions from late adolescence to adulthood. Presented at the Annual Meeting of the Behavioral Genetics Association; Edinburgh, UK. 2012. [Google Scholar]
- Friedman NP, Miyake A, Altamirano LJ, Corley RP, Young SE, Rhea SA, Hewitt JK. Stability and Change in Executive Function Abilities From Late Adolescence to Early Adulthood: A Longitudinal Twin Study. doi: 10.1037/dev0000075. (in preparation). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology. General. 2008;137(2):201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Winkler aM, Kochunov P, Almasy L, Duggirala R, Carless Ma, Blangero J. Genetic control over the resting brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(3):1223–1228. doi: 10.1073/pnas.0909969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Breeden AL, Bean SE, Vaidya CJ. Working memory-related changes in functional connectivity persist beyond task disengagement. Human Brain Mapping. 2012 doi: 10.1002/hbm.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, Vainionpää V, Reiss AL, Menon V. Persistent default-mode network connectivity during light sedation. Human Brain Mapping. 2008;29(7):839–847. doi: 10.1002/hbm.20537. Persistent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CC, Kurth F, Zhou J, Mayer Ea, Eickhoff SB, Kramer JH, Seeley WW. One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. NeuroImage. 2012 doi: 10.1016/j.neuroimage.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends in Cognitive Sciences. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain Connectivity Related to Working Memory Performance. The Journal of Neuroscience. 2006;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd SA, Banich MT, O’Reilly RC. Neural Mechanisms of Cognitive Control: An Integrative Model of Stroop Task Performance and fMRI Data. Journal of Cognitive Neuroscience. 2006;18(1):22–32. doi: 10.1162/089892906775250012. [DOI] [PubMed] [Google Scholar]
- Herd SA, O’Reilly RC, Hazy TE, Chatham CH, Brant AM, Friedman NP. Neural mechanisms underlying individual differences in the task switching component of execuitve function. 2013 [Google Scholar]
- Ivry RB, Keele SW. Timing functions of the cerebellum. Journal of Cognitive Neuroscience. 1989;1(2):136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(23):8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12968006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(41):17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U, Kliegl R. Task-set switching and long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(5):1124–1140. doi: 10.1037//0278-7393.26.5.1124. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11(1):103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Current Directions in Psychological Science. 2012;21(1):8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognitive Psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Frank MJ. Making working memory work: A computational model of learning in the prefrontal cortex and basal ganglia. Neural Computation. 2006;18(2):283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Orr JM, Banich MT. The neural mechanisms underlying internally and externally guided task selection. NeuroImage. 2014;84:191–205. doi: 10.1016/j.neuroimage.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nature Reviews. Neuroscience. 2006;7(7):511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Reviews. Neuroscience. 2004;5(3):184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Sakai K. Task set and prefrontal cortex. Annual Review of Neuroscience. 2008;31:219–245. doi: 10.1146/annurev.neuro.31.060407.125642. [DOI] [PubMed] [Google Scholar]
- Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O, Carlson S. Cognitive and motor loops of the human cerebro-cerebellar system. Journal of Cognitive Neuroscience. 2010;22(11):2663–2676. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- Schaeffer DJ, Amlung MT, Li Q, Krafft CE, Austin BP, Dyckman Ka, McDowell JE. Neural correlates of behavioral variation in healthy adults’ antisaccade performance. Psychophysiology. 2013;50(4):325–333. doi: 10.1111/psyp.12030. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Milham MP. The resting brain: unconstrained yet reliable. Cerebral Cortex (New York, N.Y.: 1991) 2009;19(10):2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex (New York, N.Y.: 1991) 2012;22(1):158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Beckmann CF. Correspondence of the brain’ s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolker HR, Depue BE, Reineberg AE, Orr JM, Banich MT. Individual differences in regional prefrontal gray matter morphometry and fractional anisotropy are associated with different constructs of executive function. Brain Structure & Function. 2014 doi: 10.1007/s00429-014-0723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cerebral Cortex (New York, N.Y.: 1991) 2010;20(8):1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65(2):280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Magnuson ME, Merritt MD, Schwarb H, Pan W-J, McKinley A, Keilholz SD. Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Human Brain Mapping. 2012 May;00 doi: 10.1002/hbm.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen Da, Greicius MD, Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cerebral Cortex (New York, N.Y.: 1991) 2010;20(11):2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage. 2004;22(4):1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wig GS, Schlaggar BL, Petersen SE. Concepts and principles in the analysis of brain networks. Annals of the New York Academy of Sciences. 2011;1224:126–146. doi: 10.1111/j.1749-6632.2010.05947.x. [DOI] [PubMed] [Google Scholar]
- Wilcox RR, Keselman HJ. Modern robust data analysis methods: measures of central tendency. Psychological Methods. 2003;8(3):254–274. doi: 10.1037/1082-989X.8.3.254. [DOI] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yntema DB. Keeping track of several things at oncce. Human Factors: The Journal of the Human Factors and Ergonomics Society. 1963;5(7):7–17. doi: 10.1177/001872086300500102. [DOI] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain’s dark energy. Nature Reviews. Neurology. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li CR. Functional networks for cognitive control in a stop signal task: independent component analysis. Human Brain Mapping. 2012;33(1):89–104. doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]