Abstract
We examined the effects of cAMP, Ca2+ and ATP on exocytosis and adipokine release in white adipocytes by a combination of membrane capacitance patch-clamp recordings and biochemical measurements of adipokine secretion. 3T3-L1 adipocyte exocytosis proceeded even in the complete absence of intracellular Ca2+ ([Ca2+]i; buffered with BAPTA) provided cAMP (0.1 mm) was included in the intracellular (pipette-filling) solution. Exocytosis typically plateaued within ∼10 min, probably signifying depletion of a releasable vesicle pool. Inclusion of 3 mm ATP in combination with elevation of [Ca2+]i to ≥700 nm augmented the rate of cAMP-evoked exocytosis ∼2-fold and exocytosis proceeded for longer periods (≥20 min) than with cAMP alone. Exocytosis was stimulated to a similar extent upon substitution of cAMP by the Epac (exchange proteins activated by cAMP) agonist 8-Br-2′-O-Me-cAMP (1 mm included in the pipette solution). Inhibition of protein kinase A (PKA) by addition of Rp-cAMPS (0.5 mm) to the cAMP-containing pipette solution was without effect. A combination of the adenylate cyclase activator forskolin (10 μm) and the phosphodiesterase inhibitor IBMX (200 μm; forsk–IBMX) augmented adiponectin secretion measured over 30 min 3-fold and 2-fold in 3T3-L1 and human subcutaneous adipocytes, respectively. This effect was unaltered by pre-loading of cells with the Ca2+ chelator BAPTA-AM and 2-fold amplified upon inclusion of the Ca2+ ionophore ionomycin (1 μm) in the extracellular solution. Adiponectin release was also stimulated by the membrane-permeable Epac agonist 8-Br-2′-O-Me-cAMP-AM but unaffected by inclusion of the membrane-permeable PKA inhibitor Rp-8-Br-cAMPS (200 μm). The adipokines leptin, resistin and apelin were present in low amounts in the incubation medium (1–6% of measured adiponectin). Adipsin was secreted in substantial quantities (50% of adiponectin concentration) but release of this adipokine was unaffected by forsk–IBMX. We propose that white adipocyte exocytosis is stimulated by cAMP/Epac-dependent but Ca2+- and PKA-independent release of vesicles residing in a readily releasable pool and that the release is augmented by a combination of Ca2+ and ATP. We further suggest that secreted vesicles chiefly contain adiponectin.
Introduction
White adipose tissue, traditionally regarded as a tissue primarily involved in lipid storage, has in recent years been increasingly recognised to be an endocrine organ that expresses and secretes a variety of bioactive molecules with auto-, para- and endocrine functions. Secreted products, collectively known as adipokines, are known to play important roles in energy homeostasis as well as in inflammatory responses (Trujillo & Scherer, 2006; Maury & Brichard, 2010). With increasing weight, adipose tissue disturbances arise resulting in dysregulated adipokine secretion. Although the secretion of almost every known adipokine is disturbed in obesity (Maury & Brichard, 2010), the molecular and cellular mechanisms involved in short-term regulation of adipokine release remain poorly characterised. Given the severe medical consequences of obesity, including type-2 diabetes, hypertension and cardiovascular disease (Hevener & Febbraio, 2009), the significance of understanding regulatory mechanisms and intracellular mediators involved in white adipocyte adipokine secretion is evident.
Adiponectin is an adipokine exclusively secreted from mature adipocytes, with insulin-sensitising, fat-burning, anti-inflammatory and anti-oxidant properties. Plasma adiponectin levels are decreased in obesity, due to disturbances of both production and secretion of the adipokine (Hoffstedt et al. 2004; Maury & Brichard, 2010; Kovacova et al. 2012). Elevated adiponectin levels have been shown to have a protective role in the development of type-2 diabetes (Spranger et al. 2003). Most investigations aimed at elucidating regulatory mechanisms involved in adipokine secretion are conducted as long-term (several hours or days) studies. Shorter-term (≤4 h) regulation has been investigated in a few studies and release of adiponectin was found to be triggered by insulin (Bogan & Lodish, 1999; Cong et al. 2007; Szkudelski, 2007; Xie et al. 2008), to involve phosphoinositide 3-kinase signalling (Cong et al. 2007) and to depend on an elevation of the cytoplasmic Ca2+ concentration ([Ca2+]i; Bogan & Lodish, 1999). Adiponectin has been suggested to be targeted to a regulatory secretory pathway (Bogan & Lodish, 1999; Xie et al. 2008) but the regulation of its secretion is inadequately investigated.
The term ‘stimulus–secretion coupling’ is used to describe events occurring in neuroendocrine cells when exposed to an immediate stimulus leading to release of a secretory product (Kits & Mansvelder, 2000). In most neuroendocrine cells, an elevation of [Ca2+]i is the trigger for secretion of hormone or neuropeptide-containing vesicles via regulated exocytosis (Burgoyne & Morgan, 2003). In addition to the triggering signal, the Ca2+-dependent secretion is modulated by several intracellular mediators such as cAMP and ATP and requires the interaction of numerous proteins involved in secretory vesicle exocytosis (reviewed in Burgoyne & Morgan, 2003; Seino & Shibasaki, 2005). However, both the cellular mechanisms and the mediators involved in white adipocyte stimulus–secretion coupling remain unknown.
Here we have used in vitro differentiated 3T3-L1 cells, an extensively characterised model of white adipocytes, as well as ex vivo primary human subcutaneous adipocytes. We report, for the first time, how white adipocyte exocytosis is affected by the intracellular mediators Ca2+, cAMP and ATP. Exocytosis, monitored as increases in membrane capacitance (Lindau & Neher, 1988), was compared to measurements of short-term (30 min) secretion of the adipokines adiponectin, leptin, resistin, apelin and adipsin. Our study is, to the best of our knowledge, the first where adipokine secretion is investigated using this methodology. We show that although exocytosis in white adipocytes shares some characteristics with archetypal endocrine cell types, there are important differences. Thus, white adipocyte exocytosis is stimulated by cAMP whilst Ca2+ and ATP exert modulatory effects. cAMP triggers exocytosis also in the complete absence of intracellular Ca2+ and stimulation occurs mainly via a PKA-independent pathway involving Epac (exchange proteins activated by cAMP). We further demonstrate that a large fraction of the vesicles may be released in an ATP-independent fashion. Our results show that exocytosis measured as increase in membrane capacitance can to a large extent be functionally correlated with release of significant quantities of adiponectin.
Methods
Ethical approval
For experiments with human adipocytes, all subjects gave their informed consent. The procedures were approved by the Regional Ethical Review Board, University of Gothenburg and were carried out in compliance with the Declaration of Helsinki.
3T3-L1 cell culture
3T3-L1 cells were maintained as subconfluent cultures in DMEM (high-glucose, 4500 mg l–1; Life Technologies, Stockholm, Sweden) containing 10% fetal bovine serum (FBS Gold; PAA laboratories) and 1% penicillin–streptomycin (medium 1; Life Technologies). Differentiation was carried out according to established procedures (Kohn et al. 1996). Briefly, cells were grown to confluence (day 0) and thereafter incubated in medium 1 supplemented with 1 μm dexamethasone, 850 nm insulin, and 0.5 mm 3-isobutyl-1-methylxanthine (IBMX). After 48 h (day 2) the medium was changed to medium 1 supplemented with insulin only. After an additional 48 h (day 4) the medium was then replaced by medium 1 alone. The medium was thereafter freshly replaced every 2 days. Experimental studies were performed in mature 3T3-L1 adipocytes (determined by occurrence of large lipid droplets; see Fig.1A) between days 8 and 10 from the start of differentiation. All cell culture reagents were purchased from Sigma-Aldrich, MO, USA, unless otherwise specified.
Figure 1. Example of differentiated 3T3-L1 adipocytes and analyses performance.
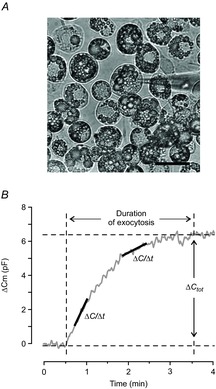
A, 3T3-L1 adipocytes 9 days after start of differentiation. Note the accumulation of large lipid droplets. Patch pipette attached to a 3T3-L1 adipocyte representative of cells chosen for experiments. Scale bar = 50 μm. B, example of a typical capacitance recording with ΔCm (delta membrane capacitance) plotted against time. Analyses were carried out as illustrated in the figure. ΔC/Δt was measured at different time points as indicated by linear fits (black lines) superimposed on the capacitance trace (grey trace). ΔCtot was measured as the difference between the start and end values of membrane capacitance, either where a plateau was obtained or at t = 10 min, as indicated.
Isolation of human adipocytes
Human adipocytes were isolated from subcutaneous adipose tissue biopsies taken during skin reduction surgery under general anaesthesia at the Sahlgrenska University Hospital, Gothenburg, Sweden. The subjects were women with a mean age of 35.3 years (range 35–36) and mean BMI of 24.9 (range 23.6–26.7). Subjects had lost weight by gastric bypass or reduced caloric intake, mean weight reduction being 35 kg (range 18–57 kg). The biopsies were transported in a heated container prior to cell isolation. Adipocyte isolation was performed as described previously (Stralfors & Honnor, 1989), with small modifications. Briefly, adipose tissue was minced and degraded using collagenase type 1 (Worthington, USA) and filtered through a nylon mesh. Adipocytes were washed by flotation in a Krebs–Ringer solution containing (in mm) 120 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 20 Hepes (pH 7.4). The solution was supplemented with 1% (w/v) fatty acid-free bovine serum albumin, 100 nm phenylisopropyladenosine, 0.5 U ml−1 adenosine deaminase with 2 mm glucose. Cells were subsequently incubated overnight (37°C and 5% CO2) in the same solution mixed with an equal volume of DMEM containing 7% (w/v) albumin, 200 nm adenosine, 20 mM Hepes, 50 U ml−1 penicillin and 50 U ml−1 streptomycin (pH 7.4).
Electrophysiology and [Ca2+]i imaging
3T3-L1 adipocytes, grown in plastic (Nunc, Denmark) or glass (MatTek Corporation, USA) Petri culture dishes, were continuously superfused during the recordings with an extracellular solution (EC) containing (in mm): 140 NaCl, 3.6 KCl, 2 NaHCO3, 0.5 NaH2PO4, 0.5 MgSO4, 5 Hepes (pH 7.4 with NaOH), 2.6 CaCl2 supplemented with 5 mm glucose.
Exocytosis, measured as increase in membrane capacitance (Lindau & Neher, 1988), was studied with the whole-cell configuration of the patch-clamp technique using an EPC-9 patch-clamp amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany) and PatchMaster software. Patch pipettes were prepared from borosilicate glass capillaries coated with Sylgard (Dow Corning, USA) and heat-polished prior to use. The pipette resistance ranged between 3 and 5 MΩ when filled with pipette-filling solution. Access and seal resistances during the recordings were typically <15 MΩ and >1 GΩ, respectively. Cells were clamped at −70 mV. The pipette-filling solutions consisted of (in mm): 125 potassium glutamate, 10 KCl, 10 NaCl, 1 MgCl2, 5 Hepes (pH 7.15 with KOH). The solutions were prepared containing different Ca2+-EGTA or -BAPTA mixtures to achieve varying [Ca2+]i and were supplemented with Mg-ATP and/or cAMP, Rp-cAMPS (Tocris) or 8-Br-2-O-Me-cAMP (Biolog Life Science Institute, GmbH, Germany) as specified.
Alterations of the intracellular Ca2+ concentration ([Ca2+]i) were recorded with dual-wavelength ratio imaging as previously described (Astrom-Olsson et al. 2012). Briefly, 3T3-L1 adipocytes were loaded for 1.5 h at room temperature with 2 μm Fura-2 AM together with 0.02% Pluronic F-127 (wt/vol, Life Technologies). A Lambda DG-4 illumination system was employed (Sutter Instrument Company, USA) together with a Nikon Diaphot 300 inverted microscope (Nikon, Japan). Image capturing was performed using a QuantEM 512SC CCD camera (Photometrics, USA) and Metafluor software (Meta imaging series 7.5, Molecular Devices, USA). The excitation wavelengths were 340 and 380 nm and emitted light was collected at 510 nm. The [Ca2+]i was determined using Eqn (5) of Grynkiewicz et al. 1985 and a Kd of 224 nm.
Measurements were carried out at 32˚C.
Adipokine secretion in 3T3-L1 adipocytes
3T3-L1 adipocytes were grown and differentiated on 6- or 12-well plates (Sarstedt). Cells were washed and pre-incubated for 30 min in EC without glucose, in the presence or absence of BAPTA-AM (Life Technologies) or Rp-8-Br-cAMPS (Biolog Life Science Institute) as indicated. Subsequently, EC supplemented with test substances as specified was added and cells were incubated with gentle shaking for 30 min. Incubations were carried out at 32˚C. At the end of the incubation the EC was removed and centrifuged (2000 rpm, 5 min) to remove non-adherent cells. Samples were aliquoted and stored at −80˚C. Secreted adiponectin, leptin and resistin were measured using mouse ELISA DuoSets for the respective adipokines (R&D Systems Europe, UK). Adipsin and apelin were analysed using pre-coated ELISA plates (Antibodies-Online GmbH). Secreted adipokine was expressed in relation to total protein content.
For measurements of protein content, the adherent cells in the wells were washed with PBS and then lysed using a PBS-solution containing protease inhibitor (1 tablet per 10 ml; Complete Mini, Roche, Germany) and SDS (2%). Cells were removed by scraping and the cell mixture was stored at −80˚C. To ensure complete cell lysis the thawed cell mixture was ultra sonicated before protein measurements were performed using Pierce BCA protein kit (Thermo Scientific, USA) according to instructions. Samples were centrifuged and the lipid layer removed.
Adiponectin release in human adipocytes
Human adipocytes (200 μl packed cells ml–1) were washed and pre-incubated in EC for 30 min at 32˚C. Subsequently, test substances were added as indicated and adipocytes were incubated with gentle shaking for 30 min at 32˚C. The incubation was terminated by separation of the cells from the media by centrifugation through diisononyl phthalate (Sigma-Aldrich, USA) and instant freezing on dry ice. Tubes were cut through the diisononyl phthalate layer at two points to separate the cells from the media. Samples were stored at −80˚C. Secreted adiponectin was measured as described above and compared to total protein content.
Measurements of cAMP content
Cyclic AMP XP Assay Kit No. 4339 (Cell Signaling, Beverly, MA, USA) was used to determine intracellular levels of cAMP. Measurements were carried out according to manufacturer's instructions.
Data analysis
In all infusion experiments the rate of capacitance increase (ΔC/Δt) was measured at two or more different time points applying linear fits as illustrated in Fig.1B. The total capacitance increase at the end of the recording (ΔCtot), reflecting the number of vesicles released, as well as the duration of the exocytotic response were also determined as illustrated in Fig.1B. As specified in Results, ΔCtot corresponds to a plateau value (when applicable) or the magnitude of the capacitance increase observed 10 min after establishment of the whole-cell configuration. The statistical significance of variance between two means was calculated using Origin Pro (OriginLab Corporation, USA) and Student's t-test, paired or unpaired as appropriate. When more than one group is compared to the same control group one-way ANOVA was applied. All data are presented as mean values ± SEM for the designated number of experiments. The free [Ca2+] of the intracellular solutions was calculated using MAXC downloads (http://www.stanford.edu/∼cpatton/maxc.html).
Results
Ca2+ dependence of exocytosis in 3T3-L1 adipocytes
The role of Ca2+ in white adipocyte exocytosis was investigated in 3T3-L1 adipocytes voltage clamped at –70 mV and infused with solutions containing different [Ca2+] as well as 3 mm ATP and 0.1 mm cAMP. Infusion of cells with 9 mm CaCl2 together with 10 mm of the Ca2+ chelator EGTA, corresponding to a free [Ca2+] of ∼1.5 μm, triggered exocytosis and the exocytotic rate (ΔC/Δt) measured during the second minute from the start of infusion averaged 26 ± 2.2 fF s−1 (Fig.2A and C). This rate is similar to what has been measured in pancreatic β-cells using the same intracellular solution (Proks et al. 1996; Barg et al. 2002; Olofsson et al. 2009). Exocytosis continued for >10 min (measured in 8 recordings with a sustained seal).
Figure 2. The exocytotic response is affected by alterations in [Ca2+]i.
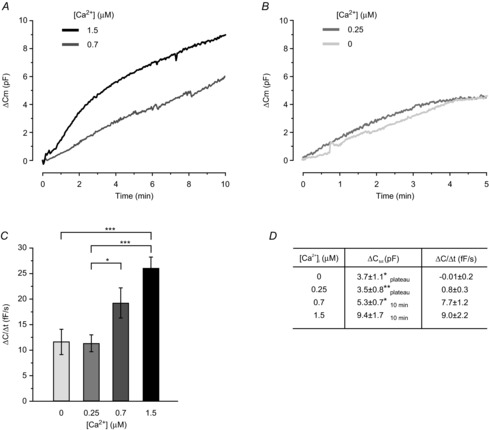
A and B, typical recordings of ΔCm after establishment of the whole-cell configuration. Cells were infused with intracellular solutions containing 3 mm ATP and 0.1 mm cAMP, together with 1.5 μm or 700 nm free Ca2+ (A) or 250 nm or 0 free Ca2+ (B). C, histogram summarising the average rate of capacitance increase (ΔC/Δt) measured at t = 2 min using intracellular solutions with different [Ca2+]. D, exocytotic plateau values or values at t = 10 min (under conditions where plateaus were not reached), as indicated, using pipette solutions containing 0 (n = 8), 250 nm (n = 7), 700 nm (n = 10) or 1.5 μm (n = 8) free Ca2+. Average starting values of Cm were 38 ± 3 pF (0 Ca2+), 35 ± 4 pF (250 nm Ca2+), 45 ± 5 pF (700 nm Ca2+) and 42 ± 2 pF (1.5 μm Ca2). Data in A–C are mean values ± SEM of 8–21 recordings. *P < 0.05; **P < 0.01; ***P < 0.001.
Exocytosis was still triggered using pipette solutions containing lower [Ca2+] of either 8 or 6 mm CaCl2 (10 mm EGTA), corresponding to free Ca2+ concentrations of ∼700 nm and ∼250 nm, respectively (3 mm ATP and 0.1 mm cAMP still included). As can be seen in Fig.2A and C, exocytosis at 700 nm free Ca2+ proceeded at a rate corresponding to ∼75% of that observed using 1.5 μm Ca2+ (P = 0.07). Again, exocytosis continued for >10 min (measured in 10 recordings). Infusion of cells with the solution containing 250 nm free Ca2+ stimulated exocytosis at a rate equal to ∼50% of that achieved with the highest Ca2+ solutions. In eight cells infused with 250 nm free Ca2+ the capacitance increase plateaued within ∼6 min, possibly signifying the depletion of a releasable vesicle pool (Burgoyne & Morgan, 2003).
We next investigated whether exocytosis could still be triggered in the absolute absence of intracellular Ca2+. To ascertain complete Ca2+ buffering, the fast Ca2+ chelator BAPTA was used (0 CaCl2 and 10 mm BAPTA; cAMP and ATP included). Exocytosis was still triggered by cAMP under Ca2+-free conditions, at a rate similar to that seen at 250 nm free Ca2+ (Fig.2B and C) and the capacitance increase again plateaued (at ∼8 min). Values of total capacitance increase (ΔCtot,) and rates at exocytotic plateaus or at t = 10 min using the different solutions are summarised in Fig.2D.
Our results demonstrate that 3T3-L1 adipocyte exocytosis may proceed for long periods in the presence of Ca2+, cAMP and ATP, possibly indicating that recruitment of vesicles for release is a Ca2+-dependent process.
The role of intracellular ATP in 3T3-L1 adipocyte exocytosis
To explore the involvement of ATP in adipocyte exocytosis, cells were infused with solutions containing or lacking the nucleotide, in the presence of 0 or 1.5 μm Ca2+ (Fig.3A–D). At 1.5 μm Ca2+, 3 mm ATP and 0.1 mm cAMP exocytosis was again triggered at a rate comparable to that seen in Fig.2. Exclusion of ATP decreased the rate of capacitance increase ∼60% (P < 0.01 vs. ATP included). Interestingly, the capacitance continued to increase for ≥10 min and did not plateau even in experiments lasting up to 20 min, regardless of the absence or presence of ATP. Our results indicate that ATP is not required to sustain exocytosis/vesicle replenishment in 3T3-L1 adipocytes.
Figure 3. Exocytosis is augmented by Ca2+ and ATP.
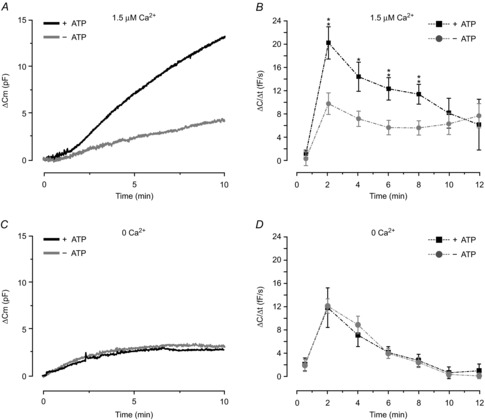
A, representative traces of ΔCm for cells infused with intracellular solutions containing 0.1 mm cAMP and 1.5 μm free Ca2+ with or without 3 mm ATP. B, average ΔC/Δt at indicated time points. The observation that rates of exocytosis are equal at times ≥10 min indicates continuous vesicle replenishment regardless of absence or presence of ATP. Note that analysis at early time points (30 s) show little evidence of net exocytosis after establishment of the whole-cell configuration, before control of the intracellular milieu by infusion of the pipette solution. C and D, as in A and B but cells dialysed with a pipette solution lacking Ca2+. The rates of exocytosis in B and D are close to 0 at 30 s, indicating negligible net exocytosis prior to infusion of the pipette solution. Data are mean values ± SEM of: 8 (+ATP) and 7 (–ATP) recordings in B, and 9 (+ATP) and 13 (–ATP) recordings in D. *P < 0.05; **P < 0.01.
We next investigated how exclusion of intracellular ATP affected Ca2+-independent exocytosis (using 10 mm BAPTA as a Ca2+ chelator; Fig.3C–D). Under these conditions, exocytosis still occurred when the intracellular solution contained 3 mm ATP and 0.1 mm cAMP; ΔC/Δt measured at 2 min averaged 12 ± 3 fF s−1, comparable to that in Fig.2. Exclusion of ATP from the pipette solution did not affect exocytotic rate measured at any time point. The total capacitance increase measured at the plateaus averaged 4.4 ± 1.1 pF in the presence of ATP and 3.8 ± 0.7 pF in the absence of the nucleotide (measured at ∼9 and ∼7 min respectively; P = 0.7).
The involvement of cAMP in 3T3-L1 adipocyte exocytosis
We next tested the effects of Ca2+ and ATP in the absence of cAMP. As shown in Fig.4A-B, exocytosis was completely abolished upon exclusion of cAMP, regardless of the presence or absence of Ca2+ (1.5 μm). Likewise, no effects of ATP were detected in the absence of cAMP. These data underscore the central role of cAMP in adipocyte exocytosis.
Figure 4. 3T3-L1 adipocyte exocytosis is triggered by cAMP via PKA-independent mechanisms.
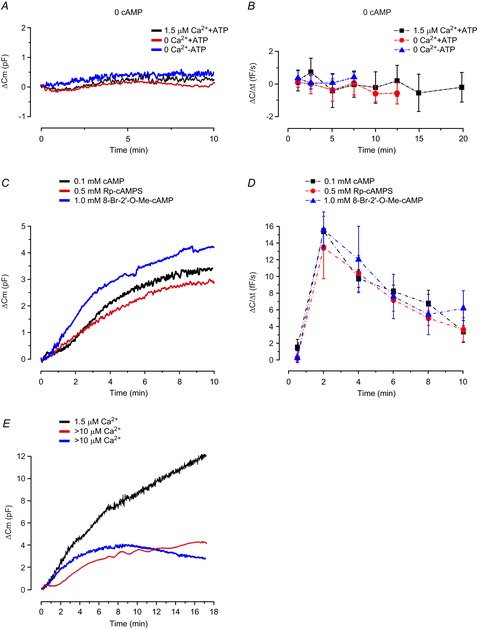
A, typical recordings of ΔCm for cells dialysed with solutions lacking cAMP in the presence or absence of 1.5 μm free Ca2+ and/or ATP (3 mm), as indicated. B, average exocytotic rates analysed at different time points for experimental series in A. C, representative traces of ΔCm for cells infused with intracellular solutions containing 0.1 mm cAMP together with 3 mm ATP, in the presence or absence of Rp-cAMPS (0.5 mm), or cells infused with a solution lacking cAMP and supplemented with 1 mm 8-Br-2′-O-Me-cAMP. D, the effect of Rp-cAMPS or 8-Br-2′-O-Me-cAMP on average ΔC/Δt analysed at designated time points. Data are mean values ± SEM of 9 (1.5 μm Ca2+), 12 (0 Ca2+) and 13 (0 Ca2+, 0 ATP) recordings in B, and 8 (0.1 mm cAMP), 7 (0.1 mm cAMP + 0.5 mm Rp-cAMPS) and 9 (0 mm cAMP + 1 mm 8-Br-2′-O-Me-cAMP) recordings in D. E, two representative traces of endocytosis in cells infused with an intracellular solutions containing >10 μm free Ca2+ together with 0.1 mm cAMP and 3 mm ATP. For comparison, a characteristic trace recorded in a cell infused with 1.5 μm free Ca2+ is shown.
Exocytosis regulated by cAMP may occur via pathways involving PKA or the cAMP receptor Epac (Seino & Shibasaki, 2005). To determine the involvement of PKA and Epac in 3T3-L1 adipocyte exocytosis, we again infused cells with the solution containing 0.1 mm cAMP (no Ca2+, 3 mm ATP) supplemented or not with 0.5 mm of the PKA inhibitor Rp-cAMPS (Fig.4C and D). In accordance with results in Fig.3, cAMP stimulated exocytosis. Inclusion of the inhibitor was without effect at any measured time point. It was verified that exocytosis was not induced by Rp-cAMPS alone (n = 4; ΔC/Δt at 2 min = 1.2 ± 0.6 fF s−1).
To further investigate the contribution of PKA-independent signalling, we infused cells with a pipette solution lacking Ca2+ and cAMP supplemented with 8-Br-2′-O-Me-cAMP, a potent and specific activator of Epac (Herfindal et al. 2013). Agonist concentrations were chosen based on the binding affinity for Epac (5 times lower than the more commonly used but less specific 8pCPT-2Me-cAMP; information from Biolog). As shown in Fig.4C and D, 1 mm 8-Br-2′-O-Me-cAMP stimulated exocytosis as potently as regular cAMP. Exocytosis was still triggered by 0.5 mm of the Epac agonist, at a rate amounting to 50% of that achieved using the higher concentration (n = 9; not shown). Taken together those results suggest that cAMP-stimulation of 3T3-L1 adipocyte exocytosis occurs predominantly via PKA-independent pathways involving Epac.
Endocytosis vs. exocytosis
Recordings of cell membrane capacitance measure the balance between exo- and endocytosis. The fact that exocytosis is abolished using a pipette solution lacking cAMP while no apparent endocytosis is observed (see Fig.4A and B) reinforces the proposal that measured alterations of membrane capacitance chiefly reflect exocytosis. Endocytosis has in several endocrine cell types been shown to be Ca2+ dependent (Eliasson et al. 1996; Mansvelder & Kits, 1998). It is therefore noteworthy that no endocytosis was detected in cells infused with a solution containing 1.5 μm free Ca2+ (cAMP absent) even in recordings lasting up to 20 min (Fig.4B).
To explore the role of Ca2+ in adipocyte endocytosis further, we infused cells with a solution containing an unphysiologically high free [Ca2+] of >10 μm. A reduction of ΔC/Δt by ∼40% at t = 2 min (15 ± 2.4 fF s−1 cf. 26 ± 2.2 fF s−1 at 1.5 μm free Ca2+ in Fig.2; P = 0.01) and by ∼70% at t = 10 min (2.9 ± 0.6 fF s−1 cf. 9.0 ± 2.2 fF s−1 using 1.5 μm Ca2+) was evident, possibly resulting from exocytosis being masked by contaminant endocytosis occurring at a higher speed at high [Ca2+]i. Interestingly, endocytosis was in some experiments apparent as ‘bursts’ of membrane retrieval resulting in a partly wave-like capacitance trace; this was never seen in experiments using a solution containing a lower [Ca2+]. In other experiments net endocytosis was evident as negative rates at later time points (examples of endocytosis depicted in Fig.4E). Our results with >10 μm free Ca2+ are in agreement with a study in pancreatic β-cells where endocytosis was first detectable upon infusion of [Ca2+] > 10 μm (Eliasson et al. 1996). Collectively, these findings make it justifiable to conclude that endocytosis interferes minimally with exocytosis triggered by the pipette solutions used in our study. It should be noted that endocytosis triggered by high [Ca2+] would, if anything, result in an underestimation of the Ca2+ dependence of exocytosis in our cells.
Exocytosis depends on cell size
Compared to most other cell types, white adipocytes are exceptionally large and the variation in cell size is immense. Adipocyte expansion is strongly associated with insulin resistance and type-2 diabetes (Jernas et al. 2006) and secretion of several adipokines has been suggested to depend on adipocyte cell size and to correlate with cell surface area (Skurk et al. 2007).
We plotted the initial cell capacitance (Cm value at the start of the recording before exocytosis was triggered) for all cells included in this study. The distribution of cell size (expressed as membrane capacitance) in a total of 155 experiments is shown in Fig.5A. Observed cell capacitance ranged between 16 pF and 129 pF. The average cell size amounted to 43 ± 1 pF and the median was 39 pF. The 8-fold variation in cell capacitance translates into a ∼3-fold variation in cell diameter, which is significantly smaller than the 20-fold variation reported in human adipocytes (Jernas et al. 2006), probably in part reflecting a selection bias (avoiding the smallest and largest cells for electrophysiology). In order to investigate the importance of cell size in our study, we plotted ΔC/Δt at t = 2 min and ΔCtot values as a function of Cm. As can be seen in Fig.5B–E, there were positive correlations between cell size and ΔC/Δt and ΔCtot both at 0 and 1.5 μm [Ca2+]i (cAMP and ATP included). Thus, exocytosis is in part a function of cell surface area. Reanalysis of ΔC/Δt and ΔCtot values in Figs2 and 3 in relation to initial cell size did not significantly affect the outcome of the experiments described.
Figure 5. Relationship between cell size and exocytotic capacity of 3T3-L1 adipocytes.
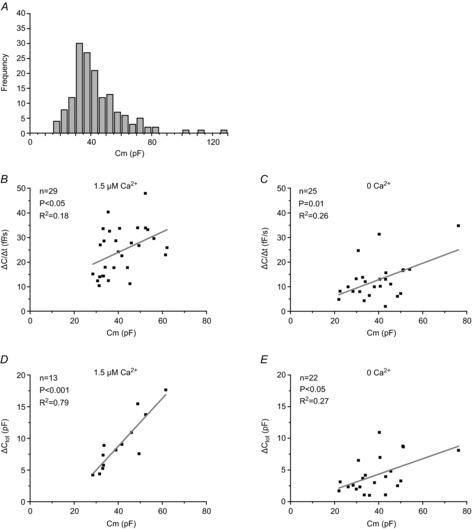
A, the distribution of cell size (measured in pF) for 155 capacitance recordings included in our study. Cell sizes have been divided in 5-pF groups. B–E, values of ΔC/Δt (B and C) and ΔCtot (D and E) as a function of Cm for cells dialysed with a pipette solution containing 3 mm ATP and 0.1 mm cAMP with 1.5 μm (B and D) or 0 (C and E) free Ca2+. Values for ΔCtot are represented by plateau values in the absence of Ca2+. In the presence of 1.5 μm free Ca2+, when plateaus were not achieved, ΔCtot represents the change in cell capacitance during the first 10 min of the recording.
It is interesting that adipocytes that are 10 times bigger than archetypal endocrine cells like pancreatic β-cells and melanotrophs, secrete at a similar rate (∼25 fF s−1; Thomas et al. 1990; Proks et al. 1996; Barg et al. 2002; Olofsson et al. 2009). However, it is worth remembering that the cytosol of white adipocytes comprises only 5% of the total cell volume while the remaining 95% of the cell interior is filled with lipids (Trujillo & Scherer, 2006). Thus, the large size of the cells is more likely to be related to their lipid storage capacity than to their secretory functions.
The role of Ca2+ and cAMP in white adipocyte adiponectin secretion
Adiponectin is released from mature white adipocytes and several studies have shown that 3T3-L1 adipocytes also secrete this adipokine (Bogan & Lodish, 1999; Blumer et al. 2008). To explore a possible association between our capacitance data and adiponectin secretion, we investigated how experimental alterations of intracellular Ca2+ and cAMP levels affected release of this adipokine. To compare the secretion results with the capacitance measurements, experiments were carried out at 32°C and secretion was measured in 30 min incubations. We argued that this would be sufficiently long to allow secretion of measurable adiponectin levels, yet short enough to permit comparison with the capacitance recordings. It should be noted that previous secretion studies have used very long (several hours or days) incubation times.
In order to investigate effects of elevated cAMP, 3T3-L1 adipocytes were incubated with 10 μm forskolin and 200 μm IBMX (forsk–IBMX). This combination elevated cytoplasmic cAMP levels >6-fold in our experiments and the elevation was unaffected by Ca2+ buffering (Fig.6A). As shown in Fig.7A, forsk–IBMX stimulated adiponectin secretion >3-fold compared to control. Forskolin has, in addition to elevating cAMP, been shown to increase [Ca2+]i in pancreatic islet cells (Gao et al. 2002) and brown adipocytes (Nakagaki et al. 2005). Ratiometric recordings of cytosolic [Ca2+] showed that forsk–IBMX elevated 3T3-L1 adipocyte [Ca2+]i, from a basal value of 92 ± 3 nm to an average peak value of 140 ± 4 nm (Fig.6B; 144 analysed cells in five separate experiments). Thus, to study the role of intracellular Ca2+ in forsk–IBMX-stimulated secretion, 3T3-L1 adipocytes were pre-incubated with membrane-permeable BAPTA-AM (50 μm; 30 min). The Ca2+ buffering effect was verified in pre-treated cells stimulated with extracellular ATP. In four separate experiments, pre-loading with BAPTA abolished the [Ca2+]i-elevating effect of 100 μm ATP (not shown). Forsk–IBMX elevated secretion of adiponectin to the same extent in BAPTA-treated cells (Fig.7A).
Figure 6. A combination of forskolin and IBMX increases adipocyte cAMP levels and elevates [Ca2+]i.
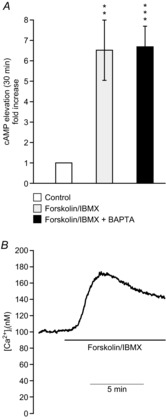
A, cytoplasmic [cAMP] in 3T3-L1 adipocytes incubated with forskolin (10 μm) + IBMX (200 μm) for 30 min in the presence or absence (pre-treatment with 50 μm BAPTA-AM) of intracellular Ca2+. B, example trace of effect of forsk–IBMX on 3T3-L1 adipocyte [Ca2+]i. **P < 0.01; ***P < 0.001.
Figure 7. cAMP stimulated adiponectin secretion is Epac dependent and potentiated by Ca2+.
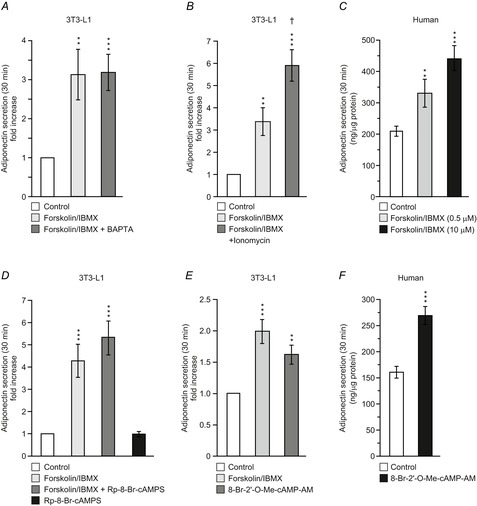
A, adiponectin secretion in 3T3-L1 adipocytes expressed as fold-increase compared to basal (5 mm glucose alone) during 30 min incubations (32°C) with forskolin (10 μm) + IBMX (200 μm) with or without BAPTA pre-treatment (50 μm). B, as in A, but cells incubated for 30 min with forsk–IBMX in the absence or presence of ionomycin (1 μm). C, adiponectin secretion during 30 min incubation of human primary subcutaneous adipocytes with forskolin (0.5 or 10 μm) together with IBMX (200 μm). D, effects of the cAMP antagonist Rp-8-Br-cAMPS (200 μm) on forsk–IBMX-stimulated adiponectin secretion in 3T3-L1 adipocytes. E, 3T3-L1 adipocyte adiponectin secretion stimulated during 30 min incubation with the Epac agonist 8-Br-2′-O-Me-cAMP-AM (20 μm). F, adiponectin secretion in human subcutaneous adipocytes stimulated with 8-Br-2′-O-Me-cAMP-AM (20 μm) for 30 min. Note that basal secretion (control) is somewhat lower than in C, but that the fold-increase produced by the agonist is of similar magnitude as that stimulated with forsk–IBMX. Data are mean values ± SEM of 14 (A), 8 (B), 10 (D) and 11 (E) experiments. Isolated adipocytes from four and three patients were used in C and F, respectively. **P < 0.01; ***P < 0.001.
To further study the role of Ca2+ in cAMP-stimulated adiponectin secretion, 3T3-L1 adipocytes were exposed to the Ca2+ ionophore ionomycin (Fig.7B). Forsk–IBMX again stimulated adiponectin secretion >3-fold, an effect that was augmented 2-fold in the presence of 1 μm ionomycin.
To verify the physiological importance of our findings, primary human subcutaneous adipocytes were incubated for 30 min together with forsk–IBMX. Forskolin was included at 0.5 or 10 μm. As shown in Fig.7C, both concentrations of forskolin (combined with 200 μm IBMX) also stimulated adiponectin release in human adipocytes.
The presence of basal adiponectin secretion under control conditions is evident in both 3T3-L1 and human adipocytes. Every hormone released by regulated secretion also has a basal background release, controlled by for the most part unknown mechanisms. In our adiponectin secretion experiments basal release can be envisaged to arise partly from the intracellular presence of some cAMP in unstimulated cells. Moreover, plasma adiponectin levels are continuously high and a notable basal secretion of this adipokine is thus expected.
The involvement of PKA-independent mechanisms in cAMP stimulation of adiponectin secretion
To elucidate the cAMP signalling pathway, we investigated the ability of forsk–IBMX to stimulate adiponectin secretion in cells treated with the membrane-permeable protein kinase A inhibitor Rp-8-Br-cAMPS (present during 30 min pre-incubation and throughout 30 min stimulations). Forsk–IBMX stimulated adiponectin secretion >4-fold in this series of experiments. As shown in Fig.7D, the stimulation was unaffected by inclusion of 200 μm Rp-8-Br-cAMPS. It was verified that Rp-8-Br-cAMPS alone was without effect on adiponectin release. The results using Rp-8-Br-cAMPS indicate that cAMP stimulates adiponectin secretion via a PKA-independent pathway. It is therefore of interest that the effect of forsk–IBMX was mimicked by the membrane-permeable Epac agonist 8-Br-2′-O-Me-cAMP-AM (20 μm; Fig.7E). In fact, the Epac agonist was only a marginally weaker stimulus than forsk–IBMX and this difference did not attain statistical significance. A higher concentration of the agonist (60 μm) had similar effects (investigated concentrations chosen based on Chepurny et al. 2010 and discussions with Biolog).
The experiment was replicated using primary human subcutaneous adipocytes (Fig.7F). When included at a concentration of 20 μm, 8-Br-2′-O-Me-cAMP-AM stimulated adiponectin release ∼2-fold above control, similar to the stimulation produced by 10 μm forskolin in the presence of IBMX (cf. Fig.7C).
Secretion of additional adipokines
White adipocytes secrete several known adipokines in addition to adiponectin whose release may contribute to observed membrane capacitance increases. Leptin and resistin are, together with adiponectin, classified as adipocyte-derived peptide hormones and have been suggested to be released via regulated exocytosis (Bogan & Lodish, 1999; Bradley & Cheatham, 1999; Roh et al. 2000; Zhong et al. 2002; Xie et al. 2008; Ye et al. 2010). We thus investigated the secretion of those adipokines in 30 min incubations. Resistin is secreted from rodent and 3T3-L1 adipocytes (Zhong et al. 2002; Ye et al. 2010) although in humans this adipokine is mainly secreted from macrophages as well as from immature adipocytes (Janke et al. 2002; Lazar, 2007). Resistin was present in the medium from cells stimulated with forsk–IBMX but amounted to only 6% of measured adiponectin (2.0 ± 0.3 ng ml−1 resistin vs. 34 ± 3 ng ml−1 adiponectin; analysed in 22 samples). The ratio between adiponectin and resistin remained constant upon inclusion of ionomycin (3.0 ± 0.5 ng ml−1 resistin vs. 50 ± 5 ng ml−1 adiponectin). The 3T3-L1 adipocytes used in this study are known to express/secrete very low levels of leptin (MacDougald et al. 1995; Norman et al. 2003); this was confirmed by measurements showing that this adipokine is secreted in small amounts in our cells (secreted leptin amounted to 2.8 ± 0.3 ng ml−1 compared to 114 ± 0.1 ng ml−1 adiponectin; analysed in 12 samples exposed to forsk–IBMX). We further investigated the content of apelin and adipsin in the same samples, two additional adipokines suggested to be abundantly released from 3T3-L1 adipocytes (Kitagawa et al. 1989; Than et al. 2012). Apelin secretion was very low (4.5 ± 0.5 ng ml−1) and thus amounted to 2% of secreted adiponectin. Adipsin was released in slightly larger amounts but the secretion was unaffected by stimulation with forsk–IBMX (13 ± 0.2 ng ml−1 in control cells compared to 18 ± 0.4 ng ml−1 apelin in cells exposed to forsk–IBMX (P = 0.3); compare with a 4-fold elevation of adiponectin release in this series from 27 ± 3 ng ml−1 under control conditions to 114 ± 0.1 ng ml−1 when incubated with forsk–IBMX).
Discussion
Here we combine electrophysiological recordings of membrane capacitance (exocytosis) with measurements of released adiponectin in white adipocytes with the aim of elucidating the mechanisms involved in short-term regulation of white adipocyte exocytosis. Neuroendocrine cells typically contain thousands of hormone or neurotransmitter-containing vesicles that need to undergo Ca2+-, ATP-, and cAMP-dependent priming steps in order to attain release competence. Primed vesicles may be directly secreted upon stimulation, usually by Ca2+, and once depleted the readily releasable vesicle pool must again be refilled by priming of new vesicles (Burgoyne & Morgan, 2003; Rorsman & Renstrom, 2003; Alvarez & Marengo, 2011). Our results show that although white adipocytes share some features of regulation of exocytosis with other endocrine cell types there are some fundamental differences. The key findings in our study, as well as certain aspects of particular interest, are discussed below.
Regulation of white adipocyte exocytosis
We here show that 3T3-L1 adipocyte exocytosis is triggered by cAMP but that this effect is influenced by both Ca2+-dependent and -independent processes. We propose that white adipocytes release adiponectin-containing vesicles according to the model summarised in Fig.8A. Our model postulates that cAMP stimulates exocytosis of a readily releasable pool of adiponectin-containing vesicles in a Ca2+- and ATP-independent manner. A combination of Ca2+ (≥700 nm) and ATP amplifies cAMP-triggered exocytosis via potentiation of vesicle release. Ca2+ is further necessary for vesicle replenishment. We base our model on the following findings. First, cAMP stimulates exocytosis (monitored as an increase in cell capacitance) at [Ca2+] ≤ 250 nm whilst adiponectin secretion (detected biochemically) is stimulated by forsk–IBMX under intracellular Ca2+-free conditions (cf. Figs2B and 7A). Second, the rate of capacitance increase evoked by cAMP in the absence of ATP is equal at early time points (t ≤ 6 min) regardless of the absence or presence of intracellular Ca2+. However, plateaus of capacitance increase are not seen when exocytosis is triggered in the presence of [Ca2+] ≥ 700 nm (cf. Figs2A and 3A), indicating a role for Ca2+ in recruitment of new vesicles for release. Third, the combination of [Ca2+] ≥ 700 nm and ATP augments cAMP-triggered capacitance increase at early time points (t = 2–8 min; see Fig.3B and schematic Fig.8B) akin to a strong potentiation of adiponectin secretion in cells incubated with ionomycin (cf. Figs2A and 7B). Further, the exocytotic rate in the presence of high Ca2+ measured at times ≥10 min equals that observed in the absence of ATP (Fig.3B), indicating that ATP exerts its effect on exocytosis at a distal step equivalent to augmentation of cAMP-stimulated vesicle release. Fourth, the potentiation of exocytosis by ATP is clearly Ca2+ dependent since ATP is without effect on exocytosis in the absence of Ca2+ (Fig.3C and D). The finding that a selective Epac agonist stimulates exocytosis and adiponectin secretion to the same extent as cAMP further indicates that the cAMP stimulation occurs largely via PKA-independent signalling mediated by the cAMP-sensing protein Epac.
Figure 8. Proposed model of regulation of white adipocyte exocytosis.
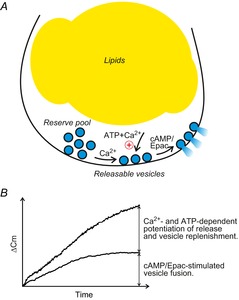
A, white adipocytes contain at least two functional pools of secretory vesicles whose release is differentially regulated by the intracellular messengers cAMP, Ca2+ and ATP. A pool corresponding to readily releasable adiponectin-containing vesicles is released by an elevation of cAMP alone. Ca2+ and ATP augment exocytosis and Ca2+-dependent mechanisms are involved in replenishment of vesicles residing in a reserve pool (see text for details). B, schematic of increases in membrane capacitance in relation to secretion of vesicles with regard to the intracellular presence of cAMP, Ca2+ and ATP.
The ultrastructural characteristics of adipokine-containing vesicles remain poorly defined. However, based on the size of known peptide hormone vesicles (Parsons et al. 1995; Olofsson et al. 2002) and the abundant presence of large (200–500 nm), electron-dense vesicle structures in close proximity to the white adipocyte plasma membrane (Ramm et al. 2000), secretion of adipokines can be envisaged to significantly contribute to membrane capacitance and account for capacitance increases amounting to several picofarads (Fig.8B).
We acknowledge that fusion of vesicles containing cargo other than adiponectin may contribute to the capacitance increases illustrated in Fig.8B. Increases in adipocyte membrane capacitance have previously been ascribed to fusion of Glut4 vesicles with the plasma membrane (Chowdhury et al. 2002). However, the small diameter of those vesicles (50–70 nm; reviewed in Stockli et al. 2011), together with the reported maximal fusion rate (0.15 vesicles min–1 μm2; Stenkula et al. 2010), suggest that Glut4 vesicle fusion only marginally (<5%) contributes to the observed increases in membrane capacitance. Our measurements indicate that the adipokines leptin, resistin and apelin are secreted at low levels compared to adiponectin (1–6%). Adipsin is released at significant levels but secretion of this adipokine was not further stimulated by forsk–IBMX, indicating that it is not released from the same vesicle population. Thus, even if we cannot exclude the possibility that release of additional adipokines contributes to the capacitance increases (if they are at all released by regulated exocytosis of a distinct vesicle population), quantitative considerations argue that the increases in cell capacitance we observe principally reflect adiponectin release. This conclusion is reinforced by the excellent correlation between measurements of cell capacitance and adiponectin secretion data.
Roles of cAMP, Ca2+ and ATP in control of exocytosis in white adipocytes and other endocrine cell types
Both the cAMP dependence as well as its Ca2+ independence distinguishes exocytosis in white adipocytes from that in archetypal endocrine cell types. Interplay between Ca2+ and cAMP is usually involved in non-neuronal cell type exocytosis but Ca2+ is typically the trigger of secretion while cAMP acts as an enhancer (Szaszak et al. 2008). In pancreatic β-cells exocytosis can be triggered by Ca2+ alone, albeit at a rate lower than observed in the presence of cAMP (Renstrom et al. 1997; Eliasson et al. 2003). However, cAMP alone is unable to stimulate β-cell exocytosis in the absence of Ca2+ (Renstrom et al. 1997). By contrast, secretion in some exocrine cell types have been shown to be stimulated by cAMP while Ca2+ has augmenting effects (Szaszak et al. 2008).
The involvement of cAMP in white adipocyte adiponectin secretion has been investigated previously. With few exceptions, these studies investigate long-term (several hours or days) effects of alterations of cAMP and report that an elevation of the nucleotide significantly decreases adiponectin release (Delporte et al. 2002; Fasshauer et al. 2003; Cong et al. 2007; Fu et al. 2007). Short-term (≤3 h) effects of cAMP elevation were in addition investigated in a couple of the studies and either show no alteration (Delporte et al. 2002) or stimulation (Juan et al. 2007) of adiponectin secretion. Our own data demonstrate, in both 3T3-L1 cells and human subcutaneous adipocytes, that the acute response to cAMP is stimulation of adiponectin secretion. The finding that the stimulatory effect of cAMP is chiefly via a PKA-independent pathway further distinguishes adipocytes from other endocrine cell types. In pancreatic β-cells only approximately 15% of the cAMP effect on vesicle replenishment is due to PKA-independent signalling (Renstrom et al. 1997). It is interesting to hypothesise that the usage of a PKA-independent pathway for control of adiponectin release is a way to differentiate regulation of secretion and lipolysis, since the latter is known to be regulated mainly via PKA-dependent mechanisms (Carmen & Victor, 2006).
The role of Ca2+ in adiponectin secretion has previously not attracted much attention. It has been reported that acute exposure of 3T3-L1 adipocytes to the Ca2+ ionophore A23187 results in a negligible elevation of adiponectin secretion at early time points but that there is a significant stimulation at times longer than 90 min (Bogan & Lodish, 1999). This effect is in agreement with our observation that Ca2+ acts at early stages in the secretory pathway including replenishment of the release-competent pool of vesicles. The effects of Ca2+ described here occur within much shorter time frames, in the capacitance measurements during the first minutes of exocytosis (provided ATP is present). We attribute those differences to the concurrent elevation of cAMP in our study making more adiponectin-containing vesicles available for Ca2+-dependent potentiation of release.
Our finding of an involvement of ATP in adipocyte exocytosis is in agreement with a study showing that a decrease of intracellular ATP levels inhibits rat adipocyte adiponectin release stimulated over 2 h (Szkudelski et al. 2011). As in other known secreting cell types, ATP may be required for phosphorylation of exocytotic proteins (Burgoyne & Morgan, 2003).
Pathophysiological significance and the way forward
In view of the important roles of cAMP and Ca2+ in control of short-term adiponectin secretion, it seems reasonable to postulate adrenergic signalling as a physiological stimulus for secretion of the adipokine. Adrenergic signalling is well known to be involved in regulation of adipocyte metabolic function and α1,2 as well as β1-3 adrenergic receptors have been identified in white adipocytes (Lafontan et al. 1997). Catecholamine stimulation elevates intracellular levels of Ca2+ and cAMP via α1 and β1–3 receptors, respectively. In contrast, activation of α2 receptors leads to decreased production of both mediators. Thus, a dynamic functional balance between different adrenergic receptor subtypes in the adipocyte can be envisaged to influence adiponectin secretion. In agreement with the above suggestion, our own results show that incubation of primary mouse subcutaneous adipocytes during 30 min in the presence of adrenaline or the β3-adrenergic agonist CL316243 elevates adiponectin release (A. M. Komai, S. Musovic & C. S. Olofsson, unpublished data). Importantly, adrenergic signalling is dysfunctional in several models of obesity and an increase in the α2 to β receptor ratio has been described in adipocytes from obese individuals (Valet et al. 2000; van Baak, 2001). According to our model of secretion (Fig. 8A), such a switch would disrupt stimulus–secretion coupling with consequent diminished adiponectin release. Previous studies consistently report that long-term (several hours or days) adrenergic stimulation results in reduced adiponectin expression/secretion (Delporte et al. 2002; Fasshauer et al. 2003; Cong et al. 2007; Fu et al. 2007). It is conceivable that the long-term effects on release are in part due to adipocyte exhaustion (adiponectin vesicle depletion), analogous to the situation in pancreatic β-cells exposed to prolonged stimulation of insulin secretion (Robertson et al. 2003). Further, an increase in adipocyte intracellular cAMP has been shown to lead to ATP depletion (Gauthier et al. 2008) which would, consistent with our model of secretion, lessen adiponectin release.
The findings that elevated levels of adiponectin reduce the risk of developing type-2 diabetes (Spranger et al. 2003), together with its ability to promote adipocyte differentiation (Fu et al. 2005), suggest adiponectin as a promising pharmacological target. Production of recombinant adiponectin has proven to be problematic and the prospect of an effective strategy for therapeutic administration is uncertain. Thus, approaches are currently aimed at increasing levels of endogenous adiponectin. The accomplishment of this is undoubtedly reliant on understanding the mechanisms controlling adiponectin secretion. Most neuroendocrine cells contain a single population of peptide hormone-containing vesicles (although they also contain smaller synaptic-like microvesicles; Kasai et al. 2012). In white adipocytes, several adipokines as well as Glut4 may be compartmentalised into distinct types of vesicles (Barr et al. 1997; Bogan & Lodish, 1999; Bradley & Cheatham, 1999; Roh et al. 2000; Xie et al. 2008; Ye et al. 2010). Insulin stimulates secretion of both leptin (Barr et al. 1997) and adiponectin (Blumer et al. 2008) as well as translocation of Glut 4-containing vesicles from an intracellular location to the plasma membrane (Liu et al. 2003; Huang et al. 2005). It is apparent that a complex control of exocytotic pathways exists in the adipocyte. Considering the well-established disturbances of adipokine secretion in obese individuals (Maury & Brichard, 2010), it is remarkable how little is known about the molecular and cellular regulation of adipokine secretion in the shorter term. Future cell physiological studies of the type we report here may help to resolve the underlying cellular mechanisms and ultimately pave the way for pharmacological correction of these defects.
Acknowledgments
We thank Seid Talavanic and Kosrat Latif for assistance with adiponectin release measurements and cell culturing and Dr Mickaël El Hachmane for help with analysis of [Ca2+]i. We thank Birgitta Odén and Dr Anna Forslöw (Discovery Sciences, AstraZenecaR&D, Mölndal) for assistance with isolation of human material. We thank Professor Patrik Rorsman for valuable discussions and reviewing of the manuscript.
Glossary
- 8-Br-2′-O-Me-cAMP
8-Bromo-2-O-methyladenosine-3′, 5′-cyclic monophosphate
- Cm
membrane capacitance
- ΔCtot
total capacitance increase
- ΔC/Δt
exocytotic rate
- EC
extracellular solution
- Epac
exchange proteins activated by cAMP
- Glut4
glucose transporter type 4
- IBMX
3-isobutyl-1-methylxanthine
- PKA
protein kinase A
- Rp-8-Br-cAMPS
8-bromoadenosine-3′, 5′-cyclic monophosphorothioate, Rp-isomer
- Rp-cAMPS
(R)-adenosine, cyclic 3′, 5′-(hydrogenphosphorothioate) triethylammonium
Key points
The molecular and cellular mechanisms involved in short-term regulation of white adipocyte adipokine release remain elusive.
Here we have examined effects of intracellular cAMP, Ca2+ and ATP on exocytosis and adipokine secretion by a combination of membrane capacitance patch-clamp recordings and biochemical measurements of secreted adipokines.
Our findings show that white adipocyte exocytosis is stimulated by cAMP/Epac (exchange proteins activated by cAMP)-dependent but Ca2+- and PKA-independent mechanisms and can largely be correlated to release of adiponectin vesicles residing in a readily releasable vesicle pool.
A combination of Ca2+ and ATP augments exocytosis/adiponectin secretion via a direct action on the release process and by recruitment of new releasable vesicles.
Our results elucidate several previously unknown cellular mechanisms involved in regulation of white adipocyte exocytosis/secretion. The well-established disturbances of adipokine secretion in obese individuals highlight the significance of understanding how white adipocyte adipokine release is controlled.
Additional information
Competing interests
None of the authors has any conflicts of interests.
Author contributions
Conception and design of the experiments: A.M.K. and C.S.O. Data collection, analysis and interpretation of data: A.M.K., C.B., S. M. and C.S.O. Drafting and revising of the manuscript: A.M.K., C.B., S. M. and C.S.O. All authors have read and approved of the final version of the manuscript. All experiments were carried out at the Department of Physiology/ Metabolic Physiology, University of Gothenburg, with the exception of isolation and incubation of human adipocytes, which were performed at AstraZeneca R&D, Mölndal.
Funding
This study was supported by Ollie and Elof Elofssons Stiftelse, the Åke Wiberg Foundation, the Magnus Bergvall Foundation, Diabetesfonden (DIA- 2011-073, DIA2012–050 and DIA2013–070), the Novo Nordisk Foundation and the Swedish Medical Research Council (Grant IDs: 521-2012-2994 and 522-2010-2656). C.O. holds a Swedish Research Council Junior Researcher position.
References
- Alvarez YD. Marengo FD. The immediately releasable vesicle pool: highly coupled secretion in chromaffin and other neuroendocrine cells. J Neurochem. 2011;116:155–163. doi: 10.1111/j.1471-4159.2010.07108.x. &. [DOI] [PubMed] [Google Scholar]
- Astrom-Olsson K, Li L, Olofsson CS, Boren J, Ohlin H. Grip L. Impact of hypoxia, simulated ischemia and reperfusion in HL-1 cells on the expression of FKBP12/FKBP12.6 and intracellular calcium dynamics. Biochem Biophys Res Commun. 2012;422:732–738. doi: 10.1016/j.bbrc.2012.05.071. &. [DOI] [PubMed] [Google Scholar]
- Barg S, Eliasson L, Renstrom E. Rorsman P. A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse beta-cells. Diabetes. 2002;51(Suppl. 1):S74–82. doi: 10.2337/diabetes.51.2007.s74. &. [DOI] [PubMed] [Google Scholar]
- Barr VA, Malide D, Zarnowski MJ, Taylor SI. Cushman SW. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology. 1997;138:4463–4472. doi: 10.1210/endo.138.10.5451. &. [DOI] [PubMed] [Google Scholar]
- Blumer RM, van Roomen CP, Meijer AJ, Houben-Weerts JH, Sauerwein HP. Dubbelhuis PF. Regulation of adiponectin secretion by insulin and amino acids in 3T3-L1 adipocytes. Metabolism. 2008;57:1655–1662. doi: 10.1016/j.metabol.2008.07.020. &. [DOI] [PubMed] [Google Scholar]
- Bogan JS. Lodish HF. Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4. J Cell Biol. 1999;146:609–620. doi: 10.1083/jcb.146.3.609. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RL. Cheatham B. Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rat adipocytes. Diabetes. 1999;48:272–278. doi: 10.2337/diabetes.48.2.272. &. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD. Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. &. [DOI] [PubMed] [Google Scholar]
- Carmen GY. Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal. 2006;18:401–408. doi: 10.1016/j.cellsig.2005.08.009. &. [DOI] [PubMed] [Google Scholar]
- Chepurny OG, Kelley GG, Dzhura I, Leech CA, Roe MW, Dzhura E, Li X, Schwede F, Genieser HG. Holz GG. PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab. 2010;298:E622–E633. doi: 10.1152/ajpendo.00630.2009. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury HH, Kreft M. Zorec R. Rapid insulin-induced exocytosis in white rat adipocytes. Pflugers Arch. 2002;445:352–356. doi: 10.1007/s00424-002-0938-2. &. [DOI] [PubMed] [Google Scholar]
- Cong L, Chen K, Li J, Gao P, Li Q, Mi S, Wu X. Zhao AZ. Regulation of adiponectin and leptin secretion and expression by insulin through a PI3K-PDE3B dependent mechanism in rat primary adipocytes. Biochem J. 2007;403:519–525. doi: 10.1042/BJ20061478. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delporte ML, Funahashi T, Takahashi M, Matsuzawa Y. Brichard SM. Pre- and post-translational negative effect of β-adrenoceptor agonists on adiponectin secretion: in vitro and in vivo studies. Biochem J. 2002;367:677–685. doi: 10.1042/BJ20020610. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson L, Ma X, Renstrom E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, Sewing S. Rorsman P. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol. 2003;121:181–197. doi: 10.1085/jgp.20028707. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson L, Proks P, Ammala C, Ashcroft FM, Bokvist K, Renstrom E, Rorsman P. Smith PA. Endocytosis of secretory granules in mouse pancreatic β-cells evoked by transient elevation of cytosolic calcium. J Physiol. 1996;493:755–767. doi: 10.1113/jphysiol.1996.sp021420. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J. Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. &. [DOI] [PubMed] [Google Scholar]
- Fu L, Isobe K, Zeng Q, Suzukawa K, Takekoshi K. Kawakami Y. β-adrenoceptor agonists downregulate adiponectin, but upregulate adiponectin receptor 2 and tumor necrosis factor-α expression in adipocytes. Eur J Pharmacol. 2007;569:155–162. doi: 10.1016/j.ejphar.2007.05.005. &. [DOI] [PubMed] [Google Scholar]
- Fu Y, Luo N, Klein RL. Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369–1379. doi: 10.1194/jlr.M400373-JLR200. &. [DOI] [PubMed] [Google Scholar]
- Gao Z, Young RA, Trucco MM, Greene SR, Hewlett EL, Matschinsky FM. Wolf BA. Protein kinase A translocation and insulin secretion in pancreatic β-cells: studies with adenylate cyclase toxin from Bordetella pertussis. Biochem J. 2002;368:397–404. doi: 10.1042/BJ20020999. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS. Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem. 2008;283:16514–16524. doi: 10.1074/jbc.M708177200. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M. Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. &. [PubMed] [Google Scholar]
- Herfindal L, Nygaard G, Kopperud R, Krakstad C, Doskeland SO. Selheim F. Off-target effect of the Epac agonist 8-pCPT-2′-O-Me-cAMP on P2Y12 receptors in blood platelets. Biochem Biophys Res Commun. 2013;437:603–608. doi: 10.1016/j.bbrc.2013.07.007. &. [DOI] [PubMed] [Google Scholar]
- Hevener AL. Febbraio MA. The 2009 Stock Conference Report: Inflammation, obesity and metabolic disease. Obes Rev. 2009;11:635–644. doi: 10.1111/j.1467-789X.2009.00691.x. &. [DOI] [PubMed] [Google Scholar]
- Hoffstedt J, Arvidsson E, Sjolin E, Wahlen K. Arner P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J Clin Endocrinol Metab. 2004;89:1391–1396. doi: 10.1210/jc.2003-031458. &. [DOI] [PubMed] [Google Scholar]
- Huang P, Altshuller YM, Hou JC, Pessin JE. Frohman MA. Insulin-stimulated plasma membrane fusion of Glut4 glucose transporter-containing vesicles is regulated by phospholipase D1. Mol Biol Cell. 2005;16:2614–2623. doi: 10.1091/mbc.E04-12-1124. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke J, Engeli S, Gorzelniak K, Luft FC. Sharma AM. Resistin gene expression in human adipocytes is not related to insulin resistance. Obes Res. 2002;10:1–5. doi: 10.1038/oby.2002.1. &. [DOI] [PubMed] [Google Scholar]
- Jernas M, Palming J, Sjoholm K, Jennische E, Svensson PA, Gabrielsson BG, Levin M, Sjogren A, Rudemo M, Lystig TC, Carlsson B, Carlsson LM. Lonn M. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–1542. doi: 10.1096/fj.05-5678fje. &. [DOI] [PubMed] [Google Scholar]
- Juan CC, Chuang TY, Chang CL, Huang SW. Ho LT. Endothelin-1 regulates adiponectin gene expression and secretion in 3T3-L1 adipocytes via distinct signaling pathways. Endocrinology. 2007;148:1835–1842. doi: 10.1210/en.2006-0654. &. [DOI] [PubMed] [Google Scholar]
- Kasai H, Takahashi N. Tokumaru H. Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol Rev. 2012;92:1915–1964. doi: 10.1152/physrev.00007.2012. &. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Rosen BS, Spiegelman BM, Lienhard GE. Tanner LI. Insulin stimulates the acute release of adipsin from 3T3-L1 adipocytes. Biochim Biophys Acta. 1989;1014:83–89. doi: 10.1016/0167-4889(89)90244-9. &. [DOI] [PubMed] [Google Scholar]
- Kits KS. Mansvelder HD. Regulation of exocytosis in neuroendocrine cells: spatial organization of channels and vesicles, stimulus–secretion coupling, calcium buffers and modulation. Brain Research Brain Research Reviews. 2000;33:78–94. doi: 10.1016/s0165-0173(00)00023-0. &. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Summers SA, Birnbaum MJ. Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. &. [DOI] [PubMed] [Google Scholar]
- Kovacova Z, Tencerova M, Roussel B, Wedellova Z, Rossmeislova L, Langin D, Polak J. Stich V. The impact of obesity on secretion of adiponectin multimeric isoforms differs in visceral and subcutaneous adipose tissue. Int J Obes (Lond) 2012;36:1360–1365. doi: 10.1038/ijo.2011.223. &. [DOI] [PubMed] [Google Scholar]
- Lafontan M, Barbe P, Galitzky J, Tavernier G, Langin D, Carpene C, Bousquet-Melou A. Berlan M. Adrenergic regulation of adipocyte metabolism. Human Reproduction. 1997;12:6–20. doi: 10.1093/humrep/12.suppl_1.6. & (Suppl. 1), [DOI] [PubMed] [Google Scholar]
- Lazar MA. Resistin- and obesity-associated metabolic diseases. Horm Metab Res. 2007;39:710–716. doi: 10.1055/s-2007-985897. [DOI] [PubMed] [Google Scholar]
- Lindau M. Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988;411:137–146. doi: 10.1007/BF00582306. &. [DOI] [PubMed] [Google Scholar]
- Liu LB, Omata W, Kojima I. Shibata H. Insulin recruits GLUT4 from distinct compartments via distinct traffic pathways with differential microtubule dependence in rat adipocytes. J Biol Chem. 2003;278:30157–30169. doi: 10.1074/jbc.M301511200. &. [DOI] [PubMed] [Google Scholar]
- MacDougald OA, Hwang CS, Fan H. Lane MD. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1995;92:9034–9037. doi: 10.1073/pnas.92.20.9034. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD. Kits KS. The relation of exocytosis and rapid endocytosis to calcium entry evoked by short repetitive depolarizing pulses in rat melanotropic cells. J Neurosci. 1998;18:81–92. doi: 10.1523/JNEUROSCI.18-01-00081.1998. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury E. Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. &. [DOI] [PubMed] [Google Scholar]
- Nakagaki I, Sasaki S, Yahata T, Takasaki H. Hori S. Cytoplasmic and mitochondrial Ca levels in brown adipocytes. Acta Physiol Scand. 2005;183:89–97. doi: 10.1111/j.1365-201X.2004.01367.x. &. [DOI] [PubMed] [Google Scholar]
- Norman D, Isidori AM, Frajese V, Caprio M, Chew SL, Grossman AB, Clark AJ, Michael Besser G. Fabbri A. ACTH and α-MSH inhibit leptin expression and secretion in 3T3-L1 adipocytes: model for a central–peripheral melanocortin–leptin pathway. Mol Cell Endocrinol. 2003;200:99–109. doi: 10.1016/s0303-7207(02)00410-0. &. [DOI] [PubMed] [Google Scholar]
- Olofsson CS, Gopel SO, Barg S, Galvanovskis J, Ma X, Salehi A, Rorsman P. Eliasson L. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic B-cells. Pflugers Arch. 2002;444:43–51. doi: 10.1007/s00424-002-0781-5. &. [DOI] [PubMed] [Google Scholar]
- Olofsson CS, Hakansson J, Salehi A, Bengtsson M, Galvanovskis J, Partridge C, SorhedeWinzell M, Xian X, Eliasson L, Lundquist I, Semb H. Rorsman P. Impaired insulin exocytosis in neural cell adhesion molecule–– mice due to defective reorganization of the submembrane F-actin network. Endocrinology. 2009;150:3067–3075. doi: 10.1210/en.2008-0475. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Coorssen JR, Horstmann H. Almers W. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. &. [DOI] [PubMed] [Google Scholar]
- Proks P, Eliasson L, Ammala C, Rorsman P. Ashcroft FM. Ca2+- and GTP-dependent exocytosis in mouse pancreatic β-cells involves both common and distinct steps. J Physiol. 1996;496:255–264. doi: 10.1113/jphysiol.1996.sp021682. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm G, Slot JW, James DE. Stoorvogel W. Insulin recruits GLUT4 from specialized VAMP2-carrying vesicles as well as from the dynamic endosomal/trans-Golgi network in rat adipocytes. Mol Biol Cell. 2000;11:4079–4091. doi: 10.1091/mbc.11.12.4079. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renstrom E, Eliasson L. Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol. 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RP, Harmon J, Tran PO, Tanaka Y. Takahashi H. Glucose toxicity in β-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–587. doi: 10.2337/diabetes.52.3.581. &. [DOI] [PubMed] [Google Scholar]
- Roh C, Thoidis G, Farmer SR. Kandror KV. Identification and characterization of leptin-containing intracellular compartment in rat adipose cells. Am J Physiol Endocrinol Metab. 2000;279:E893–E899. doi: 10.1152/ajpendo.2000.279.4.E893. &. [DOI] [PubMed] [Google Scholar]
- Rorsman P. Renstrom E. Insulin granule dynamics in pancreatic β-cells. Diabetologia. 2003;46:1029–1045. doi: 10.1007/s00125-003-1153-1. &. [DOI] [PubMed] [Google Scholar]
- Seino S. Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. &. [DOI] [PubMed] [Google Scholar]
- Skurk T, Alberti-Huber C, Herder C. Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. &. [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H. Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. &. [DOI] [PubMed] [Google Scholar]
- Stenkula KG, Lizunov VA, Cushman SW. Zimmerberg J. Insulin controls the spatial distribution of GLUT4 on the cell surface through regulation of its postfusion dispersal. Cell Metab. 2010;12:250–259. doi: 10.1016/j.cmet.2010.08.005. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockli J, Fazakerley DJ. James DE. GLUT4 exocytosis. J Cell Sci. 2011;124:4147–4159. doi: 10.1242/jcs.097063. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stralfors P. Honnor RC. Insulin-induced dephosphorylation of hormone-sensitive lipase. Correlation with lipolysis and cAMP-dependent protein kinase activity. Eur J Biochem. 1989;182:379–385. doi: 10.1111/j.1432-1033.1989.tb14842.x. &. [DOI] [PubMed] [Google Scholar]
- Szaszak M, Christian F, Rosenthal W. Klussmann E. Compartmentalized cAMP signalling in regulated exocytic processes in non-neuronal cells. Cell Signal. 2008;20:590–601. doi: 10.1016/j.cellsig.2007.10.020. &. [DOI] [PubMed] [Google Scholar]
- Szkudelski T. Intracellular mediators in regulation of leptin secretion from adipocytes. Physiol Res. 2007;56:503–512. doi: 10.33549/physiolres.931038. [DOI] [PubMed] [Google Scholar]
- Szkudelski T, Nogowski L. Szkudelska K. Short-term regulation of adiponectin secretion in rat adipocytes. Physiol Res. 2011;60:521–530. doi: 10.33549/physiolres.931971. &. [DOI] [PubMed] [Google Scholar]
- Than A, Tee WT. Chen P. Apelin secretion and expression of apelin receptors in 3T3-L1 adipocytes are differentially regulated by angiotensin type 1 and type 2 receptors. Mol Cell Endocrinol. 2012;351:296–305. doi: 10.1016/j.mce.2012.01.005. &. [DOI] [PubMed] [Google Scholar]
- Thomas P, Surprenant A. Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990;5:723–733. doi: 10.1016/0896-6273(90)90226-6. &. [DOI] [PubMed] [Google Scholar]
- Trujillo ME. Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. &. [DOI] [PubMed] [Google Scholar]
- Valet P, Grujic D, Wade J, Ito M, Zingaretti MC, Soloveva V, Ross SR, Graves RA, Cinti S, Lafontan M. Lowell BB. Expression of human α2-adrenergic receptors in adipose tissue of β3-adrenergic receptor-deficient mice promotes diet-induced obesity. J Biol Chem. 2000;275:34797–34802. doi: 10.1074/jbc.M005210200. &. [DOI] [PubMed] [Google Scholar]
- van Baak MA. The peripheral sympathetic nervous system in human obesity. Obes Rev. 2001;2:3–14. doi: 10.1046/j.1467-789x.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- Xie L, O'Reilly CP, Chapes SK. Mora S. Adiponectin and leptin are secreted through distinct trafficking pathways in adipocytes. Biochim Biophys Acta. 2008;1782:99–108. doi: 10.1016/j.bbadis.2007.12.003. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Than A, Zhao Y, Goh KH. Chen P. Vesicular storage, vesicle trafficking, and secretion of leptin and resistin: the similarities, differences, and interplays. J Endocrinol. 2010;206:27–36. doi: 10.1677/JOE-10-0090. &. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Lin CY, Clarke KJ, Kemppainen RJ, Schwartz DD. Judd RL. Endothelin-1 inhibits resistin secretion in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;296:383–387. doi: 10.1016/s0006-291x(02)00882-3. &. [DOI] [PubMed] [Google Scholar]


