Abstract
A retrospective study on the epidemiology of foot and- mouth disease (FMD) in Karnataka, India between the years 1977 and 2012-13 based on the data collected through passive and active surveillance was undertaken. A total of 11,159 outbreaks with 0.271 million cases of FMD were recorded from 30 different revenue districts of Karnataka. There was a significant difference between the years for the annual incidence of FMD (P = <0.001, F = 19.10) and also between the months (P = <0.001, F = 4.22). Cattle and buffaloes were the predominant species affected being involved in all of the outbreaks reported. A significant correlation was observed between livestock density and the number of outbreaks reported (r = 0.70, p < 0.02), and number of cases (r = 0.76, p < 0.01) for all the agro-climatic zones. The Central dry zone (n = 2257, 19.89 %) reported the highest number of outbreaks followed by the Northern dry zone (n = 1881, 16.58 %) and the Southern transition zone (n = 1761, 15.52 %), and attack rates were concentrated in the North/Northeastern/Central dry and transition zones. A large majority of the outbreaks were caused by serotype O (64.04 %), followed by Asia 1 (19.87 %) and A (12.27 %). Serotype C was not reported since 1993 in the state. In recent years, serotype O has dominated (82.59 %), with the rest of the outbreaks being almost equally caused by A (9.01 %) and Asia 1 (8.40 %). The study highlights the significance of the O serotype and cattle as the main indicator species in the epidemiology of FMD in Karnataka, India. The findings from this study can be used as baseline epidemiological data for further research to identify endemic and epidemic areas for the development of a sustainable programme for the progressive control of FMD in the state of Karnataka as well as other endemic settings.
Keywords: Foot and mouth disease, Epidemiology, Species, Agroclimatic zones, Temporal and Spatial distribution
Foot-and-mouth disease (FMD) is a highly contagious viral disease of cloven-hoofed animals including cattle, buffaloes, sheep, goats, pigs and waterbuffalo [10]. The disease is characterized by the formation of painful, serous vesicles on the tongue, lips and other tissues of the mouth, and on less stratified integumentary parts of the body such as the udder and teats, the interdigital space and the coronary band above the hooves [1]. Infected cattle and pigs usually develop typical signs whereas clinical diagnosis in sheep and goats is difficult due to mild disease manifestations.
The virus belongs to Aphthovirus genus of the family Picornaviridae, and exists as seven clinically indistinguishable serotypes: O, A, Asia-1, South African Territory-1 (SAT-1), SAT-2, SAT-3, and C, each with multiple subtypes [6]. Infection and recovery or vaccination with one serotype often fails to protect against infection from other serotypes, and sometimes against another subtype within the same serotype. Epidemiological dynamics are further compounded by the wide host range, nearly 100 per cent morbidity, multiple modes of transmission, low infectious dose of the virus, rapid rate of virus replication, high level of virus excretion by infected animals, and rapid spread [11].
Livestock sector is an important part of Indian economy, employing 8-10 per cent of the country’s labour force, and contributing to 4-6 per cent of national and 28-32 per cent of agricultural GDP. In India, FMD occurs throughout the year, and the situation is complicated due to the huge population of susceptible animals, plurality of the circulating virus serotypes and strains, unrestricted movement of animals, inapparent infection in small ruminants, and limited systematic vaccination [4]. Direct losses due to FMD alone in India have been estimated to be Rs. 200 billion (~ USD 3.5 billion) [18], 80 per cent of which is due to drop in milk production [9].
Control of FMD is significant for protecting the livestock industry and for improving livelihoods of farmers in developing countries, where FMD is endemic. Progressive risk reduction of FMD can help in progressive market access of livestock commodities. However, the success of any disease control program depends on a clear understanding of the epidemiology of the disease, especially the analysis of the distribution and patterns of spread [2, 3, 8, 12, 19]. In India, FMD was first documented in 1864 during extensive outbreaks in many parts of the country (Government of India 1868). Epidemiological studies were initiated in the earnest by the Indian Council of Agricultural Research (ICAR) in the form of “All India Co-ordinated Research Project (AICRP) for virus typing” in 1968 and expanded in 2001 to Project Directorate on Foot and Mouth Disease (PD_FMD), which has developed scientific and technical expertise in both conventional and cutting edge areas of FMD research. Currently, PD_FMD has eight regional centres and 15 network units covering the length and breadth of the country to investigate the epidemiology of FMD in terms of temporal and spatial patterns, species susceptibility, serotype prevalence, and risk factors. Further, PD_FMD also acts as the FAO Reference Centre for South-Asia, for FMD diagnosis, epidemiology and research. The epidemiological data acquired since the inception of AICRP formed the basis for the launch of the FMD control programme (FMDCP) in 2002 in 54 districts in eight states of the country covering 30 million cattle and buffaloes [4]. FMDCP now covers 221 districts in various states including Karnataka.
This paper describes the disease epidemiology in the state of Karnataka during the period 1977 to 2013 in terms of outbreaks, serotypes, spatial and temporal distribution, and other ecological factors responsible for maintenance of infection in nature. These data should be useful to formulate appropriate preventive measures.
Karnataka, the eighth largest state in India (19.1 Mha area, 5.8 % of the country), is situated between 11°40′ and 18°27′ N latitude and 74°50′ and 78°33′ E longitude, in the centre of the western peninsular India. The state has a topography ranging from the narrow stretch of coastal plains to spectacular heights of Western Ghats culminating in gentle slopes. The state has 30 revenue districts, and can be divided based on (a) rainfall pattern, quantum and distribution, (b) soil type, texture, depth and physicochemical properties, (c) elevation and topography, and (d) major crops and type of vegetation into the following agro-climatic zones: (1) Northeastern transition, (2) Northeastern dry, (3) Northern dry, (4) Central dry, (5) Eastern dry, (6) Southern dry, (7) Southern transition, (8) Northern transition, (9) Hilly, and (10) Coastal.
Data on outbreak of FMD between 1977 and 2013 were obtained from the office of the Deputy Director, Disease Surveillance and Monitoring, Department of Animal Husbandry and Veterinary Services, Government of Karnataka. The state has a passive disease surveillance system based on hierarchical reporting of cases of outbreaks by livestock owners to the local veterinarians to officials at the taluk to the district level to the Deputy Director, Disease Surveillance and Monitoring, who in turn compiles the data. The epidemiological data, annual and monthly outbreak data, and vaccine utilization data were collected, compiled and analysed. An outbreak was defined as the occurrence of one or more clinical cases of FMD in a herd in a village and each village was considered as an epidemiological unit for the purpose of defining an outbreak. Data on laboratory confirmation of FMD was obtained from the official declaring laboratory at the AICRP on FMD, Bangalore Centre, Karnataka, where confirmation was done by complement fixation till 1997, and by sandwich ELISA as per the protocol laid down by PDFMD since 1997.
The vaccination data from 2001 to 2010 was collected from the office of the Deputy Director, Disease Surveillance and Monitoring, Department of Animal Husbandry and Veterinary Services, Government of Karnataka and the data under FMD-CP was collected from the nodal officer, FMDCP. The monitoring of FMDCP was carried out by screening 200 pre- and 200 post-vaccinated serum samples collected from selected villages in each revenue district employing liquid-phase blocking (LPB)-ELISA, as recommended by OIE.
Data obtained from both reported and confirmed outbreaks of FMD were subjected to analyses. Descriptive analysis was performed using Microsoft® Office Excel, 2013. Square root transformation was carried out for normality of month wise outbreak data and ANOVA was used for transformed data. Pearsons correlation was used to investigate the relationship between the livestock density and outbreaks and number of cases in the agro- climatic zones.
The state of Karnataka has a large FMD-susceptible livestock population which includes 10.50 million cattle, 4.32 million buffaloes, 9.56 million sheep, 6.25 million goats, 0.27 million pigs and an unknown population of wild herbivores as per the 2007 census. The presumptive diagnosis of FMD at the field level is based on clinical symptoms such as fever and vesiculation progressing to erosions in the mouth, muzzle, feet and teats. In cattle, lesions are common on the tongue, dental pad, gums, soft palate, nostrils, muzzle, coronary band and the interdigital space. In pigs, vesicles are seen on the snout, face, coronary band, heel and the interdigital space.
Over the study period of 36 years, 11,159 outbreaks with 0.271 million cases of FMD were recorded from 30 different revenue districts of Karnataka. Of these, 3213 outbreaks with 0.035 million cases were reported till 1995. Unfortunately, species-wise details were unavailable during this time. Since 1996, 0.15 million cattle, 0.047 million buffaloes, 0.028 million sheep, 0.014 million goats and 289 pigs were recorded to have been clinically affected with FMD. Cattle and buffaloes were involved in majority of the outbreaks (n = 0.19 million cases), followed by sheep and goats (n = 0.042 million). Outbreak has also been confirmed in captive wild animals, especially mithun, gaur and black buck, maintained in biological parks.
Among the total of 11,159 presumptive FMD outbreaks reported since 1977, 1205 outbreaks were confirmed. Haveri (n = 855, 7.66 %), Tumkur (n = 833, 7.46 %) and Belgaum (n = 830, 7.43 %) districts reported large number of outbreaks, while the coastal districts of Dakshina Kannada and Udupi (n = 31, 0.27 % each) reported the lowest number of outbreaks. As far as number of attacks/cases are concerned, Raichur (n = 0.017 million, 10.71 %), Haveri (n = 0.015 million, 9.07 %), Gulbarga (n = 0.013 million, 7.96 %), Belgaum (n = 0.0117 million, 7.14 %) and Davanagere (n = 0.010 million, 6.36 %) accounted for more than 40 per cent of the cases. The Central dry zone (n = 2257, 20.22 %) reported the highest number of outbreaks followed by the Northern dry zone (n = 1950, 17.47 %) and the Southern transition zone (n = 1761, 15.78 %), and attack rates were concentrated in the North/Northeastern/Central dry and transition zones (Table 1). A significant correlation was observed between livestock density and the number of outbreaks reported (r = 0.70, p < 0.02), and number of cases (r = 0.76, p < 0.01) for all the agro-climatic zones.
Table 1.
FMD-susceptible livestock population and number of outbreaks in different agro-climatic zones of Karnataka, 1976-77 to 2012-2013
| Agro-climatic zone | Area (sq. km.) | Population (In millions) | Density/km2 | Reported outbreaks | Confirmed outbreaks | No of cases recorded(in millions) |
|---|---|---|---|---|---|---|
| North-eastern | 5,448 | 0.747 | 137 | 68 | 15 | 0.0016 |
| North eastern dry | 23,051 | 4.092 | 385 | 1,202 | 32 | 0.0305 |
| North dry | 55,940 | 10.625 | 1,348 | 1,950 | 116 | 0.0344 |
| Central dry | 24,961 | 5.376 | 635 | 2,257 | 167 | 0.0254 |
| Eastern dry | 24,308 | 3.022 | 675 | 1,522 | 447 | 0.0156 |
| Southern dry | 16,916 | 2.844 | 411 | 650 | 157 | 0.0135 |
| Southern transition | 22,492 | 2.680 | 409 | 1,761 | 178 | 0.0212 |
| Northern transition | 9,083 | 1.306 | 243 | 1,325 | 19 | 0.0159 |
| Hilly | 14,393 | 0.682 | 93 | 393 | 52 | 0.0049 |
| Coastal | 8,440 | 0.802 | 189 | 31 | 22 | 0.0002 |
High annual incidences of FMD were reported in 2005-06 (14 % of total outbreaks, 0.043million cases), 2000-01 (10 % of total outbreaks, 0.0330 million cases), 2002-03 (9 % of total outbreaks, 0.022 million cases), and 2007-08 (8 % of total outbreaks, 0.0288 million cases), and the lowest was recorded in 1996 (0.53 % of total outbreaks, 0.004 million cases). There was a significant difference between the years for the annual incidence of FMD (F = 19.10, P = <0.001,). The highest average incidence was reported in January (n = 18) followed by February (n = 17) and March (n = 16), together accounting for 38.63 per cent of all the outbreaks, and the lowest in May and June (n = 5). There was a significant difference in the incidence of outbreaks between the months (F = 4.22, P = <0.001,). On an average, 21 districts had outbreaks of FMD every year. The highest number of districts with outbreaks was in 2007-08 (n = 27) and 2005-06 (n = 26). There was a significant difference in the number of districts reporting FMD over the seventeen year period (F = 2.92, P = <0.001).
The confirmed outbreaks in the state of Karnataka during the study period indicated the presence of all four FMDV serotypes O, A, C and Asia-1. Serotype C was reported regularly up to 1983 and again in 1993, but not since then in the state. FMDV serotype O was detected every year. A large majority of the outbreaks were caused by serotype O (64.04 %), followed by Asia 1 (19.87 %) and A (12.27 %). However, there was a clear difference before and after 1993. Until 1993, about three fourth of the outbreaks were due to O and Asia-1 serotypes (74.02 %), both causing almost equal number of outbreaks. Since then, serotype O has dominated (82.59 %), with the rest of the outbreaks being almost equally caused by serotype A (9.01 %) and Asia 1 (8.40 %). Serotype Asia-1 was not detected from 2006-07 to 2010-11 but reappeared in 2011-12. At present all the three serotypes are circulating in the state.
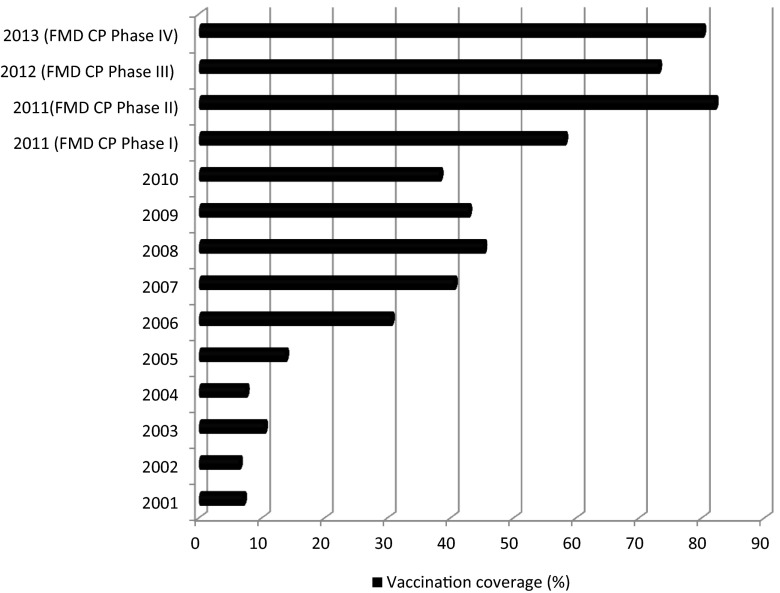
Vaccination against FMD has been carried out since the beginning of the study period but the coverage was very minimum and limited mostly to post-outbreak situations until 2006-07 when severely affected districts were brought under the ASCAD (Assistance to States for the control of Contagious Animal Diseases) program, under which, livestock were vaccinated once annually. However, there was gradual increase in coverage and the program was expanded under the FMDCP, which was implemented in Karnataka in 2011. Under FMDCP, cattle and buffaloes are being vaccinated every six months (August–September and February–March) using a trivalent oil adjuvant vaccine containing all the three serotypes O, A and Asia-1. As on March 2013, fourth round of vaccination has been carried out covering more than 80 per cent of cattle and buffaloes. Year-wise (2001 onwards) percentage populations vaccinated is given in Fig. 1. Seromonitoring following FMDCP indicated that protective antibodies had risen from 5 per cent at the beginning of the programme to 45 per cent of the population at the end of fourth phase.
Fig. 1.
FMD vaccination coverage of cattle and buffaloes in Karnataka, 2001-2013
Retrospective studies, based on the analysis of available data and passive surveillance have contributed greatly to the understanding of the epidemiology of FMD in other states of India [3, 15, 19] and in other endemic countries such as Bhutan [7], Laos [8], and Mali [14]. This report is the first to describe the status of FMD in the state of Karnataka, India. The study showed that the disease is endemic in the state.
The analysis indicated that the disease is reported from all the agro-climatic zones, but not uniformly distributed. Lower number of outbreaks were reported in the hilly and coastal regions, and this could be attributed to factors such as (a) low livestock population and density (i.e., more scattered), (b) the higher altitudes limiting the movement and hence contact between animals, and (c) the climate being a deterrent for the natural spread of the virus. On the other hand, large numbers of outbreaks were reported from the dry zones and transition zones where the majority of the susceptible livestock population is located.
The regions where the disease was more prevalent constitute the semiarid ecosystems with an average annual rainfall of 100–800 mm and relative humidity of 60-80 per cent. Maximum outbreaks were reported from the districts with relative humidity of 60-70 per cent. The incidence of FMD was high in winter months (November to February) than rainy (July to September) or summer months (April to June). The number of outbreaks increased during late monsoon and winter, probably due to the favourable environmental conditions of dry weather, dry winds and moderately high relative humidity. This is also the time of the year when migration and movement of livestock is common, especially in rural livestock fairs where cross-bred milch cattle are traded mainly from the southern districts of Karnataka to other districts and across the country, and buffaloes and draught animals are traded from the northern districts. Large-scale congregation of animals at these fairs, typically held after October, are highly congenial for virus transmission. Illegal trading, lack of monitoring, ignorance of the farmers, and lack of quarantine policy for the newly purchased animals also play a major role in precipitation of the disease [3, 5, 16, 19]. The extreme hot weather during summer may be the reason for less number of outbreaks reported during this season [17]. Indeed, all the states bordering Karnataka, viz., Tamil Nadu, Andhra Pradesh, Kerala and Maharashtra, have also been reporting FMD every year and the outbreaks in the southern states including Karnataka constitutes 31.5 per cent of the total outbreaks in the country [17]. In fact, in Karnataka state, Asia-1 was detected for the first time after five years in 2011-12, mainly in the northern districts bordering Maharashtra where Asia-1 was dominant in that year (PDFMD ANNUAL REPORT 2011-12) [13]. The serotype subsequently spread to southern district of Karnataka, indicating unrestricted animal movement across the state border and within the state, and pointing to this being the single-most important factor influencing the changing scenario of FMD outbreaks in the southern states.
In this study, livestock density was found to be positively correlated with disease incidence and number of cases for all the agroclimatic zones. Similar findings have been reported in countries and states with similar livestock management systems [8, 19]. Hence, it would be necessary to prioritize disease control activities in zones with high livestock populations to optimize the use of resources. As far as species are concerned, cattle and buffaloes were the most affected (82.43 % of the cases). This was not surprising since disease symptoms are clearly observed in these animals. On the other hand, cases in sheep and goats constituted only 17.57 per cent of the cases recorded and 0.14 per cent of the susceptible population even though sheep and goats constituted 51.00 per cent of the total livestock population. While this is most probably due to the asymptomatic nature of FMD in these species, it is worth noting that outbreaks were confirmed from them in the state of Karnataka. Nevertheless, small ruminants do not seem to play a major role in the epidemiology of FMD in Karnataka.
It is noteworthy that only 10.8 per cent of the clinically recorded outbreaks were confirmed by laboratory tests. Ignorance of the farmers, wrong presumptive diagnosis, not collecting samples, improper/insufficient/mistimed sample collection, improper storage or transport of the sample could all contribute to the low confirmatory diagnosis, and this suggests that reporting, sample collection and forwarding systems need to be strengthened.
The 1-D gene–based nucleotide sequence analyses have established complex molecular epidemiological scenario with the circulation of different genotypes/lineages/strains of FMD virus O, A and Asia1 in India. In the early 1980s, Pan-Asia O serotype was circulating in the country. In 2001, Ind2001 with high genetic divergence from PanAsia emerged in the southern states and remerged in 2008 causing majority of outbreaks. However, the current vaccine strain for serotype O is antigenically well matched with all these strains. Serotype A found to be genetically and antigenically most heterogeneous in nature. Since 2001, genotype 18 has been exclusively responsible for all the field outbreaks. At present, a divergent of this lineage VP359 deletion group is dominating the field outbreaks. However, all the outbreak strains are antigenically close to the vaccine strain currently in use. (PDFMD Annual reports 2008-09 to 2012-13) [13]. Serotype Asia-1 virus isolates of India are genetically more stable with less than 20 per cent nucleotide divergence among them. Lineage C has been in circulation in the country since 2005, and all Asia-1 outbreaks recorded during 2006–2011 were caused by this lineage virus [17]. Whether the predominance of serotype O in causing field outbreaks is due to more diversity of strains of serotype O, or higher susceptibility of animals to this serotype compared to that to serotypes A and Asia-1, or fitness of serotype O when co-circulating together with the other serotypes, or better and longer protection afforded by infection or vaccination by the other serotypes needs to be investigated.
In endemic countries like India, FMD can be controlled only by systematic preventive vaccination of susceptible animals, restriction of animal movement, and zoosanitary measures in the event of outbreaks. Preventive vaccination against FMD was initiated in Karnataka in 2006-07 under ASCAD programme and stepped up in 2011 under FMDCP. The latter has increased the coverage and built up herd immunity to a considerable extent as outbreaks have been observed only in few unvaccinated cattle in recent times. A similar situation has been observed in the states like Punjab and Haryana where only a few sporadic cases of FMD have been recorded following regular vaccination under FMDCP [4]. An interesting observation from the annual outbreak data is that the outbreaks appeared to peak every 2-3 years (2001-02, 2002-03, 2005-06, 2007-08). It is tempting to suggest that natural infection is able to protect susceptible populations for 2-3 years and immunity wanes after that. Alternatively, this pattern could be due to a new generation of animals being available for the virus to attack. The cyclical peak is especially the case with serotype O, which has dominated since 1994. Interestingly, high activities of serotypes A and/or Asia-1 appear to be random, peaking when activity of serotype is O relatively low (1999-2000 or 2000-2001) or in parallel with high activity of serotype O (2005-06 and 2007-08). In any case, although the numbers of outbreaks have varied, the attack rates have dwindled after the introduction of vaccination.
In conclusion, the study highlights the significance of the O serotype and cattle as the main indicator species in the epidemiology of FMD in Karnataka, India. The findings from this study can be used as baseline epidemiological data for further research to identify primary endemic areas (virus maintenance areas), secondary endemic areas (areas of virus propagation) and epidemic areas (areas of explosive outbreak) for the development of a sustainable programme for the progressive control of FMD in the state of Karnataka as well as other endemic settings.
Acknowledgments
We would like to thank the Project Director, PD- FMD (ICAR), for providing all the logistics to conduct this study. We would also like to thank Department of Animal Husbandry and Veterinary Services, Government of Karnataka for providing the outbreak data. We wish to thank Dr. Nagendra R. Hegde for critically reviewing the manuscript.
References
- 1.Alexandersen S, Zhang Z, Donaldson AI, Garland AJ. The pathogenesis and diagnosis of foot-and-mouth disease. J Comp Pathol. 2003;129:1–36. doi: 10.1016/S0021-9975(03)00041-0. [DOI] [PubMed] [Google Scholar]
- 2.Ayebazibwe, C., TjØrnehØj, K, Mwiine, FN, Muwanika, VB, Ademun Okurut, AR, Siegismund, HR, and Alexandersen, S, 2010: Patterns, risk factors and characteristics of reported and perceived foot-and-mouth disease (FMD) in Uganda. Tropical Animal Health and Production, 1–13. [DOI] [PubMed]
- 3.Bhattacharya S., Banerjee R, Ghosh R, Chattopadhayay AP, and Chatterjee A, 2005: Studies of the outbreaks of foot and mouth disease in West Bengal, India, between 1985 and 2002. Revue Scientifique et Technique—Office International des Epizooties. 24, 945–952. [PubMed]
- 4.Biswal JK, Sanyal A, Rodriguez LL, Subramaniam S, Arzt J, Sharma GK, Hammond JM, Parida S, Mohapatra JK, Mathapati BS, Dash BB, Ranjan R, Rout M, Venketaramanan R, Misri Jyoti, Krishna Lal, Prasad Gaya, Pathak KML, Pattnaik B. Foot-and-mouth disease: global status and Indian perspective. Indian Journal of Animal Sciences. 2012;82:109–131. [Google Scholar]
- 5.Chowdhury B, Bhattacharya UK, Bhattacharya HM. Geo-climatic variation influencing foot and mouth disease outbreaks in West Bengal. Indian J. anim. Health. 1986;25:171–174. [Google Scholar]
- 6.Domingo E, Escarmis C, Baronowski E, Ruiz-Jarabo CM, Carrillo E, Nunez JI. Evolution of foot-and-mouth disease virus. Virus Res. 2003;91:47–63. doi: 10.1016/S0168-1702(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 7.Dukpa K, Robertson ID, Edwards JR, ell Ellis TM. A retrospective study on the epidemiology of foot-and-mouth disease in Bhutan. Trop Anim Health Prod. 2011;43:495–502. doi: 10.1007/s11250-010-9722-z. [DOI] [PubMed] [Google Scholar]
- 8.Khounsy S, Conlan JV, Gleeson LJ, Westbury HA, Colling A, Paton DJ, Knowles NJ, Ferris NR, Blacksell SD. Foot and mouth disease in the Lao People’s Democratic Republic: i. A review of recent outbreaks and lessons from control programmes. Scientific and Technical Review. Office International des Epizooties. 2008;27:839–849. doi: 10.20506/rst.27.3.1840. [DOI] [PubMed] [Google Scholar]
- 9.Mathew L, Menon GD. Economic Impact of FMD in Chazhoor Panchayath District Veterinary Centre, Thrissur. Kerala State. Veterinary World. 2008;1:5–6. [Google Scholar]
- 10.OIE, 2012. Foot and Mouth Disease. Chapter 2.1.5. In: Manual of Diagnostic Tests & Vaccines for Terrestrial Animals 6th Edition . World Organisation for Animal Health (OIE) Paris: France; 2008. [Google Scholar]
- 11.Pattnaik B, Subramaniam S, Sanyal A, Mohapatra JK, Dash BB, Ranjan R and Rout M, 2012: Agric. Res. DOI 10.1007/s40003-012-0012-z.
- 12.Perry BD, Gleeson LJ, Khounsey S, Bounma P, Blacksell SD. The dynamics and impact of foot and mouth disease in smallholder farming systems in South-East Asia: a case study in Laos. Scientific and Technical Review. Office International des Epizooties. 2002;21:663–673. doi: 10.20506/rst.21.3.1354. [DOI] [PubMed] [Google Scholar]
- 13.PD FMD Annual reports 2008-09 to 2012-13. www.pdfmd.ernet.in.
- 14.Sangare O, Dungu B, Bastos ADS. Foot and mouth disease in Mali: the current situation and proposed control strategies. Scientific and Technical Review. Office International des Epizooties. 2004;23:863–872. doi: 10.20506/rst.23.3.1525. [DOI] [PubMed] [Google Scholar]
- 15.Sarma DK, and Sutopa D, 2003: FMD outbreaks and virus types in Assam and NE States, India between 1998 and 2001. Blue Cross Book, 16–18.
- 16.Sivakumar AT, Thennarasu, Rajkumar JSI. Effect of season on the incidence of infectious diseases of bovine in Tamilnadu. Elixir Meteorology. 2012;47:8874–8875. [Google Scholar]
- 17.Subramaniam S, Pattnaik B, Sanyal A, Mohapatra JK, Pawar SS, Sharma GK, Das B, Dash BB. Status of Foot-and-mouth Disease in India. Transboundary and Emerging Diseases. 2013;60:197–203. doi: 10.1111/j.1865-1682.2012.01332.x. [DOI] [PubMed] [Google Scholar]
- 18.Venkataramanan, R., Hemadri D, Bandyopadhyay S. K, and Taneja V. K, 2006: Foot-and mouth Disease in India: Present status. Paper presented at a workshop on Global Roadmap for improving the tools to control foot-and-mouth disease in endemic settings. 29 Nov-1 Dec 2006, Agra, India.
- 19.Verma AK, Pal BC, Singh CP, Udit J, Yadav SK, Mahima Studies of the outbreaks of foot and mouth disease in Uttar Pradesh, India, between 2000 and 2006. Asian Journal of Epidemiology. 2008;1:40–46. doi: 10.3923/aje.2008.40.46. [DOI] [Google Scholar]