Abstract
To assess the cell mediated and mucosal immune responses in chicks to Newcastle disease vaccine, expression levels of certain genes encoding cytokines and chemokines were quantified by q-PCR. The utility of cytokine and chemokine gene expression profile in estimating the cell mediated and humoral immune response has been established. The cell mediated immune response was assessed by quantifying the IFN-γ gene expression in splenocytes and compared with colorimetric blastogenesis assay. The mucosal immune response was assessed by quantifying the expression of IL-8, IL1-β, MIP1-β, K60 and K203 in the intestinal cells and compared with IgA ELISA. On 14th day post vaccination, the expression of IFN-γ was upregulated by 12-folds in the Group I, which have received oral pellet vaccine and fourfolds in the Group II where birds have received live thermostable vaccine as occulonasal instillation. 3 and 7 days after receiving booster, the same cytokine gene was upregulated by 12-folds and 27-folds respectively in the Group III, where birds have received live thermostable ND vaccine as priming vaccine and oral pellet vaccine as booster. On 21st day post vaccination the expression of IL-8 was upregulated by 42.8-folds in Group I and 3.3-folds in the Group II. The expression of IL-1β was upregulated by eightfolds on 3rd day post vaccination and 23-folds on 21st day post vaccination in Group I. The expression of macrophage inflammatory protein-1β (MIP-1β) was upregulated by 16-folds in Group I and 70-folds in Group II on 14th day post vaccination. No significant change in expression of chemokine genes K60 and K203 in vaccinated birds. The results were comparable with the results of conventional tests and proved the utility of qPCR in estimating the cellular and mucosal immune responses.
Electronic supplementary material
The online version of this article (doi:10.1007/s13337-014-0230-z) contains supplementary material, which is available to authorized users.
Keywords: Newcastle disease; Cellular, humoral and mucosal immune response; Cytokine and chemokine genes; Real time PCR; Colorimetric blastogenesis assay; IgA ELISA
Introduction
Newcastle disease (ND) has been reported to be an economically important and highly contagious disease of poultry caused by Newcastle disease virus (NDV), a member of the family Paramyxoviridae [12]. It has been reported that, despite the availability of vaccines, good husbandry practices and biosecurity protocols the disease still remains as a potential threat with regular and severe outbreaks [41]. Hence, control measures are focussed towards regular vaccination and continuous sero-monitoring. Cell mediated immune response (CMI) has been considered to be important for conferring protection following vaccination [22] and responsible for the early protection [3] [9]. However, CMI alone was not reported to be sufficient to protect birds against virulent NDV [27]. Mucosal immune response was also found to be produced in the chickens in both the respiratory and intestinal tracts [5]. In chicken, bile, lachrymal fluid [19] and Harderian gland [34] were found to be major source of IgA [4]. It has also been proved that the mucosal immunity represented by IgA plays an important role in the development of protection in chickens vaccinated against ND vaccine [38]. Measurement of cellular, humoral and mucosal immune response has been reported to improve the understanding of the immune response generated by experimental vaccines. While the techniques to assess the humoral immune response have been well standardized, the techniques commonly used to assess cell mediated and mucosal immune responses like colorimetric blastogenesis assay (CBA) and agglutination reactions respectively are either time consuming, lack sensitivity or the results are not reproducible. Of late, the availability of new technologies like real time quantitative PCR (qPCR) have been reported to be used in the quantification of cytokine mRNA [44] and thus have been a method to determine mechanisms of pathogenesis and immune response [18]. To characterise host response to vaccination, profile of genes whose expression is affected by vaccination is very important. While several studies have been conducted to measure cytokine mRNA using qPCR in many species of animals, such a type of work was found to be very minimal in chickens. In the present study, 3 week old chicken, free of maternal antibodies were vaccinated with two different vaccines and expression of cytokine genes like IFN-γ and interleukin-1β and chemokine genes like interleukin-8, macrophage inflammatory protein-1β, K60 and K203 were quantified in different time points post vaccination by qPCR with β-actin gene as reference. The cytokine IFN-γ is reported to be involved in macrophage activation [6], inhibition of virus replication [15] [14] [28] [27], augmenting expression of major histocompatibility class I and II antigens (MHC-I and MHC-II) and Fc receptor [8], class switching of immunoglobulins [43] and tumour control [36]. We have also identified certain cytokine gene namely IL-1β, and chemokine genes like IL-8, MIP-1β, K60 and K203 to assess the mucosal immune response in the present work. IL-8 functions as a chemotactic factor for leukocytes [45], MIP-1β is involved in activation of acute and chronic inflammatory host responses during infection by recruiting pro-inflammatory cells [39] [24], K60 function as a chemotactic factor for heterophils [37] and K203 is involved in recruitment of macrophages to site of infection [18]. The importance of these genes in the assessment of immune responses is discussed.
Materials and methods
Vaccines
Two vaccines namely thermostable live Newcastle disease vaccine (D58 strain) and an oral pellet vaccine (D58 strain) were used. They were administered into separate groups of birds on 21st day of age by oral and occulonasal routes respectively. Each bird was administered a dose of 106.5 EID50/0.1 ml.
Experimental design
Chicks (Nandanam B3 broiler variety) with no history of hatchery vaccination were housed in the Centralized Animal House at Madras Veterinary College. They were provided with unmedicated feed and water ad libitum. They were divided into three experimental groups—Group I, Group II and Group III of 12 birds each and one group—Group IV of unvaccinated control with eight birds. Group I were given the oral pellet vaccine, Group II received live thermostable Newcastle disease vaccine as occulonasal instillation and Group III received live thermostable Newcastle disease vaccine as occulonasal instillation followed by oral pellet vaccine on 21st day post vaccination as booster. Group IV was maintained as unvaccinated controls.
Sampling
Samples for the assessment of gene expression and IgA ELISA namely spleen, intestine (caecal tonsils), intestinal washings, bile [33], tracheal washings, lachrymal fluid [42] and Harderian gland [2] were collected from both vaccinated and unvaccinated control on 0, 3, 7, 14, 21, 24 and 28 days post vaccination. The peripheral lymphocytes were collected from blood, purified [29] and used for CBA.
Extraction of tissue RNA and cDNA synthesis
RNA was extracted from cells of spleen and intestine using TRIZOL (Cat # 15596018, Invitrogen) as per the manufacture’s instruction and the concentration was estimated at 260/280 Å using spectrophotometer (Biophotometer Plus, Eppendorf) [35].
The synthesis of cDNA was carried out with ~400 ng of total RNA using cDNA synthesis kit (Cat # K1612, Fermentas) following manufacturer’s instruction. For each gene, 10 µl cDNA was synthesized using random hexamer. The cDNA was quantified, diluted to have a final concentration of 100 ng/µl and used.
Primers
The primers used for the amplification of cellular/cytokine genes are provided as supplementary material.
Quantitative real time PCR and gene expression data analysis
Quantitative PCR (qPCR) was carried out using SYBR® Green (Cat # 4309155, TaKaRa) following the manufacturer’s instruction in a real time thermocycler (Mastercycler® Eprealplex, model # 22331, Eppendorf). The qPCR conditions included an initial denaturation at 94 °C for 3 mins followed by 45 cycles of three temperature (denaturation 94 °C for 15 s, annealing for 20 s as per temperature mentioned in the above table for respective genes and extension at 72 °C for 20 s) and final extension at 72 °C for 5 mins followed by a melting curve analysis. The fold increase/decrease in expression of cellular/cytokine genes in response to vaccines was estimated by relative standard curve method [21]. Briefly, the Ct values were transformed into “Sample ng” by applying the following formula after estimating the slope and intercept using mean Ct values obtained from standards.
Then the sample ng values were normalised with endogenous gene control (β actin) by applying the formula
The expression ratio is obtained by dividing the sample signal that is normalised to the endogenous control to control sample signal that is normalised to the endogenous control.
The expression percentage is obtained by the following formula
Colorimetric blastogenesis assay
Colorimetric blastogenesis assay [29] was carried out to estimate the CMI response, which was also used to validate qPCR results. The CMI response was reported as stimulation index (SI) whereas SI = (Mean absorbance of stimulated culture) − (Mean absorbance of unstimulated culture)/Mean absorbance of unstimulated culture.
Indirect ELISA for IgA
Indirect ELISA [13] [40] was performed to estimate IgA response. The optimum dilutions of coating antigen (purified NDV), samples and conjugate were determined by checkerboard titration [31]. The optimum dilution of coating antigen and conjugate were 5 ng/µl and 1:5,000 respectively. Bile, lachrymal fluid was used in 1:1,000 dilution and intestinal washings, tracheal washings and Harderian gland was used in 1:10 dilution.
Results
Cell mediated immune response
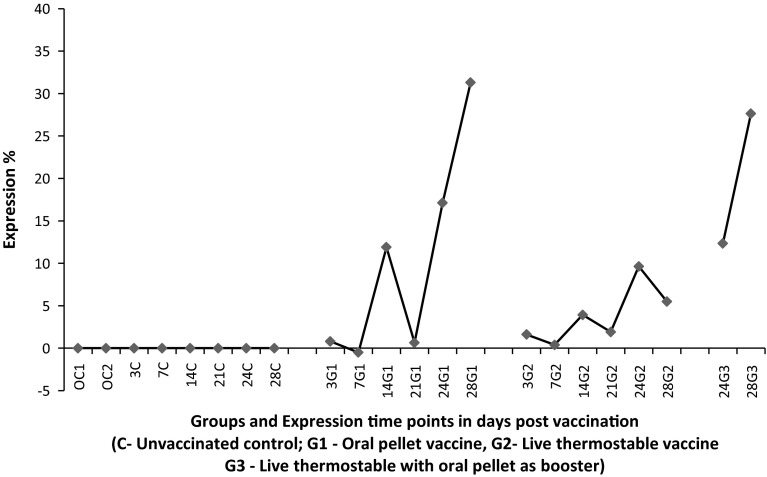
In the present study, the CMI response was assessed using qPCR for the quantification of IFN-γ gene. As a comparative measure, results have been compared with CBA. The expression of IFN-γ was upregulated by 12-folds from 14th day post vaccination in the Group I, which have received oral pellet vaccine. Stimulation Index (SI) value of this group during the same time period was 0.324. In the Group II, where birds have received live thermostable vaccine as occulonasal instillation, the same gene was upregulated by fourfolds from 14th day post vaccination. Further, the SI value of this group during the same time period was 0.302. In Group III, where birds have received live thermostable ND vaccine as priming vaccine and oral pellet vaccine as booster, upregulation was noticed by 12-folds and 27-folds on 3 and 7 days after receiving booster respectively. However, SI value of this group during the same time period was 0.275 and 0.068 respectively. The expression ratios of above gene during the same time points are also graphically depicted in Fig. 1. The SI values during various time points in different groups are provided in Table 1.
Fig. 1.
Expression pattern of IFN-γ gene in splenocytes in chickens vaccinated against NDV to assess cell mediate immune response
Table 1.
Stimulation index values in peripheral lymphocytes from blood in chickens vaccinated against NDV to assess cell mediate immune response
| Days post-vaccination/ Groups | 0 | 3 | 7 | 14 | 21 | 24 | 28 |
|---|---|---|---|---|---|---|---|
| Group IV | 0.019 | −0.09 | −0.098 | −0.064 | 0.028 | 0.013 | −0.105 |
| Group I | 0.019 | 0.103 | 0.046 | 0.324 | 0.267 | 0.019 | |
| Group II | 0.019 | 0.016 | −0.042 | 0.302 | 0.025 | −0.029 | |
| Group III | 0.019 | ND | 0.275 | 0.068 |
Mucosal immune response
The mucosal immune response against live thermostable vaccine and oral pellet vaccine was assessed by quantitative real time PCR (qPCR) against five genes namely IL-8, IL1-β, MIP1-β, K60 and K203 in the intestinal cells. The details of expression pattern at various time points for these genes are provided in Table 2. The genes mentioned above were not expressed in Group IV, which did not receive any vaccine. In Group I, which have received oral pellet vaccine upregulation of IL-8, IL1-β and MIP1-β was noticed by approximately 43-, 23- and 14-folds respectively on 21st day post vaccination. Neither up nor down regulation of K60 and K203 genes was noticed in this group. In Group II, which have received live thermostable Newcastle disease vaccine as occulonasal instillation, only MIP1-β gene was upregulated. In Group III, where birds have received live thermostable ND vaccine as priming vaccine and oral pellet vaccine as booster, neither up nor down regulation of these genes were noticed after receiving booster. IgA response could be detected only in bile and intestinal wash in the Group I. Only in this group significant increase in OD values could be observed. IgA could not be detected in lachrymal fluid, tracheal washings and Harderian gland. The IgA values are provided in Table 3.
Table 2.
Expression of IL-8, IL-1β, MIP-1β, K60 and K203 genes in intestinal cells in chickens vaccinated against NDV to assess mucosal immune response
| Time points | IL-8 | IL-1β | MIP-1β | Time points | K60 | K203 |
|---|---|---|---|---|---|---|
| 0G4 | 0.00 | 0.00 | 0.00 | 0G4 | 0.00 | 0.00 |
| 0G4 | 0.00 | 0.00 | 0.00 | 0G4 | 0.00 | 0.00 |
| 3G4 | 0.00 | 0.00 | 0.00 | 3G4 | 0.00 | 0.00 |
| 7G4 | 0.00 | 0.00 | 0.00 | 7G4 | 0.00 | 0.00 |
| 14G4 | 0.00 | 0.00 | 0.00 | 14G4 | 0.00 | 0.00 |
| 21G4 | 0.00 | 0.00 | 0.00 | 21G4 | 0.00 | 0.00 |
| 24G4 | 0.00 | 0.00 | 0.00 | 24G4 | 0.00 | 0.00 |
| 28G4 | 0.00 | 0.00 | 0.00 | 28G4 | 0.00 | 0.00 |
| 3G1 | 1.12 | 8.19 | 0.58 | 3G1 | 0.39 | −0.01 |
| 7G1 | −0.38 | −0.29 | −0.68 | 7G1 | −1.51 | −1.13 |
| 14G1 | −0.56 | −0.82 | 16.76 | 14G1 | 1.69 | −0.24 |
| 21G1 | 42.80 | 23.21 | 14.81 | 21G1 | 0.85 | −2.12 |
| 24G1 | 0.11 | −0.61 | −0.56 | 28G1 | 0.16 | |
| 28G1 | 0.23 | 1.50 | −0.74 | |||
| 3G2 | −0.12 | −0.32 | −0.44 | 3G2 | 0.13 | −0.54 |
| 7G2 | 0.54 | −0.81 | −0.72 | 7G2 | −0.89 | −1.02 |
| 14G2 | 0.62 | −0.36 | 70.83 | 14G2 | 1.02 | 0.07 |
| 21G2 | 3.33 | 0.26 | −0.04 | 21G2 | 0.33 | −1.47 |
| 24G2 | −0.68 | −0.68 | −0.82 | 28G2 | 1.55 | ND |
| 28G2 | −0.20 | −0.51 | −0.86 | |||
| 24G3 | 0.07 | 5.92 | ||||
| 24G3 | −0.61 | −0.50 | −0.78 | 28G3 | −0.03 | −21.09 |
| 28G3 | −0.92 | −0.90 | −0.97 |
Values expressed as percentage fold increase
G4 unvaccinated control, G1 oral pellet vaccine, G2 live thermostable vaccine, G3 live thermostable vaccine with oral pellet as booster, ND not done
Table 3.
IgA response in chickens against NDV in different secretion as optical density (OD) values
| Days | 3 | 7 | 14 | 21 | 24 | 28 |
|---|---|---|---|---|---|---|
| Group IV | ||||||
| Bile | 0.255 | 0.331 | 0.248 | 0.284 | 0.259 | 0.259 |
| Lacrymal fluid | 0.228 | 0.226 | 0.222 | 0.235 | 0.223 | 0.151 |
| Intestinal wash | 0.265 | 0.265 | 0.189 | 0.172 | 0.197 | 0.123 |
| Tracheal wash | 0.214 | 0.275 | 0.302 | 0.092 | 0.188 | 0.188 |
| Hardererian gland | 0.302 | 0.314 | 0.197 | 0.096 | 0.166 | 0.166 |
| Group I | ||||||
| Bile | 0.301 | 0.504 | 0.345 | 0.412 | 0.338 | 0.125 |
| Lacrymal fluid | 0.260 | 0.293 | 0.170 | 0.159 | 0.161 | 0.122 |
| Intestinal wash | 0.230 | 0.304 | 0.310 | 0.099 | 0.186 | 0.186 |
| Tracheal wash | 0.281 | 0.281 | 0.158 | 0.136 | 0.163 | 0.104 |
| Hardererian gland | 0.231 | 0.205 | 0.182 | 0.137 | 0.167 | 0.114 |
| Group II | ||||||
| Bile | 0.327 | 0.329 | 0.390 | 0.287 | 0.261 | 0.116 |
| Lacrymal fluid | 0.343 | 0.328 | 0.158 | 0.124 | 0.176 | 0.101 |
| Intestinal wash | 0.288 | 0.253 | 0.213 | 0.110 | 0.212 | 0.143 |
| Tracheal wash | 0.242 | 0.285 | 0.158 | 0.117 | 0.205 | 0.111 |
| Hardererian gland | 0.388 | 0.336 | 0.170 | 0.121 | 0.187 | 0.132 |
| Group III | ||||||
| Bile | 0.261 | 0.130 | ||||
| Lacrymal fluid | 0.176 | 0.146 | ||||
| Intestinal wash | 0.212 | 0.132 | ||||
| Tracheal wash | 0.205 | 0.115 | ||||
| Hardererian gland | 0.187 | 0.114 | ||||
OD values in bold font indicate significant increase than control values
Discussion
Both cellular and humoral immune responses were reported to play an important role in both the respiratory and intestinal tracts [5] in the host defense against NDV [16] [29] [30]. However, estimation of these are difficult due to limited sensitivity and poor reproducibility of many assays. Hence, in the present study was contemplated to study these responses by quantifying the expression of genes responsible for them. Of the methods used to study the expression of genes, qPCR has been reported to be a sensitive assay. It is used to study the CMI response against cancer antigens [10], to study the mucosal immune response to Eimera tenella and Eimeria maxima [18], to find out the immunogenicity of virulent and vaccine ND viruses [32], to find out in vivo and in vitro transcriptional response against ND [7] [17] [26] and to find out the suitability of chicken line for strong immune response against ND [1]. However, to the extent possible to us, we could not come across a paper dealing with utility of qPCR in quantification of response of genes of certain cytokines and chemokines and comparing it with conventional test used for assessment of mucosal immune response and literature on utility of this test in estimating the CMI is also minimal. On perusal of literature, upregulation of IFN-γ gene and its association with CMI has been established against many pathogens including NDV [32] [7] [26] and infectious bursal disease virus (IBDV) [25]. In the present study, the SI values were comparable with the fold increase in expression of IFN-γ gene. This proves the utility of estimating the expression of IFN-γ gene in quantifying the CMI response. Variations observed in SI values could be due to limited sensitivity of CBA. Further, the CBA test could not identify the increase in CMI 3rd and 7th day post booster vaccine in Group III. This could be due to the poor sensitivity of CBA. The absence of variation in expression of IFN-γ gene makes this test better than CBA.
Unlike adaptive immune responses like cellular and humoral, quantification of mucosal immune response is difficult due to poor sensitivity of tests used and lack of IgA mediated anamnestic response. The IgA ELISA has been reported to be the simplest assay in assessing mucosal immune response. However, this test has been reported to be able to detect IgA response efficiently in bile and intestine only [13]. Further, the response was interpreted in terms of increase in optical density (OD) values and not in titres. Hence as an alternative, we have attempted to quantify certain cytokine genes responsible for local response in intestinal cells using qPCR. On perusal of literature, we could identify the utility of qPCR in the quantification of mucosal immune response against Eimeria tenella and Eimeria maxima [18] and not against any other organism. The available literature pertaining to NDV [32] [7] [26] mostly discussed the transcription profile or the ability of virulent NDV to induce strong upregulation of certain cytokine genes.
IL-8 has been reported to be a member of groups of small structurally related cytokines with chemotactic activity for specific leukocyte types [45]. This chemokine has been reported to be an avian orthologue for human IL-8 and has been termed as chicken chemotactic and angiogenic factor (CAF) [23]. Two functions have been associated with this chemokine namely increase in influx of heterophils [11] and initiation of wound healing cascade in vivo [23]. Hence, the upregulation of this gene in the present study is an indication of the influx of heterophils and initiation of wound healing cascade and mitogenicity of fibroblasts. Though the influx of heterophils can damage intestinal epithelium, it could not be so in this case as the upregulation was only on 14th day post vaccination and not earlier than that. The birds were apparently normal during this with no signs of diarrhoea or others. Hence, it could be attributed that influx of heterophils mediated by this chemokine is a beneficial feature in chicken in removal of NDV that replicate in epithelium. It could also be attributed that initiation of wound healing cascade and mitogenesis of fibroblasts are also beneficial to the chicken.
It has been reported that chicken IL-1β, an activator of immune system in an acute phase response is functionally similar to mammalian IL-1β. An increase in expression has been reported to attract macrophages and T lymphocytes. Induction of macrophages and T lymphocytes are essential in stimulation of immune response as the former is a good antigen presenting cell (APC) and the latter is an important factor converted to Th1 or Th2 cells. In the present study, elevation of this cytokine was reported in Group I, which has received oral pellet vaccine and not in Group II. This could be due to the simple fact that Group II birds have received vaccine as occulonasal instillation. The initial increase of eightfolds on day 3 and 23-folds on day 21 is clear indication of induction of immune response in intestine. Same type results have been observed 7th day post infection in Eimeria infection [18].
Macrophage inflammatory proteins have been reported to be responsible for eliciting immune response. They have been reported to be produced by all cells including T and B lymphocytes. Further they have also been reported to increase production of other cytokines namely tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β) and interferon-Gamma (IFN-γ). They have also been reported to have chemotactic for monocytes, dendritic cells and natural killer (NK) cells. Hence, it could be concluded that upregulation of this gene in intestinal mucosa is important in eliciting immune response locally. In the present study also strong upregulation up to the extent of 16-folds was observed on day 14th post vaccination in Group I. Interestingly, during same time point, Group II, despite of receiving vaccine through occulonasal route has shown strong upregulation of 70-folds. Hence it could be concluded that the vaccine causes upregulation of MIP-1β irrespective of route of inoculation and produces strong pro-inflammatory response which are essential to produce immune response locally.
The chemokines K60 and K203 are CXC and CC chemokines respectively and have been reported to have the function of chemo-attractivity towards heterophils and macrophages respectively. In the present study, K60 and K203 chemokines were more or less constantly expressed. Same finding was also reported by [26], whereas, upregulation of K203 was reported on 7th day of Eimeria infection [14]. Though these two genes were not upregulated, it could be concluded that the development of immune response may not be affected as the other cytokine like, IL-1β and chemokines like IL-8, and MIP-1β could strongly attract heterophils, monocytes and lymphocytes to sites of viral replication. The upregulation of K203 in Group III post booster could be due to fact that in chicken, IL-1β and IFN-γ induces the upregulation of K203 [37].
In the present study, IgA ELISA has been performed with bile, intestinal wash, tracheal wash, Harderian gland and lachrymal fluid. Though, the test could identify the presence of IgA in all these samples, only in bile and intestinal wash in the group vaccinated with oral pellet vaccine (Group I) significant increase in OD values could be observed. Whereas, in all other time points in Group I and in other groups in all time points the increase in OD value was not significant. This could be due to the poor sensitivity of IgA ELISA. The earlier workers have reported the same and the same test in their experiments could detect IgA response efficiently in bile and intestine only [13] [40]. No significant increase in the OD value could be observed days 3, 7 after booster. Though the main purpose of the study was to assess the cell mediated and mucosal immune response, assessment of humoral response was also performed as a comparative measure (data not provided). Hence, the results of humoral response are not discussed.
In the present study we could clearly establish the utility of qPCR in estimating the mucosal immune. This test could overcome the drawbacks mentioned for IgA ELISA. The reliability of qPCR is certainly better as the estimation of immune response could be compared with the genes responsible for the same.
Electronic supplementary material
References
- 1.Ahmed KA, Saxena VK, Ara A, Singh KB, Sundaresan NR, Saxena M, Rasool TJ. Immune response to Newcastle disease virus in chicken lines divergently selected for cutaneous hypersensitivity. Int J Immunogenet. 2007;34:445–455. doi: 10.1111/j.1744-313X.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 2.Aitken ID, Survashe BD. A procedure for location and removal of lachrymal and Harderian glands of avian species. Comp Biochem Physiol. 1976;53:193. doi: 10.1016/S0300-9629(76)80054-0. [DOI] [PubMed] [Google Scholar]
- 3.Allan WH, Gough RE. A comparison between the haemagglutination inhibition and complement fixation tests for Newcastle disease. Res Vet Sci. 1976;20:101–103. [PubMed] [Google Scholar]
- 4.Baba T, Kawata T, Musumoto K, Kajikawa T. Role of the Harderian gland in IgA production in chicken lachrymal fluid. Res Vet Sci. 1990;49:20. [PubMed] [Google Scholar]
- 5.Beard CW, Max Brugh JR. Immunity to Newcastle disease. Am J Vet Res. 1975;36:509. [PubMed] [Google Scholar]
- 6.Djeraba A, Musset E, Bernarder N, Vern YL, Quere P. Similar pattern of iNOS expression, NO production and cytokine response in genetic and vaccination acquired resistance to Marek’s disease. Vet Immunol Immunopathol. 2002;85:63. doi: 10.1016/S0165-2427(01)00412-3. [DOI] [PubMed] [Google Scholar]
- 7.Ecco R, Brown C, Susta L, Cagle C, Cornax I, Jackwood MP, Miller PJ, Afonso CL. In vivo transcriptional cytokine responses and association with clinical and pathological outcomes in chickens infected with different Newcastle disease virus isolates using formalin-fixed paraffin-embedded samples. Vet Immunol Immunopathol. 2011;141:221–229. doi: 10.1016/j.vetimm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg M, Linda S, Elkowsky, Barry RB. Regulation of macrophage function by interferon-γ. J Clin Invest. 1990;85:563–569. doi: 10.1172/JCI114473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gough RE, Alexander DJ. The speed of resistance to challenge induced in chickens vaccinated by different routes with a B1 strain of live NDV. Vet Rec. 1973;92:563–564. doi: 10.1136/vr.92.21.563. [DOI] [PubMed] [Google Scholar]
- 10.Hempel DM, Smith KA, Claussen KA, Perricone MA. Analysis of cellular immune responses in the peripheral blood of mice using real-time RT-PCR. J Immunol Methods. 2002;259:129–138. doi: 10.1016/S0022-1759(01)00502-6. [DOI] [PubMed] [Google Scholar]
- 11.Henderson SC, Bounous DI, Lee MD. Early events in the pathogenesis of avian salmonellosis. Infec Immun. 1999;67:3580–3586. doi: 10.1128/iai.67.7.3580-3586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Committee for Taxonomy of Viruses (ICTV)—2011 Classification. www.ictvonline.org.
- 13.Jayawardane GW, Spradbrow PB. Mucosal immunity in chickens vaccinated with the V4 strain of Newcastle disease virus. Vet Microbiol. 1995;46:69–77. doi: 10.1016/0378-1135(95)00073-J. [DOI] [PubMed] [Google Scholar]
- 14.Jeurissen SHM, Boonstra-Blom AG, Al-Garib SO, Hartog L, Koch G. Defence mechanisms against viral infection in poultry. Vet Q. 2000;22:204–208. doi: 10.1080/01652176.2000.9695059. [DOI] [PubMed] [Google Scholar]
- 15.Karupiah G, Xie QW, Buller RM, Nathan C, Duarte C, Macmicking JD. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 16.Kirubaharan JJ, Vel Murugan R, Kumanan K, Albert A. Inactivated vaccines against Newcastle disease virus. Humoral immune response 2. Indian Vet J. 2007;7:563. [Google Scholar]
- 17.Kumar R, Kirubaharan JJ, Chandran NDJ, Gnanapriya N. Transcriptional response of chicken embryo cells to Newcastle disease virus (D58 strain) infection. Indian J Virol. 2013;24(2):278–283. doi: 10.1007/s13337-013-0148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurent F, Mancassola R, Lacroix S, Menezes R, Naciri M. Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect Immun. 2001;69:2527–2534. doi: 10.1128/IAI.69.4.2527-2534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JS, Hanson RP. Effects of bile and gastro-intestinal secretions in the infectivity of Newcastle disease virus. Infect Immunol. 1975;11:692. doi: 10.1128/iai.11.4.692-697.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li YP, Handberg KJ, Juul Madsen HR, Zhang MF, Jorgensen PH. Transcriptional profile of chicken embryo cell cultures following infection with infectious bursal disease virus. Arch Virol. 2007;152:463–478. doi: 10.1007/s00705-006-0878-9. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen DT. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Marino OC, Hanson RP. Cellular and humoral response of in ovo-bursectomized chickens to experimental challenge with velogenic Newcastle disease virus. Avian Dis. 1987;31:293. doi: 10.2307/1590875. [DOI] [PubMed] [Google Scholar]
- 23.Martins-Green M, Feugate JE. The 9E3/CEF4 gene product is a chemotactic and angiogenic factor that can initiate the wound healing cascade in vivo. Cytokine. 1998;10:522–535. doi: 10.1006/cyto.1997.0311. [DOI] [PubMed] [Google Scholar]
- 24.Maurer M, Von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36(10):1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Mo CW, Cao YC, Lim BL. The in vivo and in vitro effects of chicken interferon—alpha on infectious bursal disease virus and Newcastle disease virus infection. Avian Dis. 2001;45:389–399. doi: 10.2307/1592978. [DOI] [PubMed] [Google Scholar]
- 26.Munir S, Sharma JM, Kapur V. Transcriptional response of avian cells to infection with Newcastle disease virus. Virus Res. 2005;107:103–108. doi: 10.1016/j.virusres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Premraj A, Sreekumar E, Rasool TJ. Cloning and biological characterization of buffalo (Bubalus bubalis) interferon-γ. Mol Immunol. 2006;43:717. doi: 10.1016/j.molimm.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Rautenschlein S, Sharma JM, Winslow BJ, Mcmillen J, Junker D, Cochran M. Embryo vaccination of turkeys against Newcastle disease infection with recombinant fowlpox virus constructs containing interferons as adjuvants. Vaccine. 2000;18:426. doi: 10.1016/S0264-410X(99)00254-6. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds DL, Maraqa AD. Protective immunity against Newcastle disease: the role of cell-mediated immunity. Avian Dis. 2000;44:145–154. doi: 10.2307/1592518. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds DL, Maraqa AD. Protective immunity against Newcastle disease: the role of antibodies specific to Newcastle disease virus polypeptides. Avian Dis. 2000;44:138–144. doi: 10.2307/1592517. [DOI] [PubMed] [Google Scholar]
- 31.Rose RN, Folds DJ, Clifford H, Nakamura MR. Manual of clinical laboratory immunology. 5. Washington: American Society for Microbiology; 1997. [Google Scholar]
- 32.Rue CA, Susta L, Cornax I, Brown CC, Kapczynski DR, Suarez DL, King DJ, Miller PJ, Afonso CL. Virulent Newcastle disease virus elicits a strong innate immune response in chickens. J Gen Virol. 2011;92:931–939. doi: 10.1099/vir.0.025486-0. [DOI] [PubMed] [Google Scholar]
- 33.Russell PH. Newcastle disease virus vaccines: differences between line c and line 151 chickens with respect to virus replication and IgA responses in the gut and Harderian gland. Vet Immunol Immunopathol. 1993;42:357. doi: 10.1016/0165-2427(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 34.Russell P, Koch G. Local antibody forming cell responses to the Hitchner B1 and Ulster strains of Newcastle disease virus. Vet Immun Immunopathol. 1993;37(2):165–180. doi: 10.1016/0165-2427(93)90063-A. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook J, Russell DN. Molecular cloning: a laboratory manual. 3. New York: Cold Spring Harbour; 2001. [Google Scholar]
- 36.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 37.Sick C, Schneider K, Staeheli P, Weining KC. Novel chicken CXC and CC chemokines. Cytokine. 2000;12(3):181–186. doi: 10.1006/cyto.1999.0543. [DOI] [PubMed] [Google Scholar]
- 38.Takada A, Kida H. Protective immune response of chickens against Newcastle disease, induced by the intranasal vaccination with inactivated virus. Vet Microbiol. 1996;50:17–25. doi: 10.1016/0378-1135(96)00004-1. [DOI] [PubMed] [Google Scholar]
- 39.Taub DD, Conlon K, Lioyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–388. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 40.Varalakshmi S. Assessment of cell mediated and mucosal immune response in chickens vaccinated with live Newcastle disease vaccines. M.V.Sc thesis, 2005. Tamil Nadu Veterinary and Animal Sciences University.
- 41.Westbury H. Commentary Newcastle disease virus: an evolving pathogen. Avian Pathol. 2001;30:5. [DOI] [PubMed]
- 42.Wight PAL, Burns RB, Rothwell B, Mackenzie GM. Harderian gland of domestic fowl I. Histology with reference to genesis of plasma cells and Russel bodies. J Anat. 1971;110:307 [PMC free article] [PubMed]
- 43.Wigley Kaiser. Avian cytokines. Braz J Poult Sci. 2003;5(1):1–14. [Google Scholar]
- 44.Wong ML, Medrano JF. Real time PCR for mRNA quantitation. Biotechniques. 2005;39(1):75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- 45.Wuyts A, Proost P, Van Damme J. Interleukin-8 and other CXC chemokines. In: Thomson AW, editor. The cytokine handbook. 3. San Diego: Academic Press; 1998. pp. 229–269. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.