Abstract
In 1960s, potato spindle tuber was thought to be a viral disease. In 1971, the agent of the disease was characterised as a low-molecular weight infectious ribonucleic acid (RNA), which was named as ‘viroid’, specifically Potato spindle tuber viroid (PSTVd)). Since then, more than 30 plant diseases in horticultural and ornamental plants have been shown to be caused by different viroids globally. Viroids are single-stranded RNA, covalently closed circular molecule, without any protein coat. They are the smallest known plant pathogen containing RNA genome ranging from 246 nucleotides (Coconut cadang–cadang viroid) to 399 nucleotides (Chrysanthemum chlorotic mottle viroid). Some viroids are located in the plant cell nucleus (pospiviroids) and others in the chloroplast (avsunviroids). With the recognition of pathogenic nature of viroid, specific detection methodologies were developed, which enabled detection of PSTVd in seed-potato tubers prior to their planting in the field, and thus PSTVd was prevented from spreading the disease. As a result, PSTVd was eradicated from Canada in late 1980s. Viroids similar to PSTVd (Pospiviroid) have been discovered and they are detected in symptomless ornamental plants. Although, PSTVd has been eradicated from Canada, there is a strong possibility of viroid introduction from other plants besides potato and tomato and causing PSTVd like diseases.
Keywords: Potato Spindle tuber viroid, Discovery of viroid, Eradication of PSTVd
Introduction
Viroids disguised as “viruses” have probably existed as long as the plant viruses, considering that they do not differ significantly from viruses in their pathological effects on plants or in their modes of natural transmission [77]. Relative to virus, viroid is a newly described plant pathogen. Discovery of a novel class of pathogen is quite rare. Economic devastation through crop failures or significant losses in quality of agricultural products for human consumption takes place prior to attracting the attention of researchers such as plant pathologists. The discovery of viroids was no exception in that their presence remained undetected until they had plenty of impact on agricultural and fruit crops. Approximately a century ago (1917), Werner in Nebraska [91] was working on a degeneration disease of potato (probably potato spindle tuber). However, the first report of the disease and the use of name “spindle tuber” are credited to Martin [34]. Unaware of the naming of the disease by Martin, Schultz and Folsom [53] demonstrated the transmissibility of what they termed “spindling tuber” disease (Fig. 1). This disease was studied in New Brunswick, Canada, as early as 1925 by MacLeod [33]. Potatoes in the USA and Canada have suffered the outbreaks of potato spindle tuber virus (PSTV) with infection as high as 25–50 %. Low yields and long-pointed tubers with an excessive number of deep eyes were characteristic symptoms of the novel disease as described from the several areas of USA and Canada. In Canada potatoes exhibiting characteristic symptoms were observed from 1918 to 1921 mainly in the province of New Brunswick and in subsequent years in other provinces of Canada. Besides North America, in the 1960s and 1970s over half of the plants in some stands were infected in certain states of the Ukraine [27] and in China [42]. However, it was the high rejection rate of the potato-seed fields due to PSTV in the mid-1950s in Canada [32] which led to the renewed interest in the study of PSTV in Canadian and US laboratories.
Fig. 1.

Potato plant with PSTVd infection. Upright growth and upward pointed leaves are the identifying symptoms in potato plants. The tubers are spindly with deep cracks and unfit for human consumption
On a personal note, my journey into the world of PSTV began in 1962 as I was accepted into PhD program at North Dakota State University, Fargo, USA and was assigned the PSTV as my research subject. By mid 1960s, two government laboratories with long-time virus-researchers [13, 59] and four universities with PhD students [1, 28, 68, 92], were investigating the potato spindle tuber disease in the northeast USA and Canada. In 1964 and 1966, investigators from Nebraska and North Dakota in USA claimed that rod-shaped [1] or spherical-particles [68] were associated with the spindle tuber disease. Although, both claims later turned out to be wrong, these failures compelled us to try other approaches. Finally, the PSTV was identified as a unique phytopathogen named as Potato spindle tuber viroid (PSTVd). In this review, the discovery of PSTVd and its eradication in Canada are summerasied.
Viroid discovery
After completing my studies at North Dakota State University in early 1966, I joined the Canada Department of Agriculture as a National Research Council, post-doctorate fellow, to continue work on PSTV with Dr. R.H. Bagnall. By 1967–1968, it became clear to us, that the agent causing potato spindle tuber disease was not a conventional “virus” but a unencapsidated free ribonucleic acid (RNA) [13, 59]. Continued investigations in the two laboratories, one in USA [11] and the other in Canada [62], using the density-gradient centrifugation and the mobility in polyacrylamide gel electrophoresis showed that the infectious entity of PSTV was an extremely low-molecular weight RNA, compared to the conventional viral RNA [11, 62]. For the infectious RNA, names like ‘viroid’ [11] or “low-molecular weight RNA” [62] or “metavirus” [25] were used. After a few discussions at the Potato Association of America meetings, the “viroid” name was accepted.
Viroid discovery controversy
Viroid, being one of the newest discovered plant pathogen, was extensively reviewed in the early 1970–1980 s. For example, within the first 12 years of discovery there were 48 review articles dealing with viroid [58]. About 80 % of these articles were contributed by two groups, namely; T.O. Diener and associates (60 %) (USA) and H. L. Sänger and associates (20 %) (Germany). As high as 10 reviews were published in the years 1979 and 1983. However, most of the reviews by Diener have made an issue of the viroid-discovery aspect. Although the viroid or low-molecular weight nature of PSTVd was demonstrated by Diener [11], and Singh and Clark [62] in 1971, and has been referred in some books and review articles dealing with viroids and additionally pointed out to Diener the “real” circumstances of discovery in exchanges of Letter to the Editor to Plant Disease [56, 57], the treatment of our work has been either omitted or distorted by Diener. For example, in his review article 30 years later [12], he made misleading statements and quoted non-existent ‘Singh personal communication’ as the evidence. Finally, he has eliminated the reference of our 1971 paper [62] altogether from the Table 1 in the same 2001 review article by his ‘arbitrary’ decision. Therefore, the circumstances dealing with the viroid discovery have been elaborated below.
Table 1.
Families, Genera and Species of viroids
| Families | Genera | Species |
|---|---|---|
| Avsunviroidae | Avsunviroid | Avocado sunblotch viroid |
| Elaviroid | Eggplant latent viroid | |
| Pelamoviroid | Chrysanthemum chlorotic mottle viroid Peach latent mosaic viroid | |
| Pospiviroidae | Apscaviroid | Apple dimple fruit viroid |
| Applefruit crinkle viroid | ||
| Apple scar skin viroid | ||
| Australian grapevine viroid | ||
| Citrus bent leaf viroid | ||
| Citrus viroid III | ||
| Citrus viroid V | ||
| Citrus viroid original source | ||
| Grapevine yellow speckle viroid 1 | ||
| Grapevine yellow speckle viroid 2 | ||
| Grapevine yellow speckle viroid 3 | ||
| Pear blister canker viroid | ||
| Persimmon viroid | ||
| Cocadviroid | Coconut cadang-cadang viroid | |
| Coconut tinangaja viroid | ||
| Citrus bark cracking viroid | ||
| Hop latent viroid | ||
| Coleviroid | Coleus blumei viroid I | |
| Coleus blumei viroid II | ||
| Coleus blumei viroid III | ||
| Coleus blumei viroid IV | ||
| Coleus blumei viroid V | ||
| Coleus blumei viroid VI | ||
| Hostuviroid | Hop stunt viroid | |
| Pospiviroid | Chrysanthemum stunt viroid | |
| Citrus exocortis viroid | ||
| Columnea latent viroid | ||
| Irsine viroid 1 | ||
| Mexican Papita viroid | ||
| Pepper chat fruit viriod | ||
| Potato spindle tuber viroid | ||
| Tomato apical stunt viroid | ||
| Tomato planta macho viroid | ||
| Tomato chlorotic dwarf viroid |
Adopted from, J. TH J. Verhoven 2010, with permission
Our first paper reporting the isolation of RNA from PSTVd infected-plants was accepted for publication in Phytopathology in 1967 [59]. It was in reviewer’s hand before the publication of Diener and Raymer [13] paper. The reviewer of our paper, in this case happen to “see” a galley-proof of Diener and Raymer’s [13] paper. To our amazement, this reviewer recommended to the editor of the Phytopathology that “…… Singh and Bagnall, (1967) manuscript [59] is acceptable for publication (29 November 1967) but “I” would strongly urge that the authors withhold submitting until they had an opportunity to evaluate more recent results….. the results which I have mentioned”. This “unusual recommendation” by a reviewer of Phytopathology clearly shows that our work [59] was done independently and at the same time as Diener and Raymer’s [13].
Again in 1971, our continuing research demonstrating that the PSTVd was a low- molecular weight infectious RNA [62], was presented at a meeting of the American Phytopathological Society on August 17, 1971, in Philadelphia, USA. The abstract of this paper could have been available to the society members earlier than the 17th of August. The fact that Diener was aware of our work in advance of its publication was shown in US press release (source, Washington UPI) and published in Toronto Telegram (Canada), and St. Luis Post-Dispatch (USA), dated August 14, 1971. The release stated: “Agriculture officials (USDA) hurried the announcement of Diener’s discovery [11] yesterday because a similar announcement is expected from a Canadian scientist next week. Officials said Dr. R. P. Singh of the Canadian Department of Agriculture was planning to deliver a paper on the subject at a meeting in Philadelphia.” In light of the above statement, it is unfortunate that later reviews by Diener put a different slant on the discovery of viroid.
Viroid, a novel phytopathogen
At present more than 30 plant diseases [16–18] have been recognized as being caused by viroids in agricultural, horticultural and ornamental plants throughout the world (Table 1).
Viroids are molecularly a novel class of plant pathogen compared to viruses, where they were previously grouped. Viroids are considered as the most efficient and sophisticated ‘molecular plant parasites’ apparently shedding the need to synthesize structural or functional proteins by manipulating host enzymes for their replication and are capable of destroying trees, including coconut palms [44]. Viroids are highly contagious, particularly members of the genus Pospiviroid [77]. Some viroids have a wide host-range and are transmitted readily by mechanical means, including foliage contact, handling during cultivation, and contamination of cutting knives or other tools, e.g., 80 % of potato plants were infected by PSTVd when infected leaves were rubbed in the field against healthy plant leaves [40]. All viroids are transmissible by grafting, while a number of reports are for transmission through seeds and pollen of the infected plants [3, 55, 65, 71, 72, 73, 87]. There are a few reports of transmission of pospiviroid by insects [10, 21]. Despite the free RNA nature of viroids, PSTVd can survive in freeze-dried tomato leaves at room temperature for several years [66] or in true potato seeds for over 20 years [72]. Most cultivated plants have no natural resistance to viroid diseases and transgenic plant approaches have had limited success [8].
Although viroids multiply faster at high temperatures and under high light-intensities [8, 48], however, repeated exposures to freezing temperature has also been found to eliminate PSTVd from potato tubers [61] and Tomato chlorotic dwarf viroid (TCDVd), which is closely related to PSTVd, can flourish in symptomless plants of Vinca minor at subzero temperatures (−12 °C) [65].
Besides PSTVd, the low-molecular weight nature of citrus exocortis viroid (CEVd) was reported in 1972 in Germany [47]. Within 5 years of viroid-discovery in North America, the German researchers elucidated the complete nucleotide sequence of PSTVd and titled their paper as “Viroids are single stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures” [49]. By the year 1982 the German team had described other structural properties of viroids [22–24, 26, 45]. They showed that the viroids consist of a single unit of single-stranded, covalently closed, circular RNA that ranges in size from 246 to 399 nucleotides, depending on the viroid species. The nucleotide chain length of various strains of a viroid could vary 2–5 nucleotides in length, for example in PSTVd [26], CEVd [86] and hop stunt (HSVd) [50].
The German team also provided the details of viroid structure and function. They demonstrated that due to the extensive regions of intra-molecular complementarity a viroid molecule under native conditions is double-stranded and rod-like, but when denatured, it becomes a circular, single-stranded molecule. The circles have been shown to elongate and form a defective double-helix, in which double helical segments are separated by short unpaired stretches. In conditions where free energy is low, viroids form a rod-like native conformation as a secondary structure, in which loops and bulges of unpaired nucleotides separate double-stranded regions. The secondary structure is assumed to be the key for the biological activity by being functional as such or by providing binding signals to specific host factors.
The rod-like conformation has been shown to contain five structural domains based on nucleotide sequence analysis [30]. These domains are referred to as central (CD), pathogenic (PD), variable (VD), terminal left (TLD) and terminal right (TRD). Initially, the domains were presumed to have individual functional roles. However, later it was shown [80] that pathogenicity was not only controlled by PD but also by TLD, VD and TRD. In our studies, a seven-nucleotide substitution in the TRD has been correlated with severe stunting symptoms of potato and tomato plants (Fig. 2) infected with a strain of TCDVd [80]. In addition, it has been shown that even substitutions of single nucleotide in the lower CD could have a dramatic impact on pathogenicity [88]. Besides the rod-like structure, members of the family Pospiviroidae can form metastable secondary structures, e.g., hairpins, tetra-loops and stems. Many members of Pospiviroidae have been shown to contain an internal loop in the CCR, which appears similar to the loop E of the eukaryotic 5S rRNA [2, 6]. The E-loop is involved in the synthesis and transport of 5S rRNA. A similar role has been assumed for the E-loop-like structure of the Pospiviroidae [2].
Fig. 2.

Tomato chlorotic dwarf viroid in a greenhouse tomato production system (From Singh et al. 1999)
An orderly grouping of different viroids in genera and families, based on their genome structure and replication features has been established [9]. Viroid species have been divided into Pospiviroidae and Avsunviroidae families. The viroids, which possess specific sequences within their molecular structure, known as central conserved region (CCR) and are located in the plant cell nucleus, are in the Pospiviroidae and those viroids which possess a hammerhead ribozyme structure and are located in the plant chloroplast are in the family Avsunviroidae. The PSTVd has been recognized as a distinct species of viroid under the genus Pospoviroid, class Pospiviroinae and family Pospiviroidae.
Both groups of viroids have different replication modes. In Pospiviroidae the replication takes place in the nucleus. The replication pathway is an asymmetrical cycle, and the enzyme involved is DNA dependent RNA polymerase II and no self-cleavage is involved. The steps involved are as follows: The (+) circular RNA is transcribed into an oligomeric linear (-) strand RNA. The (-) strands then serve as intermediate for the synthesis of oligomeric (+) strand RNAs, which are cleaved into unit length monomers and then ligated into circles, the active viroid molecule [5]. Members of the family Avsunviroidae have a quasi-rod like or branched-secondary structure. They replicate in chloroplast by a symmetric rolling circle mechanism using chloroplastic encoded RNA polymerase and are self-cleaved via hammerhead structures [9]. Beside this simple outline, different enzymes are involved in transcription, cleavage and ligation and have not been confirmed.
Methodologies for viroid detection
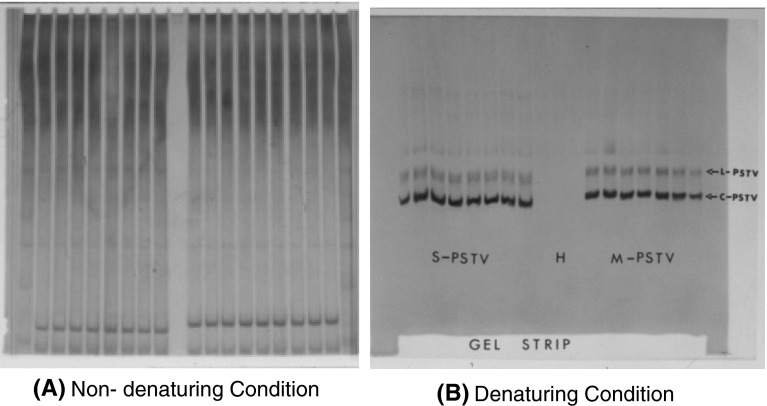
Unlike viruses, viroids are devoid of a protein-coat and they do not contain the machinery for protein synthesis. Therefore, the serological detection methods commonly used for viruses are not applicable to viroids. The polyacrylamide gel electrophoresis (PAGE), which initially enabled successful separation of viroids from other cellular constituents or from host RNAs [11, 62] was modified for large-scale diagnosis of PSTVd in various laboratories [19, 36, 37, 40, 41, 52, 60, 69, 73]. Extensive use of PAGE technique for viroid detection resulted in many modifications [52, 60, 69, 73]. The latter two modifications are known as “return” polyacrylamide gel electrophoresis (R-PAGE) [69, 73]. In R-PAGE the viroid molecules are first separated on 5 % non-denaturing gels in high salt buffer (Fig. 6). After the first electrophoresis, the buffer is exchanged for a heated (87–90 °C) low-salt buffer [69]. The polarity is reversed and a second electrophoresis is performed at 70–71 °C. The separation of viroid from other nucleic acids is achieved by the slower mobility of the denatured viroid molecules (Fig. 3). Extensive evaluation of R-PAGE using mild and severe PSTVd strains-infected potato leaves, tubers, pollen and true-potato seeds, and seeds from coleus infected with coleus viroids, showed that viroids can be reliably detected from individual seed despite the small seed size [19, 68, 73]. The R-PAGE detection technology was also adopted in Brazil [43], China [65] and India [39] to survey for the viroids from crop plants and ornamentals.
Fig. 6.
Four symptomless Verbena selections, infected with chrysanthemum stunt, citrus exocortis or iresine viroids (top panel), when transferred to potato plants (only two potato plants are shown), caused severe stunting symptoms. Tubers were badly deformed, spindly and small (Nie et al. 2005)
Fig. 3.
Return-Polyacrylamide Gel Electrophoresis. First electrophoresis run is in non-denaturing gel (a). Lower part of gel-strip is cut out and placed in the bottom of the gel (b). Polarity is reversed and electrophoresis is run as before but at higher temperature
Although a sensitive technique for the detection of viroids was available [90] based on nucleic acid hybridization using radioactive-labelled probes, the use of radio-labelled probe is limited to centers with facilities which can handle storage and waste disposal of radioactive material. Therefore, highly sensitive digoxigenin-labelled DNA or RNA probes were developed [4, 75]. However, in addition to the R-PAGE and radiolabelled or digoxigenin probes, at present RT-PCR based methods for viroid detection are available [64, 78]. These RT-PCR methods can detect several members of a group. For this purpose RT-PCR based primer pairs are designed which can amplify more than one viroid [78] irrespective of the host species and viroid family (Fig. 4). The effectiveness of the primer pair for members of genus Pospiviroid has been demonstrated by the detection of PSTVd and TCDVd in potato; CSVd and IrVd in Verbena and Vinca species and CEVd from Impatiens [78]. Similar methods are also available in other countries [64]. To facilitate and simplify the viroid extraction from plant parts without organic solvents, NaOH-EDTA solution based protocol for viroid preparation from crop and ornamental plants has been developed. In this protocol plant tissues are homogenized in NaOH-EDTA (50 mM + 25 mM EDTA) with a tissue to solution ratio of 1:4 (W/V) and incubated at room temperature for 15 min to settle the coarse plant material then the supernatant is used for RT-PCR [78].
Fig. 4.
A universal Pospiviroid primer pair, designed to amplify most viroids of the group (Bostan et al. [4])
Eradication of PSTVd from Canada
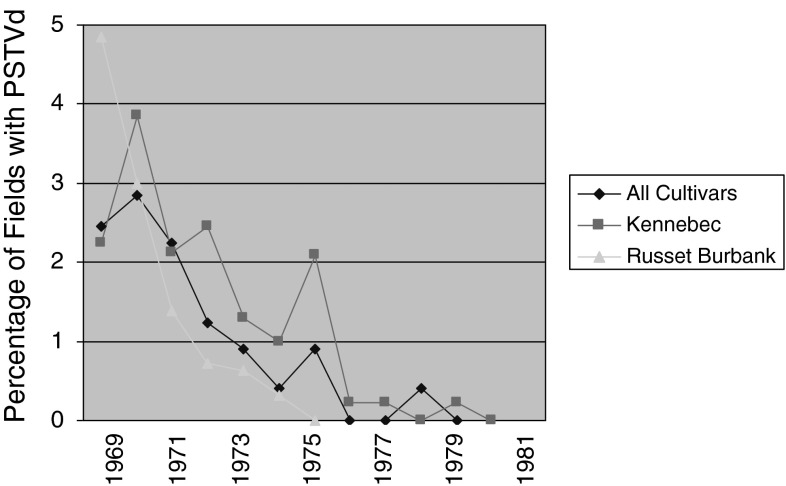
In Canada, a multifaceted eradication methodology was employed involving following steps: (1) Compulsory pre-planting testing of seed-potatoes for viroid to be used in commercial planting. (2) Establishment of specialized laboratories to carry out R-PAGE testing of seed potatoes throughout the country. (3) Requirement of viroid-testing of all parental material prior to their use in potato-breeding programs and (4) Viroid-freedom of the new cultivars before their release to potato seed growers. As a result PSTVd was eradicated from potato crops in Canada [64, 70] (Fig. 5). An analysis of the field inspection data over a period of 1969–1983 of seed potato crop in selected areas of Canada showed that the incidence of PSTVd by late 1980s had decreased to the point where it could not be detected by visual observation as well as by R-PAGE test. This eradication of PSTVd in the seed potato crop was attributed to the higher standards and stricter regulations in seed certification programs, use of viroid-free seed multiplied at Elite seed farms, enactment of provincial disease eradication acts and strict planting requirements by processing and seed growers in the region. With the eradication of PSTVd trade to customer countries resumed in the late 1980s.
Fig. 5.
Eradication of the PSTVd from Canada. Since visual observation of PSTVd is not reliable in all potato cultivars, three types of leaf samples were tested. All the samples were tested in the prescribed laboratories [64]
Looming threats to crops from viroids in symptomless ornamental plants
Although PSTVd has been eradicated from Canada, there is a strong possibility of viroid introduction from other plants besides potato and tomato and causing PSTVd like diseases. For example, CEVd [76], and a viroid from Nematanthuswettsteinii plants, closely related to CLVd [74] have been shown to cause PSTVd like symptoms in potatoes. Recently, another pospiviroid, named Tomato chlorotic dwarf viroid (TCDVd), a viroid closely related to PSTVd was identified from the commercial greenhouse tomato Fig. 2 crops in Canada [76]. Finding of TCDVd in Canada was soon confirmed in other countries. For example TCDVd, infecting tomatoes in the Netherlands [84], Japan [35], USA [31] France [7] and Norway [20]. In addition to the infection of tomato plants, TCDVd has been isolated from symptomless ornamental plants from India [79], Netherlands [85], UK [29] and Japan [54] in the last few years. These rapid findings of TCDVd in tomato greenhouse crops and in open fields in ornamental plants pose serious problems in keeping crops like potato and tomato free of viroids. Particularly the occurrences of TCDVd in tomato greenhouses could be due to the cultivation practices used at present in the greenhouses throughout the world. For example, continuous tomato production year round, tall plants, repeated pruning of new plant shoots during cultivation, high humidity and high temperature in the greenhouses provide ample chances of viroid contamination, survival and spread. However, the findings of TCDVd in symptomless ornamental plants [15, 29, 54, 65, 85] needs additional studies to determine the sources of viroids in the area. As shown (Table 2) at present the known pospiviroids have been detected from many more ornamental plants compared to the crop plants. It has also been shown that compared to PSTVd, the CLVD strains from ornamental plants can cause significant losses of potato tuber yields. Although most of the ornamental plants do not show symptoms when infected with viroids, they do cause severe symptoms, when transferred to potato and tomato plants and tubers (Fig. 6).
Table 2.
Pospiviroids and their economic and ornamental host plants
| Viroids | Economic hosts | Ornamental hosts |
|---|---|---|
| Citrus exocortis viroid (CEVd) | Citrus | Glandularia pulchella |
| Impatiens sp. | ||
| Solanum jasminoides | ||
| Verbena sp. | ||
| Potato spindle tuber viroid (PSTVd) | Potato | Brumansia × candida |
| Tomato | Brugmansia × flava | |
| Brugmansia sanguinea | ||
| Brugmansia suaveolens | ||
| Calibrachoa sp | ||
| Datura sp. | ||
| Lycianthes rantonnetii | ||
| Petunia sp. | ||
| Solanum jasminoides | ||
| Streptosolen jamesonii | ||
| Tomato apical stunt viroid (TASVd) | Tomato | Cestrum sp. |
| Lycianthes rantonnetii | ||
| Solanum jasminoides | ||
| Solanum psecapsicumuedo | ||
| Streptosolen jamesonii | ||
| Tomato chlorotic dwarf viroid (TCDVd) | Tomato | Brugmansia sanguinea |
| Pittosporum tobira | ||
| Verbena sp. | ||
| Vinca minor |
Adopted from, J. TH J. Verhoven 2010, with permission
Viroid as molecular tool
Attempts to isolate viroids from their host plant-tissues resulted in a new method of R-PAGE [52], which is increasingly used for viroid detection from plants [39, 43, 69, 73]. Similarly, another method of gel-electrophoresis, known as temperature-gradient gel electrophoresis (TGGE) [46] developed during the characterization of PSTVd, is used for determining the viroid conformal transactions, sequence variations and protein-nucleic acid interactions [46]. These by-products of viroid research have become the new tools for viroid characterization. Similarly, viroid replication strategies in their hosts have shown that both Pospiviroids and Avsunviroids, have to be transported into nucleus (Pospiviroids) and to the chloroplast (Avsunviroids) prior to their replication. In addition, the enzymes involved in viroid replication are host-coded. How the viroids manage these significantly important aspects of their movement and replication using enzymes from the host plants needs elucidation of the mechanisms. Understanding of this mode of pathogenicity would be a new tool for the plant molecular biology at large. Knowledge of how viroids accomplish these feats would go long way to show their real uniqueness as a plant pathogen. On these aspects, questions have been posed by viroid researchers dealing with the movement and replication [38]. What are the molecular signals from viroids, which make host enzymes to accept viroids as templates for the synthesis of complementary RNAs? [38]. It is worth noting, that the evidence of RNA-directed DNA methylation was discovered in tobacco plants, that contained multimeric genome-integrated copies of PSTVd cDNA [5], which is another tool to expand our understanding of viroid pathogenicity and may extend to other pathogens and to gene-silencing in host plants.
Concluding remarks
Transition from the dark phases of PSTV characterization in 1960s, to the viroid discovery in 1970s, created a competitive environment in viroid research. Just to illustrate how high was the “publication” pressure during that period, that some researchers were not thoroughly confirming their results before publication. For example, when one reads the variable molecular weight values of PSTVd from the same laboratory, one gets the idea of hurried publication path. For example, the molecular weight of PSTVd in 1971was 50,000 daltons [14], in 1973 it was 75,000–85,000 daltons [15], or 80–90,000 daltons [81] in North American publications. However, it was finally shown to be 127,000 daltons by European researchers in 1976 using novel technology [22]. The knowledge about the viroids has increased many-fold since then. However, more has to be done to learn how viroids manipulate the host plants to their advantage. Such knowledge could open up new avenues of disease management.
References
- 1.Allington WB, Ball EM, Galvez G. Potato spindle tuber caused by a strain of potato virus X. Plant Dis Rep. 1964;48:597–598. [Google Scholar]
- 2.Baustark T, Schröder ARW, Riesner D. Viroid processing: switch from cleavage to ligation is driven by a change from tetra-loop to a loop E conformation. EMBO J. 1997;16:510–599. doi: 10.1093/emboj/16.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson AP, Singh RP. Seed transmission of potato spindle tuber virus in tomato. Am Potato J. 1964;41:293. doi: 10.1007/BF02854871. [DOI] [Google Scholar]
- 4.Bostan H, Nie X, Singh RP. An RT-PCR primer pair for the detection of pospiviroid and its application in surveying ornamental plants for viroids. J Virol Methods. 2004;166:189–193. doi: 10.1016/j.jviromet.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Branch AD, Robertson HD. A replication cycle for viroids and small infectious RNAs. Science. 1984;223:450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- 6.Branch AD, Benenfeld BJ, Robertson HD. Ultraviolet light-induced cross-linking reveals a unique region of tertiary structure in potato spindle viroid and Hela 5S RNA. Proc Nat Acad Sci USA. 1985;82:6590–6594. doi: 10.1073/pnas.82.19.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Candresse T, Marais A, Tassus X, Suhard I, Renaudin I, Leguay A, Poliakoff F, Blancard D. First report of Tomato chlorotic dwarf viroid in tomato in France. Plant Dis. 2010;94:633. doi: 10.1094/PDIS-94-5-0633B. [DOI] [PubMed] [Google Scholar]
- 8.DaGraca JV, Van Vuuren SP. Use of high temperature to increase the rate of avocado sunblotch symptom development in indicator plant. Plant Dis. 1981;65:46–47. doi: 10.1094/PD-65-46. [DOI] [Google Scholar]
- 9.Daròs JA, Marcos JF, Hernández C, Flores R. Replication of avocado sunblotch viroid: evidence for a symmetric pathway with two rolling circles and hammerhead ribozyme processing. Proc Nat Acad Sci USA. 1994;91:1281–12813. doi: 10.1073/pnas.91.26.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bokx JA, Piron PGM. Transmission of potato spindle tuber viroid by aphids. Neth J Plant Pathol. 1981;87:31–34. doi: 10.1007/BF01976653. [DOI] [Google Scholar]
- 11.Diener TO. Potato spindle tuber “virus”.IV. A replicating, low molecular weight RNA. Virology. 1971;45:411–428. doi: 10.1016/0042-6822(71)90342-4. [DOI] [PubMed] [Google Scholar]
- 12.Diener TO. The viroid. Biological oddity or evolutionary fossil? Adv Virus Res. 2001;57:137–184. doi: 10.1016/S0065-3527(01)57003-7. [DOI] [PubMed] [Google Scholar]
- 13.Diener TO, Raymer WB. Potato spindle tuber virus: a plant virus with properties of a free nucleic acid. Science. 1967;158:378–381. doi: 10.1126/science.158.3799.378. [DOI] [PubMed] [Google Scholar]
- 14.Diener TO, Smith DR. Potato spindle tuber viroid. VI. Monodisperse distribution after electrophoresis in 20 % polyacrylamide gels. Virology. 1971;46:498–499. doi: 10.1016/0042-6822(71)90052-3. [DOI] [PubMed] [Google Scholar]
- 15.Diener TO, Smith DR. Potato spindle tuber viroid. IX. Molecular weight determination by gel electrophoresis of formylated RNA. Virology. 1973;53:359–365. doi: 10.1016/0042-6822(73)90214-6. [DOI] [PubMed] [Google Scholar]
- 16.Flores R, Randles JW, Owens RA. Classification. In: Hadidi A, Flores R, Randle JW, Semanick JS, editors. Viroids. Collingwood: CSIRO Publishing; 2003. pp. 71–75. [Google Scholar]
- 17.Flores R, Delgado S, Gas ME, Carbonell A, Molina D, Gago S, De la Pena M. Viroids: the minimum non-coding RNAs with autonomous replication. FEBS Lett. 2004;567:42–48. doi: 10.1016/j.febslet.2004.03.118. [DOI] [PubMed] [Google Scholar]
- 18.Flores R, Hernández C, de Martinez Alba AE, Daròs J-A, DI Serio F. Viroids and viroid host interactions. Anu Rev Phytopath. 2005;43:117–139. doi: 10.1146/annurev.phyto.43.040204.140243. [DOI] [PubMed] [Google Scholar]
- 19.Fonseca MEN, Boiteux LS, Singh RP, Kitajima EW. A small viroid in coleus species from Brazil. Fitopatol Bras. 1989;14:94–96. [Google Scholar]
- 20.Fox A, Daly M, Nixon T, Brurberg MB, Blystad DR, Harju V, Skelton A, Adams IP. First report of Tomato chlorotic dwarf viroid (TCDVD) in tomato in Norway and subsequent eradication. New Dis Rep. 2013;27:8. doi: 10.5197/j.2044-0588.2013.027.008. [DOI] [Google Scholar]
- 21.Galindo J, Lopez M, Aguilar T. Significance of Myzus persicae in the spread of tomato planta macho viroid. Fitopatol Brasileira. 1986;11:400–410. [Google Scholar]
- 22.Gross HJ, Domdey H, Lossow C, Jank P, Raba M, Alberty H, Sänger HL. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature (London). 1978;203(273):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- 23.Gross HJ, Liebl U, Alberty H, Krupp G, Domdey H, Ramm K, Sänger HL. A severe and mild potato spindle tuber viroid isolate differ in 3 nucleotide exchanges only. Biosci Rep. 1981;1:235–241. doi: 10.1007/BF01114910. [DOI] [PubMed] [Google Scholar]
- 24.Gross HJ, Krupp G, Domdey H, Raba M, Alberty H, Lossow CH, Ramm K, Sänger HL. Nucleotide sequence and secondary structure of citrus exocortis and chrysanthemum stunt viroid. Eur J Biochem. 1982;121:249–257. doi: 10.1111/j.1432-1033.1982.tb05779.x. [DOI] [PubMed] [Google Scholar]
- 25.Hanneman RE, Singh RP. Seed production in the virus indicator plant Scopolia sinensis. Can Plant Dis Surv. 1972;52:60–61. [Google Scholar]
- 26.Herold T, Hass B, Singh RP, Boucher A, Sänger HL. Sequence analysis of five new field isolates demonstrates that the chain length of potato spindle tuber viroid (PSTVd) is not strictly conserved but as variable as in other viroids. Plant Mol Biol. 1992;19:329–333. doi: 10.1007/BF00027356. [DOI] [PubMed] [Google Scholar]
- 27.Hrama DP. Distribution and infectivity of potato gothic in the Ukraine. Microbiol J. 1969;31:12–45. [PubMed] [Google Scholar]
- 28.Hunter JE, Rich AE. The effect of potato spindle tuber virus on growth and yield of Saco potatoes. Am Potato J. 1964;41:113–116. doi: 10.1007/BF02855019. [DOI] [Google Scholar]
- 29.James T, Mulholland V, Jefferies C, Chard J. First report of tomato chlorotic dwarf viroid infecting commercial petunia stocks in the United Kingdom. Plant Pathol. 2008;57:400. doi: 10.1111/j.1365-3059.2007.01727.x. [DOI] [Google Scholar]
- 30.Keese P, Symons RH. Domains in viroids: evidence of intra-molecular RNA arrangements and their contribution to viroid evolution. Proc Natl Acad Sci USA. 1985;82:4582–4886. doi: 10.1073/pnas.82.14.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling KS, Verhoeven JThJ, Singh RP, Brown JK. First report of Tomato chlorotic dwarf viroid in greenhouse tomatoes in Arizona. USA Plant Dis. 2009;93:1076. doi: 10.1094/PDIS-93-10-1075B. [DOI] [PubMed] [Google Scholar]
- 32.MacLachlan DS. Potato spindle tuber in Eastern Canada. Am Potato J. 1960;37:13–17. doi: 10.1007/BF02855586. [DOI] [Google Scholar]
- 33.MacLeod, DS. Rept. of Dominion Field Laboratory of Plant Pathology, Fredericton, N.B.-Rept. Dominion Botanist for the year 1925. Div. of Botany, Canada Dept. of Agriculture. 1926; pp 42–51.
- 34.Martin, WH. Spindle tuber, a new potato trouble. Hints: to potato growers, NJ State Potato Assoc. 1922; 3(8).
- 35.Matsushita Y, Kanda A, Usugi T, Shinya T. First report of a Tomato chlorotic dwarf viroid disease on tomato plants in Japan. J Gen Plant Pathol. 2008;74:182–184. doi: 10.1007/s10327-008-0076-6. [DOI] [Google Scholar]
- 36.Morris TJ, Wright NS. Detection on Polyacrylamide gel of a diagnostic nucleic acid from tissue infected with potato spindle tuber viroid. Am Potato J. 1975;52:57–63. doi: 10.1007/BF02852039. [DOI] [Google Scholar]
- 37.Mosch WHM, Huttinga H, Matt DZ, Treur A. Development of a standard method for detection of potato spindle tuber viroid in potato plants. Neth J Plant Pathol. 1982;88:113–122. doi: 10.1007/BF01976358. [DOI] [Google Scholar]
- 38.Owen RA. Molecular understanding of viroid replication cycles and identification of targets for disease management. In: Punza ZK, De Boer SH, Sanfacon H, editors. Biotechnology and Plant Disease Management. Wallingford: CABI; 2007. pp. 125–145. [Google Scholar]
- 39.Owens RA, Diener TO. Sensitive and rapid diagnosis of potato spindle tuber viroid disease by nucleic acid spot hybridization. Science. 1981;213:670–672. doi: 10.1126/science.213.4508.670. [DOI] [PubMed] [Google Scholar]
- 40.Pfannenstiel MA, Slack SA. Response of potato cultivars to infection by the potato spindle tuber viroid. Phytopathology. 1980;70:922–926. doi: 10.1094/Phyto-70-922. [DOI] [Google Scholar]
- 41.Pfannenstiel MA, Slack SA, Lane SC. Detection of potato spindle tuber viroid in field-grown potatoes by an improved electrophoretic assay. Phytopathology. 1980;70:1015–1018. doi: 10.1094/Phyto-70-1015. [DOI] [Google Scholar]
- 42.Po T, Li PH, Dale T. Potato degeneration research in China. Am Potato J. 1982;59:13–17. doi: 10.1007/BF02854883. [DOI] [Google Scholar]
- 43.Ramachandran P, Kumar D, Varma A, Pandey PK, Singh RP. Coleus viroid in India. Current Sci. 1992;62:271–272. [Google Scholar]
- 44.Randles JW, Rodriguez MJB. Coconut cadang cadang viroid. In: Hadidi A, Flores R, Randle JW, Semanick JS, editors. Viroids. Collingwood: CSIRO Publishing; 2003. pp. 242–245. [Google Scholar]
- 45.Riesner D, Henco K, Rokohl U, Klotz G, Kleinschmidt AK, Domdey H, Jank P, Gross HJ, Sänger HL. Structure and structure formation of viroids. J Mol Biol. 1979;133:85–115. doi: 10.1016/0022-2836(79)90252-3. [DOI] [PubMed] [Google Scholar]
- 46.Riesner D, Steger G, Zimmat R, Owens RA, Wagenhöfer M, Hillen W, Vollbach S, Henco K. Temperature –gradient gel electrophoresis of nucleic acids: analysis of conformational transactions, sequence variations and protein-nucleic acid interactions. Electrophoresis. 1989;10:377–389. doi: 10.1002/elps.1150100516. [DOI] [PubMed] [Google Scholar]
- 47.Sänger HL. An infectious and replicating RNA of low molecular weight: the agent of the exocortis disease of citrus. Adv Bio Sci. 1972;8:103–116. [Google Scholar]
- 48.Sänger HL, Ramm K. Radioactive labeling of viroid RNA. In: Markham R, Davies DR, Hapwood DA, Home RW, (eds.). Modifications of the information content of Plant Cells. 1975; 230-253.
- 49.Sänger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viriods are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Nat Acad Sci USA. 1976;73:3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sano T, Haytaya T, Terai Y, Shikata E. Hop stunt viroid strains from dapple fruit disease of plum and peach in Japan. J Gen Virol. 1989;70:1311–1319. doi: 10.1099/0022-1317-70-6-1311. [DOI] [PubMed] [Google Scholar]
- 51.Sano T, Candresse T, Hammond RW, Diener TO, Owens RA. Identification of multiple structural domains regulating viroid pathogenecity. Proc Nat Acad Sci USA. 1992;89:10104–10108. doi: 10.1073/pnas.89.21.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmacher J, Meyer N, Riesner D, Weidamann HL. Diagnostic procedure for detection of viroids and viruses with circular RNAs by ‘return’-gel electrophoresis. J Phytopathol. 1986;115:332–343. doi: 10.1111/j.1439-0434.1986.tb04346.x. [DOI] [Google Scholar]
- 53.Schultz ES, Folsom DA. A “spindling tuber” disease of Irish potatoes. Science. 1923;57:149. doi: 10.1126/science.57.1466.149. [DOI] [PubMed] [Google Scholar]
- 54.Shiraishi T, Maejima K, Komatsu K, Hashimoto M, Okano Y, Kitazawa Y, Yamaji Y, Namba SJ. First report of tomato chlorotic dwarf viroid isolated from symptomless petunia plants (Petunia spp.) in Japan. Gen Plant Pathol. 2009;79:3. [Google Scholar]
- 55.Singh RP. Seed transmission of potato spindle tuber virus in tomato and potato. Am Potato J. 1970;47:225–227. doi: 10.1007/BF02872303. [DOI] [Google Scholar]
- 56.Singh RP. Viroid discovery. Plant Dis. 1980;64:418. [Google Scholar]
- 57.Singh RP. Viroid discovery. Plant Dis. 1980;64:964. [Google Scholar]
- 58.Singh RP. Bibliography of viroid reviews through 1983. Plant Dis Surv. 1984;64:15–16. [Google Scholar]
- 59.Singh RP, Bagnall RH. Infectious nucleic acid from host tissues infected with potato spindle tuber virus. Phytopathology. 1968;58:696–699. [Google Scholar]
- 60.Singh RP, Boucher A. Electrophoresis separation of a severe from mild strains of potato spindle tuber viroid. Phytopathology. 1987;77:1588–1591. doi: 10.1094/Phyto-77-1588. [DOI] [Google Scholar]
- 61.Singh RP, Boucher A. Loss of potato spindle tuber viroid from tissues after repeated freezing. Am Potato J. 1988;65:283–287. doi: 10.1007/BF02854054. [DOI] [Google Scholar]
- 62.Singh RP, Clark MC. Infectious low-molecular weight ribonucleic acid from tomato. Biochem Biophys Res Commun. 1971;44:1077–1082. doi: 10.1016/S0006-291X(71)80195-X. [DOI] [PubMed] [Google Scholar]
- 63.Singh RP, Clark MC. Similarity of host response to both potato spindle tuber and citrus exocortis viruses. FAO Plant Prot Bull. 1973;21:121–125. [Google Scholar]
- 64.Singh RP, Crowley CF. Successful management of potato spindle tuber viroid in seed potato crop. Can Plant Dis Surv. 1985;65:9–10. [Google Scholar]
- 65.Singh RP, Dilworth AD. Tomato chlorotic dwarf viroid in the ornamental plant Vinca minor and its transmission through tomato seed. Eur J Pl Pathol. 2009;123:111–116. doi: 10.1007/s10658-008-9344-8. [DOI] [Google Scholar]
- 66.Singh RP, Finnie RE. Stability of potato spindle tuber viroid in freeze-dried leaf powder. Phytopathology. 1977;67:283–286. doi: 10.1094/Phyto-67-283. [DOI] [Google Scholar]
- 67.Singh RP, Jaime AT Da Silva. Ornamental plants: silent carrier of evolving viroids, floriculture, ornamental and plant biotechnology. Global Science Books. 2006; vol III.
- 68.Singh RP, Benson AP, Salama FM. Purification and electron microscopy of potato spindle virus. Phytopathology. 1966;56:901–902. [Google Scholar]
- 69.Singh RP, Boucher A, Seabrook JEA. Detection of the mild strains of potato spindle tuber viroid from single true potato seed by return electrophoresis. Phytopathology. 1988;8:663–667. doi: 10.1094/Phyto-78-663. [DOI] [Google Scholar]
- 70.Singh RP, DeHaan T-L, Jaswal AS. A survey of the incidence of potato spindle tuber viroid in Prince Edward Island using two testing methods. Can J Plant Sci. 1988;68:1229–1236. doi: 10.4141/cjps88-152. [DOI] [Google Scholar]
- 71.Singh RP, Boucher A, Singh A. High incidence of transmission and occurrence of a viroid in commercial seeds of Coleus in Canada. Plant Dis. 1991;75:184–187. doi: 10.1094/PD-75-0184. [DOI] [Google Scholar]
- 72.Singh RP, Boucher A, Wang RG. Detection, distribution and long-term persistence of potato spindle tuber viroid in true potato seed from Heilongjiang China. Am Potato J. 1991;68:65–74. doi: 10.1007/BF02893342. [DOI] [Google Scholar]
- 73.Singh RP, Boucher A, Somerville TH. Detection of potato spindle tuber viroid in the pollen and various parts of potato plant pollinated with viroid-infected pollen. Plant Dis. 1992;76:951–953. doi: 10.1094/PD-76-0951. [DOI] [Google Scholar]
- 74.Singh RP, Lakshman DK, Boucher A, Tavantzis SM. A viroid from Nematanthus wettsteinii plants closely related to Columnea latent viroid. J Gen Virol. 1992;73:2769–2774. doi: 10.1099/0022-1317-73-11-2769. [DOI] [PubMed] [Google Scholar]
- 75.Singh RP, Boucher A, Lakshman DK, Tavantzis SM. Multimeric non-radioactive cRNA probes improve detection of potato spindle tuber viroid (PSTVd) J Virol Methods. 1999;49:221–234. doi: 10.1016/0166-0934(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 76.Singh RP, Nie X, Singh M. Tomato chlorotic dwarf viroid: an evolutionary link in the origin of pospiviroid. J Gen Virol. 1999;80:2823–2828. doi: 10.1099/0022-1317-80-11-2823. [DOI] [PubMed] [Google Scholar]
- 77.Singh RP, Ready KFM, Nie X. Biology. In: Hadidi A, Flores R, Randle JW, Semancik JS, editors. Viroids. Collinwood: CSIRO Publishing; 2003. pp. 30–48. [Google Scholar]
- 78.Singh RP, Dilworth AD, Singh M, Babcock KM. An alkaline solution simplifies nucleic acid preparation for RT-PCR and infectivity assays of viroids from crude sap and spotted membrane. J Virol Methods. 2006;132:204–211. doi: 10.1016/j.jviromet.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 79.Singh RP, Dilworth AD, Baranwal VK, Gupta KN. Detection of Citrus exocortis viroid, Iresine viroid, and Tomato chlorotic dwarf viroid in new ornamental host plants in India. Plant Dis. 2006;90:1457. doi: 10.1094/PD-90-1457A. [DOI] [PubMed] [Google Scholar]
- 80.Singh RP, Dilworth AD, Xaoping AO, Singh M, Misra S. Molecular and biological characterization of a severe isolate of Tomato chlorotic dwarf viroid containg a novel terminal right (TR) domain sequence. Eur J Pl Pathol. 2010;127:63–72. doi: 10.1007/s10658-009-9571-7. [DOI] [Google Scholar]
- 81.Sogo JM, Koller T, Diener TO. Potato spindle tuber viroid. X. Visualization and size determination by electron microscopy. Virology. 1973;55:70–80. doi: 10.1016/S0042-6822(73)81009-8. [DOI] [PubMed] [Google Scholar]
- 82.Tabler M, Tagris M. Viroids: petite RNA pathogens with distinguished talents. Trends in Plant Sci. 2004;9:348–399. doi: 10.1016/j.tplants.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 83.Verhoeven JThJ. Identification and epidemiology of Pospiviroids. Thesis submitted in fulfillment of the requirements for the degree of doctor, Wageningen University, 2010; p 135.
- 84.Verhoeven JThJ, Jansen CCC, Willemen TM, Kox LFF, Owens RA, Roenhorst JW. Natural infections of tomato by Citrus exocortis viroid, Columnea latent viroid, Potato spindle tuber viroid and Tomato chlorotic dwarf viroid. Eur J Plant Pathol. 2004;110:823–831. doi: 10.1007/s10658-004-2493-5. [DOI] [Google Scholar]
- 85.Verhoeven JThJ, Jansen CCC, Werkman AW, Roenhorst JW. First report of Tomato chlorotic dwarf viroid in Petunia hybrida from United States of America. Plant Dis. 2007;91:324. doi: 10.1094/PDIS-91-3-0324B. [DOI] [PubMed] [Google Scholar]
- 86.Visvader JE, Symons RH. Eleven new sequence variants of citrus exocortis viroid and the correlation of sequence with pathogenicity. Nucleic Acid Res. 1985;13:2907. doi: 10.1093/nar/13.8.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wallace JM, Drake RJ. A high rate of seed transmission of avocado sunblotch virus from symptomless trees and the origin of such trees. Phytopathology. 1962;52:237–241. [Google Scholar]
- 88.Wassenegger M, Spieker RL, Thalmeir S, Gast FU, Riedel L, Sänger HL. A single nucleotide substitution converts potato spindle tuber viroid (PSTVd) from non-infectious to an infectious RNA for Nicotiana tabacum. Virology. 1996;226:191–197. doi: 10.1006/viro.1996.0646. [DOI] [PubMed] [Google Scholar]
- 89.Wassenegger M, Heimes S, Riedel L, Sanger HL. RNA–directed de novo methylation of sequences in plants. Cell 1994;76:567–76. [DOI] [PubMed]
- 90.Welnicki M, Hiruki C. Highly sensitive digoxigenin-lablled DNA probe for the detedtion of potato spindle tuber viroid. J Virol Methods. 1992;39:91–99. doi: 10.1016/0166-0934(92)90128-Z. [DOI] [PubMed] [Google Scholar]
- 91.Werner HO. Relation of environment to spindle tuber symptoms. Proc Potato Assoc AM Annu. 1924;11:102–106. [Google Scholar]
- 92.Whitney ED, Peterson LC. An improved technique for inducing diagnostic symptoms in tomato infected by potato spindle tuber virus. Phytopathology. 1963;53:893. [Google Scholar]