Abstract
The recombinant baculoviruses were constructed to investigate the necessity of VSV-G pseudotyping for mammalian cell transduction. The viruses were designed to express green fluorescent protein (GFP) gene under the control of cytomegalovirus promoter, with or without pseudotyping with VSV-G. VSV-G was placed under the control of polyhedrin promoter that is recognized by insect cells, allowing the formation of pseudotyped baculovirus. The study findings demonstrate that the pseudotyping of baculovirus significantly enhanced transduction efficiency compared to non-pseudotyped baculovirus, resulting in consequent distinction in the expression of GFP in mammalian cells. The results confirmed that pseudotyping is important for baculovirus mediated gene delivery. Further, when full-length VSV-G pseudotyping was compared with truncated VSV-G containing GED domain (G-stem of ectodomain in conjunction with the TM and CT domains of the glycoprotein), latter was relatively less efficient in transducing mammalian cells. This study demonstrated that pseudotyping with full-length VSV-G had better transduction efficiency in mammalian cells. However, at higher multiplicity of infection, both full-length and truncated VSV-G showed equivalent transduction. This study established the significance of pseudotyping of baculovirus with full-length VSV-G for efficient transduction of mammalian cells, utilizing the highly sensitive GFP marker system. These findings have significant implications in designing of baculovirus vector based antigen delivery for developing new generation vaccines.
Keywords: Baculovirus vector, VSV-G pseudotyping, Gene delivery, Mammalian cells
Introduction
Virus vectored gene delivery systems such as adenovirus, poxvirus, lentivirus and baculovirus, are useful in development of novel antigen delivery systems. Among these, baculovirus Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is gaining importance because it does not replicate in vertebrate hosts and thus with no health risk. AcMNPV are enveloped viruses having circular double stranded DNA (134 kbp) contained in 15–40 × 200–300 nm size rod shaped nucleocapsids [8]. They belong to genus Alpha baculovirus of Baculoviridae, that specifically infect insects in the order Lepidoptera [10]. The AcMNPV can however transduce mammalian cells, though its replication is restricted to insect cells [3]. Further, due to its high CpG content, baculovirus can stimulate TLR-9 mediated host antiviral innate immune responses [1, 17]. Therefore, the baculoviruses are safe to use as vectors for antigen gene delivery.
AcMNPV has envelope glycoprotein GP64, which plays role in binding to host cell receptors [20]. The GP64 has been utilized to pseudotype lentiviral vectors to transduce a variety of cell lines in vitro [15]. The GP64 mediated transduction was found to be efficient and therefore pseudotyping of baculovirus vector was reported to be redundant [14]. However, several researchers [9, 19] have reported the utility of vesicular stomatitis virus glycoprotein (VSV-G, 511 amino acids long) pseudotyping of baculovirus for gene delivery into mammalian systems. Furthermore, while some researchers have utilized full-length VSV-G for pseudotyping of baculovirus [9, 19], there are also reports that truncated VSV-G, termed as VSV-GED, having a 21-amino-acid G-stem of ectodomain in conjunction with the transmembrane (TM) and C-terminal (CT) domain, was sufficient and increased the transduction efficiency of baculovirus-mediated gene delivery [4, 11]. Thus far, there is no systematic comparative study on the identical platform investigating if VSV-G pseudotyping of the baculovirus is redundant given that the virus already has a similar functional protein, GP64. Also it is unclear whether the truncated VSV-G is sufficient for the pseudotyping. Therefore, to address these ambiguities, transduction efficiencies of baculovirus pseudotyped with full-length VSV-G and truncated VSV-G were evaluated in comparison with non-pseudotyped baculovirus, for the first time utilizing the sensitive green fluorescent protein marker system.
Materials and methods
Cells
Sf-9 and Sf-21 insect cells derived from Spodoptera frugiperda were grown in SF900-II medium (Invitrogen, USA) supplemented with 5 % fetal bovine Serum (FBS) (Life tech, USA) at 27 °C. Tn-5 (Trichoplusia ni) insect cells were cultured in SF900-II medium. BHK-21 cells were grown in Glasgow’s Modified Eagle’s Medium (GMEM) (SAFC Biosciences, USA) supplemented with 10 % FBS, at 37 °C with 5 % CO2. HEK 293 cells, PK-15 cells, A549 and CHO cells were grown in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10 % FBS, at 37 °C with 5 % CO2.
Antibody
Rabbit anti-VSV-G antibody (Sigma, USA) raised against a synthetic peptide corresponding to amino acids 497–511 of vesicular stomatitis virus glycoprotein, was used in the study.
Generation of baculovirus transfer vectors
At first the open reading frame (ORF) of AcGFP (Aequorea coerulescens green fluorescent protein) (Genbank accession#AB255038.1) was cloned into pcDNA 3.1 (+) (Invitrogen) under the cytomegalovirus (CMV) promoter. The generated pcDNA-AcGFP plasmid was digested with NruI and PsiI restriction enzymes to obtain the CMVp-AcGFP fragment having AcGFP between cytomegalovirus (CMV) promoter and bovine growth hormone poly A tail. The CMVp-AcGFP was ligated into AvrII treated and blunt ended pFastBacI vector (Invitrogen, USA), to obtain pFBac-AcGFP plasmid. For generating pseudotyped baculovirus, the pFBAc-AcGFP was used as background vector to construct pFBac-VSVG-AcGFP or pFBac-VSVG(T)-AcGFP, by inserting VSV-G ORF (GenBank accession#J02428.1) or its truncated version VSVG(T), respectively, under the control of polyhedrin promoter. The VSVG(T) contained amino acid residues 1–45 and 421–511, covering signal sequence and a 44-amino-acid ectodomain in conjunction with the TM and CT domain. The numbering of amino acid residues of VSV-G is with reference to unprocessed form of the protein containing signal sequence of 16 amino acid residues.
To monitor the expression of the CMVp-gene cassette by fluorescence microscopy, DNA (2 μg) from each of the pFBac-AcGFP, pFBac-VSVG-AcGFP and pFBac-VSVG(T)-AcGFP constructs was used to transfect 2 × 105 BHK-21 cells in a six-well tissue culture plate (Corning, India) by using Lipofectamine 2000 (Invitrogen, USA). All the three transfer plasmids produced green fluorescence in BHK-21 cells (data not shown), indicating the functionality of the AcGFP under the CMVp. To generate recombinant baculoviruses, the pFBac-VSVG-AcGFP, pFBac-AcGFP and pFBac-VSVG(T)-AcGFP transfer vectors were transformed into Max efficiency DH10 bac (Invitrogen) cells and recombinant bacmids were isolated from the larger white colonies. The bacmids after authentication by gene specific PCR amplification, were transfected into Sf-9 cells using Cellfectin II (Invitrogen) reagent. Baculovirus stocks were amplified several folds by infecting Sf-21 insect cell monolayer culture at multiplicity of infection (MOI) of 0.1 and harvesting the virus 72 h post-infection. Recombinant baculovirus stocks were titrated by plaque assay and characterized by PCR and western blotting.
Western blotting
To confirm the expression of VSV-G protein by the pseudotyped recombinant baculoviruses, they were infected in Tn-5 insect cells for 3 days and lysate from the infected cells were analysed by western blotting using VSV-G antibody, following the protocol described previously [2].
Transduction studies
To study the transduction efficiency, cells were seeded at a concentration of 2.5 × 105 cells/well in a six well tissue culture plate. The cells were infected with pseudotyped or non-pseudotyped recombinant baculoviruses at a MOI of 5. The cells were incubated for 2 h at 27 °C, replaced by fresh medium and incubated further at 37 °C. After 72 h of infection, the cells were visualized under fluorescence microscopy to image GFP expression. Further, the cells were trypsinized and resuspended in PBS. The cells were then charged into a haemocytometer to count the number of green fluorescent and the total cells under a fluorescence microscope. The percentage of fluorescent cells was calculated for each of the recombinant baculovirus. A similar protocol was followed for experiments using BHK-21, HEK293, PK-15, A549 and CHO cells. The percent fluorescent cells were expressed as mean ± standard error. The significance of differences in the means was determined by Student’s t test.
Results
Characterization of pseudotyped recombinant baculoviruses
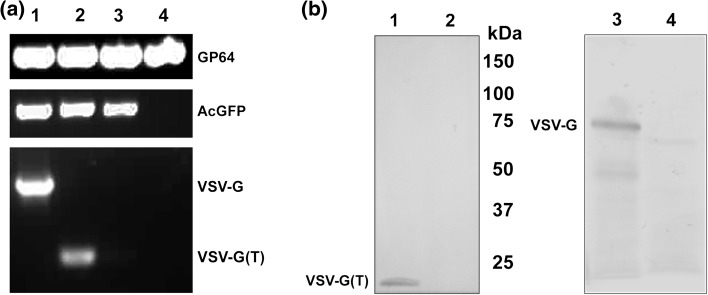
Recombinant bacmids were confirmed by PCR analysis. Pseudotyped and non-pseudotyped baculoviruses were generated by transfecting the bacmids into Sf-9 cells. Viral DNA purified from recombinant baculoviruses was analysed by PCR using gene specific primers. DNA from native AcMNPV virus was used as a negative control. The PCR analysis revealed an amplification of positive control GP64 specific amplicon (0.68 kb) in all the baculoviruses, as expected. AcGFP specific amplicon (0.7 kb) was seen only in recombinant baculoviruses, while the VSV-G specific amplicons (full-length, 1.5 kb and truncated, 0.4 kb) were observed only in pseudotyped baculoviruses (Fig. 1a). Thus it was confirmed that the pseudotyped baculoviruses carried either full-length or truncated VSV-G gene.
Fig. 1.
Characterization of recombinant baculoviruses. (a) Agarose gel (1 %) electrophoresis of gene specific PCR products from DNA of recombinant baculoviruses. Lane 1 AcGFP pseudotyped with VSV-G, lane 2 AcGFP pseudotyped with VSV-G (T), lane 3 non-pseudotyped AcGFP, and lane 4 native AcMNPV. (b) Western blotting of Tn5 cell lysates infected with recombinant baculoviruses. Left panel: Lane 1 VSV-G (T) pseudotyped AcGFP, lane 2 native AcMNPV; right panel: lane 3 VSV-G pseudotyped AcGFP, and lane 4 non-pseudotyped AcGFP. The positions of protein molecular sizes as well as VSV-G and VSV-G(T) are indicated
The expression of VSV-G protein by the pseudotyped recombinant baculoviruses was confirmed by SDS-PAGE and western blotting analysis of lysate of 3 day infected Tn-5 insect cells. Western blot analysis by probing with rabbit antibody raised against a synthetic peptide corresponding to amino acids 497–511 of VSV-G revealed a 65 kDa band corresponding to full-length VSV-G pseudotyped baculovirus, while the truncated version produced a band at 15 kDa (Fig. 1b).
Transduction efficiency of pseudotyped and non-pseudotyped baculoviruses
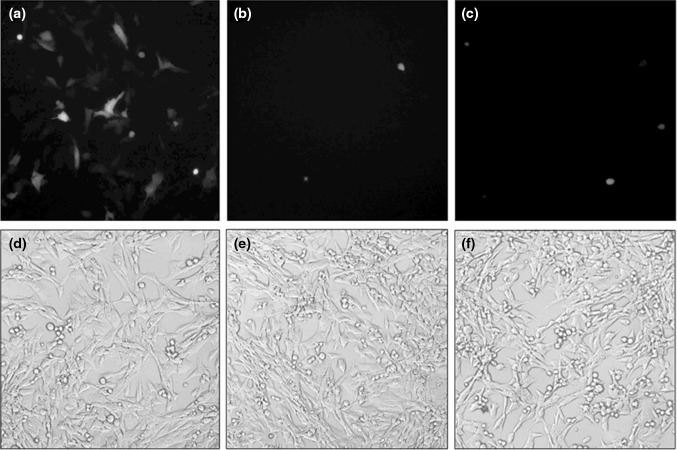
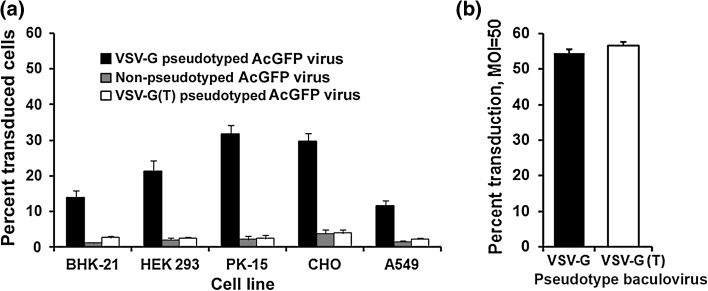
The transduction efficiency of the pseudotyped and non-pseudotyped recombinant baculoviruses was compared by incubating the baculoviruses with BHK-21, HEK293, PK-15 and CHO cells at an MOI of 5. The fluorescence microscopy of BHK-21 cells infected for 72 h, with pseudotyped and non-pseudotyped baculoviruses encoding AcGFP, revealed the expression of green fluorescence in the cells transduced by the three recombinant viruses. However, number of green cells was significantly higher in cells transduced with full-length VSV-G pseudotyped recombinant baculovirus. Low percentages of cells were transduced using non-pseudotyped (GP64 only) AcMNPV and truncated VSV-G pseudotyped baculovirus (Fig. 2). Similar pattern was seen with transduction experiments in HEK 293, PK-15, A549 and CHO cells. Further, following trypsinization, number of fluorescent as well as the total cells were counted using a haemocytometer under a florescence microscope. It was observed that full-length VSV-G pseudotyped baculovirus could transduce 13.8, 21.3, 31.65, 29.7 and 11.5 % of BHK-21, HEK 293, PK-15, CHO and A549 cells, respectively. However, the truncated VSV-G pseudotyped baculovirus could transduce only 2.69, 2.51, 2.43, 3.92 and 2.25 % of BHK-21, HEK 293, PK-15, CHO and A549 cells. The non pseudotyped baculovirus virus exhibited transduction in 1.21, 2.07, 2.19, 3.78 and 1.5 % of BHK-21, HEK 293, PK-15, CHO and A549 cells (Fig. 3a). Statistical analysis clearly showed that the full-length VSV-G pseudotyped baculovirus transduced the mammalian cells at a higher efficiency compared to the non-pseudotyped or truncated VSV-G pseudotyped baculovirus. However, when multiplicity of infection of 50 was used, both full-length and truncated VSV-G showed similar transduction efficiency in BHK-21 cells (Fig. 3b).
Fig. 2.
Fluorescence microscopy of BHK-21 cells infected with pseudotyped or non-pseudotyped recombinant baculoviruses. Top panel (a) VSV-G pseudotyped AcGFP, (b) non-pseudotyped AcGFP, (c) VSV-G(T) pseudotyped AcGFP. Bottom panel Bright field microscopy images- (d), (e) and (f) respectively correspond to (a), (b) and (c) of top panel
Fig. 3.
Bar diagram representing transduction efficiency of the pseudotyped and non-pseudotyped recombinant AcGFP baculoviruses. (a) Transduction efficiency in indicated mammalian cells at MOI of 5. (b) Transduction efficiency in BHK-21 cells at MOI 50. The percentage (mean ± SEM) of transduction (cells showing green fluorescence among total cells), 72 h post infection, is shown
Discussion
Development of new generation vaccines using viral vectors is gaining considerable importance. In this regard, baculoviruses are of special interest in gene delivery of vaccine antigens, because of their ability to stimulate innate immune responses. This study compared the transduction efficiency of baculovirus with or without pseudotyping with VSV-G, a glycoprotein of vesicular stomatitis virus, to evaluate if VSV-G pseudotyping of the baculovirus is dispensable given that the virus has GP64 glycoprotein with similar function. Two versions of baculovirus expressing marker protein, GFP controlled by CMV promoter, were employed in this study. One was pseudotyped with VSV-G, while other was not. The non-pseudotyped baculovirus could no doubt transduce the mammalian cells, but less efficiently compared to the VSV-G pseudotyped baculovirus. These results indicate that VSV-G has added effect to GP64 mediated transduction and thereby significantly enhancing the expression of GFP in mammalian cells, emphasizing the significance of pseudotyping baculovirus with VSV-G, corroborating earlier report [18]. The enhanced effect in VSV-G mediated transduction could possibly be due to similar mechanism of viral entry mediated by GP64 and VSV-G as suggested previously [12]. It is also likely that widespread expression of LDL receptor and its family members that serve as major cell entry port for VSV [7], may provide a positive effect in VSV-G pseudotyped viral vectors. The observed variation in VSV-G mediated transduction efficiency in different cell types could be due to difference in receptor(s) expression.
After confirming that VSV-G pseudotyping enhanced the transduction of mammalian cells by the baculovirus, it was investigated if VSV-GED, a truncated version of VSV-G, comprising of partial G-stem of ectodomain in juxtaposition with the TM and CT domains of VSV-G, was sufficient enough for increasing baculovirus-mediated gene delivery, as reported earlier [4, 11]. Therefore the transduction was evaluated for recombinant baculovirus encoding AcGFP under CMV promoter, pseudotyped with either full-length VSV-G or its truncated version. It was found that pseudotyping with full-length VSV-G was superior in terms of number of cells transduced and the level of the reporter gene expression compared to the baculovirus pseudotyped with truncated VSV-G. The transduction efficiency and expression of marker GFP protein by truncated VSV-G pseudotyped baculovirus were no different from non-pseudotyped baculovirus. The possible reasons for the inefficiency of the truncated VSV-G pseudotyping in the present study could be due to lack of fusion domain (aa residues 69-188) comprising of bipartite fusion loops containing conserved hydrophobic residues [13, 16], and charged residues 145–164, that are involved in membrane interaction and fusion [6]. Also, the truncated VSV-G is devoid of H76 and H176 that form a cluster of three histidine residues (H76, H176 and H423), that triggers the movement of the fusion domain towards the target membrane, during the pre-fusion state [5]. These may make the truncated VSV-G inefficient of receptor binding. It was earlier reported that VSV-GED had strong improvement of transduction efficiency over GP64 control [11]. To understand if the disparity between the observation in this study and the previous study [11] was due to difference in MOI used, a transduction was carried out in BHK-21 cells using the high MOI of 50 like in the previous study. It was observed that at the higher MOI, both full-length and truncated VSV-G pseudotyped virus constructs show similar transduction efficiency. This could be explained by saturation of the VSV-G interacting cellular receptors by the high amount of transducing viruses, thus resulting in a similar effect.
In summary, the study for the first time using a more sensitive GFP marker system, confirmed the essentiality of VSV-G pseudotyped baculovirus in mediating gene transduction into mammalian cells. Further, it was demonstrated that at low MOI, the baculovirus pseudotyped with full-length VSV-G was found to be superior to the truncated VSV-G pseudotyped baculovirus or the non-pseudotyped baculovirus. However, at higher MOI, pseudotyping with either full-length or truncated VSV-G made no significant difference. These findings have important implications in designing of baculoviral vectors for new generation vaccines against viral diseases. The baculovirus vector has several advantages when compared to other viral vectors as it can potentiate innate immune response against viral infections [1]. Also, the pseudotyped baculovirus can trigger a protective immune response in mice models [4, 9]. Considering these promising results, it is desirable to effectively harness the potential of pseudotyped baculovirus as a viral vector for vaccine antigen delivery.
Acknowledgments
The authors thank the Director, ICAR-Indian Veterinary Research Institute (IVRI), Izatnagar and Joint Director of IVRI Bangalore, for facilitating this work. SMK acknowledges JRF received from Indian Council of Agricultural Research. Authors also thank Dr. B.P. Sreenivasa for useful discussion.
References
- 1.Abe T, Matsuura Y. Host innate immune responses induced by baculovirus in mammals. Curr Gene Ther. 2010;10:226–231. doi: 10.2174/156652310791321279. [DOI] [PubMed] [Google Scholar]
- 2.Basagoudanavar SH, Hosamani M, Tamil Selvan RP, Sreenivasa BP, Saravanan P, Chandrasekhar Sagar BK, Venkataramanan R. Development of a liquid-phase blocking ELISA based on foot-and-mouth disease virus empty capsid antigen for seromonitoring vaccinated animals. Arch Virol. 2013;158:993–1001. doi: 10.1007/s00705-012-1567-5. [DOI] [PubMed] [Google Scholar]
- 3.Brusca J, Summers M, Couch J, Courtney L. Autographa californica nuclear polyhedrosis virus efficiently enters but does not replicate in poikilothermic vertebrate cells. Intervirology. 1986;26:207–222. doi: 10.1159/000149703. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, Lu Z, Sun P, Fu Y, Tian F, Hao X, Bao H, Liu X, Liu Z. A pseudotype baculovirus expressing the capsid protein of foot-and-mouth disease virus and a T-cell immunogen shows enhanced immunogenicity in mice. Virol J. 2011;8:77. doi: 10.1186/1743-422X-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carneiro FA, Stauffer F, Lima CS, Juliano MA, Juliano L, Da Poian AT. Membrane fusion induced by vesicular stomatitis virus depends on histidine protonation. J Biol Chem. 2003;278:13789–13794. doi: 10.1074/jbc.M210615200. [DOI] [PubMed] [Google Scholar]
- 6.Carneiro FA, Vandenbussche G, Juliano MA, Juliano L, Ruysschaert JM, Da Poian AT. Charged residues are involved in membrane fusion mediated by a hydrophilic peptide located in vesicular stomatitis virus G protein. Mol Membr Biol. 2006;23:396–406. doi: 10.1080/09687860600780892. [DOI] [PubMed] [Google Scholar]
- 7.Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci USA. 2013;110:7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grein TA, Michalsky R, Vega Lopez M, Czermak P. Purification of a recombinant baculovirus of Autographa californica M. nucleopolyhedrovirus by ion exchange membrane chromatography. J Virol Methods. 2012;183:117–124. doi: 10.1016/j.jviromet.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Huang H, Xiao S, Qin J, Jiang Y, Yang S, Li T, Gao Y, Li Z, Su X, Ruan Y, Xu F, Wang H, Chen H, Xia X. Construction and immunogenicity of a recombinant pseudotype baculovirus expressing the glycoprotein of rabies virus in mice. Arch Virol. 2011;156:753–758. doi: 10.1007/s00705-010-0909-4. [DOI] [PubMed] [Google Scholar]
- 10.Jehle JA, Blissard GW, Bonning BC, Cory JS, Herniou EA, Rohrmann GF, Theilmann DA, Thiem SM, Vlak JM. On the classification and nomenclature of baculoviruses: a proposal for revision. Arch Virol. 2006;151:1257–1266. doi: 10.1007/s00705-006-0763-6. [DOI] [PubMed] [Google Scholar]
- 11.Kaikkonen MU, Raty JK, Airenne KJ, Wirth T, Heikura T, Yla-Herttuala S. Truncated vesicular stomatitis virus G protein improves baculovirus transduction efficiency in vitro and in vivo. Gene Ther. 2006;13:304–312. doi: 10.1038/sj.gt.3302657. [DOI] [PubMed] [Google Scholar]
- 12.Mangor JT, Monsma SA, Johnson MC, Blissard GW. A GP64-null baculovirus pseudotyped with vesicular stomatitis virus G protein. J Virol. 2001;75:2544–2556. doi: 10.1128/JVI.75.6.2544-2556.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roche S, Rey FA, Gaudin Y, Bressanelli S. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein G. Science. 2007;315:843–848. doi: 10.1126/science.1135710. [DOI] [PubMed] [Google Scholar]
- 14.Sarkis C, Serguera C, Petres S, Buchet D, Ridet JL, Edelman L, Mallet J. Efficient transduction of neural cells in vitro and in vivo by a baculovirus-derived vector. Proc Natl Acad Sci USA. 2000;97:14638–14643. doi: 10.1073/pnas.260472897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schauber CA, Tuerk MJ, Pacheco CD, Escarpe PA, Veres G. Lentiviral vectors pseudotyped with baculovirus gp64 efficiently transduce mouse cells in vivo and show tropism restriction against hematopoietic cell types in vitro. Gene Ther. 2004;11:266–275. doi: 10.1038/sj.gt.3302170. [DOI] [PubMed] [Google Scholar]
- 16.Sun X, Belouzard S, Whittaker GR. Molecular architecture of the bipartite fusion loops of vesicular stomatitis virus glycoprotein G, a class III viral fusion protein. J Biol Chem. 2008;283:6418–6427. doi: 10.1074/jbc.M708955200. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Chang MO, Kitajima M, Takaku H. Baculovirus activates murine dendritic cells and induces non-specific NK cell and T cell immune responses. Cell Immunol. 2010;262:35–43. doi: 10.1016/j.cellimm.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Tani H, Nishijima M, Ushijima H, Miyamura T, Matsuura Y. Characterization of cell-surface determinants important for baculovirus infection. Virology. 2001;279:343–353. doi: 10.1006/viro.2000.0699. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Fang L, Fan H, Jiang Y, Pan Y, Luo R, Zhao Q, Chen H, Xiao S. Construction and immunogenicity of pseudotype baculovirus expressing GP5 and M protein of porcine reproductive and respiratory syndrome virus. Vaccine. 2007;25:8220–8227. doi: 10.1016/j.vaccine.2007.09.069. [DOI] [PubMed] [Google Scholar]
- 20.Wu C, Wang S. A pH-sensitive heparin-binding sequence from Baculovirus gp64 protein is important for binding to mammalian cells but not to Sf9 insect cells. J Virol. 2011;86:484–491. doi: 10.1128/JVI.06357-11. [DOI] [PMC free article] [PubMed] [Google Scholar]