Abstract
Canine distemper virus (CDV) is a morbillivirus related to measles virus that infects dogs and other carnivores. CDV has a significant global impact on animal health; however, there is no current antiviral treatment for CDV infection. In recent years, it has been demonstrated that sulfated polysaccharides exhibit antiviral properties both in vivo and in vitro, despite their low cytotoxicity to host cells. Fucoidan is a sulfated polysaccharide found in the cell wall matrix of brown algae. In this study, we evaluated in vitro anti-CDV activity of fucoidan, which was derived from Cladosiphon okamuranus. Fucoidan actively inhibited CDV replication in Vero cells at a 50 % inhibitory concentration (IC50) of 0.1 µg/ml. The derived selectivity index (SI50) was >20,000. This polysaccharide likely inhibits viral infection by interference in the early steps and by inhibiting CDV-mediated cell fusion. Fucoidan may be useful in development of pharmacological strategies to treat and control CDV infection.
Keywords: Fucoidan, CDV, Antiviral, Cladosiphon okamuranus, Morbillivirus
Introduction
Canine distemper virus (CDV) is a negative-stranded RNA morbillivirus related to measles virus. CDV infection causes severe morbidity and mortality in dogs and other carnivores and significantly affects animal health worldwide [22]. Despite an increasing demand from veterinarians and pet owners, an antiviral treatment for CDV infection is not available; but research is in progress. Ribavirin inhibits CDV replication in vitro in a dose- and time-dependent manner [7]. However, ribavirin exhibited low selectivity indexes, so it is unsuitable for clinical treatment of CDV-infected animals.
Marine algae produce metabolites that have been recognized as promising biologically active compounds for use as antiviral drugs. Fucoidans are complex sulfated fucosylated polysaccharides produced by brown seaweeds and have a wide spectrum of pharmacological properties (e.g., anti-inflammatory, anti-angiogenic, anti-coagulant, and antiviral) [2, 5, 14, 19]. Because fucoidans are present in large quantities in dietary brown seaweed products consumed in Asian countries, these compounds may already have an important role in disease prevention [6].
Cladosiphon okamuranus is an edible brown alga that is commercially cultured around Okinawa Island, Japan. Fucoidan is prepared from this alga on an industrial scale and is used as an additive for health foods, drinks, and cosmetics. Fucoidan extracted from C. okamuranus possesses α-1-3-linked l-fucosyl residues that are substituted with d-glucuronic acid at C-2 and with sulfate groups at C-4 of the l-fucosyl residues. The average branched chain structure consists of one sulfate group for every two molecules of fucose and one glucuronic residue for every six molecules of fucose [16]. The polysaccharide also contains xylose as a minor monosaccharide constituent. Using a room temperature extraction, Tako et al. reported an acetylfucoidan yield of 2.3 % (w/w) based on the wet alga weight. The total contents of carbohydrates, d-glucuronic acid, sulfuric acid, ash, and moisture were 69, 13.5, 13.6, 23 and 3.2 %, respectively, and the molar ratio of l-fucose:d-xylose:d-glucuronic acid:acetic acid:sulfuric acid was estimated to be 4.0:0.03:1.0:2.0:2.0 [24]. In contrast with other sulfated polysaccharides, Cladosiphon fucoidan does not promote an inflammatory response in vitro [21] and lacks anti-angiogenic and anti-coagulant activities [4]. Cladosiphon okamuranus fucoidan is not toxic to hepatocytes and has no adverse effects on the serum electrolytes of Wistar rats [10]. However, similar doses potently inhibit dengue virus type 2 (DEN2) replication [12] and significantly inhibit the proliferation of HTLV-1-infected T-cells [11]. In the present study, we examined the in vitro anti-CDV activity of fucoidan derived from C. okamuranus, and compared this activity to ribavirin.
Materials and methods
Fucoidan and ribavirin
Fucoidan was purchased as a dried powder from Kadoya & Co., Kobe, Japan (lot A03012) and extracted from cultured kelp C. okamuranus harvested off the coast of Okinawa Island, Japan [24]. The polysaccharide preparation was certified to contain 90.4 % fucoidan (anthrone-sulfuric acid method) and to have a mean molecular weight of 92.1 kDa (HPLC method). The fucose and sulfate contents of fucoidan were 38.6 and 15.9 %, respectively, with ash comprising 19.6 % of the content and other sugars comprising 23 % (glucuronic acid and traces of xylose). Dried samples of fucoidan were suspended in Dulbecco’s modified Eagle’s medium (DMEM) at a concentration of 2.5 mg/ml and filtered through a 0.22 mm membrane filter. Ribavirin (Vilonapediatrica, Valeant, México) was used as an antiviral control.
Cells culture and virus
African green monkey kidney cells (Vero cells) were grown as monolayers in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal calf serum (FCS) and 1 % penicillin and streptomycin. Vero cells were cultured at 37 °C in a 5 % CO2 incubator. Canine distemper virus (CDV; Onderstepoort strain) adapted to Vero cells was titrated by plaque forming units (PFU). Virus aliquots were stored at -70 °C until use.
Cytotoxicity assay
The conventional MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method [15], with some modifications, was used to evaluate cytotoxicity. Vero cells were seeded in 96-well plates at an initial density of 5 × 103 cells per well. After the cells were incubated for 20 h at 37 °C, various concentrations of the antiviral compounds fucoidan (1, 10, 100, 500 and 1,000 μg/ml) or ribavirin (0.1, 1, 10 and 100 μg/ml) were added to the growth medium, with six wells for each concentration. The cultures were then incubated for an additional 72 h. MTT solution (20 μl well) at a concentration of 5 mg/ml was added to each well and cells were incubated at 5 % CO2 and 37 °C for 4 h. The medium and excess MTT were removed from each well, and 100 μl dimethyl sulfoxide was added to dissolve the formazan crystals. The optical density was measured at 570 and 690 nm using a Microplate Autoreader EL311 (BIOTEK Instruments Inc., USA). Each experiment was repeated three times. Cytotoxicity was expressed as 50 % cytotoxic concentration (CC50), which was the concentration of the test substances that inhibited up to 50 % of the growth of Vero cells.
Virus plaque reduction assays
Fucoidan was tested for anti-viral activity against CDV with a plaque reduction assay using Vero cell monolayers in six-well culture plates. The assay was performed by adding the compound during all assay (72 h). For a typical assay, 100 plaque forming units (PFUs) were incubated with increasing concentrations of fucoidan (0.1, 1 and 10 μg/ml) for 1 h at room temperature. Virus was allowed to adsorb to the cells for 1 h at 37 °C. The residual inoculum was then discarded and infected cells were overlaid with medium containing 0.6 % agar (MP Biomedicals, Inc) and the indicated concentrations of the sulfated polysaccharide. Each concentration was performed in triplicate. After incubation of 72 h at 37 °C to allow for plaque formation, the cells were fixed with methanol and stained with 1 % crystal violet. Plaques in the Vero cell monolayer were counted. Using dose–response curves, plaque reduction by the test compounds was determined by, and expressed as, the test compound concentration that inhibited plaque formation by 50 % (IC50).
Time of addition assays
In order to analyze the inhibitory mechanism of fucoidan against CDV, infected cells were treated with increasing concentrations (0.001–10 μg/ml) for various periods of time prior to, during, and after infection with CDV. Fucoidan antiviral activity was compared with ribavirin in post-adsorption assays.
Drug treatment before virus infection or concurrent with infection
Vero cells were treated with increasing concentrations of fucoidan (0.001–10 μg/ml) for 1 h at 37 °C prior to CDV infection. After incubation, the cells were washed three times with phosphate buffered saline to ensure the complete removal of fucoidan from the media, then infected with 100 PFU per well of CDV and incubated at 37 °C for 1 h. The infected cell monolayer was then washed three times with phosphate buffered saline, overlaid with medium containing 0.6 % agar (MP Biomedicals, Inc.), and incubated for 72 h at 37 °C. A second assay was performed with cells infected at the same time as fucoidan treatment. The virus suspension was mixed with the indicated concentration of fucoidan before adding it to the cell monolayer. Vero cells were treated with the mix for 1 h at 37 °C, then removed from cell media by extensively washed and the monolayer was cultured for plaque assay as previously described.
Drug treatment after virus adsorption
Cells were infected with CDV for 1 h at 37 °C. After incubation, the infected cell monolayer was then washed three times with phosphate buffered saline and increasing concentrations of fucoidan or ribavirin were then added and cells were incubated at 37 °C for 1 h. The infected cell monolayer was then washed three times with phosphate buffered saline, overlaid with medium containing 0.6 % agar, and incubated for 72 h at 37 °C.
Viral syncytium inhibition assay
Virus-induced cell fusion assays were used to examine the ability of fucoidan to inhibit CDV induced syncytia formation. Vero cells were cultured in 6-well plates in DMEM, supplemented with 10 % FCS. Cell monolayers were infected with CDV at 100 PFU per well. After adsorption for 1 h, the inoculum was replaced by DMEM with 2 % FCS and dilutions of fucoidan in medium without FCS were added. At 24 h post infection, the cells were fixed and stained with 1 % (w/v) crystal violet in 100 % methanol. The number of nuclei was counted for 20 syncytia per well, and inhibition of syncytium size was determined.
Data analysis
Data were analyzed using SPSS 10 software (IBM, Armonk, New York, USA). Probit regression analysis was used to determine CC50, which was the concentration of the test substance that reduced the optical density by 50 %, compared with the controls. The IC50 was the concentration that reduced PFU number by 50 %. The selectivity index (SI) was the ratio CC50/IC50. The CC50 and IC50 values were estimated from the mean and standard deviations or standard errors (time of addition assays) of the mean values from three independent experiments.
Results
Cytotoxicity assay
The cytotoxicity of fucoidan and ribavirin to Vero cells was evaluated. As shown in Fig. 1, although the no effect on cytotoxicity was detected for fucoidan at concentrations up to 10 µg/ml (CC50 = 2,089 μg/ml ± 6 SD). However, 10 μg/ml ribavirin was toxic to Vero cells (CC50 = 88.9 μg/ml ± 6 SD). For the remainder of the studies, the antiviral activity of these compounds was assessed at concentrations below 10 μg/ml to rule out non-specific inhibition due to cell cytotoxicity.
Fig. 1.
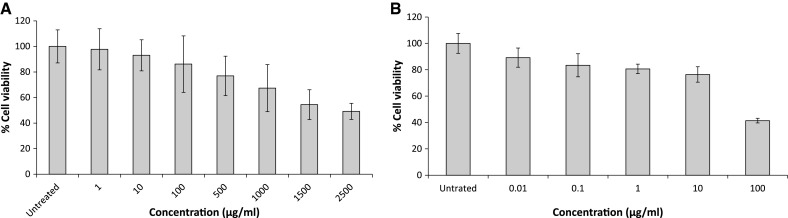
Evaluation of fucoidan (A) and ribavirin (B) cytotoxicity to Vero cells using the MTT assay. Control: cells without treatment. Bars represent means, with vertical lines indicating standard deviations, n = 9, *p ≤ 0.05
Fucoidan inhibits CDV replication
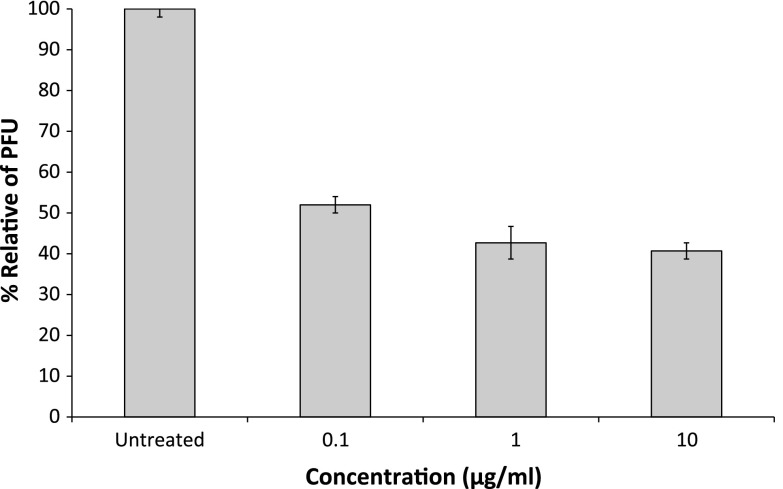
To assess fucoidan inhibition of CDV replication, Vero cell monolayers were treated with increasing concentrations of fucoidan that was added at the same time, each well received 100 PFUs of CDV. The same concentration of fucoidan was maintained in the plaque assay overlay medium throughout the infection. Antiviral activity was evaluated by observing a reduction in the number of CDV plaques. The results demonstrated significant and reproducible antiviral activity of fucoidan against CDV (Fig. 2). Fucoidan treatment reduced not only the number but also the size of plaques (data not shown). Fucoidan inhibition resulted in an IC50 < 0.1 μg/ml. The resulting selectivity index (SI; >20,000 CC50/IC50) indicated that fucoidan potently inhibited CDV at a very low drug concentration, and was not cytotoxic.
Fig. 2.
Evaluation of antiviral activity of fucoidan against canine distemper virus in Vero cells by plaque reduction. Infected cells were cultured in the absence of drug, or with the indicated concentration of fucoidan. Bars represent mean values and vertical lines represent the standard deviations from the mean (n = 9, *p < 0.05)
Effect of fucoidan on virus adsorption and entry
Vero cells were pretreated with various concentrations of fucoidan (0.001–10 μg/ml), which was removed before incubation with CDV by extensively washed. The results indicated that they were protected from CDV infection in a manner that was not strictly dose-dependent (Fig. 3a). Additional assays were performed with fucoidan added directly to virus suspensions. Under these conditions, the apparent efficacy of fucoidan was slightly increased.
Fig. 3.
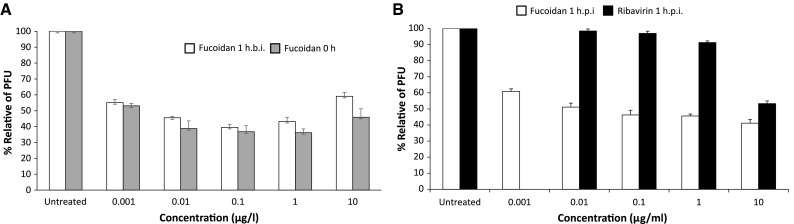
Effect of fucoidan on adsorption and entry of canine distemper virus (CDV). The fucoidan was added to and incubated for 1 h with cells either “before infection (1 h b.i.)” or “during infection (0 h)” of cell infection with CDV (100 PFU) (A). The fucoidan or ribavirin was added and incubated for 1 h with cells after 1 h period of cell infection with CDV (100 PFU) “post-infection (1 h p.i.)” (B), following 72 h incubation. Control cells were infected with CDV (100 PFU), but not treated with fucoidan. Bars represent mean values and vertical lines represent the standard error from the mean (n = 9, *p < 0.05)
After virus adsorption, the unadsorbed virus was removed and Vero cells were treated with various concentrations of fucoidan (0.001–10 μg/ml) or ribavirin (0.01–10 μg/ml) as described in methodology, then washed to ensure the complete removal of test compounds. Virus yield was determined by plaque assay. Fucoidan displayed anti-CDV activity when the cells were treated after virus adsorption (Fig. 3b). However, this activity was slightly lower than the inhibition that occurred when cells were treated at the time of infection (Fig. 3a). Fucoidan-treated Vero cells were much better protected from CDV infection than ribavirin-treated cells (Fig. 3b). These experimental results suggest that fucoidan has antiviral activity via the inhibition of virus binding and/or penetration.
Antiviral efficacy versus cytotoxicity
Assessment of cytotoxicity is clearly an important part of the evaluation of any potential antiviral agent. As shown in Fig. 4, fucoidan effectively inhibits the replication of CDV even at drug concentrations that showed no cytotoxicity. In contrast, ribavirin was cytotoxic at drug concentrations that inhibit CDV replication (SI < 8.9, a > 2000-fold difference; Fig. 4).
Fig. 4.
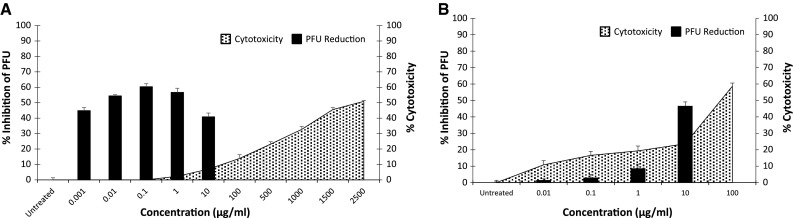
Fucoidan and ribavirin antiviral efficacy. Percent inhibition of plaque forming units versus percent cytotoxicity. Test results based on treatment with fucoidan, concurrent with infection (A) and test results based on ribavirin treatment after adsorption (B). Bars represent mean values and vertical lines represent the standard error from the mean (n = 9, *p < 0.05)
Fucoidan reduces cell-to-cell spread of CDV
F and H proteins of CDV are necessary for fusion of adjacent cell membranes. The expression of CDV F protein on the cell surface results in cell–cell fusion (syncytia) and subsequent cell-to-cell spread of progeny viruses [23]. Vero cells infected with CDV were incubated in increasing concentrations (0.01–10 μg/ml) of fucoidan. Syncytia size was markedly reduced by fucoidan treatment in a dose-dependent manner (Fig. 5).
Fig. 5.
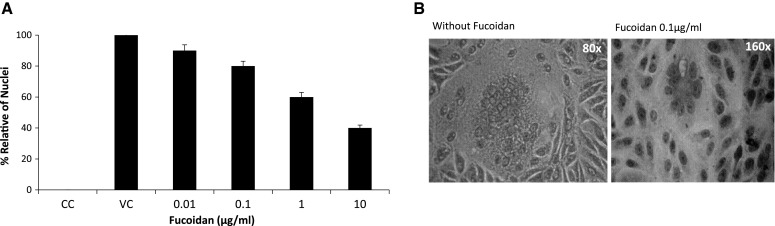
Effect of fucoidan on syncytium formation. (A) Circles represent mean values and vertical lines represent standard deviation from the mean. The mean ± standard deviation (%) for 20 syncytia per well was determined in three different experiments (*p < 0.05). (B) The effect of fucoidan on syncytium size (i.e., the number of nuclei per syncytium)
Discussion
The aim of the present study was to test and evaluate the antiviral activity of fucoidan against CDV. Fucoidan is a polysaccharide isolated from the brown seaweed Cladosiphon okamuranus, since other similar polysaccharides have been shown to have a wide array of pharmacological activities. This study also sought to determine the mechanism(s) by which fucoidan blocked CDV infection. Understanding the mechanism of antiviral activity of this algal polysaccharide is important for its future development as antiviral drugs for clinical or veterinary use.
The major undesirable side effect of sulfated polysaccharides is their well-known anticoagulant activity. This adverse effect can be avoided by selecting sulfated polymers, such as fucoidan from C. okamuranus, which exhibit virtually no anticoagulant activity [4]. Cumashi et al. suggested that the high presence of glucuronic acid branches is the most likely feature responsible for the lack of anticoagulant activity by C. okamuranus fucoidan, as the less active compounds are characterized by a low degree of sulfation and a high presence of 2-O-α-d-glucuronyl substituents along the linear polysaccharide backbone (characteristics fucoidan from C. okamuranus) [4]. By contrast, it has been proposed that the antiviral activity of fucoidan is related to the concentration of fucose and uronic acids. Hidari et al. found that the antiviral properties of fucoidan from C. okamuranus against dengue virus type 2 vanished when the glucuronic acid was carboxyl-reduced [12]. In the present study, we evaluated the antiviral activity of fucoidan from C. okamuranus extracted by the method of Tako et al. with a significant degree of uronic acids, and investigated its possible mechanism of action in Vero cells [24].
Sulfated polysaccharides act primarily through inhibition of the entry of enveloped viruses into host cells [5, 9, 14]. Marine polysaccharides from different sources can block virus infection by interfering with the virus adsorption process. The antiviral action of fucoidan seems to stem from inhibiting the binding of the virus particles to the host cell and interferes with the adsorption process [1]. Certain sulfated marine polysaccharides, can interfere with virus internalization and the subsequent uncoating by blocking the allosteric process of virus particles [26]. Our results support the ability of fucoidan to block the early stages of CDV infection by blocking viral adsorption and maybe one or more post binding penetration steps.
Fucoidan inhibited the first steps of the viral infection cycle and strongly suppressed the formation of CDV-induced syncytia in infected cells perhaps by direct action on the proteins responsible for CDV fusion (F or H) or by inhibiting its expression. We previously found that fucoidan of C. okamuranus both blocking of post-binding entry steps of NDV to cells and strongly suppresses syncytia formation when fucoidan was added before cleavage of the fusion protein, feasibly indicating a specific interaction between fucoidan and the F0 protein [8]. These apparent multifaceted mechanisms of fucoidan action are similar to the mechanisms of action of other sulfated polysaccharides [3, 18, 20, 25]. Our results agree with the results of Hoshino et al. and Nyberg et al., who demonstrated that the mechanisms of antiviral activity of low molecular weight sulfated polysaccharides include inhibition of the entry of enveloped virions into host cells and inhibition of cell-to-cell spread of the virus [13, 17]. Cytolytic viruses, such as CDV, spread through a cell culture by producing infectious particles and by lateral cell-to-cell fusion. Thus, virus production observed in our studies with fucoidan might reflect predominantly on secondary spread rather than rapid lateral transmission, resulting in a greatly reduced syncytia number and plaque size.
Elia et al. reported that ribavirin was active against CDV in vitro [7]. In this study, ribavirin showed lower antiviral activity than fucoidan, (ribavirin SI < 9, fucoidan SI > 20,000). Assessment of cytotoxicity is clearly an important part of the evaluation of a potential antiviral agent since a useful compound should not show acute or long-term toxicity against the host. In the study by Gideon and Rengasamy, rats treated with C. okamuranus exhibited no necropsy or other pathological changes in organs or changes in histopathological morphology, consistent with the lower toxicity possessed by sulfated polysaccharides, specifically fucoidan from C. okamuranus [10].
This is the first report of a sulfated polysaccharide with antiviral activity against CDV. The high selectivity of antiviral action reported for fucoidan >20,000 is clinically promising.
Acknowledgments
We greatly appreciate the gift of Canine distemper virus (Onderstepoort strain) provided by Dr. Raymundo Iturbe (Departamento de Microbiología, Facultad de Medicina Veterinaria of Universidad Nacional Autónoma de México). This work was supported by Grants from Consejo Nacional de Ciencia y Tecnología (CONACYT) México (No. 99862).
References
- 1.Baba M, Snoeck R, Pauwels R, de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988;32:1742–1745. doi: 10.1128/AAC.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berteau O, Mulloy B. Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology. 2003;13:29R–40R. doi: 10.1093/glycob/cwg058. [DOI] [PubMed] [Google Scholar]
- 3.Chen MZ, Xie HG, Yang LW, Liao ZH, Yu J. In vitro anti-influenza virus activities of sulfated polysaccharide fractions from Gracilaria lemaneiformis. Virol Sin. 2010;25:35–341. doi: 10.1007/s12250-010-3137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI, Usov AI, Ustyuzhanina NE, Grachev AA, Sanderson CJ, Kelly M, Rabinovich GA, Lacobelli S, Nifantiev NE, Consorzio Interuniversitario Nazionale per la Bio-Oncologia, Ital A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17:541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- 5.Damonte EB, Matulewicz MC, Cerezo AS. Sulfated seaweed polysaccharides as antiviral agents. Curr Med Chem. 2004;11:2399–2419. doi: 10.2174/0929867043364504. [DOI] [PubMed] [Google Scholar]
- 6.Doh-Ura K, Kuge T, Uomoto M, Nishizawa K, Kawasaki Y, Iha M. Prophylactic effect of dietary seaweed fucoidan against enteral prion infection. Antimicrob Agents Chemother. 2007;51:2274–2277. doi: 10.1128/AAC.00917-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elia G, Belloli C, Cirone F, Lucente MS, Caruso M, Martella V, Decaro N, Buonavoglia C, Ormas P. In vitro efficacy of ribavirin against canine distemper virus. Antiviral Res. 2008;77:108–113. doi: 10.1016/j.antiviral.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Elizondo-Gonzalez R, Cruz-Suarez LE, Ricque-Marie D, Mendoza-Gamboa E, Rodriguez-Padilla C, Trejo-Avila LM. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against newcastle disease virus. Virol J. 2012;9:307. doi: 10.1186/1743-422X-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh T, Pujol CA, Damonte EB, Sinha S, Ray B. Sulfated xylomannans from the red seaweed Sebdenia polydactyla: structural features, chemical modification and antiviral activity. Antivir Chem Chemother. 2009;19:235–242. doi: 10.1177/095632020901900603. [DOI] [PubMed] [Google Scholar]
- 10.Gideon TP, Rengasamy R. Toxicological evaluation of fucoidan from Cladosiphon okamuranus. J Med Food. 2008;11:638–642. doi: 10.1089/jmf.2007.0127. [DOI] [PubMed] [Google Scholar]
- 11.Haneji K, Matsuda T, Tomita M, Kawakami H, Ohshiro K, Uchihara JN, Masuda M, Takasu N, Tanaka Y, Ohta T, Mori N. Fucoidan extracted from Cladosiphon okamuranus Tokida induces apoptosis of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Nutr Cancer. 2005;52:189–201. doi: 10.1207/s15327914nc5202_9. [DOI] [PubMed] [Google Scholar]
- 12.Hidari KI, Takahashi N, Arihara M, Nagaoka M, Morita K, Suzuki T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem Biophys Res Commun. 2008;376:91–95. doi: 10.1016/j.bbrc.2008.08.100. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino T, Hayashi T, Hayashi K, Hamada J, Lee JB, Sankawa U. An antivirally active sulfated polysaccharide from Sargassum horneri (TURNER) C. AGARDH. Biol Pharm Bull. 1998;21:730–734. doi: 10.1248/bpb.21.730. [DOI] [PubMed] [Google Scholar]
- 14.Li B, Lu F, Wei X, Zhao R. Fucoidan: structure and bioactivity. Molecules. 2008;13:1671–1695. doi: 10.3390/molecules13081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Nagaoka M, Shibata H, Kimura-Takagi I, Hashimoto S, Kimura K, Makino T, Aiyama R, Ueyama S, Yokokura T. Structural study of fucoidan from Cladosiphon okamuranus TOKIDA. Glycoconj J. 1999;16:19–26. doi: 10.1023/A:1006945618657. [DOI] [PubMed] [Google Scholar]
- 17.Nyberg K, Ekblad M, Bergström T, Freeman C, Parish CR, Ferro V, Trybala E. The low molecular weight heparan sulfate-mimetic, PI-88, inhibits cell-to-cell spread of herpes simplex virus. Antiviral Res. 2004;63:15–24. doi: 10.1016/j.antiviral.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Preeprame S, Hayashi K, Lee JB, Sankawa U, Hayashi T. A novel antivirally active fucan sulfate derived from an edible brown alga, Sargassum horneri. Chem Pharm Bull. 2001;49:484–485. doi: 10.1248/cpb.49.484. [DOI] [PubMed] [Google Scholar]
- 19.Pomin VH, Mourão PA. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology. 2008;18:1016–1027. doi: 10.1093/glycob/cwn085. [DOI] [PubMed] [Google Scholar]
- 20.Schaeffer DJ, Krylov VS. Anti-HIV activity of extracts and compounds from algae and cyanobacteria. Ecotoxicol Environ Saf. 2000;45:208–227. doi: 10.1006/eesa.1999.1862. [DOI] [PubMed] [Google Scholar]
- 21.Shibata H, Kimura-Takagi I, Nagaoka M, Hashimoto S, Aiyama R, Iha M, Ueyama S, Yokokura T. Properties of fucoidan from Cladosiphon okamuranus tokida in gastric mucosal protection. BioFactors. 2000;11:235–245. doi: 10.1002/biof.5520110402. [DOI] [PubMed] [Google Scholar]
- 22.Silin D, Lyubomska O, Ludlow M, Duprex WP, Rima BK. Development of a challenge-protective vaccine concept by modification of the viral RNA-dependent RNA polymerase of canine distemper virus. J Virol. 2007;81:13649–13658. doi: 10.1128/JVI.01385-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singethan K, Hiltensperger G, Kendl S, Wohlfahrt J, Plattet P, Holzgrabe U, Schneider-Schaulies J. N-(3-cyanophenyl)-2-phenylacetamide, an effective inhibitor of morbillivirus-induced membrane fusion with low cytotoxicity. J Gen Virol. 2010;91:2762–2772. doi: 10.1099/vir.0.025650-0. [DOI] [PubMed] [Google Scholar]
- 24.Tako M, Yoza E, Tohma S. Chemical characterization of acetyl fucoidan and alginate from Commercially Cultured Cladosiphonokamuranus. Bot Mar. 2000;43:393–398. doi: 10.1515/BOT.2000.040. [DOI] [Google Scholar]
- 25.Tong XK, Qiu H, Zhang X, Shi LP, Wang GF, Ji FH, Ding HY, Tang W, Ding K, Zuo JP. WSS45, a sulfated alpha-d-glucan, strongly interferes with Dengue 2 virus infection in vitro. Acta Pharmacol Sin. 2010;31:585–592. doi: 10.1038/aps.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Wang SX, Guan HS. The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]