Abstract
Ca2+/calmodulin-dependent regulation of voltage-gated CaV1-2 Ca2+ channels exhibits extraordinary modes of spatial Ca2+ decoding and channel modulation1–6, vital for many biological functions6–9. A single calmodulin (CaM) constitutively associates with the channel carboxy tail3,10–13, and Ca2+ binding to the C- and N-terminal lobes of CaM can each induce distinct channel regulations2,14. As expected from close channel proximity, the C-lobe responds to the ~100 µM Ca2+ pulses driven by the associated channel15,16, a behavior defined as ‘local Ca2+ selectivity.’ Conversely, all prior observations indicate that the N-lobe somehow senses the far weaker signals from distant Ca2+ sources2,3,17,18. This ‘global Ca2+ selectivity’ satisfies a general signaling requirement, enabling a resident molecule to remotely sense cellular Ca2+ activity, which would otherwise be overshadowed by Ca2+ entry through the host channel5,6. Here, we report that the spatial Ca2+ selectivity of N-lobe CaM regulation is not invariably global, but can be switched by a novel Ca2+/CaM binding site within the amino terminus of channels (NSCaTE, N-terminal Spatial Ca2+ Transforming Element). Native CaV2.2 channels lack this element, and display N-lobe regulation with a global selectivity. Upon introducing NSCaTE into these channels, spatial Ca2+ selectivity transforms from a global to local profile. Given this effect, we examine CaV1.2/CaV1.3 channels, which naturally contain NSCaTE, and find that their N-lobe selectivity is indeed local. Disruption of this element produces a global selectivity, confirming the native function of NSCaTE. Thus differences in spatial selectivity between advanced CaV1 and CaV2 channel isoforms are explained by the presence or absence of NSCaTE. Beyond functional effects, the position of NSCaTE on the channel amino terminus indicates that CaM can bridge the amino and carboxy termini of channels. Finally, the modularity of NSCaTE offers practical means to understand the basis of global Ca2+ selectivity19.
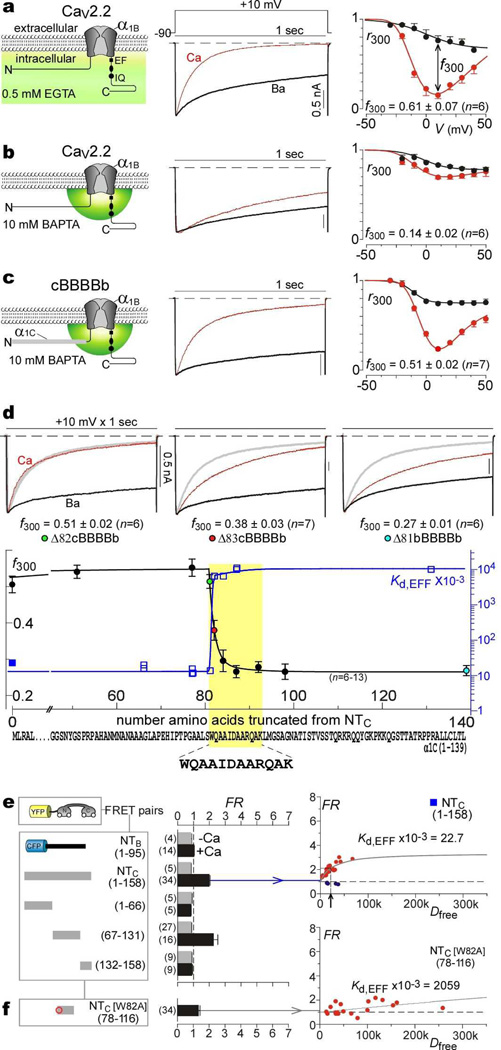
Figs. 1a, b display a prototypic example of global Ca2+ regulation induced by Ca2+ binding to the N-lobe of CaM, here driving Ca2+-dependent inactivation (CDI) of CaV2.2 channels3. Upon channel activation by a depolarizing voltage step (Fig. 1a), this CDI manifests as the accelerated decay of Ca2+ (middle, red trace) versus Ba2+ current (black trace). The baseline decay in Ba2+, which binds poorly to CaM2, reflects a separate voltage-dependent inactivation process7. In population data (right), the fraction of peak Ca2+ current remaining after a 300 ms depolarization, r300, is a U-shaped function of voltage (red circles), providing a hallmark of CDI20. The corresponding Ba2+ relation (black circles) shows a weak monotonic decline, and the difference between Ca2+ and Ba2+ relations (f300) quantifies pure CDI20. In accord with a thus far invariant rule3,6, N-lobe regulation of Ca2+ channels is selective for global Ca2+ elevation arising from spatially distant sources. Such global crosstalk is permitted under modest intracellular Ca2+ buffering18 that approximates physiological conditions (Fig. 1a, left, shading with 0.5 mM EGTA). Thus, strong buffering that localizes Ca2+ to channel nanodomains15,16 (Fig. 1b, left, small hemisphere in 10 mM BAPTA) nearly eliminates CDI (Fig. 1b, middle and right).
Figure 1. Transformation of spatial Ca2+ selectivity in CaV2.2 channels.
a, CaV2.2 CDI, low buffering. Left, cartoon of global Ca2+ elevation. Middle, exemplar traces in Ca2+ (red) and Ba2+ (black). Throughout, current bar references Ca2+ trace; Ba2+ trace normalized to Ca2+ peak. Right, average CDI; f300 at 10 mV; data, mean ± SEM throughout; cell number in parentheses. b, CaV2.2 CDI, high buffering. Left, cartoon of local Ca2+ signaling. c, CDI of cBBBBb. d, Localizing spatial Ca2+ transforming element (yellow highlight) within NTC. Top, exemplar currents for cBBBBb with amino-terminal deletions, (Δ82cBBBBb, removal of first 81 aa of cBBBBb; Δ81bBBBBb, removal of entire amino terminus of CaV2.2). Bottom, f300 (high buffering) versus residues deleted from cBBBBb; left axis and circles with colors corresponding to exemplars above. Kd,EFF is also plotted on same abscissa; blue right axis and blue squares, with filled symbol corresponding to e. Extreme bottom, localized sequence for both CDI transformation and Ca2+/CaM binding within NTC (yellow highlight).
e, f, FRET for YFP–CaM versus channel amino-terminal segments tagged with CFP. Left, construct schematics. Middle, FRET ratios (FR). Right, exemplar binding curves, with red symbols for Ca2+/CaM, and blue symbols for apoCaM (e, arrow shows Kd,EFF).
Previous splice variations, mutations, and chimeras have at best altered the strength of such CDI2,3,21, making global Ca2+ preference appear immutable. Here, however, when the amino terminus of CaV1.2 channels (NTC) was substituted into CaV2.2, the resulting ‘cBBBBb’ chimera exhibited strong N-lobe CDI in high buffering (Fig. 1c, Supplementary Information 1.1). Introducing NTC into CaV2.1 produced an analogous result (data not shown), thus generalizing the effect across the CaV2 family. This conversion to local Ca2+ selectivity is unprecedented, especially given the preponderance of known structural determinants for CDI on the carboxy termini of channels22.
To explore the basis of this effect, we undertook progressive amino-terminal deletions from the NTC segment within the cBBBBb chimera. Deleting the first 81 residues (Δ82cBBBBb) completely spared CDI in high buffering (Fig. 1d, top, left), where the gray trace shows the full-length NTC profile. By contrast, removing just one more residue (82W) significantly reduced CDI (Fig. 1d, top, middle), and additional deletion further suppressed CDI (Fig. 1d, top, right). Explicit correlation of CDI strength with NTC deletion corroborated these trends (Fig. 1d, bottom, left axis, circles), localizing the impact to a short contiguous region (yellow highlight).
What could be the essential mechanistic ingredient of this locus? We reasoned that this segment might orchestrate special molecular interactions with cytoplasmic channel loops or modulatory ligands, and thus probed for such associations using a live-cell FRET two-hybrid assay13. Using this approach, with EYFP fused to CaM (EYFP–CaM), and ECFP to various portions of NTC or the CaV2.2 amino terminus (NTB), we found a unique ability of NTC to bind Ca2+/CaM within the intracellular milieu of HEK293 cells (Fig. 1e). To start, NTB failed to display FRET interaction with CaM, either in Ca2+-free or Ca2+-bound states (Fig. 1e, middle column, topmost bars). Specifically, the optical parameter FR was ~1, signifying the absence of FRET13. By contrast, NTC clearly interacted with Ca2+/CaM (FR ~2), but not apoCaM (Ca2+-free CaM). Trisection of NTC localized Ca2+/CaM binding to the middle third (Fig. 1e, middle column, residues 67–131), and further deletions identified a sharp decrease in FRET upon removal of W82 (Supplementary Information 1.2). Since FR depends on the fractional binding between interacting partners, cell-to-cell variation in expression permitted resolution of binding curves, each specifying a relative dissociation constant Kd,EFF13 (Fig. 1e, right column, arrow). Alignments of these NTC fragments and their Kd,EFF values (Fig. 1d, squares, Supplementary Information 1.2) localized a CaM-binding segment that coincides well with the critical CDI segment (Fig. 1d, yellow highlight). Notably, a W82A point mutation suppressed Ca2+/CaM interaction with NTC (Fig. 1f), fitting with the drop in CDI of the corresponding channel truncation (Fig. 1d, top row, middle). Thus, the transformation of spatial Ca2+ selectivity correlates with a novel Ca2+/CaM interaction site within NTC.
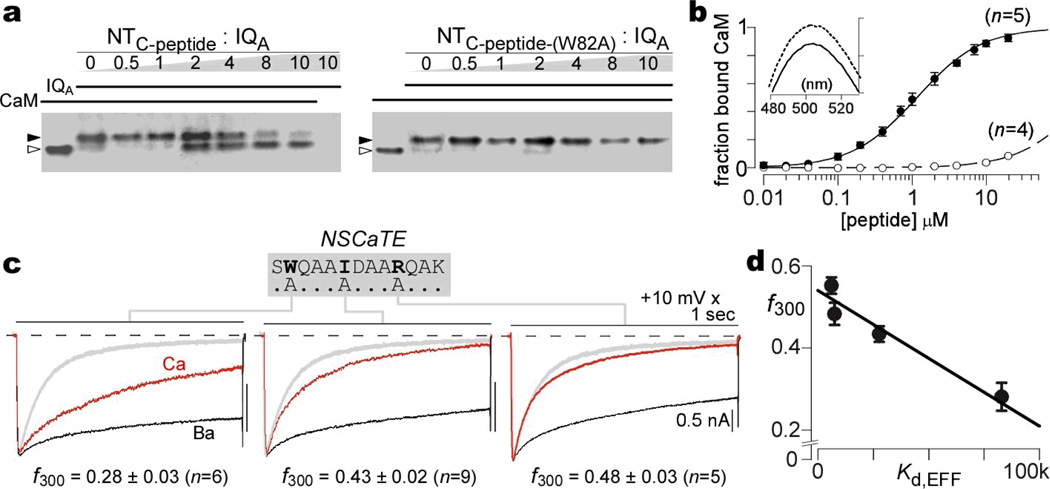
This connection was deepened in two ways (Fig. 2). First, the in situ FRET approach was confirmed by in vitro assays, pairing purified Ca2+/CaM with a synthetic peptide (SWQAAIDAARQAKLMGSA) spanning the key NTC binding region. Without peptides, CaM migrated rapidly under non-denaturing PAGE2 (Fig. 2a, left gel, lane 1, open triangle). When fully complexed with the IQ domain peptide of CaV2.1 (IQA), CaM exhibited a known slowing of mobility2 (lane 2, filled triangle). Increasing NTC-peptide produced progressive conversion to the faster mobility species (lanes 3–8), indicating competitive binding to CaM. By contrast, a mutant W82A peptide (NTC-peptide-(W82A), right gel) was unable to bind CaM. More quantitatively, NTC-peptide depressed the emission spectrum of dansylated CaM (Fig. 2b, inset), allowing resolution of a 1:1 binding curve with a Kd of 1.2 µM (Fig. 2b, black symbols). NTC-peptide-(W82A) exhibited no such interaction (Fig. 2b, open symbols). These in vitro assays established direct Ca2+/CaM binding to the NTC core region.
Figure 2. Direct CaM binding and mapping of key NSCaTE residues.
a, Competitive gel mobility shift assay, confirming Ca2+/CaM interaction with NTC-peptide (left), not mutant NTC-peptide-(W82A) (right). b, Dansylated Ca2+/CaM spectrofluorometry. Binding with NTC-peptide (filled circles), not with mutant NTC-peptide-(W82A) (open circles), both plotted as mean ± SEM. Inset, NTC-peptide decreases emission spectrum. c, CDI in high buffering, for Δ78cBBBBb channels with point mutations as labeled. Format as in Fig. 1a; gray trace shows baseline Δ78cBBBBb profile. d, Close correlation of CDI in high buffering with NTC module affinity for Ca2+/CaM34 (Supplemmentary Information 2.1).
Second, further in-depth mapping refined the correlation between spatial Ca2+ selectivity and CaM binding to NTC. Alanine point mutations were introduced into the NTC domain of cBBBBb channels, targeting key sites inferred from a preliminary report19. These manipulations reduced CDI (in high buffering) with the rank order: W82A > I86A > R90A (Fig. 2c). Reassuringly, these changes in CDI (f300) correlated closely with the relative dissociation constant (Kd,EFF) for CaM interaction with corresponding mutant NTC peptides, as determined by FRET (Fig. 2d, Supplementary Information 2.1). Given the strong function and affiliation with CaM binding to the core NTC locus, we named this element NSCaTE (Fig. 2c, top N-terminal Spatial Ca2+ Transforming Element).
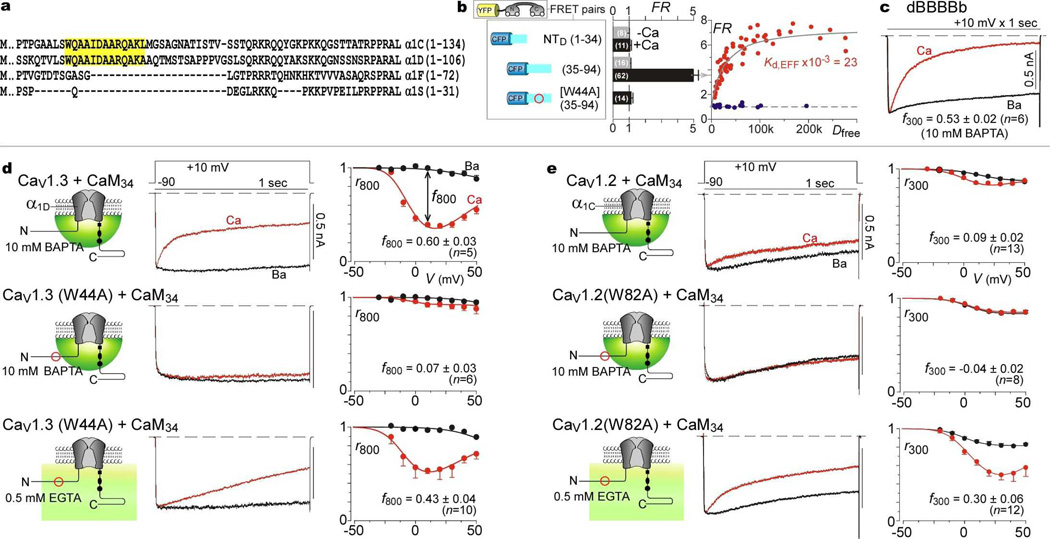
While this module impacts chimeric channels, does NSCaTE function within naturally occurring channels? Alignments of various CaV1-2 Ca2+ channels (Fig. 3a) revealed that NSCaTE is present not only in CaV1.2 (α1C), but also CaV1.3 (α1D). Our attention was foremost drawn to NSCaTE in CaV1.3, as these channels exhibit two distinct and robust forms of CDI, each selectively triggered by Ca2+ binding to a different lobe of CaM14. As a preliminary test for the functionality of the CaV1.3 NSCaTE, we checked for Ca2+/CaM interaction (Fig. 3b). FRET assays showed selective Ca2+/CaM interaction with the midsection of the CaV1.3 amino terminus (Fig. 3b, NTD-(35–94)), yielding a Kd,EFF on par with NTC analogs (Fig. 1e). Consistent with W82A effects in NTC (Fig. 1f), mutating the analogous tryptophan in NTD (NTD-W44A-(35–94)) eliminated Ca2+/CaM interaction (Fig. 3b, Supplementary Information 3.1). Substituting NTD into CaV2.2 (yielding the dBBBBb chimera) also produced strong CDI in high buffering (Fig. 3c, Supplementary Information 3.1), confirming the transforming capacity of the CaV1.3 NSCaTE.
Figure 3. NSCaTE transforms spatial Ca2+ selectivity in native CaV1 channels.
a, α1 alignment, CaV1 channels. b, FRET assays. Format as in Fig. 1e. c, CDI of dBBBBb in high buffering. d, Impact of native NSCaTE upon N-lobe CDI of CaV1.3. Top, CDI persists in high buffering. Middle, CDI elimination by W44A mutation. Bottom, CDI reappearance in W44A mutant under low buffering. Format as in Fig. 1a, except for 800-msec metrics. e, Native NSCaTE effects in CaV1.2. Format as in d, with 300-msec metrics.
Encouraged by these preliminary results, we asked whether the intrinsic NSCaTE imparts local selectivity to the native CaV1.3, contrary to the current dogma that the N-lobe invariably senses global Ca2+. In CaV1.3, the N-lobe form of CDI can be isolated by coexpresing a mutant CaM (CaM34) in which Ca2+ binding is restricted to the N-lobe2,20. Thus far, such CDI has only been studied under modest Ca2+ buffering14. Here, even under high buffering, N-lobe CDI was pronounced (Fig. 3d, top row). More telling, a W44A mutation within CaV1.3 nearly eliminated this CDI (middle row), as did deletion of this region (Supplementary Information 3.2). To exclude indiscriminate disruption of CDI, we confirmed that CDI resurfaced under modest buffering permissive of global signaling (Fig. 3d, bottom row, Supplementary Information 3.2). Importantly, CDI was entirely CaM-mediated under reduced buffering14 (Supplementary Information 3.3); and C-lobe CDI was unaffected by like mutations19. Hence, the native CaV1.3 NSCaTE endows N-lobe CDI with a local selectivity, and reducing Ca2+/CaM binding to NSCaTE switches this selectivity towards a global profile.
Returning to native CaV1.2, coarse identification of Ca2+/CaM binding to the amino terminus of these channels has been reported, but the functional consequences have been unclear23. In particular, the CaM-mediated CDI of these channels has been attributed only to the C-lobe of CaM7,20; apparently absent has been an N-lobe form of CDI, the target of NSCATE effects. Here, however, upon co-transfection of CaV1.2 with CaM34, to fully isolate a potential N-lobe component, small but unmistakable CDI is seen during prolonged 1-sec depolarization, even in high buffering (Fig. 3e, top row). As well, a W82A mutation abolished this CDI (middle row), but spares it under global Ca2+ signaling (bottom row). A more drastic deletion of the native NSCaTE produced an identical effect (Supplementary Information 3.4). Again, the globally signaled CDI remains entirely CaM dependent (Supplementary Information 3.5). Hence, NSCaTE functions similarly in native CaV1.2 and CaV1.3; the weaker N-lobe CDI of CaV1.2 is expected from its lower open probability19,24. These results generalize the lessons of chimeric channels to the operation of native channels and constitute the first examples where the N-lobe of CaM shows local Ca2+ selectivity.
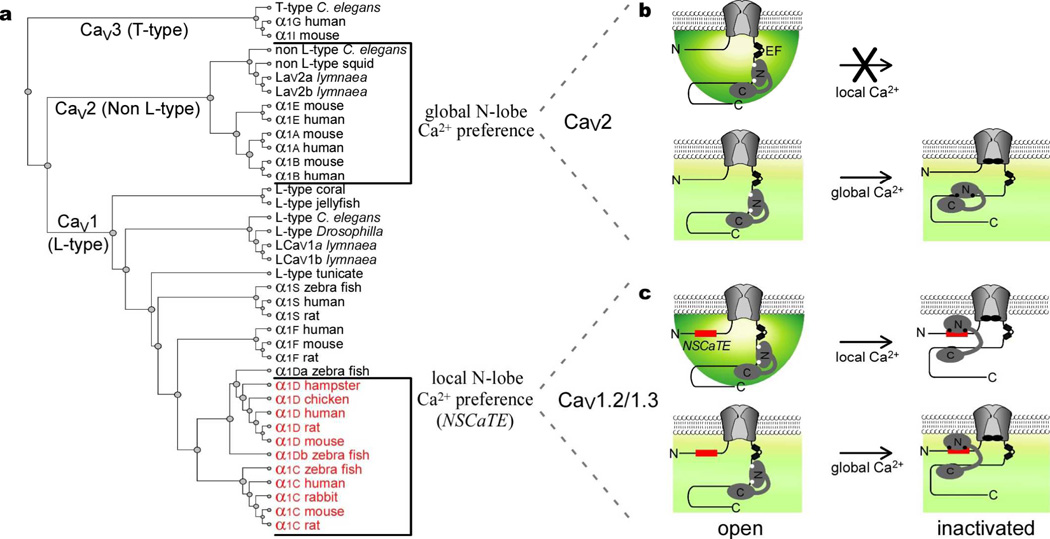
In all, the traditionally global spatial Ca2+ selectivity of channel regulation by the N-lobe3,6 is not invariant, but can be transformed towards a local selectivity by NSCaTE, a novel Ca2+/CaM binding site unrecognized by current motif-detection algorithms25. Four perspectives emerge. First, the presence of NSCaTE in CaV1.2 and CaV1.3—along with its absence in CaV2 channels—underlies the contrasting spatial Ca2+ preferences of N-lobe CDI between these two channel clades (Fig. 4a). Previously, N-lobe regulation had been viewed as always having a global Ca2+ selectivity3,6, which is true for CaV2 channels (Fig. 4b). We now recognize that the analogous N-lobe CDI of CaV1.2/CaV1.3 channels14 exhibits local selectivity, owing to an intrinsic NSCaTE (Fig. 4c). Second, NSCaTE promises vital adjustments of spatial selectivity in diverse biological contexts. NSCaTE would customize CaV1.2/1.3 regulation for local Ca2+ signaling in cardiac dyads4,7, and for neuronal Ca2+ entry whose precise regulation is essential for long-term synaptic plasticity26,27. Of further intrigue, a naturally occurring CaV1.2 splice variant features a premature stop codon just after the channel amino terminus21. The predicted protein product contains NSCaTE, and may exert a dominant negative effect. Although coexpressing NTC and CaV1.3 left CDI unchanged in HEK293 cells (data not shown), additional mechanisms may exist in native tissues to render this splice product active. Third, unlike most functional motifs, which are pervasive from bacteria through advanced mammals25, NSCaTE is only found in a subset of CaV1 channels from advanced species (Fig. 4a, red), and in multiple bacterial proteins (Supplementary Information 4). A prokaryotic NSCaTE from Xanthomonas may in fact bind endogenous CaM-like molecules (Supplementary Information 4), hinting that channel and bacterial motifs share a common heritage. Finally, NSCaTE furnishes valuable structural and mechanistic insights. For structure, we deduce that CaM can bridge the carboxy and amino termini of CaV1.2/CaV1.3 channels (Fig. 4c, right), as follows. This study establishes Ca2+-bound N-lobe interaction with NSCaTE on the channel amino terminus. By contrast, prior reports emphasize that regulation by the C-lobe involves its Ca2+-bound and Ca2+-free interactions with the channel carboxy terminus12,20,28. Moreover, a single resident CaM orchestrates both C- and N-lobe regulation10,11, where both coexist in CaV1.2/CaV1.3 channels14,20. Finally, channel amino and carboxy termini are in close proximity, and move with channel gating29. Hence, this bridging appears probable, expanding a theme where the lobes of CaM crosslink separate parts of a molecule30. As for mechanism, the modularity of NSCaTE indicates that spatial Ca2+ preference is not hopelessly intertwined within holochannel structure. Manipulating this compact element promises valuable insights into the elusive mechanism underlying spatial Ca2+ selectivity6,19.
Figure 4. Functional and structural properties for NSCaTE switching of spatial Ca2+ selectivity.
a, CaV1-2 dendrogram, based on α1 subunits (Supplementary Information 4.1). Red, NSCaTE-containing channels. b, Schematic of N-lobe CDI in CaV2 channels. Absence of NSCaTE yields global Ca2+ selectivity. Black circles on CaM, Ca2+ ions. c, N-lobe CDI in CaV1 channels that contain NSCaTE (red) exhibit local Ca2+ selectivity. CaM bridges amino and carboxy termini of inactivated channels (right).
Methods
Molecular Biology
The α1cBBBBb subunit was the published α1cBBBBb/pcDNA3 construct31. PCR was used to truncate the amino terminus of this construct: the forward primer, containing a unique EcoRI site and Kozak start, determined the extent of truncation; the reverse primer annealed downstream of a unique AgeI site. PCR fragments were ligated into α1cBBBBb/pcDNA3 via EcoRI/AgeI. Mutations to NSCaTE were introduced into the α1Δ78cBBBBb truncation construct (starts with 78th residue of α1C), using forward primers as above, but containing desired mutations. For chimeric α1dBBBBb, PCR introduced a silent and unique BsiWI site at residue 83 of α1B/pcDNA3, yielding α1B_BsiWI+. The amino terminus of α1D32 was PCR amplified, then ligated into unique EcoRI and BsiWI sites of α1B_BsiWI+, yielding α1dBBBBb. The α1B (CaV2.2) used here is a variant of the human α1B reported previously33; it is identical but for V354M and R369S variations. This variant backbone was used for all constructs, including α1dBBBBb and the previously published α1cBBBBb/pcDNA331. For completeness, we confirmed that transforming NSCaTE effects were also present using the more common α1B backbone for α1cBBBBb and α1dBBBBb. A 141-residue amino-terminal truncation of the CaV1.2 α1C subunit (α1C[NTΔ]) was generated by PCR amplifying residues 142–611 from α1C34, followed by ligation into the parental α1C via unique KpnI and StuI sites. A 44-residue amino-terminal truncation of the CaV1.3 α1D subunit (α1D[NTΔ]) was similarly constructed by PCR amplifying residues 45–180 from α1D32, followed by ligation into α1D via unique NheI and BsiWI sites. The mutant α1C[W82A] was made by PCR amplifying α1C residues 1–87, using a reverse primer containing a W82A mutation. This product was ligated into α1C via unique HindIII and ClaI sites. The mutant α1D[W44A] subunit was as described19.
For FRET, all CFP–NTB/C/D constructs were made by PCR of desired segments of α1cBBBBb or α1D, followed by substitution of CaMWT in a published CFP–CaMWT/pcDNA3 clone13, using unique Not I and Xba I sites. For CFP fusion to the NSCaTE of the TonB Dependent Receptor (TBDR, amino acids 464–484 of YP_242632.1), annealed synthetic primers encoding this motif (mammalian optimized) were ligated into unique NotI/XbaI sites of CFP–CaMWT/pcDNA3. Throughout, all PCR amplified segments were fully verified by sequencing.
Electrophysiology
HEK293 cells were transiently transfected using a calcium phosphate protocol20. Cells were co-transfected with 8 µg of rat brain β2a35, 8 µg of rat brain α2δ36, and 1–8 µg of Ca2+ channel α1 subunit. All α1C and α1D constructs were co-transfected with 2 µg of SV40 T antigen to enhance expression; 8 µg of cDNA for rat brain CaM12, CaM34, or CaM1234 was added as required20.
Room temperature, whole-cell recordings were performed 1–3 days after transfection, using Axopatch 200A/B amplifiers (Axon Instruments). P/8 leak subtraction was used, with series resistances of 1–2 MΩ after >70% compensation. Currents were filtered at 2 kHz (4-pole Bessel), and sampled at 10 kHz. For high-buffer experiments, internal solutions contained, (in mM): CsMeSO3, 114; CsCl2, 5; MgCl2, 1; MgATP, 4; HEPES (pH 7.4), 10; and BAPTA, 10; at 295 mOsm adjusted with CsMeSO3. For low-buffer experiments, (in mM): CsMeSO3, 135; CsCl2, 5; MgCl2, 1; MgATP, 4; HEPES (pH 7.4), 10; and EGTA, 0.5. In all experiments, except those with CaV1.2/1.3, external solutions contained (in mM): TEA-MeSO3, 140; HEPES (pH 7.4), 10; and CaCl2 or BaCl2, 5; at 300 mOsm, adjusted with TEA-MeSO3. Due to lower CaV1.2 expression, 20 mM CaCl2 or BaCl2 was used during high internal buffering, and 10 mM during low buffering. CaV1.3 recordings were made in 40 mM CaCl2 or BaCl2. A holding potential of −90 mV and 60 s repetition interval were used throughout. Data were analyzed by custom MATLAB software (Mathworks, MA), with average data shown as mean ± SEM.
FRET two-hybrid assay
Fluorescence resonance energy transfer (FRET) two-hybrid experiments were carried out in HEK293 cells and analyzed as described13. During imaging, the bath solution was a Tyrode’s buffer containing 10 mM Ca2+, where 5 µM ionomycin (Sigma-Aldrich, MO) was added for Ca2+/CaM experiments. Concentration-dependent spurious FRET was subtracted from the raw data prior to binding-curve analysis37.
Gel mobility shift assay
We used a non-denaturing, 13% acrylamide resolving gel, coupled with a 4% acrylamide stacking gel. 100 µM CaCl2 was added to the gel to maintain Ca2+/CaM binding. Peptide samples were freshly solubilized into a sample buffer: 20 mM Tris-Cl (pH=6.8), 150 mM NaCl, 2% glycerol, 10 mM CaCl2, 0.001% bromphenol blue dye, and 2 µg purified CaM protein7. Binding reactions (×1h) and electrophoresis were performed at ~0–4°C in 25 mM Tris (pH=8.4) and 200 mM glycine, with parameters of 50 V × 20 min, followed by 100 V × 2 h. Gels were stained over heat × 1 h with Commassie R-250 (Bio-Rad Laboratories, CA), and destained overnight20. Peptides were synthesized by the Synthesis and Sequencing Facility at the Johns Hopkins School of Medicine (Baltimore, MD). The wildtype NSCaTE peptide NTC-peptide was SWQAAIDAARQAKLMGS (rabbit α1C sequence), and the mutant was SAQAAIDAARQAKLMGS. The IQA peptide was KIYAAMMIMEYYRQSKAKKLQ (based on human α1A subunit2). IQA peptide was added to Ca2+/CaM in a molar ratio of 8:1 to produce a maximal binding shift. NSCaTE peptide was then added in varying amounts (Fig. 2a).
Dansyl CaM experiments
Purified recombinant CaM protein was dansylated with 0.5 mM dansyl-Cl under alkaline conditions at room temperature (0.3 mM CaCl2 × 4 hours). The reaction was terminated with 5 mM hydroxylamine, and Tris buffer added before dialysis against 10 mM MOPS (pH 7.2) at 4°C38. Peptides were as above. Spectrofluorometry was conducted at 25°C in 5 mM CaCl2, 150 mM NaCl, and 50 mM Tris-Cl (pH=7.5). Peptide stocks were freshly prepared and used within 6 h. 100 nM dansyl-CaM was excited at 340 nm, and fluorescence recorded from 400–650 nm (slit size, 3 nm excitation, 15 nm emission; integration time, 0.1 s), using a Fluorolog-3 spectrofluorometer (Horiba Jobin Yvon). No detectable bleaching was observed in controls. Binding curves were determined from the average of five 490-nm measurements (F490) at each peptide concentration. Subtraction of background fluorescence utilized a correction factor (mpeptide) derived from measurements of peptide alone (F490,bg-corrected = F490 – mpeptide · [peptide]). We calculated F490,full-corrected = F490,bg-corrected · Vtot / Vinitial (where Vtot is the final volume, and Vinitial was 2.5 mL), so as to correct for dilution by successive peptide additions. Fractional binding (B) was (F490,full-corrected,max - F490,full-corrected) / (F490,full-corrected,max - F490,full-corrected,min); least-square fits were performed using B = [peptide]k / ([peptide]k + Kdk), with resulting k ~1.
Supplementary Material
Acknowledgements
We thank Drs. Heather Agler and Masayuki Mori for early characterization of the cBBBBb chimeric channel, Drs. Carrie Iwema and Jonathan Pevsner for bioinformatics advice; King-Wai Yau, Eric Young, and members of the CSL lab for valuable comments. Supported by grants from the NINDS (to I.E.D.), NIGMS (to M.R.T.), and from the NIMH and NHLBI (to D.T.Y.)
References
- 1.Lee A, Scheuer T, Catterall WA. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J Neurosci. 2000;20:6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 3.Liang H, et al. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–960. doi: 10.1016/s0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- 4.Bootman MD, Lipp P, Berridge MJ. The organisation and functions of local Ca(2+) signals. J Cell Sci. 2001;114:2213–2222. doi: 10.1242/jcs.114.12.2213. [DOI] [PubMed] [Google Scholar]
- 5.Evans RM, Zamponi GW. Presynaptic Ca2+ channels--integration centers for neuronal signaling pathways. Trends Neurosci. 2006;29:617–624. doi: 10.1016/j.tins.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Dunlap K. Calcium channels are models of self-control. J Gen Physiol. 2007;129:379–383. doi: 10.1085/jgp.200709786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proc Natl Acad Sci U S A. 2002;99:17185–17190. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46:633–645. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 10.Mori MX, Erickson MG, Yue DT. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science. 2004;304:432–435. doi: 10.1126/science.1093490. [DOI] [PubMed] [Google Scholar]
- 11.Yang PS, Mori MX, Antony EA, Tadross MR, Yue DT. A single calmodulin imparts distinct N- and C-lobe regulatory processes to individual CaV1.3 channels (abstr.) Biophys. J. 2007;354a(Supplement) 1669-Platform. [Google Scholar]
- 12.Pitt GS, et al. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J Biol Chem. 2001;276:30794–30802. doi: 10.1074/jbc.M104959200. [DOI] [PubMed] [Google Scholar]
- 13.Erickson MG, Liang H, Mori MX, Yue DT. FRET two-hybrid mapping reveals function and location of L-type Ca2+ channel CaM preassociation. Neuron. 2003;39:97–107. doi: 10.1016/s0896-6273(03)00395-7. [DOI] [PubMed] [Google Scholar]
- 14.Yang PS, et al. Switching of Ca2+-dependent inactivation of Ca(v)1.3 channels by calcium binding proteins of auditory hair cells. J Neurosci. 2006;26:10677–10689. doi: 10.1523/JNEUROSCI.3236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- 16.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhuri D, Issa JB, Yue DT. Elementary Mechanisms Producing Facilitation of Cav2.1 (P/Q-type) Channels. J Gen Physiol. 2007;129:385–401. doi: 10.1085/jgp.200709749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song LS, Sham JS, Stern MD, Lakatta EG, Cheng H. Direct measurement of SR release flux by tracking 'Ca2+ spikes' in rat cardiac myocytes. J. Physiol. (Lond.) 1998;512:677–691. doi: 10.1111/j.1469-7793.1998.677bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tadross MR, Dick IE, Yue DT. Mechanism of Ca2+ decoding by the CaM/CaV channel complex (abstr.) Biophys. J. 2007;(Supplement):354a. 1670-Plat. [Google Scholar]
- 20.Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 21.Tang ZZ, et al. Transcript scanning reveals novel and extensive splice variations in human l-type voltage-gated calcium channel, Cav1.2 alpha1 subunit. J Biol Chem. 2004;279:44335–44343. doi: 10.1074/jbc.M407023200. [DOI] [PubMed] [Google Scholar]
- 22.Van Petegem F, Chatelain FC, Minor DL., Jr Insights into voltage-gated calcium channel regulation from the structure of the CaV1.2 IQ domain-Ca2+/calmodulin complex. Nat Struct Mol Biol. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanina T, Blumenstein Y, Shistik E, Barzilai R, Dascal N. Modulation of L-type Ca2+ channels by gbeta gamma and calmodulin via interactions with N and C termini of alpha 1C. J Biol Chem. 2000;275:39846–39854. doi: 10.1074/jbc.M005881200. [DOI] [PubMed] [Google Scholar]
- 24.de Leon M, et al. Essential Ca(2+)-binding motif for Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Science. 1995;270:1502–1506. doi: 10.1126/science.270.5241.1502. [DOI] [PubMed] [Google Scholar]
- 25.Rhoads AR, Friedberg F. Sequence motifs for calmodulin recognition. FASEB J. 1997;11:331–340. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- 26.Toescu EC, Verkhratsky A. The importance of being subtle: small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell. 2007;6:267–273. doi: 10.1111/j.1474-9726.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 27.Moosmang S, et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans J, Erickson MG, Anderson MJ, Yue DT. FRET-based mapping of calmodulin preassociation with P/Q-type Ca channels (abstr.) Biophys J. 2003;(Supplement) 2615-Pos. [Google Scholar]
- 29.Kobrinsky E, Schwartz E, Abernethy DR, Soldatov NM. Voltage-gated mobility of the Ca2+ channel cytoplasmic tails and its regulatory role. J Biol Chem. 2003;278:5021–5028. doi: 10.1074/jbc.M211254200. [DOI] [PubMed] [Google Scholar]
- 30.Drum CL, et al. Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature. 2002;415:396–402. doi: 10.1038/415396a. [DOI] [PubMed] [Google Scholar]
references below in Methods
- 31.Agler HL, et al. G protein-gated inhibitory module of N-type (CaV2.2) Ca2+ channels. Neuron. 2005;46:891–904. doi: 10.1016/j.neuron.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Xu W, Lipscombe D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams ME, et al. Structure and functional expression of an omega-conotoxin- sensitive human N-type calcium channel. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- 34.Wei XY, et al. Heterologous regulation of the cardiac Ca2+ channel alpha 1 subunit by skeletal muscle beta and gamma subunits. Implications for the structure of cardiac L-type Ca2+ channels. J. Biol. Chem. 1991;266:21943–21947. [PubMed] [Google Scholar]
- 35.Perez-Reyes E, et al. Cloning and expression of a cardiac/brain beta subunit of the L- type calcium channel. J. Biol. Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- 36.Tomlinson WJ, et al. Functional properties of a neuronal class C L-type calcium channel. Neuropharmacology. 1993;32:1117–1126. doi: 10.1016/0028-3908(93)90006-o. [DOI] [PubMed] [Google Scholar]
- 37.Stratton J, Evans J, Erickson MG, Alvania RS, Yue DT. The nature of concentration-dependent spurious FRET arising from CFP and YFP (abstr.) Biophys J. 2004;86:317a. [Google Scholar]
- 38.Kincaid RL, Billingsley ML, Vaughan M. Preparation of fluorescent, cross-linking, and biotinylated calmodulin derivatives and their use in studies of calmodulin-activated phosphodiesterase and protein phosphatase. Methods Enzymol. 1988;159:605–626. doi: 10.1016/0076-6879(88)59058-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.