Abstract
Dietary sodium affects function of the beta-2 adrenoceptor (ADRB2). We tested the hypothesis that haplotype variation in the ADRB2 gene would influence the cardiovascular and regional vasodilator responses to sympathoexcitatory manoeuvres following low, normal and high sodium diets, and ADRB2-mediated forearm vasodilation in the high sodium condition. Seventy-one healthy young adults were grouped by double homozygous haplotypes: Arg16+Gln27 (n = 31), the rare Gly16+Gln27 (n = 10) and Gly16+Glu27 (n = 30). Using a randomized cross-over design, subjects were studied following 5 days of controlled low, normal and high sodium with 1 month or longer between diets (and low hormone phase of the menstrual cycle). All three visits utilized ECG and finger plethysmography for haemodynamic measures, and the high sodium visit included a brachial arterial catheter for forearm vasodilator responses to isoprenaline with plethysmography. Lymphocytes were sampled for ex vivo analysis of ADRB2 density and binding conformation. We found a main effect of haplotype on ADRB2 density (P = 0.03) with the Gly16+Glu27 haplotype having the greatest density (low, normal, high sodium: 12.9 ± 0.9, 13.5 ± 0.9 and 13.6 ± 0.8 fmol mg−1 protein, respectively) and Arg16+Gln27 having the least (9.3 ± 0.6, 10.1 ± 0.5 and 10.3 ± 0.6 fmol mg−1 protein, respectively), but there were no sodium or haplotype effects on receptor binding conformation. In the mental stress trial, there was a main effect of haplotype on cardiac output (P = 0.04), as Arg16+Gln27 had the lowest responses. Handgrip and forearm vasodilation yielded no haplotype differences, and no correlations were present for ADRB2 density and haemodynamics. Our findings support cell-based evidence that ADRB2 haplotype influences ADRB2 protein expression independent of dietary sodium, yet the haemodynamic consequences appear modest in healthy humans.
Introduction
In the circulatory system, the beta-2 adrenoceptor (ADRB2) is a major contributor to heart rate, myocardial contractility and peripheral vasodilation (Eisenach & Wittwer, 2010). ADRB2 function is also modulated by dietary sodium intake. It has long been established that in hypertension, ADRB2-mediated vasodilation is reduced, which is corrected by a low sodium diet (Feldman et al. 1984, 1987; Feldman, 1990; Naslund et al. 1990). Parallel to these findings, the functional relevance of variation in the ADRB gene has been characterized over the last 20 years (Green et al. 1993). Single nucleotide polymorphisms (SNPs) in the ADRB2 gene that have been suggested to affect physiological function include amino acid position 16, which contains either glycine or arginine (major/minor allele: Gly16/Arg), and amino acid position 27, which contains either glutamine or glutamic acid (Gln27/Glu).
Over the last decade our laboratory has conducted a series of experiments examining the cardiovascular pleiotropic effects of ADRB2 gene variation in relation to dietary sodium. First, we established that individuals homozygous for Gly16 possess a greater forearm vasodilator response to intra-arterial graded infusions of the beta-agonist isoprenaline – when compared to Arg 16 homozygotes – after 5 days of a controlled normal sodium diet (Garovic et al. 2003). Subsequently, we demonstrated that the greater forearm vasodilator response to isoprenaline in Gly16 homozygotes was no longer present following 5 days of a controlled low sodium diet (Eisenach et al. 2006). Next, in support of the regional blood flow studies, we infused the beta-2 agonist terbutaline intravenously during baroreflex inhibition with trimethaphan to ‘isolate’ the systemic vasodilator response, and found that terbutaline evoked greater systemic vasodilation (lower systemic vascular resistance, SVR) in Gly16 vs. Arg16 homozygotes; this study was conducted after a normal sodium diet (Hesse et al. 2010). We also found that Gly16 was associated with a greater density of ADRB2s on lymphocytes, whereas the ADRB2 high and low affinity binding conformation was similar between genotype groups (Hesse et al. 2010).
A separate series of studies examining the ADRB2 SNPs in response to sympathoexcitatory manoeuvres demonstrated that Gly16 homozygotes have a lower resting heart rate (HR) and greater HR and cardiac output (CO) response to isometric handgrip (Eisenach et al. 2004, 2005). During head-up tilt, Arg16 homozygotes have a greater HR, greater SVR and greater arterial plasma noradrenaline response than Gly16 homozygotes (Wittwer et al. 2011). Haemodynamic and forearm vasodilator responses to mental stress were not dependent on Gly16/Arg genotype (Liu et al. 2006). Importantly, these latter studies did not control for dietary sodium intake prior to the sympathoexcitation protocols.
Summarizing these findings, evidence suggests that the Gly16 and Glu27 SNPs may be associated with augmented peripheral vasodilator function and myocardial function (Tang et al. 2003; Snyder et al. 2006b). Clinically, these phenotypes may confer favourable outcomes in patients with acute coronary syndrome (Lanfear et al. 2005) and heart failure (Kaye et al. 2003; de Groote et al. 2005; Metra et al. 2010). Conversely, the Arg16 and Gln27 alleles appear to harbour unfavourable characteristics in cardiovascular and pulmonary health, owing to blunted indices of cardiac function, vasodilatation and perhaps post-exercise bronchodilatation, as recently reviewed (Eisenach & Wittwer, 2010). The two commonly tested SNPs in the ADRB2 gene are in linkage disequilibrium, such that Glu27 homozygotes are homozygous for Gly16, and Arg16 homozygotes are homozygous for Gln27 (Drysdale et al. 2000; Hawkins et al. 2006). Therefore, if an individual is homozygous for both positions 16 and 27, that individual will contain one of three homozygous haplotype combinations: Arg16+Gln27, Gly16+Gln27 or Gly16+Glu27. Thus, it is reasonable to postulate that if the individual SNPs functionally impact ADRB2 function and exist in linkage disequilibrium, then individuals with homozygous forms of the haplotypes should demonstrate the most contrasting physiological function.
Hypertension as a modifiable risk factor for cardiovascular disease has reached global epidemic proportions (Sliwa et al. 2011). High dietary sodium increases blood pressure (BP), cardiovascular morbidity and mortality (Aaron & Sanders, 2013). Therefore, dietary sodium has emerged as a major focus of cardiovascular disease prevention, with ongoing evidence to support widespread recommendations calling for reduced sodium intake (Cook et al. 2014). However, the intermediate physiological effects of dietary sodium on cardiovascular control with respect to genetic variation in key regulatory pathways are indeterminate. The functional relevance of individual SNPs in the ADRB2 gene have been characterized with mixed results. Even less is known on how these SNPs interact in haplotype combinations.
With this information as background, in the present study we tested the overall hypothesis that dietary sodium affects cardiovascular control in an ADRB2 haplotype-dependent manner. In a randomized cross-over design, we administered 5 days of controlled low, normal and high dietary sodium with an interval of 1 month or greater between diets. Our aims were: (1) to determine the effect of dietary sodium and ADRB2 genotype on lymphocyte ADRB2 density and binding conformation; (2) to determine the effect of dietary sodium on the haemodynamic and forearm vasodilator responses to mental stress; (3) to determine the effect of dietary sodium on the haemodynamic responses to isometric handgrip; (4) to determine the influence of ADRB2 gene variation on these cardiovascular responses, and whether genotype-dependent responses would no longer be present after beta-blockade with propranolol. As a follow-up to our prior forearm blood flow (FBF) protocols during normal and low sodium diets, (5) our final aim was to determine whether ADRB2 genotype influenced the forearm vasodilator response to isoprenaline in the high dietary sodium condition.
Methods
Ethical approval
This study was approved by the Mayo Clinic IRB and conformed to the standards set by the latest revision of the Declaration of Helsinki. Written, informed consent was obtained from each participant at the screening visit prior to study participation.
Subjects
This study was conducted between 2009 and 2013. The duration of study completion was dependent on recruitment of individuals who were identified as homozygous for both ADRB positions 16 and 27. Of the 71 participants, approximately half of the subjects were recruited from community-wide genotyping efforts performed by our laboratory from 2000 to 2010 (n = 2226 samples). The remainder were recruited from the Mayo Clinic Centre for Individualized Medicine's Biobank repository in 2009–2012 (n = 1000 samples). To control for race and ethnicity, enrolment was limited to white Caucasians. The subjects were non-obese (body mass index ≤28), non-smokers, non-diabetic and not currently taking anti-hypertensive or other medications except for oral contraceptives. Candidates were considered ineligible if they were men over age 40, women over age 50 (or post-menopausal), used tobacco products, or had any acute or chronic disorders associated with alterations in cardiovascular structure or function (such as hypertension or diabetes). Female volunteers had a negative pregnancy test within 48 h of being studied and on each visit, and were studied during the low hormone phase of the menstrual cycle or the placebo phase of oral contraceptives. Prior to the study protocol, each subject was evaluated by one of the investigators who reviewed the subject's medical history and performed a physical examination. Subjects were considered ineligible if they participated in strenuous regular physical activity such as training for competitive distance events.
Diets
In randomized order separated by 1 month or longer, subjects were placed on one of three diets: the low sodium diet contained 10 mmol (0.23 g Na+; 0.6 g salt) of sodium daily; normal sodium diet contained 150 mmol (3.45 g Na+; 8.6 g salt) of sodium daily; and high sodium diet contained 400 mmol (9.2 g Na+; 23 g salt) of sodium daily. The diets provided constant daily amounts of protein (1.4 g (kg body weight)−1 day−1), potassium (100 mmol day−1) and calcium (1100 mg day−1). The caloric content of the diet was adjusted using the Harris Benedict equation to maintain constant body weight, and no more than 35% of calories were provided by fat. All meals were prepared in the dietary kitchen of the Mayo Clinical Research Unit (CRU). On day 5, urine was collected for 24 h for measurement of sodium, potassium and creatinine excretion. All study visits were conducted between 07:00 and 16:00 h. Subjects remained fasting on the morning of each study except for water until the study measurements were completed. If an individual was studied in the afternoon, a light breakfast administered by the research kitchen consistent with the dietary condition was allowed to be consumed more than 4 h before data collection.
Protocol
From this trial, two recent interim analyses on the effects of dietary sodium on HR variability and sex differences in sodium sensitivity describe the details of the study visit protocols (Eisenach et al. 2012; Allen et al. 2014). The data included in this report have not been reported on all 71 participants with respect to the interactions between ADRB2 genotype, ADRB2 lymphocyte density, cardiovascular haemodynamics during stress manoeuvres, FBF and dietary sodium. Briefly, the study visit days after the low and normal sodium diets were identical. Subjects were placed in a semi-recumbent chair and an intravenous (i.v.) catheter was placed in the non-dominant arm and used for blood sampling and systemic propranolol infusion. On the study day following the high sodium diet, subjects were initially placed in the supine position. A 20-gauge brachial arterial catheter was placed under local anaesthesia in the non-dominant arm for BP measurement and the forearm drug infusion protocol as detailed previously (Eisenach et al. 2012). FBF was measured with venous occlusion plethysmography. Upon completion of the forearm protocol, subjects were allowed a toilet break and ad libitum intake of water, and were transferred to the semi-recumbent chair. An i.v. catheter and finger plethysmography cuff were placed in the non-dominant arm. The remainder of the protocol on the high sodium day was identical to the other visits.
Measurements
HR measured with a three-lead ECG. BP was measured non-invasively with finger plethysmography (NexFin, Edwards Lifesciences, Irvine, CA, USA) and verified by brachial cuff oscillometric sphygmomanometry prior to each data collection period. Stroke volume (SV), CO and SVR were derived from pulse contour analysis of the finger plethysmography waveform according to NexFin algorithms (Critoph et al. 2013). NexFin has shown low numbers for within-patient variability or tracking errors and reliable tracking of rapid BP changes compared to intra-arterial data (Martina et al. 2012; Truijen et al. 2012). On the high sodium visit, the brachial arterial catheter provided BP measures, and the NexFin was calibrated to the arterial pressure and recorded to allow for SV, CO and SVR comparisons across dietary conditions. Following each sodium condition, data were recorded at 1000 Hz and analysed on LabChart (AD Instruments, Colorado Springs, CO, USA). Haemodynamic variables were averaged during pre-stress baseline and during the entire period of stress for mental stress and isometric handgrip. FBF was averaged during the 2 min baseline (pre-drug infusion) and final 1 min of each vasodilator dose.
Forearm drug infusion protocol
The forearm vasodilation protocol was conducted after the high sodium diet and conducted in identical fashion to our previous report in the low sodium condition (Eisenach et al. 2006). To measure endothelium-independent vasodilation, sodium nitroprusside (NTP) was infused for 2 min at 1.0 μg (100 ml limb volume)−1 min−1. To measure ADRB2-mediated vasodilation, isoprenaline was infused for 2 min at 1.0, 3.0, 6.0 and 12.0 ng (100 ml limb volume)−1 min−1. To measure endothelium-dependent vasodilation, acetylcholine (ACh) was infused for 2 min at 4.0 μg (100 ml limb volume)−1 min−1. Following a 20 min washout period, the NO synthase inhibitor NG-monomethyl-l-arginine (l-NMMA; 50 mg) was infused over 10 min, followed by a maintenance dose of 1 mg min−1 for the remainder of the forearm protocol. The vasodilator drugs were repeated in reverse order, to allow determination that the NO component of endothelium-dependent dilation was markedly inhibited in response to ACh.
Laboratory stressors
After baseline recording for 2 min, instructions were narrated into participant headphones followed by automated subtraction problems for a total time of 5 min with 81 problems in random order and difficulty (Allen et al. 2014). The audio track verbalized random extraneous numbers and was interrupted with a narration urging subjects to improve their performance. A study team member stood behind the subject and recorded the number of incorrect responses. The test concluded with a 2 min recovery period. A mental stress survey developed by Reims et al. (2004) was administered. To prevent familiarity with the tests across diets, three maths files were created and randomly selected on each study visit. After 15 min quiet rest and 2 min of baseline recording, subjects performed isometric handgrip at 40% of maximal voluntary contraction (determined on screening visit) until exhaustion. To ensure maximal effort, subjects were encouraged to continue squeezing until fatigue was reached, defined as the inability to maintain force within 10% of the target. After a 10 min rest, an i.v. loading dose of propranolol (0.15 mg kg−1) was infused at a rate of 1 mg min−1, followed by a maintenance infusion of 0.004 mg kg−1 min−1 for systemic beta-adrenoceptor blockade (Hjemdahl et al. 1983; Freyschuss et al. 1988). Ten minutes later, the mental stress and handgrip protocols were completed, and the subjects were de-instrumented and discharged.
Blood samples for lymphocyte ADRB2 density and binding affinity
At the beginning of low and normal sodium visits, 80 ml of venous blood was withdrawn from the i.v. catheter. At the beginning of the high sodium visit, 80 ml of arterial blood was withdrawn from the arterial catheter. Lymphocyte ADRB2 density and binding conformation assays were conducted as previously described (Hesse et al. 2010). These assays were performed in yearly batches, and each participant's samples collected from the three sodium conditions were assayed together.
Statistical analysis
Subject characteristics in each sodium condition were compared between haplotype groups using ANOVA. Haemodynamic variables’ changes with stress (stress – baseline) were analysed using mixed-effect linear models (repeated measures analysis) taking into account the longitudinal study design. The mixed-effect modelling was performed using PROC MIXED (SAS version 9.3, SAS Institute, Cary, NC, USA). For these models, the change in the given haemodynamic variable with stress was the dependent variable, and the explanatory variables included haplotype group as a between-subject effect, and diet and propranolol as within-subject effects. The diet-by-haplotype interaction terms were included in the models to assess whether dietary differences in the haemodynamic response to stress were dependent on haplotype. FBF responses to isoprenaline, NTP and ACh were analysed using mixed linear models with FBF as the dependent variable. For these models, haplotype was a between-subject effect, drug dose was a within-subject effect and the analysis was repeated using the data collected before and after the administration of l-NMMA.
The comparison between the common haplotypes Arg16+Gln27 and Gly16+Glu27 was of primary interest, with an approximate sample size of 30 individuals per group based on previous studies comparing the CO response to isometric exercise, and FBF responses to isoprenaline between groups that were homozygous for the Arg16 or Gly16 variant (Garovic et al. 2003; Eisenach et al. 2006). Given our final sample size, the statistical power (two-sided, α = 0.05) to detect differences between Arg16+Gln27 and Gly16+Glu27 was 81% for a difference of 0.75 SD, 97% for a difference of 1.0 SD and 99% for 1.25 SD. Individuals with Gly16+Gln27 haplotype were recruited with a sample size goal of 15 to provide a statistical power of 63, 86 and 97% for a difference of 0.75, 1.0 and 1.25 SD, respectively, in comparison with the other two groups. However, only 10 individuals were enrolled due to the rare occurrence of the homozygous form of this haplotype. All data are presented means±SEM. P values of <0.05 were considered statistically significant.
Results
Demographics, lymphocyte ADRB2 density and binding conformation
Subject characteristics based on haplotype and dietary condition are shown in Table1. Because there was a greater proportion of males in the Gly16+Gln27 group, the P values generated in this table included sex as a covariate. There was a main effect of dietary sodium on weight for all subjects irrespective of genotype, as the mean ± SEM weight in low, normal and high sodium conditions was 70.1 ± 1.4, 71.5 ± 1.5 and 72.2 ± 1.4 kg, respectively. There was a main effect of dietary sodium on HR, as low sodium was associated with a greater HR, but this was not dependent on haplotype. For BP, there was a trend toward main effects of sodium and haplotype on diastolic BP (DBP). Within haplotype groups DBP was greater in the low sodium condition, but there was no interaction effect, suggesting that DBP did not respond differently to dietary sodium based on haplotype. Urine volume was dependent on both sodium and haplotype, with the Gly16+Gln27 haplotype displaying greater urine output than the other haplotypes. As expected, urine sodium excretion was dependent on dietary sodium condition. Potassium and creatinine excretion were not affected by diet or haplotype (data not shown). Finally, there was a main effect of sodium and haplotype on ADRB2 density on lymphocytes, with the Arg16+Gln27 haplotype displaying a lower density than the other groups (Fig.1). There was no sodium-by-haplotype interaction, suggesting that ADRB2 density did not react differently from the sodium conditions based on haplotype. Because the ADRB2 density assays were performed in yearly batches over the 4 years of the study, we repeated the analysis with time as a covariate and the effects remained significant (Table1). Finally, there was no evidence to suggest that ADRB2 high and low affinity binding conformation was influenced by dietary sodium or haplotype (data not shown).
Table 1.
Subject characteristics by genotype and dietary sodium condition (low, normal, high)
| Arg16 ± Gln27 (n = 31: 20F, 11M) |
Gly16 ± Gln27 (n = 10: 4F, 6M) |
Gly16 ± Glu27 (n = 30: 21F, 9M) |
P values |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Low | Normal | High | Low | Normal | High | Low | Normal | High | Psodium | Phaplotype | Pinteraction |
| Weight (kg) | 70 ± 2 | 71 ± 2 | 72 ± 2 | 80 ± 5 | 79 ± 5 | 80 ± 5 | 66 ± 2 | 69 ± 2 | 69 ± 2 | <0.001 | 0.26 | 0.08 |
| HR (b.p.m.) | 76 ± 2 | 73 ± 2 | 70 ± 2 | 70 ± 3 | 71 ± 5 | 64 ± 4 | 78 ± 2 | 71 ± 2 | 71 ± 2 | <0.001 | 0.71 | 0.55 |
| SBP (mmHg) | 118 ± 2 | 117 ± 2 | 118 ± 2 | 114 ± 4 | 119 ± 3 | 115 ± 4 | 115 ± 2 | 117 ± 2 | 116 ± 3 | 0.37 | 0.66 | 0.63 |
| DBP (mmHg) | 72 ± 2 | 69 ± 1 | 69 ± 1 | 67 ± 4 | 64 ± 3 | 62 ± 4 | 69 ± 2 | 67 ± 2 | 66 ± 1 | 0.06 | 0.06 | 0.86 |
| MAP (mmHg) | 87 ± 2 | 85 ± 1 | 85 ± 1 | 83 ± 4 | 83 ± 2 | 79 ± 3 | 84 ± 2 | 84 ± 1 | 83 ± 1 | 0.23 | 0.13 | 0.79 |
| 24 h urine volume (ml) | 1646 ± 127 | 1695 ± 126 | 2450 ± 160 | 2255 ± 252 | 2605 ± 435 | 3385 ± 901 | 1504 ± 122 | 1589 ± 168 | 2025 ± 157 | <0.001 | <0.01 | 0.45 |
| 24 h Na+ excretion (mmol) | 20 ± 2 | 116 ± 6 | 343 ± 18 | 22 ± 3 | 117 ± 7 | 323 ± 32 | 21 ± 3 | 101 ± 6 | 314 ± 21 | <0.001 | 0.34 | 0.40 |
| ADRB2 receptor density on lymphocytes | 9.3 ± 0.6 | 10.1 ± 0.5 | 10.3 ± 0.6 | 10.6 ± 1.2 | 13.5 ± 1.6 | 11.8 ± 1.9 | 12.9 ± 0.9 | 13.5 ± 0.9 | 13.6 ± 0.8 | <0.01 | 0.03 | 0.11 |
Values are means ± SEM. Haemodynamic values were obtained on arrival for each study visit after 5 days of each sodium diet. Urinary sodium (Na+) was obtained from a 24 h urine collection on the final day of the diets. HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure.
Figure 1. Lymphocyte density of beta-2 adrenoceptor (ADRB2) based on haplotype and dietary sodium condition.
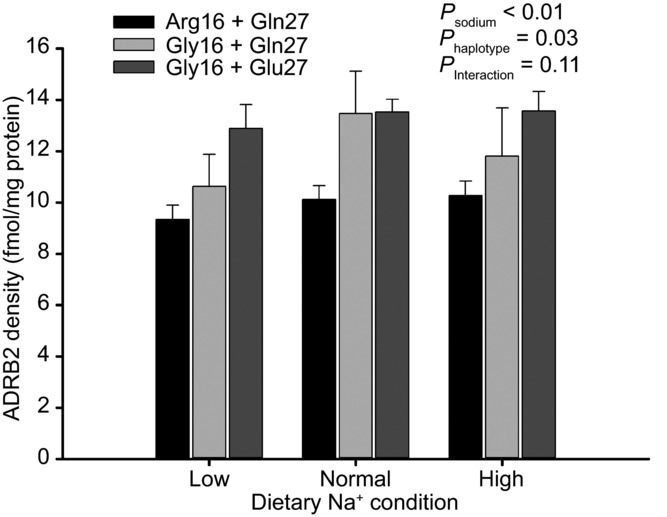
There was a main effect of sodium and haplotype on receptor density, as the Gly16+Glu27 haplotype was associated with greater density than the other groups. Error bars denote SEM.
Dietary sodium and the haemodynamic and forearm vasodilator responses to mental stress
Table2 gives the cardiovascular haemodynamics in each dietary condition, at rest (immediately before mental stress) and during mental stress, before and after propranolol. There was a main effect of dietary sodium on the change in HR (stress–baseline), such that the incremental sodium restriction (from high to low sodium) evoked a decrease in the HR response. A similar pattern was generally present for CO. For BP, there was no main effect of sodium on systolic BP (SBP), DBP or mean arterial pressure (MAP), but there was a sodium-by-propranolol interaction for MAP, suggesting that dietary sodium altered the MAP response to beta-blockade. There was also a main effect of sodium on SVR, as SVR was greatest after high sodium, followed by low sodium and least after normal sodium. Dietary sodium did not affect the change in forced vital capacity (FVC), but similar to MAP, there was a significant sodium-by-propranolol interaction, indicating that dietary sodium altered the FVC response to beta-blockade. The Reims perceived stress scores that were obtained immediately after the mental stressor were not affected by dietary sodium (between sodium visits). However, within each study visit (under similar sodium conditions), the Reims stress scores were reduced after propranolol, probably as a consequence of test familiarity on a given study day.
Table 2.
Mental stress variables before and after propranolol by genotype and dietary sodium condition
| Arg16+Gln27 |
Gly16+Gln27 |
Gly16+Glu27 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Normal | High | Low | Normal | High | Low | Normal | High | P values | ||
| HR (b.p.m.) | Psodium | <0.01 | |||||||||
| Pre-propranolol | Phaplotype | 0.15 | |||||||||
| Rest | 67 ± 2 | 64 ± 2 | 64 ± 2 | 65 ± 3 | 65 ± 4 | 61 ± 4 | 68 ± 2 | 65 ± 2 | 62 ± 3 | Pprop. | <0.001 |
| Mental stress | 81 ± 2 | 80 ± 2 | 79 ± 2 | 81 ± 6 | 87 ± 7 | 82 ± 7 | 84 ± 3 | 82 ± 3 | 82 ± 3 | Psodium*prop. | 0.12 |
| Post-propranolol | Phaplotype*prop. | 0.27 | |||||||||
| Rest | 57 ± 1 | 55 ± 1 | 55 ± 1 | 55 ± 2 | 55 ± 3 | 52 ± 3 | 58 ± 1 | 55 ± 1 | 55 ± 1 | Psodium*haplotype | 0.65 |
| Mental stress | 62 ± 1 | 61 ± 1 | 60 ± 1 | 59 ± 2 | 61 ± 3 | 60 ± 4 | 63 ± 2 | 61 ± 2 | 61 ± 2 | Psodium*haplotype*prop. | 0.59 |
| SBP (mmHg) | Psodium | 0.96 | |||||||||
| Pre-propranolol | Phaplotype | 0.34 | |||||||||
| Rest | 121 ± 2 | 120 ± 2 | 124 ± 2 | 121 ± 3 | 124 ± 5 | 126 ± 5 | 115 ± 4 | 121 ± 3 | 121 ± 3 | Pprop. | <0.001 |
| Mental stress | 128 ± 12 | 128 ± 2 | 134 ± 2 | 130 ± 4 | 135 ± 6 | 139 ± 8 | 128 ± 2 | 128 ± 2 | 134 ± 3 | Psodium*prop. | 0.07 |
| Post-propranolol | Phaplotype*prop. | 0.57 | |||||||||
| Rest | 118 ± 2 | 117 ± 2 | 123 ± 2 | 118 ± 2 | 117 ± 2 | 128 ± 5 | 116 ± 3 | 120 ± 3 | 125 ± 3 | Psodium*haplotype | 0.054 |
| Mental stress | 124 ± 2 | 124 ± 3 | 127 ± 2 | 123 ± 3 | 126 ± 3 | 134 ± 4 | 124 ± 2 | 126 ± 3 | 131 ± 3 | Psodium*haplotype*prop. | 0.89 |
| DBP (mmHg) | Psodium | 0.99 | |||||||||
| Pre-propranolol | Phaplotype | 0.39 | |||||||||
| Rest | 73 ± 1 | 72 ± 1 | 75 ± 1 | 75 ± 2 | 73 ± 3 | 76 ± 3 | 70 ± 2 | 72 ± 1 | 74 ± 2 | Pprop. | <0.01 |
| Mental stress | 79 ± 1 | 77 ± 1 | 82 ± 1 | 80 ± 2 | 80 ± 3 | 83 ± 4 | 78 ± 2 | 77 ± 2 | 82 ± 2 | Psodium*prop. | 0.14 |
| Post-propranolol | Phaplotype*prop. | 0.34 | |||||||||
| Rest | 74 ± 1 | 71 ± 2 | 76 ± 1 | 73 ± 3 | 74 ± 2 | 79 ± 2 | 71 ± 2 | 73 ± 2 | 77 ± 2 | Psodium*haplotype | 0.62 |
| Mental stress | 78 ± 1 | 75 ± 1 | 79 ± 1 | 78 ± 3 | 81 ± 2 | 84 ± 3 | 76 ± 2 | 77 ± 2 | 80 ± 2 | Psodium*haplotype*prop. | 0.91 |
| MAP (mmHg) | Psodium | 0.79 | |||||||||
| Pre-propranolol | Phaplotype | 0.39 | |||||||||
| Rest | 91 ± 1 | 90 ± 1 | 94 ± 2 | 92 ± 2 | 92 ± 3 | 96 ± 4 | 90 ± 2 | 91 ± 2 | 94 ± 2 | Pprop. | <0.001 |
| Mental stress | 98 ± 1 | 97 ± 2 | 102 ± 2 | 99 ± 2 | 102 ± 4 | 105 ± 5 | 98 ± 2 | 98 ± 2 | 102 ± 2 | Psodium*prop. | 0.050 |
| Post-propranolol | Phaplotype*prop. | 0.90 | |||||||||
| Rest | 91 ± 1 | 89 ± 2 | 94 ± 1 | 90 ± 2 | 91 ± 2 | 98 ± 3 | 89 ± 2 | 91 ± 2 | 96 ± 2 | Psodium*haplotype | 0.48 |
| Mental stress | 96 ± 1 | 94 ± 2 | 98 ± 1 | 96 ± 3 | 99 ± 2 | 104 ± 3 | 96 ± 2 | 97 ± 2 | 100 ± 3 | Psodium*haplotype*prop. | 0.95 |
| CO (l min−1) | Psodium | <0.01 | |||||||||
| Pre-propranolol | Phaplotype | 0.04 | |||||||||
| Rest | 6.4 ± 0.2 | 6.3 ± 0.2 | 6.4 ± 0.2 | 6.2 ± 0.4 | 6.8 ± 0.4 | 6.4 ± 0.4 | 6.4 ± 0.2 | 6.6 ± 0.2 | 6.2 ± 0.3 | Pprop. | <0.001 |
| Mental stress | 7.4 ± 0.3 | 7.8 ± 0.2 | 7.8 ± 0.2 | 7.9 ± 0.6 | 9.0 ± 0.8 | 8.6 ± 0.6 | 8.1 ± 0.3 | 8.3 ± 0.3 | 8.0 ± 0.3 | Psodium*prop. | 0.58 |
| Post-propranolol | Phaplotype*prop. | <0.01 | |||||||||
| Rest | 5.2 ± 0.2 | 5.4 ± 0.2 | 5.3 ± 0.2 | 5.4 ± 0.3 | 5.3 ± 0.2 | 5.4 ± 0.2 | 5.4 ± 0.2 | 5.4 ± 0.2 | 5.3 ± 0.2 | Psodium*haplotype | 0.34 |
| Mental stress | 5.5 ± 0.2 | 5.9 ± 0.1 | 5.8 ± 0.2 | 5.6 ± 0.3 | 5.7 ± 0.2 | 5.9 ± 0.3 | 5.9 ± 0.2 | 6.0 ± 0.2 | 5.8 ± 0.2 | Psodium*haplotype*prop. | 0.65 |
| SV (ml) | Psodium | 0.27 | |||||||||
| Pre-propranolol | Phaplotype | 0.11 | |||||||||
| Rest | 100 ± 4 | 104 ± 4 | 102 ± 3 | 97 ± 4 | 107 ± 4 | 107 ± 4 | 96 ± 4 | 104 ± 4 | 100 ± 3 | Pprop. | <0.01 |
| Mental stress | 98 ± 4 | 108 ± 7 | 101 ± 3 | 99 ± 4 | 107 ± 4 | 108 ± 5 | 99 ± 4 | 103 ± 4 | 100 ± 3 | Psodium*prop. | 0.16 |
| Post-propranolol | Phaplotype*prop. | 0.03 | |||||||||
| Rest | 97 ± 3 | 99 ± 2 | 99 ± 3 | 100 ± 6 | 98 ± 4 | 104 ± 2 | 96 ± 3 | 101 ± 3 | 98 ± 3 | Psodium*haplotype | 0.06 |
| Mental stress | 94 ± 3 | 99 ± 2 | 98 ± 3 | 86 ± 11 | 95 ± 3 | 100 ± 3 | 96 ± 3 | 100 ± 3 | 98 ± 3 | Psodium*haplotype*prop. | 0.13 |
| SVR (units) | Psodium | <0.01 | |||||||||
| Pre-propranolol | Phaplotype | 0.38 | |||||||||
| Rest | 1196 ± 48 | 1155 ± 32 | 1216 ± 46 | 1209 ± 73 | 1105 ± 63 | 1212 ± 32 | 1157 ± 39 | 1130 ± 39 | 1250 ± 44 | Pprop. | <0.001 |
| Mental stress | 1105 ± 47 | 1003 ± 29 | 1077 ± 36 | 1050 ± 72 | 942 ± 66 | 998 ± 48 | 1005 ± 40 | 986 ± 40 | 1061 ± 43 | Psodium*prop. | 0.62 |
| Post-propranolol | Phaplotype*prop. | 0.10 | |||||||||
| Rest | 1491 ± 84 | 1343 ± 44 | 1450 ± 41 | 1360 ± 90 | 1402 ± 70 | 1474 ± 58 | 1346 ± 47 | 1378 ± 46 | 1513 ± 92 | Psodium*haplotype | 0.59 |
| Mental stress | 1508 ± 96 | 1292 ± 35 | 1375 ± 39 | 1379 ± 79 | 1394 ± 62 | 1424 ± 69 | 1322 ± 51 | 1317 ± 44 | 1441 ± 91 | Psodium*haplotype*prop. | 0.80 |
| FVC (units) | Psodium | 0.26 | |||||||||
| Pre-propranolol | Phaplotype | 0.29 | |||||||||
| Rest | 1.9 ± 0.1 | 2.1 ± 0.1 | 2.9 ± 0.2 | 2.0 ± 0.3 | 2.7 ± 0.4 | 2.3 ± 0.3 | 2.3 ± 0.2 | 2.2 ± 0.2 | 3.1 ± 0.2 | Pprop. | <0.001 |
| Mental stress | 3.3 ± 0.2 | 3.5 ± 0.3 | 5.3 ± 0.5 | 3.5 ± 0.8 | 5.2 ± 0.9 | 4.5 ± 0.7 | 3.8 ± 0.4 | 4.4 ± 0.5 | 5.6 ± 0.4 | Psodium*prop. | 0.02 |
| Post-propranolol | Phaplotype*prop. | 0.93 | |||||||||
| Rest | 2.0 ± 0.1 | 2.1 ± 0.1 | 3.4 ± 0.8 | 1.7 ± 0.2 | 2.5 ± 0.4 | 2.4 ± 0.3 | 1.9 ± 0.1 | 2.2 ± 0.2 | 2.6 ± 0.2 | Psodium*haplotype | 0.60 |
| Mental stress | 3.0 ± 0.2 | 2.8 ± 0.2 | 3.7 ± 0.3 | 2.3 ± 0.2 | 3.3 ± 0.5 | 3.4 ± 0.4 | 3.2 ± 0.5 | 3.0 ± 0.3 | 3.6 ± 0.3 | Psodium*haplotype*prop. | 0.21 |
| Perceived stress scores | Psodium | 0.49 | |||||||||
| Pre-propranolol | 20.4 ± 0.8 | 19.8 ± 0.8 | 19.9 ± 0.8 | 18.9 ± 0.9 | 19.6 ± 1.4 | 18.9 ± 1.6 | 21.4 ± 1.1 | 21.1 ± 0.9 | 21.9 ± 0.8 | Phaplotype | 0.47 |
| Post-propranolol | 18.5 ± 0.9 | 18.3 ± 0.8 | 18.2 ± 1.0 | 17.3 ± 1.5 | 17.5 ± 1.8 | 18.7 ± 1.4 | 19.3 ± 1.1 | 19.4 ± 1.0 | 21.0 ± 0.7 | Pprop. | <0.001 |
Values are means ± SEM. Values at rest were recorded immediately prior to beginning handgrip. HR, heart rate; MAP, mean arterial pressure; CO, cardiac output; SV, stroke volume; SVR, systemic vascular resistance; prop., propranolol.
ADRB2 haplotype and mental stress responses
Also shown in Table2, there were modest effects of haplotype on the study variables. For CO, there was a main effect of haplotype and a haplotype-by-propranolol interaction, indicating that the difference between haplotypes in CO response to mental stress was attenuated with propranolol. Interestingly, SV was significant for a haplotype-by-propranolol interaction and a tendency for a sodium-by-haplotype interaction, indicating that propranolol and sodium independently affected SV based on haplotype. However, the three-way interaction was not significant. For regional vasodilator responses to mental stress, there was no evidence to suggest FVC differed based on haplotype.
Dietary sodium and the haemodynamic responses to isometric handgrip
Table3 displays the cardiovascular haemodynamics in each dietary condition, at rest (immediately before handgrip) and during handgrip, before and after propranolol. Consistent with the mental stress trial, there was a main effect of dietary sodium on HR, CO and SVR. Dietary sodium did not affect blood pressure (SBP, DBP, MAP) or SV. For ADRB2 haplotype during handgrip, there was no evidence to suggest that the haemodynamic and forearm variables were influenced by haplotype.
Table 3.
Handgrip variables before and after propranolol by haplotype and dietary sodium condition
| Arg16 ± Gln27 |
Gly16 ± Gln27 |
Gly16 ± Glu27 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Normal | High | Low | Normal | High | Low | Normal | High | P values | ||
| HR (b.p.m.) | Psodium | <0.001 | |||||||||
| Pre-propranolol | Phaplotype | 0.54 | |||||||||
| Rest | 67 ± 1 | 64 ± 1 | 64 ± 1 | 65 ± 3 | 63 ± 3 | 59 ± 4 | 67 ± 2 | 66 ± 2 | 64 ± 2 | Pprop. | <0.001 |
| Handgrip | 85 ± 2 | 87 ± 4 | 85 ± 2 | 83 ± 5 | 81 ± 5 | 82 ± 5 | 83 ± 2 | 81 ± 2 | 83 ± 2 | Psodium*prop. | 0.91 |
| Post-propranolol | Phaplotype*prop. | 0.13 | |||||||||
| Rest | 58 ± 1 | 56 ± 1 | 55 ± 1 | 55 ± 2 | 55 ± 2 | 52 ± 3 | 58 ± 1 | 55 ± 1 | 56 ± 1 | Psodium*haplotype | 0.44 |
| Handgrip | 69 ± 1 | 68 ± 1 | 70 ± 1 | 66 ± 3 | 67 ± 3 | 68 ± 4 | 69 ± 2 | 67 ± 2 | 70 ± 2 | Psodium*haplotype*prop. | 0.49 |
| SBP (mmHg) | Psodium | 0.58 | |||||||||
| Pre-propranolol | Phaplotype | 0.95 | |||||||||
| Rest | 119 ± 2 | 120 ± 2 | 123 ± 2 | 121 ± 3 | 117 ± 2 | 121 ± 7 | 119 ± 2 | 121 ± 3 | 126 ± 3 | Pprop. | 0.01 |
| Handgrip | 135 ± 4 | 138 ± 3 | 140 ± 3 | 140 ± 5 | 132 ± 2 | 137 ± 7 | 136 ± 3 | 138 ± 4 | 142 ± 3 | Psodium*prop. | 0.69 |
| Post-propranolol | Phaplotype*prop. | 0.79 | |||||||||
| Rest | 115 ± 2 | 117 ± 2 | 122 ± 2 | 115 ± 3 | 116 ± 3 | 120 ± 2 | 116 ± 3 | 119 ± 3 | 124 ± 2 | Psodium*haplotype | 0.58 |
| Handgrip | 134 ± 3 | 136 ± 2 | 142 ± 3 | 133 ± 5 | 134 ± 3 | 137 ± 4 | 136 ± 3 | 137 ± 3 | 142 ± 3 | Psodium*haplotype*prop. | 0.61 |
| DBP (mmHg) | Psodium | 0.29 | |||||||||
| Pre-propranolol | Phaplotype | 0.68 | |||||||||
| Rest | 74 ± 1 | 73 ± 1 | 75 ± 1 | 75 ± 2 | 73 ± 2 | 76 ± 3 | 73 ± 1 | 73 ± 2 | 76 ± 1 | Pprop. | <0.001 |
| Handgrip | 87 ± 2 | 88 ± 2 | 88 ± 1 | 90 ± 3 | 87 ± 2 | 88 ± 3 | 86 ± 2 | 86 ± 2 | 89 ± 2 | Psodium*prop. | 0.26 |
| Post-propranolol | Phaplotype*prop. | 0.94 | |||||||||
| Rest | 72 ± 1 | 72 ± 2 | 76 ± 1 | 70 ± 4 | 74 ± 2 | 76 ± 2 | 72 ± 2 | 73 ± 2 | 75 ± 2 | Psodium*haplotype | 0.74 |
| Handgrip | 89 ± 1 | 87 ± 2 | 92 ± 2 | 87 ± 4 | 89 ± 2 | 92 ± 2 | 87 ± 2 | 87 ± 2 | 90 ± 2 | Psodium*haplotype*prop. | 0.65 |
| MAP (mmHg) | Psodium | 0.10 | |||||||||
| Pre-propranolol | Phaplotype | 0.83 | |||||||||
| Rest | 92 ± 1 | 91 ± 2 | 94 ± 1 | 93 ± 1 | 91 ± 2 | 93 ± 4 | 91 ± 1 | 92 ± 2 | 96 ± 2 | Ppropranolol | 0.03 |
| Handgrip | 108 ± 2 | 109 ± 2 | 110 ± 2 | 111 ± 3 | 106 ± 2 | 108 ± 4 | 107 ± 2 | 108 ± 3 | 111 ± 2 | Psodium*prop. | 0.54 |
| Post-propranolol | Phaplotype*prop. | 0.99 | |||||||||
| Rest | 89 ± 1 | 89 ± 2 | 94 ± 1 | 87 ± 3 | 90 ± 2 | 94 ± 2 | 89 ± 2 | 91 ± 2 | 94 ± 2 | Psodium*haplotype | 0.54 |
| Handgrip | 107 ± 2 | 106 ± 2 | 112 ± 2 | 105 ± 4 | 107 ± 2 | 110 ± 2 | 106 ± 2 | 107 ± 2 | 111 ± 2 | Psodium*haplotype*prop. | 0.65 |
| CO (l min–1) | Psodium | <0.001 | |||||||||
| Pre-propranolol | Phaplotype | 0.99 | |||||||||
| Rest | 6.1 ± 0.2 | 6.3 ± 0.2 | 6.4 ± 0.2 | 6.4 ± 0.4 | 6.2 ± 0.3 | 5.8 ± 0.4 | 6.4 ± 0.2 | 6.3 ± 0.3 | 6.3 ± 0.2 | Pprop. | <0.001 |
| Handgrip | 7.3 ± 0.3 | 7.8 ± 0.2 | 8.1 ± 0.3 | 7.7 ± 0.5 | 7.6 ± 0.5 | 7.8 ± 0.5 | 7.7 ± 0.4 | 7.7 ± 0.3 | 7.9 ± 0.3 | Psodium*prop. | 0.65 |
| Post-propranolol | Phaplotype*prop. | 0.39 | |||||||||
| Rest | 5.3 ± 0.2 | 5.3 ± 0.2 | 5.2 ± 0.2 | 5.5 ± 0.3 | 5.1 ± 0.2 | 5.1 ± 0.2 | 5.3 ± 0.2 | 5.4 ± 0.1 | 5.4 ± 0.2 | Psodium*haplotype | 0.17 |
| Handgrip | 5.7 ± 0.2 | 6.1 ± 0.2 | 6.0 ± 0.2 | 5.8 ± 0.3 | 5.7 ± 0.3 | 5.9 ± 0.3 | 6.0 ± 0.2 | 5.9 ± 0.2 | 6.3 ± 0.3 | Psodium*haplotype*prop. | 0.54 |
| SV (ml) | Psodium | 0.67 | |||||||||
| Pre-propranolol | Phaplotype | 0.31 | |||||||||
| Rest | 98 ± 4 | 100 ± 2 | 102 ± 3 | 100 ± 6 | 102 ± 4 | 100 ± 5 | 97 ± 4 | 102 ± 4 | 101 ± 3 | Pprop. | <0.001 |
| Handgrip | 92 ± 4 | 96 ± 2 | 97 ± 3 | 95 ± 5 | 96 ± 3 | 98 ± 3 | 94 ± 3 | 98 ± 3 | 97 ± 3 | Psodium*prop. | 0.053 |
| Post-propranolol | Phaplotype*prop. | 0.19 | |||||||||
| Rest | 96 ± 3 | 98 ± 2 | 97 ± 3 | 102 ± 6 | 93 ± 4 | 100 ± 2 | 94 ± 3 | 99 ± 3 | 99 ± 3 | Psodium*haplotype | 0.71 |
| Handgrip | 87 ± 3 | 91 ± 2 | 88 ± 2 | 90 ± 6 | 87 ± 4 | 88 ± 2 | 88 ± 3 | 93 ± 3 | 92 ± 3 | Psodium*haplotype*prop. | 0.18 |
| SVR (units) | Psodium | <0.001 | |||||||||
| Pre-propranolol | Phaplotype | 0.90 | |||||||||
| Rest | 1252 ± 54 | 1181 ± 35 | 1204 ± 40 | 1202 ± 77 | 1192 ± 69 | 1316 ± 59 | 1175 ± 45 | 1257 ± 97 | 1242 ± 42 | Ppropranolol | <0.001 |
| Handgrip | 1266 ± 66 | 1135 ± 38 | 1132 ± 42 | 1191 ± 70 | 1168 ± 85 | 1144 ± 68 | 1168 ± 47 | 1174 ± 52 | 1169 ± 47 | Psodium*prop. | 0.95 |
| Post-propranolol | Phaplotype*prop. | 0.76 | |||||||||
| Rest | 1389 ± 55 | 1361 ± 41 | 1473 ± 43 | 1313 ± 114 | 1473 ± 109 | 1492 ± 55 | 1368 ± 47 | 1378 ± 44 | 1458 ± 84 | Psodium*haplotype | 0.36 |
| Handgrip | 1563 ± 60 | 1425 ± 41 | 1520 ± 45 | 1495 ± 125 | 1550 ± 110 | 1539 ± 79 | 1468 ± 54 | 1499 ± 74 | 1492 ± 94 | Psodium*haplotype*prop. | 0.47 |
Values are means ± SEM. Values at rest were recorded immediately prior to beginning handgrip. HR, heart rate; MAP, mean arterial pressure; CO, cardiac output; SV, stroke volume; SVR, systemic vascular resistance; prop., propranolol.
Forearm vasodilation in the high sodium condition
The forearm vasodilator responses in the high sodium condition are displayed in Table4. As shown in Fig.2, there was no effect of haplotype on the FBF response to isoprenaline. Moreover, there was no effect of haplotype on the FBF responses to NTP and ACh. As expected, l-NMMA evoked a reduction in baseline FBF and the FBF responses to isoprenaline and ACh.
Table 4.
Forearm blood flow following high dietary sodium
| Arg16 ± Gln27 |
Gly16 ± Gln27 |
Gly16 ± Glu27 |
P values |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-l-NMMA | Post-l-NMMA | Pre-l-NMMA | Post-l-NMMA | Pre-l-NMMA | Post-l-NMMA | Pre-l-NMMA | Post-l-NMMA | ||
| Isoprenaline | |||||||||
| Baseline | 2.3 ± 0.3 | 1.8 ± 0.1 | 2.3 ± 0.3 | 1.8 ± 0.3 | 2.4 ± 0.2 | 1.6 ± 0.1 | Pdose | <0.001 | <0.001 |
| 1.0 | 6.3 ± 0.4 | 3.2 ± 0.2 | 7.0 ± 1.1 | 3.6 ± 0.7 | 6.7 ± 0.4 | 3.1 ± 0.3 | Phaplotype | 0.88 | 0.89 |
| 3.0 | 7.5 ± 0.6 | 4.3 ± 0.3 | 7.7 ± 0.7 | 4.6 ± 1.0 | 7.5 ± 0.4 | 4.2 ± 0.4 | Pdose*haplotype | 0.98 | 0.42 |
| 6.0 | 9.7 ± 0.7 | 5.7 ± 0.4 | 10.1 ± 1.7 | 7.1 ± 1.8 | 10.3 ± 0.7 | 6.0 ± 0.7 | |||
| 12.0 | 12.3 ± 0.9 | 8.0 ± 0.7 | 13.0 ± 2.1 | 7.6 ± 1.1 | 13.1 ± 0.8 | 7.9 ± 0.7 | |||
| Nitroprusside | Pdose | <0.001 | <0.001 | ||||||
| Baseline | 2.3 ± 0.1 | 1.8 ± 0.1 | 2.3 ± 0.3 | 1.8 ± 0.3 | 2.2 ± 0.1 | 1.7 ± 0.1 | Phaplotype | 0.77 | 0.63 |
| 1.0 | 15.1 ± 0.8 | 15.8 ± 0.9 | 14.4 ± 1.5 | 14.2 ± 1.7 | 15.9 ± 1.0 | 16.4 ± 1.1 | Pdose*haplotype | 0.58 | 0.50 |
| Acetylcholine | Pdose | <0.001 | <0.001 | ||||||
| Baseline | 2.9 ± 0.3 | 1.8 ± 0.1 | 3.1 ± 0.5 | 1.8 ± 0.3 | 2.9 ± 0.2 | 1.6 ± 0.1 | Phaplotype | 0.80 | 0.40 |
| 4.0 | 19.8 ± 1.4 | 16.1 ± 1.3 | 17.2 ± 3.0 | 12.1 ± 2.2 | 19.5 ± 1.9 | 16.3 ± 1.7 | Pdose*haplotype | 0.65 | 0.31 |
Forearm blood flow is expressed as mean ± SE, in ml (100 ml limb volume)−1 min−1. Doses for isoprenaline are in ng (100 ml limb volume)−1 min−1, acetylcholine are in μg (100 ml limb volume)−1 min−1 and nitroprusside are in μg (100 ml limb volume)−1 min−1.
Figure 2. During the high sodium condition, forearm blood flow (FBF) response to administration of isoprenaline via the brachial artery in individuals homozygous for both amino acid positions 16 and 27 in the beta-2 adrenoceptor.
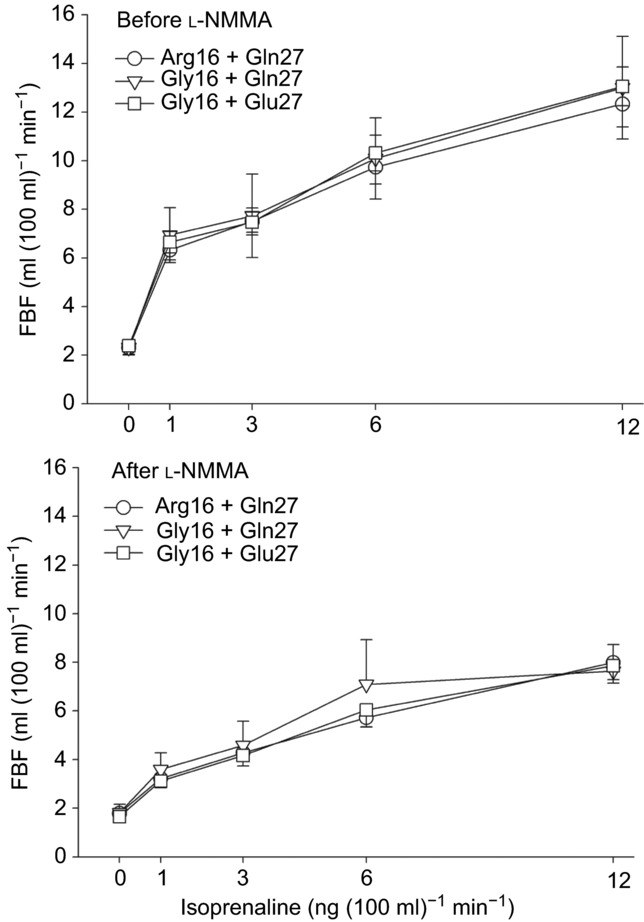
Baseline FBF did not differ between groups. Isoprenaline increased FBF and this was not different between haplotypes. In the lower panel, NO synthase inhibition with l-NMMA decreased FBF and significantly blunted the FBF response to isoprenaline, but this was not dependent on haplotype.
Polymorphic variation based on position 16
To determine the effect of SNP position 16 alone on the study variables, we repeated all analyses with individuals grouped as either Arg16 (n = 31) vs. Gly16 (n = 40). For ADRB2 density on lymphocytes, the Gly16 group demonstrated a significantly greater density across all sodium conditions. Additionally, the low sodium condition was associated with the least ADRB2 density within groups. In the mental stress trial, similar to the haplotype analysis, there was a sodium-by-propranolol interaction effect for SBP, DBP and MAP, but these were not influenced by position 16. Also for mental stress, there was a main effect of position 16 genotype on CO, and a significant genotype-by-propranolol interaction, consistent with the haplotype analysis. For handgrip, no variables were significant based on SNP position 16.
Discussion
The purpose of this investigation was to determine the interaction between three distinct levels of dietary sodium intake and functional ADRB2 gene haplotypes. Our first finding was that ADRB2 density on circulating lymphocytes was dependent on ADRB2 haplotype and SNP position 16 alone. Analysis of lymphocyte ADRB2 density and function has been correlated with receptor properties in less accessible tissues such as the heart and vasculature (Fraser et al. 1981; Aarons & Molinoff, 1982; Feldman et al. 1984). For example, the density of the ADRB2s on lymphocytes by radioligand binding has been shown to correlate with the density of ADRB2s in cardiac tissue using positron emission tomography (Qing et al. 1997). In this context, lymphocyte ADRB2 density or agonist binding affinity may predict the influence of ADRB2 haplotype variation on receptor function in vivo. Furthermore, ex vivo analysis of ADRB2 function and polymorphic variation using lymphocytes has been described but the influence of the common genetic variations in the ADRB2 gene has been inconsistent among studies (Aziz et al. 1999; Lipworth et al. 2002; Bao et al. 2005; Oostendorp et al. 2005; Snyder et al. 2006b). The present study is the third in a series of studies at our institution to demonstrate that ADRB2 density is greater in Gly16 homozygotes (Snyder et al. 2006b; Hesse et al. 2010), which appears to be the dominant effect when comparing position 16 + 27 haplotypes. Importantly, 64 of the current 71 subjects had not participated in previous trials. Our findings are consistent with formative work by Drysdale et al. (2000), who first reported the importance of ADRB2 haplotype and transfected the corresponding haplotypes into HEK293 cells, resulting in approximately 50% greater mRNA and ADRB2 density in Gly16+Glu27 than Arg16+Gln27. Taken together, these reports lend further evidence that ADRB2 gene variation affects ADRB2 cell membrane density.
Our finding that dietary sodium restriction decreased lymphocyte ADRB2 density and sodium loading increased ADRB2 density in all subjects, regardless of genotype, is consistent with a similarly designed trial in healthy young adult normotensive men over three decades ago (Fraser et al. 1981). Our rationale to determine the percentage of ADRB2 receptor in high and low affinity binding conformation was based on seminal work showing that the high affinity conformation is altered by dietary sodium restriction and loading in a small study of hypertensive and normotensive subjects (Naslund et al. 1990). It is unclear why we were unable to demonstrate either a sodium or a haplotype effect on ADRB2 binding conformation. However, this may explain the statistically non-significant interactions between sodium and haplotype on the haemodynamic results during mental stress and handgrip.
We recently reported an interim analysis of HR variability on the present cohort, showing the effects of dietary sodium on HR and cardiac autonomic modulation (Allen et al. 2014), but in the present analysis these effects were independent of haplotype (data not shown). Sodium loading increases total blood volume and reduces resting HR (McNeely et al. 2008), while sodium restriction increases sympathetic activity and circulating catecholamines (Graudal et al. 2012). In the present study, resting HR was inversely associated with sodium intake, but this was not dependent on haplotype. Dietary sodium did not change resting BP in our cohort, which is consistent with other studies of short-term dietary sodium manipulation in healthy normotensives (Wedler et al. 1992; DuPont et al. 2013). Furthermore, there was no effect of haplotype on BP in any of the sodium conditions, consistent with a recent meta-analysis that failed to associate positions 16 and 27 with essential hypertension (Lou et al. 2010). It is unclear why 24 h urine volume was dependent on haplotype, even after sex was included as a covariate due to the greater proportion of men in the Gly16+Gln27 group. The haplotype variants may influence renal sodium handling (Snyder et al. 2006c), although in the present study this is purely speculative as 24 h sodium excretion was unaffected by haplotype.
The next major finding was a main effect of dietary sodium on HR and CO for the mental stress trial, probably related to circulating blood volume and total body water, as evidenced by sodium-related weight changes. It is of note that resting SVR was greatest after high sodium, followed by low sodium and least after normal sodium, which remained in this general pattern during mental stress before and after propranolol. The mechanism for elevated SVR during sodium loading is postulated to be a result of elevated cerebral spinal fluid sodium concentration, which increases hypothalamic ouabain release and promotes a cascade of events to increase sympathetic nerve activity (Blaustein et al. 2012). In acute sodium restriction, blood volume decreases, which activates baroreceptors and sympathetic activity, concomitant with renin–angiotensin–aldosterone system activation (Graudal et al. 2012). Thus, both extremes of sodium intake evoke diverse mechanisms to increase SVR, while adaptive mechanisms during sodium restriction promote a reduction in SVR and BP over time (Makela et al. 2008). Therefore, our findings support the previous mechanistic work, in that dietary sodium extremes evoked perturbations in cardiovascular control (HR, CO and SVR) to a greater extent than in normal sodium conditions.
A major hypothesis in this study was that the sympathoexcitatory manoeuvres would reveal haplotype-dependent differences in haemodynamic responsiveness. In the mental stress trial, the main effects of haplotype were present for CO but not HR or stroke volume. The haplotype-by-propranolol interaction for CO reflects that beta blockade is altering CO in a haplotype-dependent manner. This finding was also present for position 16 alone. The interesting distinction with SV is that the haplotype effect did not reach significance (P = 0.11) but displayed interactions between haplotype and propranolol (P = 0.03), and a trend toward significance between haplotype and sodium (P = 0.06). Other investigators have shown that Gly16 homozygotes have greater fractional shortening, ejection fraction, mid-wall shortening and stress-corrected mid-wall shortening compared to both heterozygotes and Arg16 homozygotes using echocardiography (Tang et al. 2003). In a separate cohort from our institution, Gly16 homozygotes demonstrated greater CO and SV at rest compared to Arg16 homozygotes, as measured by the open-circuit acetylene wash-in method (Snyder et al. 2006b). During low and high intensity exercise, the Gly16 homozygotes also had greater CO and SV compared to Arg16 homozygotes (Snyder et al. 2006a). Together with our findings, this suggests that haplotype variation may mediate SV that is influenced by dietary sodium and is affected differentially by beta blockade.
While few haplotype effects were present in the mental stress trial, there were no variables influenced by haplotype in the handgrip trial. These statistically non-significant findings were in contrast to our hypothesis which was based on experimental evidence that the Arg16/Gly SNP influences the HR and CO response to handgrip (Eisenach et al. 2004, 2005) in separate study cohorts from nearly a decade ago. We also reasoned that forearm ADRB2-mediated vasodilation would be greater in the individuals homozygous for Gly16 and/or Glu27, as these variants have been associated with augmented regional vasodilator responsiveness (Cockcroft et al. 2000; Dishy et al. 2001; Garovic et al. 2003) and systemic vasodilator responsiveness during ganglionic blockade (Hesse et al. 2010). While it may remain that Gly16 and/or Glu27 is associated with augmented ADRB2 vasodilation during the normal sodium condition, we are confident that these effects are not apparent in the low sodium and high sodium dietary conditions. Furthermore, while ADRB2 lymphocyte density was different based on haplotype, linear regression analysis did not demonstrate correlations between density and the haemodynamic variables.
The strength of this investigation was our strategy to improve the predictive power of gene variation by studying the interaction between SNP positions 16 and 27 by recruiting only subjects who were double homozygous at both sites. This in turn served to classify our subjects according to the common haplotypes originally described by Drysdale et al. (2000). Despite our efforts to recruit these genetically specific individuals from a large pool of samples from the general population, the limitations are centred on the possibility that there are functionally relevant variants in the ADRB2 gene beyond ADRB2 positions 16 and 27. Along these lines, a more recent study constructed eight common ADRB2 haplotypes derived from 26 polymorphisms in the coding and non-coding regions of the gene, and performed whole-gene transfection in COS-7 cells to reveal haplotype differences in ADRB2 protein expression (Panebra et al. 2010). Of those eight haplotypes, only one contains Gly16+Glu27, and this was the haplotype with the greatest ADRB2 protein expression, which is in accord with the Gly16+Glu27 subjects in our study. Unfortunately, in Panebra's study the haplotype with the second-highest ADRB2 expression was one of the five haplotypes that included Arg16+Gln27, which underlines the authors’ conclusions that extended additional variations in extended haplotypes may provide greater discrimination of phenotype (Panebra et al. 2010). In this context, we acknowledge that phenotyping based on individual SNPs is probably inadequate to characterize functional gene variation, but alternative strategies must be discovered to allow for practical and comprehensive phenotyping that can be replicated in human populations. Finally, despite the large genotyping recruitment effort, we were unable to complete enrolment of the Gly16+Gln27 haplotype; therefore, we acknowledge that the lack of difference in haemodynamics between this rare haplotype group and the others could be due the low sample size (type II error). In addition, beyond the primary aims of this study, there were analyses of secondary outcomes that were performed without adjustment for multiple comparisons. These secondary analyses should be considered exploratory given the increased possibility of type I error.
In conclusion, we have explored the interactions among sympathoexcitatory manoeuvres, adrenergic agonists and antagonist infusions, and dietary sodium intake to generate a detailed picture of how ADRB2 gene variation influences physiological responses pertinent to the development of hypertension and cardiovascular disease. Most notably the Gly16+Glu27 haplotype and Gly16 alone is associated with greater ADRB2 receptor density on lymphocytes than Arg16 and Gln27. Moreover, we have shown that CO is affected by ADRB2 haplotype and position 16 alone during the mental stress trial but was no longer apparent in the handgrip trial. Finally, we found no evidence to suggest that the interaction between ADRB2 gene variation and the physiological responses to sympathoexcitatory stress are influenced by dietary sodium. In the overall context of ADRB2 gene variation, dietary sodium and cardiovascular control, this high-resolution phenotyping trial suggests that common ADRB2 polymorphisms influence ADRB2 density on circulating lymphocytes, but only modest effects on haemodynamics.
Acknowledgments
We thank Pamela A. Engrav for her efforts in volunteer recruitment and scheduling. Pamela I. Hammond, Jodie L. Van De Rostyne, Carolyn C. Allred and Dr. Adrian Vella were instrumental in our genotyping efforts over the last decade. Finally, we thank the enthusiastic participation of our volunteers, including one volunteer whose tragic death is held in our memory.
Glossary
- ACh
acetylcholine
- ADRB2
beta-2 adrenoceptor
- Arg
arginine
- BP
blood pressure
- CO
cardiac output
- DBP
diastolic blood pressure
- FBF/FVC
forearm blood flow/conductance
- Gln
glutamine
- Glu
glutamate
- Gly
glycine
- l-NMMA
NG-monomethyl-l-arginine
- MAP
mean arterial pressure
- NTP
sodium nitroprusside
- SBP
systolic blood pressure
- SNP
single nucleotide polymorphism
- SV
stroke volume
- SVR
systemic vascular resistance
Key points
Common single nucleotide polymorphisms in the beta-2 adrenoceptor (ADRB2) gene influence cardiovascular function, but they exist in combinations (haplotypes), so it is crucial to characterize the haplotypes to improve the functional predictive power of ADRB2 gene variation.
Dietary sodium affects ADRB2 function.
In a large randomized cross-over phenotyping trial that administered low, normal and high dietary sodium, we determined the interactions between ADRB2 haplotype, receptor density on lymphocytes, cardiovascular haemodynamics during stress manoeuvres, forearm blood flow vasodilator responsiveness and dietary sodium.
Healthy young adults were recruited based on the homozygous haplotypes of the ADRB2 gene: Arg16+Gln27, the rare Gly16+Gln27 and Gly16+Glu27.
Independent of dietary sodium, the Gly16+Glu27 haplotype had the greatest ADRB2 density and Arg16+Gln27 had the least, suggesting that ADRB2 haplotype influences ADRB2 protein expression, yet the haemodynamic consequences appear modest in healthy humans, necessitating larger trials that explore variation in multiple candidate genes.
Additional information
Competing interests
None declared.
Author contributions
All authors participated in the (1) conception and design, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; (3) final approval of the version to be published. The experiments were conducted at the Mayo Clinic in Rochester, Minnesota, USA.
Funding
This project was supported by NIH R-01 HL-089331, and by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- Aaron KJ. Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013;88:987–995. doi: 10.1016/j.mayocp.2013.06.005. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarons RD. Molinoff PB. Changes in the density of beta adrenergic receptors in rat lymphocytes, heart and lung after chronic treatment with propranolol. J Pharmacol Exp Ther. 1982;221:439–443. &. [PubMed] [Google Scholar]
- Allen AR, Gullixson LR, Wolhart SC, Kost SL, Schroeder DR. Eisenach JH. Dietary sodium influences the effect of mental stress on heart rate variability: a randomized trial in healthy adults. J Hypertens. 2014;32:374–382. doi: 10.1097/HJH.0000000000000045. &. [DOI] [PubMed] [Google Scholar]
- Aziz I, McFarlane LC. Lipworth BJ. Comparative trough effects of formoterol and salmeterol on lymphocyte beta2-adrenoceptor – regulation and bronchodilatation. Eur J Clin Pharmacol. 1999;55:431–436. doi: 10.1007/s002280050652. &. [DOI] [PubMed] [Google Scholar]
- Bao X, Mills PJ, Rana BK, Dimsdale JE, Schork NJ, Smith DW, Rao F, Milic M, O'Connor DT. Ziegler MG. Interactive effects of common beta2-adrenoceptor haplotypes and age on susceptibility to hypertension and receptor function. Hypertension. 2005;46:301–307. doi: 10.1161/01.HYP.0000175842.19266.95. &. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J. Wier WG. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1031–1049. doi: 10.1152/ajpheart.00899.2011. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft JR, Gazis AG, Cross DJ, Wheatley A, Dewar J, Hall IP. Noon JP. β2-Adrenoceptor polymorphism determines vascular reactivity in humans. Hypertension. 2000;36:371–375. doi: 10.1161/01.hyp.36.3.371. &. [DOI] [PubMed] [Google Scholar]
- Cook NR, Appel LJ. Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129:981–989. doi: 10.1161/CIRCULATIONAHA.113.006032. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critoph CH, Patel V, Mist B, Thomas MD. Elliott PM. Non-invasive assessment of cardiac output at rest and during exercise by finger plethysmography. Clin Physiol Funct Imaging. 2013;33:338–343. doi: 10.1111/cpf.12032. &. [DOI] [PubMed] [Google Scholar]
- de Groote P, Lamblin N, Helbecque N, Mouquet F, Mc Fadden E, Hermant X, Amouyel P, Dallongeville J. Bauters C. The impact of beta-adrenoreceptor gene polymorphisms on survival in patients with congestive heart failure. Eur J Heart Fail. 2005;7:966–973. doi: 10.1016/j.ejheart.2004.10.006. &. [DOI] [PubMed] [Google Scholar]
- Dishy V, Sofowora GG, Xie HG, Kim RB, Byrne DW, Stein CM. Wood AJ. The effect of common polymorphisms of the β2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001;345:1030–1035. doi: 10.1056/NEJMoa010819. &. [DOI] [PubMed] [Google Scholar]
- Drysdale CM, McGraw DW, Stack CB, Stephens JC, Judson RS, Nandabalan K, Arnold K, Ruano G. Liggett SB. Complex promoter and coding region β2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci U S A. 2000;97:10483–10488. doi: 10.1073/pnas.97.19.10483. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB. Edwards DG. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens. 2013;31:530–536. doi: 10.1097/HJH.0b013e32835c6ca8. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach JH, Barnes SA, Pike TL, Sokolnicki LA, Masuki S, Dietz NM, Rehfeldt KH, Turner ST. Joyner MJ. The Arg16/Gly β2-adrenergic receptor polymorphism alters the cardiac output response to isometric exercise. J Appl Physiol. 2005;99:1776–1781. doi: 10.1152/japplphysiol.00469.2005. &. [DOI] [PubMed] [Google Scholar]
- Eisenach JH, Gullixson LR, Kost SL, Joyner MJ, Turner ST. Nicholson WT. Sex differences in salt sensitivity to nitric oxide dependent vasodilation in healthy young adults. J Appl Physiol. 2012;112:1049–1053. doi: 10.1152/japplphysiol.01197.2011. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach JH, McGuire AM, Schwingler RM, Turner ST. Joyner MJ. The Arg16/Gly β2-adrenergic receptor polymorphism is associated with altered cardiovascular responses to isometric exercise. Physiol Genomics. 2004;16:323–328. doi: 10.1152/physiolgenomics.00152.2003. &. [DOI] [PubMed] [Google Scholar]
- Eisenach JH, Schroeder DR, Pike TL, Johnson CP, Schrage WG, Snyder EM, Johnson BD, Garovic VD, Turner ST. Joyner MJ. Dietary sodium restriction and β2-adrenergic receptor polymorphism modulate cardiovascular function in humans. J Physiol. 2006;574:955–965. doi: 10.1113/jphysiol.2006.112102. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach JH. Wittwer ED. β-Adrenoceptor gene variation and intermediate physiological traits: prediction of distant phenotype. Exp Physiol. 2010;95:757–764. doi: 10.1113/expphysiol.2009.048330. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RD. Defective venous β-adrenergic response in borderline hypertensive subjects is corrected by a low sodium diet. J Clin Invest. 1990;85:647–652. doi: 10.1172/JCI114487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RD, Lawton WJ. McArdle WL. Low sodium diet corrects the defect in lymphocyte β-adrenergic responsiveness in hypertensive subjects. J Clin Invest. 1987;79:290–294. doi: 10.1172/JCI112797. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RD, Limbird LE, Nadeau J, Robertson D. Wood AJ. Leukocyte β-receptor alterations in hypertensive subjects. J Clin Invest. 1984;73:648–653. doi: 10.1172/JCI111255. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J, Nadeau J, Robertson D. Wood AJ. Regulation of human leukocyte β receptors by endogenous catecholamines: relationship of leukocyte β receptor density to the cardiac sensitivity to isoproterenol. J Clin Invest. 1981;67:1777–1784. doi: 10.1172/JCI110217. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyschuss U, Hjemdahl P, Juhlin-Dannfelt A. Linde B. Cardiovascular and sympathoadrenal responses to mental stress: influence of beta-blockade. Am J Physiol. 1988;255:H1443–1451. doi: 10.1152/ajpheart.1988.255.6.H1443. &. [DOI] [PubMed] [Google Scholar]
- Garovic VD, Joyner MJ, Dietz NM, Boerwinkle E. Turner ST. β2-Adrenergic receptor polymorphism and nitric oxide-dependent forearm blood flow responses to isoproterenol in humans. J Physiol. 2003;546:583–589. doi: 10.1113/jphysiol.2002.031138. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graudal NA, Hubeck-Graudal T. Jurgens G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review) Am J Hypertens. 2012;25:1–15. doi: 10.1038/ajh.2011.210. &. [DOI] [PubMed] [Google Scholar]
- Green SA, Cole G, Jacinto M, Innis M. Liggett SB. A polymorphism of the human β2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J Biol Chem. 1993;268:23116–23121. &. [PubMed] [Google Scholar]
- Hawkins GA, Tantisira K, Meyers DA, Ampleford EJ, Moore WC, Klanderman B, Liggett SB, Peters SP, Weiss ST. Bleecker ER. Sequence, haplotype, and association analysis of ADRβ2 in a multiethnic asthma case-control study. Am J Respir Crit Care Med. 2006;174:1101–1109. doi: 10.1164/rccm.200509-1405OC. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse C, Schroeder DR, Nicholson WT, Hart EC, Curry TB, Penheiter AR, Turner ST, Joyner MJ. Eisenach JH. β2-Adrenoceptor gene variation and systemic vasodilatation during ganglionic blockade. J Physiol. 2010;588:2669–2678. doi: 10.1113/jphysiol.2010.190058. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjemdahl P, Akerstedt T, Pollare T. Gillberg M. Influence of beta-adrenoceptor blockade by metoprolol and propranolol on plasma concentrations and effects of noradrenaline and adrenaline during i.v. infusion. Acta Physiol Scand Suppl. 1983;515:45–53. &. [PubMed] [Google Scholar]
- Kaye DM, Smirk B, Williams C, Jennings G, Esler M. Holst D. Beta-adrenoceptor genotype influences the response to carvedilol in patients with congestive heart failure. Pharmacogenetics. 2003;13:379–382. doi: 10.1097/00008571-200307000-00002. &. [DOI] [PubMed] [Google Scholar]
- Lanfear DE, Jones PG, Marsh S, Cresci S, McLeod HL. Spertus JA. β2-Adrenergic receptor genotype and survival among patients receiving beta-blocker therapy after an acute coronary syndrome. JAMA. 2005;294:1526–1533. doi: 10.1001/jama.294.12.1526. &. [DOI] [PubMed] [Google Scholar]
- Lipworth B, Koppelman GH, Wheatley AP, Le Jeune I, Coutie W, Meurs H, Kauffman HF, Postma DS. Hall IP. β2 adrenoceptor promoter polymorphisms: extended haplotypes and functional effects in peripheral blood mononuclear cells. Thorax. 2002;57:61–66. doi: 10.1136/thorax.57.1.61. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Barnes SA, Sokolnicki LA, Snyder EM, Johnson BD, Turner ST, Joyner MJ. Eisenach JH. Beta-2 adrenergic receptor polymorphisms and the forearm blood flow response to mental stress. Clin Auton Res. 2006;16:105–112. doi: 10.1007/s10286-006-0329-4. &. [DOI] [PubMed] [Google Scholar]
- Lou Y, Liu J, Huang Y, Wang Z, Liu Y, Li Z, Li Y, Xie Y. Wen S. A46G and C79G polymorphisms in the β2-adrenergic receptor gene (ADRB2) and essential hypertension risk: a meta-analysis. Hypertens Res. 2010;33:1114–1123. doi: 10.1038/hr.2010.151. &. [DOI] [PubMed] [Google Scholar]
- Makela P, Vahlberg T, Kantola I, Vesalainen R. Jula A. The effects of a 6-month sodium restriction on cardiac autonomic function in patients with mild to moderate essential hypertension. Am J Hypertens. 2008;21:1183–1187. doi: 10.1038/ajh.2008.272. &. [DOI] [PubMed] [Google Scholar]
- Martina JR, Westerhof BE, van Goudoever J, de Beaumont EM, Truijen J, Kim YS, Immink RV, Jobsis DA, Hollmann MW, Lahpor JR, de Mol BA. van Lieshout JJ. Noninvasive continuous arterial blood pressure monitoring with NexfinR. Anesthesiology. 2012;116:1092–1103. doi: 10.1097/ALN.0b013e31824f94ed. &. [DOI] [PubMed] [Google Scholar]
- McNeely JD, Windham BG. Anderson DE. Dietary sodium effects on heart rate variability in salt sensitivity of blood pressure. Psychophysiology. 2008;45:405–411. doi: 10.1111/j.1469-8986.2007.00629.x. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metra M, Covolo L, Pezzali N, Zaca V, Bugatti S, Lombardi C, Bettari L, Romeo A, Gelatti U, Giubbini R, Donato F. Dei Cas L. Role of beta-adrenergic receptor gene polymorphisms in the long-term effects of beta-blockade with carvedilol in patients with chronic heart failure. Cardiovasc Drugs Ther. 2010;24:49–60. doi: 10.1007/s10557-010-6220-5. &. [DOI] [PubMed] [Google Scholar]
- Naslund T, Silberstein DJ, Merrell WJ, Nadeau JH. Wood AJ. Low sodium intake corrects abnormality in beta-receptor-mediated arterial vasodilation in patients with hypertension: correlation with beta-receptor function in vitro. Clin Pharmacol Ther. 1990;48:87–95. doi: 10.1038/clpt.1990.121. &. [DOI] [PubMed] [Google Scholar]
- Oostendorp J, Postma DS, Volders H, Jongepier H, Kauffman HF, Boezen HM, Meyers DA, Bleecker ER, Nelemans SA, Zaagsma J. Meurs H. Differential desensitization of homozygous haplotypes of the β2-adrenergic receptor in lymphocytes. Am J Respir Crit Care Med. 2005;172:322–328. doi: 10.1164/rccm.200409-1162OC. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panebra A, Wang WC, Malone MM, Pitter DR, Weiss ST, Hawkins GA. Liggett SB. Common ADRB2 haplotypes derived from 26 polymorphic sites direct β2-adrenergic receptor expression and regulation phenotypes. PLoS One. 2010;5:e11819. doi: 10.1371/journal.pone.0011819. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing F, Rahman SU, Hayes MJ, Rhodes CG, Ind PW, Jones T. Hughes JM. Effect of long-term β2-agonist dosing on human cardiac beta-adrenoceptor expression in vivo: comparison with changes in lung and mononuclear leukocyte beta-receptors. J Nucl Cardiol. 1997;4:532–538. doi: 10.1016/s1071-3581(97)90012-x. &. [DOI] [PubMed] [Google Scholar]
- Reims HM, Sevre K, Fossum E, Hoieggen A, Eide I. Kjeldsen SE. Plasma catecholamines, blood pressure responses and perceived stress during mental arithmetic stress in young men. Blood Press. 2004;13:287–294. doi: 10.1080/08037050410016474. &. [DOI] [PubMed] [Google Scholar]
- Sliwa K, Stewart S. Gersh BJ. Hypertension: a global perspective. Circulation. 2011;123:2892–2896. doi: 10.1161/CIRCULATIONAHA.110.992362. &. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Beck KC, Dietz NM, Eisenach JH, Joyner MJ, Turner ST. Johnson BD. Arg16Gly polymorphism of the β2-adrenergic receptor is associated with differences in cardiovascular function at rest and during exercise in humans. J Physiol. 2006a;571:121–130. doi: 10.1113/jphysiol.2005.098558. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Hulsebus ML, Turner ST, Joyner MJ. Johnson BD. Genotype related differences in beta2 adrenergic receptor density and cardiac function. Med Sci Sports Exerc. 2006b;38:882–886. doi: 10.1249/01.mss.0000218144.02831.f6. &. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Turner ST, Joyner MJ, Eisenach JH. Johnson BD. The Arg16Gly polymorphism of the β2-adrenergic receptor and the natriuretic response to rapid saline infusion in humans. J Physiol. 2006c;574:947–954. doi: 10.1113/jphysiol.2006.107672. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Devereux RB, Kitzman DW, Province MA, Leppert M, Oberman A, Hopkins PN. Arnett DK. The Arg16Gly polymorphism of the β2-adrenergic receptor and left ventricular systolic function. Am J Hypertens. 2003;16:945–951. doi: 10.1016/s0895-7061(03)01001-x. &. [DOI] [PubMed] [Google Scholar]
- Truijen J, van Lieshout JJ, Wesselink WA. Westerhof BE. Noninvasive continuous hemodynamic monitoring. J Clin Monit Comput. 2012;26:267–278. doi: 10.1007/s10877-012-9375-8. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedler B, Brier ME, Wiersbitzky M, Gruska S, Wolf E, Kallwellis R, Aronoff GR. Luft FC. Sodium kinetics in salt-sensitive and salt-resistant normotensive and hypertensive subjects. J Hypertens. 1992;10:663–669. &. [PubMed] [Google Scholar]
- Wittwer ED, Liu Z, Warner ND, Schroeder DR, Nadeau AM, Allen AR, Murillo CJ, Elvebak RL, Aakre BM. Eisenach JH. β-1 and β-2 adrenergic receptor polymorphism and association with cardiovascular response to orthostatic screening. Auton Neurosci. 2011;164:89–95. doi: 10.1016/j.autneu.2011.07.004. &. [DOI] [PMC free article] [PubMed] [Google Scholar]


