Abstract
Abstract
The search for effective therapeutic strategies for irritable bowel syndrome (IBS) is hampered by an incomplete understanding of its underlying pathophysiology. Stress and altered plasma cytokine profiles indicative of immune activation are characteristic of the disorder. The neuromodulatory effects of interleukin-6 (IL-6) and corticotropin-releasing factor receptor (CRFR) 1 in visceral pain and stress-induced defecation in the Wistar Kyoto (WKY) rat model of IBS were investigated. Sprague Dawley and WKY rats were administered anti-IL-6 receptor antibodies (xIL-6R, 0.5 mg kg−1 i.p) with or without the CRFR1 antagonist antalarmin (10 mg kg−1 i.p). Post-intervention, the pain threshold to colorectal distension and stress-induced faecal output were compared and changes in colonic mucosal protein expression were investigated. The neuro-stimulatory effects of IBS plasma on the myenteric plexus is mediated by IL-6, IL-8 and CRF. The stimulatory effects of these soluble factors on myenteric neuron excitability and colonic contractility were additive. Moreover, inhibition of IL-6 and CRF1 receptors in vivo in the WKY IBS rat model normalized stress-induced defecation (P < 0.01) and visceral pain sensitivity (P < 0.001) with associated changes in protein expression of the tight junction proteins occludin and claudin 2, the visceral pain-associated T-type calcium channel CaV3.2 and intracellular signalling molecules STAT3, SOCS3 and ERK1/2. These studies demonstrate the additive effects of immune and stress factors on myenteric neuronal excitability. Moreover, combined targeting of peripheral IL-6 and CRF1 receptors is effective in alleviating IBS-like symptoms in the WKY rat. Thus, crosstalk between stress and immune factors during IBS flares may underlie symptom exacerbation.
Introduction
Irritable bowel syndrome (IBS) is a highly prevalent functional gastrointestinal (GI) disorder affecting up to 15–20% of the population (Lovell & Ford, 2012). Characterized by chronic episodic bouts of abdominal pain, bloating and altered bowel habit (Longstreth et al. 2006), IBS substantially impairs sufferers’ quality of life and confers a heavy economic and social burden on society. The underlying pathophysiology of this functional bowel disorder is poorly understood and a lack of reproducible biomarkers hampers diagnosis and therapeutic target identification. Nonetheless it is generally accepted that dysfunction of the bidirectional communication system between the brain and the gut contributes to symptom manifestation (Shanahan & Anton, 1988; Cryan & O'Mahony, 2011). Integral to the initiation, prolongation and persistence of IBS symptom flares is activation of the hypothalamic–pituitary–adrenal (HPA) stress axis (Bohmelt et al. 2005; Dinan et al. 2006; Chang et al. 2009). Indeed, mimicking the effects of stress, the hypothalamic hormone corticotropin-releasing factor (CRF) induces pathophysiological changes in GI function (Mawdsley & Rampton, 2005). CRF exerts its biological effects through activation of the CRF1 and CRF2 receptors (CRFR1 and CRFR2), which are expressed in both the CNS and the enteric nervous system of the GI tract (Tache & Bonaz, 2007; O'Malley et al. 2010b, 2011b).
The importance of immune system activation in the development of IBS is well recognized with a bout of inflammatory gastroenteritis conferring increased susceptibility to develop IBS (Spiller & Garsed, 2009). IBS mucosal biopsies display more immune infiltrates such as T-cells, intra-epithelial lymphocytes and mast cells (Chadwick et al. 2002) and soluble mediators secreted from IBS biopsies have excitatory actions on human enteric neurons (Buhner et al. 2009). Moreover, we previously demonstrated that colonic secretions from the Wistar Kyoto (WKY) rat model of IBS stimulated naïve submucosal neurons to a greater extent than secretions from low anxiety Sprague Dawley (SD) controls, an effect mediated in part by the pro-inflammatory cytokine interleukin-6 (IL-6) (O'Malley et al. 2011a). IL-6, along with other cytokines such as IL-1β and tumour necrosis factor α, are elevated in IBS peripheral mononuclear cells (Liebregts et al. 2007) and circulating levels of IL-6 and IL-8 have reproducibly been found to be raised in IBS patients (Dinan et al. 2006; Liebregts et al. 2007; Dinan et al. 2008). However, more than simply being a biomarker for IBS (Clarke et al. 2009), IL-6 activates submucosal secretomotor neurons (Xia et al. 1999; O'Malley et al. 2011c) and modulates mucosal ion transport and epithelial permeability (Natale et al. 2003; Kindt et al. 2010) suggesting a causative role for IL-6 in this disorder.
Both psychological stress and immune activation have been proposed as contributory factors in symptom flares, and therefore crosstalk between these systems may exacerbate symptoms (O'Malley et al. 2011d, 2013). In support of this, low-grade inflammatory changes correlate with alterations in the stress axis in IBS (Heitkemper et al. 1996; Bohmelt et al. 2005; Dinan et al. 2006) and IBS patients often exhibit concurrent increases in markers of a hyperactive stress response and immune upregulation (Dinan et al. 2006). To test the hypothesis that crosstalk between stress and immune factors results in exacerbation of IBS-like symptoms, such as visceral pain and altered bowel habit, the WKY rat model of IBS was treated with monoclonal anti-IL-6 receptor (xIL-6R) antibodies with and without the CRFR1 antagonist antalarmin.
Methods
Ethical approval
All experiments involving animals were conducted in full accordance with the European Community Council Directive (86/609/EEC) following University College Cork animal ethics committee approval.
The study protocol (APC020, 2009) for collecting blood samples from IBS patients and healthy volunteers was approved by the University College Cork Clinical Research Ethics committee of the Cork University Hospital. Informed consent was obtained from all participants.
Animals and chronic treatments
SD and WKY rats (200–250 g), purchased from Harlan, Derbyshire, UK, were group-housed six per cage and maintained on a 12/12 h light–dark cycle (08:00–20:00 h) with a room temperature of 22 ± 1°C. Rats were handled for at least 5 days by the same researcher prior to behavioural assessment. Food and water were available ad libitum. For the in vivo open field and colorectal distension (CRD) studies, SD and WKY rats were allocated to one of three groups: control, xIL-6R or xIL-6R plus antalarmin (n = 12 per group). All animal groups received an i.p. injection of anti-IgG (Jackson Immunoresearch, Westgrove, PA, USA, 1 mg kg−1) on day 0 to induce antibody tolerance. Group 1 were administered saline and acted as the control group. Group 2 received an i.p. injection of monoclonal xIL-6R antibodies (1 mg kg−1 on day 0 and 0.5 mg kg−1 on days 3 and 10). Group 3 also received xIL-6R on days 0, 3 and 10 but additionally received the CRFR1 antagonist antalarmin (10 mg kg−1) 1 h prior to the open field trial on day 10 and CRD on day 14. Following CRD, all animals were killed by decapitation. Trunk blood samples were centrifuged at 1600 g. and plasma samples were stored at −80 °C for later analysis. Sections of colon (2 cm long) were excised and snap frozen in liquid nitrogen for Western blotting analysis. Tissue was stored at −80 °C for later processing.
Study participants and plasma samples
IBS patients aged 18–65 years who satisfied Rome II criteria for the diagnosis of IBS (Thompson et al. 1999) were recruited from gastroenterology clinics at Cork University Hospital, Cork, Ireland. Healthy controls were recruited from the research institute (Alimentary Pharmabiotic Centre) or hospital staff. Bowel habit for IBS patients was defined as constipation-predominant (IBS-C), diarrhoea-predominant (IBS-D) or alternating (IBS-A). Individuals with a history of psychiatric illness, inflammatory bowel disease, celiac disease, lactose intolerance, immunodeficiency or abdominal surgery were excluded. No patient was categorized as having post-infectious IBS. Each individual was evaluated with a full review of their family history, details of current and recent medications, a physical examination and documentation of body mass index. Plasma samples were selected from a larger study previously published (McKernan et al. 2011b).
Twenty millilitres of venous blood was donated between 11:00 and 13:00 h to avoid diurnal variations. Whole blood (15 ml) was added to an equal volume of Histopaque 1077 (Sigma, St Louis, MO, USA) and centrifuged at 400 g (30 min, room temperature). Consistent with previous studies in the laboratory (Dinan et al. 2006,2008), plasma was collected and stored at −80 °C. To maintain a consistency of response, pooled plasma samples (n = 6 samples in each group) from both healthy volunteers and IBS patients (IBS-D, IBS-C and IBS-A) were applied to the neurons and the responses collated.
Behavioural measurements
Open field trial
As previously described (O'Mahony et al. 2009, 2010a), animals were exposed to the psychological stress of being placed in the centre of a brightly illuminated (∼800 lux), white open field (OF) arena (0.9 m in diameter, 38 cm in height) for a 10 min trial. Trials were conducted daily between 09:00 and 13:00 h and the arena was cleaned with 70% ethanol between trials. The number of faecal pellets excreted during the 10 min exposure was recorded as an indication of stress-induced defecation. Trials were recorded and the distance and velocity of movement was analysed offline.
Colorectal distension
Rats were fasted for 24 h before CRD and acclimatized to the testing room for 30 min prior to being lightly anaesthetized (Isoflurane, Abbott Animal Research, Ireland). As previously described (O'Mahony et al. 2009), a latex balloon (6 cm in length, Durex, Union, NJ, USA) was inserted into the colon, 1 cm from the anus. The animals were permitted a recovery time of 10 min before the CRD procedure was initiated. A ramp distension protocol was used where the balloon was distended from 0 to 80 mmHg over an 8 min period (increasing by 10 mmHg each minute). During this period two parameters were measured. The first was the threshold pressure that evoked visibly identifiable pain behaviours. Behaviours included were stretching, abdominal retractions and/or abdominal withdrawal reflex as previously described (O'Mahony et al. 2010). Secondly, the cumulative number of visceral pain behaviours over the course of the trial was counted. The animals were tested in a random fashion by a single blinded investigator.
Immunoassays
Western blotting
As previously described (O'Malley et al. 2011b), the supernatants from homogenized colonic mucosal samples taken from rats 1 h post-CRD were separated on 12% SDS polyacrylamide gels. Proteins were electro-transferred to polyvinylidene fluoride (PVDF) membranes, which were blocked and incubated with primary antibodies against phosphoERK1/2, total ERK1/2, phosphoSTAT3, total STAT3 (all Cell Signaling Technology, Danvers, MA, USA), occludin (Abcam, Cambridge, UK), claudin 2 (Santa Cruz Biotechnology, Dallas, TX, USA) (all at 1:1000), suppressor of cytokine signalling 3 (SOCS3; Everest Biotech, Upper Heyford, UK) (1:500) and CaV3.2 (Merck Millipore, Temecula, CA, USA) (1:200, overnight at 4 °C). Complimentary horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch) (1:5000) and an ECL detection system (Pierce ECL Western Blotting Substrate, Rockford, IL, USA) were used to visualize the protein bands. β-Actin (Cell Signaling Technology) (1:1000) was used as a reference protein for occludin, claudin 2, SOCS3 and CaV3.2. Images were captured using a GelDoc Image Reader (Las3000; Fujifilm, Tokyo, Japan) and analysed using Multigauge v2.2 (Fujifilm) software. Protein densitometry was used to measure expression levels in arbitrary units as a ratio of the loading controls. Protein bands are comparable only between each sub-group which was analysed at the same time, under identical conditions. Protein samples for each experimental group (SD saline, WKY saline, WKY xIL-6R, WKY xIL-6R & antalarmin) were run in a single tank and probed under identical conditions.
ELISA
A sandwich ELISA (Legend Max™ Rat IL-6 ELISA kit; BioLegend, San Diego, CA, USA) was carried out to determine IL-6 levels in plasma samples from control WKY (n = 7) and SD (n = 7) animals, and from WKY rats treated with either anti-IL-6R (n = 8) or anti-IL-6R and antalarmin (n = 12), according to the manufacturer's guidelines. The test was carried out in duplicate and the plate read on a fluorescent plate reader (Biotek Synergy HT plate reader, Gen5 data analysis software). A standard curve was generated from known IL-6 concentrations and sample IL-6 concentrations were extrapolated from the curve.
Calcium imaging in myenteric neurons
During an action potential, Ca2+ enters the neuron via voltage-gated channels and at the post-synaptic membrane neurotransmitters induce an influx of calcium via receptor-mediated receptors such as N-methyl-d-aspartate (NMDA) receptors, thereby contributing to dendritic action potentials. Thus, neuronal changes in calcium levels as imaged using a calcium-sensitive dye (Fura-2AM) are indicative of changes in neuronal excitability. Changes in intracellular calcium were recorded from myenteric neuronal cell bodies as reported (O'Malley et al. 2011c), SD rats were killed via an overdose of CO2 and the distal colon (<4 cm from the anus) was excised and placed in ice-cold, 95% O2/5% CO2 bubbled Krebs saline solution consisting of (mmol l−1): NaCl, 117; KCl, 4.8; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; and d-glucose (11 with 1 μm nifedipine to inhibit smooth muscle contractions). Whole mount preparations of longitudinal muscle myenteric plexus (LMMP) were prepared by removing the mucosa and circular muscle, pinning the tissue out in Sylgard-lined petri dishes and loading with Fura-2AM (7 μm, 1 h). Images were acquired at 3 Hz using a xenon/mercury arc burner (Olympus, Melville, NY, USA), a charge-coupled device digital camera (F-view II; Olympus Soft Imaging Solutions, Munster, Germany) and a 40× water-immersion objective on a fixed stage upright microscope (Olympus BX51WI). Ganglionic neurons were identified based on morphology and responsiveness to 75 mm KCl. Cell R software (Olympus Soft Imaging Solutions, 1986–2009) was used to record excitation and emission wavelengths of 340/380 and 510 nm, respectively, permitting ratiometric imaging. Responses are reported as a change in ratio. Responding neurons were defined as those with increases in intracellular calcium [Ca2+]i greater than two standard deviations from baseline (calculated as the average ratio during the 150 s preceding stimulus application). A perfusion system continuously superfused the colonic tissue with carbogen-bubbled Krebs-buffered saline.
Antibody neutralization of IBS plasma (pooled from all IBS subtypes) with human neutralizing anti-IL-6 (1:100), anti-IL-8 (1:250), anti-C-reactive protein (anti-CRP, 1:200) and anti-IgG (1:200, all from R&D systems, Minneapolis, MN, USA) antibodies (1 h at 37 °C) was carried out prior to neuronal application.
Colonic contractile function
To measure circular muscle contractile activity from SD distal colons, mucosa was removed and, with the circular muscle orientated lengthwise, the tissue was suspended from a tension transducer under 1 g of tension in a bath of carbogen-bubbled Krebs saline at 37°C and allowed to equilibrate (20–30 min). Changes in tension were amplified, recorded and analysed using Powerlab and LabChart7 (both AD Instruments, Colorado Springs, CO, USA). Responses are reported as a percentage of the maximal response evoked by the muscarinic agonist carbachol (100 μm) in each experiment.
Statistics
Data are represented as mean ± SEM. Student's t-tests and repeated measures one-way or two-way ANOVAs with Neumann Keuls post-hoc tests and Chi squared tests were used where appropriate. P ≤ 0.05 was considered significant.
Results
IL-6 and CRF activate myenteric neurons and stimulate colonic contractility
We have previously demonstrated that the pro-inflammatory cytokine IL-6 stimulates increases in [Ca2+]i in the submucosal plexus (O'Malley et al. 2011c) and that this is further modulated by the presence of CRF (O'Malley et al. 2013). We have interpreted this rise in Ca2+, which regulates the release of synaptic neurotransmitters and post-synaptic excitability, as an indicator of neuronal excitability. In total, 46% of LMMP neurons (n = 35 neurons from three rats) prepared from naïve SD rats were activated by IL-6 (1 nm, 3 min). Moreover, CRF (100 nm, 3 min), which has a higher affinity for CRFR1, activated 34% of the same neurons and co-application of IL-6 plus CRF activated 54% of these neurons and evoked a larger calcium response on average than either reagent alone (Fig.1A).
Figure 1. IL-6 and CRF activate myenteric neurons and stimulate colonic contractility.
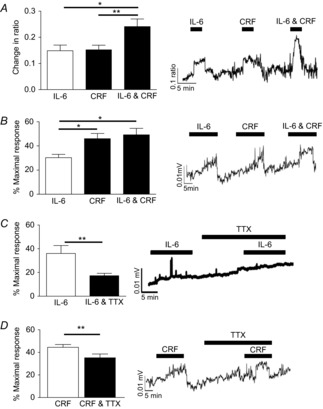
A, bar chart and representative trace illustrating calcium responses in myenteric neurons from SD distal colon in response to IL-6, CRF and IL-6 & CRF (n = 35). B–D, bar charts and representative traces illustrating changes in circular muscle contractile activity evoked by IL-6, CRF and IL-6 & CRF (B, n = 4), IL-6 (C, n = 4) CRF (D, n = 4) in the absence or presence of TTX, as a percentage of a maximal contraction evoked by carbachol (100 μm). Asterisks indicate *P < 0.05 and **P < 0.01.
The functional consequences of activating myenteric neurons on colonic contractility were investigated. Control experiments using the cholinergic agonist carbachol (100 μm) at the beginning and the end of the experiment demonstrated that the evoked contractions were not diminished following multiple stimulations (0.074 ± 0.004 vs. 0.076 ± 0.008, P > 0.05). IL-6- and CRF-evoked contractions were expressed as a percentage of this maximal carbachol response. IL-6 (10 nm, 20 min) induced a small but robust increase in circular muscle contractile activity (n = 5), whereas CRF evoked a larger contraction (n = 4). However, unlike the results in the LMMP, addition of both agonists did not have an additive effect on muscle contractility (n = 4, P > 0.05, Fig.1B). Consistent with a neuronal contribution to contractile activity, the Na+ channel blocker TTX (100 nm, 20 min) diminished the IL-6 (n = 5, P < 0.01, Fig.1C) and CRF (n = 4, P < 0.01, Fig.1D) effects, although incomplete abolition of the responses suggests additional direct actions of IL-6 and CRF on the musculature.
IL-8 activates myenteric neurons and stimulates colonic contractility
The chemokine IL-8 is also elevated in IBS plasma (Dinan et al. 2006; McKernan et al. 2011b) and like IL-6 it also has functional effects on colonic neurons. IL-8 (1 nm, 3 min) increased [Ca2+]i in 58% of myenteric neurons (n = 33) tested. The amplitude of the response was similar to CRF-mediated effects (P > 0.05). In total, 34% of neurons responded to CRF alone, whereas 71% of neurons were activated by the co-application of IL-8 and CRF, resulting in a significantly enhanced response (P < 0.01, Fig.2A). When compared with recombinant IL-6, IL-8 evoked a calcium response of similar amplitude but the number of neurons sensitive to IL-8 (58%, n = 33) was greater than the percentage sensitive to IL-6 (46%; χ2 = 4.8, d.f. = 2, P < 0.05). However, the combination of IL-8 and IL-6 activated an even greater percentage of neurons (65%; χ2 = 6.2, d.f. = 2, P < 0.05) and increased the amplitude of the calcium response compared with either IL-6 (P < 0.001) or IL-8 (P < 0.01, Fig.2B) alone. Application of IL-6, IL-8 and CRF stimulated 55% of the neurons (n = 33) and, although the amplitude of the response was greater than IL-6 plus IL-8 (P < 0.05), it was similar to the response evoked by IL-8 plus CRF (P > 0.05).
Figure 2. IL-8 activates myenteric neurons and stimulates colonic contractility.
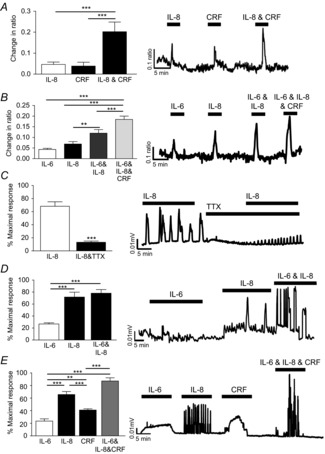
A and B, bar charts and traces illustrating calcium responses in SD myenteric neurons following exposure to IL-8 and/or CRF (A, n = 24) and IL-6 and/or IL-8 and/or CRF (B, n = 33). C and D, bar charts and original traces illustrating circular muscle contractile responses to IL-8 in the absence or presence of TTX (C, n = 5) and IL-6 ± IL-8 (D, n = 5) as a percentage of a maximal contraction evoked by carbachol (100 μm). E, bar chart and original trace illustrating circular muscle contractile responses to IL-6, IL-8, CRF or all three (n = 4). Asterisks indicate **P < 0.01 and ***P < 0.001.
IL-8 evoked large, repetitive colonic contractions (n = 5, Fig.2C). While the amplitude of contractions was attenuated in the presence of TTX, the frequency of contractions increased from 0.48 ± 0.1 to 0.84 ± 0.1 Hz (n = 5, P < 0.01). The amplitude of IL-8-evoked contractions (n = 5) was greater than IL-6-evoked contractions (n = 5, P < 0.001) but no further change was noted when IL-8 and IL-6 were co-applied (n = 5, P > 0.05, Fig.2D). However, the amplitude of contractions evoked by co-application of IL-6, IL-8 and CRF together (n = 4) was larger than IL-6 (P < 0.001), IL-8 (P < 0.001) or CRF (P < 0.001) responses alone (Fig.2E).
Soluble factors in IBS plasma activate myenteric neurons
Thus far, we have demonstrated neuro-immune and neuro-endocrine interactions between IL-6, IL-8 and CRF in myenteric neurons and on colonic contractile activity. To relate these findings to human IBS, SD rat myenteric neurons were exposed to patient and healthy control plasma samples. Soluble factors in plasma samples from healthy controls (n = 6 pooled samples) induced a small increase in [Ca2+]i in naïve rat myenteric neurons (n = 43, Fig.3A). However, IBS plasma (n = 6 pooled samples from all subtypes) evoked a larger response (n = 43, P < 0.001, Fig.3A).
Figure 3. Immune factors in human IBS plasma activates myenteric neuron.
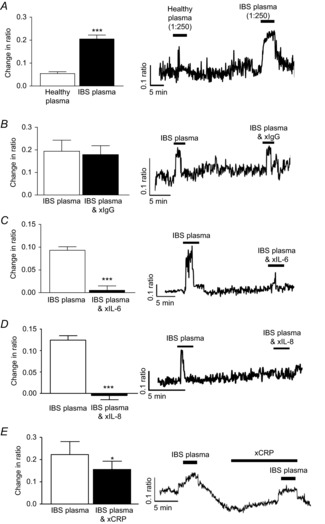
A, bar chart and original trace showing calcium responses in SD myenteric neurons to pooled plasma samples from healthy controls and IBS patients (n = 43). B–E, bar charts and original traces showing the effects of antibody neutralization of IgG (B, n = 45), IL-6 (C, n = 71), IL-8 (D, n = 70) and CRP (E, n = 38) on IBS plasma-evoked calcium responses in SD myenteric neurons. Asterisks indicate *P < 0.05 and ***P < 0.001.
As cytokine profiles in plasma from IBS patients are altered (McKernan et al. 2011b), we investigated whether the aforementioned immune molecules contributed to the neuroexcitatory effect of IBS plasma. Control experiments, where IgG was neutralized, had no effect on the calcium responses evoked by IBS plasma (n = 45, P > 0.05, Fig.3B). However, neutralization of IL-6 (n = 71, P < 0.001, Fig.3C) and IL-8 (n = 70, P < 0.001, Fig.3D) attenuated the initial IBS plasma-evoked response. The inflammatory marker CRP is also elevated in these plasma samples (McKernan et al. 2011a) and neutralizing antibodies directed against this protein also reduced the IBS-evoked response (n = 38, P < 0.05, Fig.3E) although to a lesser extent than the cytokines. Antalarmin (1 μm, 10 min), the CRFR1 antagonist, blocked IBS plasma-evoked neuronal activation (n = 44, P < 0.001, Fig.4A), whereas astressin 2B, a CRFR2 inhibitor, had no effect (n = 53, P > 0.05, Fig.4B).
Figure 4. Stress factors in human IBS plasma activate myenteric neurons.
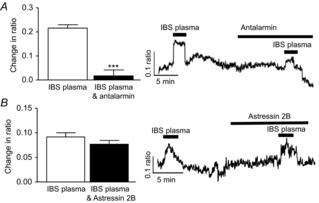
Bar charts and original traces illustrating the effects of the CRFR1 antagonist antalarmin (A, n = 44) and the CRFR2 antagonist astressin 2B (B, n = 53) on IBS plasma-evoked calcium responses in SD myenteric neurons. ***P < 0.001.
Incubation of myenteric neurons with the specific STAT3 inhibitor WP1006 (10 μm, 10 min) attenuated the IBS plasma-evoked response (n = 48, P < 0.01, Fig.5A) as did the extracellular signal-regulated kinase-mitogen activated protein kinase (ERK-MAPK) inhibitor PD98059 (10 μm, 10 min, n = 40, P < 0.001, Fig.5B). In contrast, the phosphatidyl 3-kinase inhibitor wortmannin (10 μm, 10 min) had no effect on the IBS plasma responses (n = 48, P > 0.05, Fig.5C). Finally the role of nuclear factor κ-light-chain-enhancer of activated B cells (NFκB) was tested using the NFκB antagonist IκB Kinase2 (IKK2) (5 μm, 10 min). Interestingly, IKK2 stimulated an increase in the amplitude of the IBS plasma-evoked calcium response (n = 37, P < 0.05, Fig.5D).
Figure 5. Intracellular signalling molecules activated by IBS plasma.
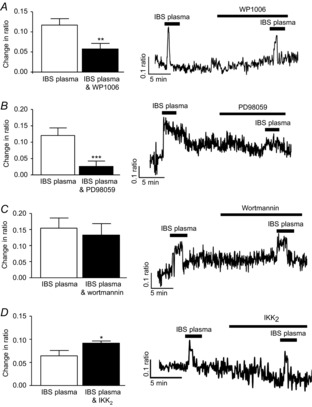
Bar charts and original traces illustrating the effects of the STAT3 inhibitor WP1006 (A, 10 μm, n = 48), the ERK-MAPK inhibitor PD98059 (B, 10 μm, n = 40), the PI 3-kinase inhibitor wortmannin (C, 10 μm, n = 48) and the NFκB antagonist IKK2 (D, 5 μm, n = 37) on calcium signals evoked by IBS plasma in SD myenteric neurons. Asterisks indicate *P < 0.05, **P < 0.01 and ***P < 0.001.
Monoclonal xIL-6R antibodies and antalarmin inhibit defecation in WKY rats
The above studies demonstrate a functional role for IL-6 and CRF at a cellular level, but in vivo studies were necessary to determine if these observations had translational potential. Thus, stress-sensitive WKY rats, which defecate more in the anxiogenic OF arena compared with SD controls (O'Malley et al. 2010a) and display visceral hypersensitivity to CRD (Gunter et al. 2000; O'Mahony et al. 2010) were treated with neutralizing xIL-6R antibodies and antalarmin. Control IgG- and saline-treated WKY rats excreted more boli in the OF than SD rats (n = 12, P < 0.001, Fig.6A). The previously described anxious phenotype of WKY rats (O'Malley et al. 2010a) was further evidenced by decreased exploration of the exposed inner zone of the OF arena compared with SD (P < 0.001). Targeting IL-6 signalling with xIL-6R monoclonal antibodies did not affect stress-induced defecation in SD rats in the OF, but the number of faecal pellets excreted by WKY rats was reduced (n = 12, P < 0.05). However, this remained higher than the low-anxiety SD comparator (n = 12, P < 0.01, Fig.6A). Co-application of xIL-6R with the CRFR1 antagonist antalarmin, which can modulate GI function (O'Malley et al. 2013), decreased faecal output further (n = 12, P < 0.001) to levels equivalent to SD controls (P > 0.05, Fig.6A).
Figure 6. IL-6R neutralization and antalarmin inhibit defecation in WKY rats.
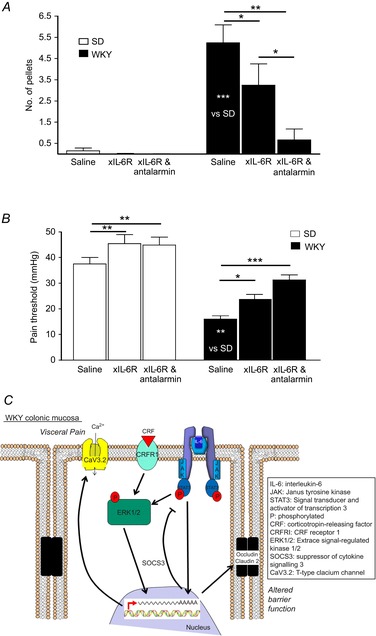
A, bar chart illustrating the number of faecal pellets excreted by SD (n = 7, 7 and 9) and WKY (n = 10, 9 and 11) rats in the OF when administered saline, anti-IL-6 receptor antibodies (xIL-6R) or xIL-6R and antalarmin. B, bar chart showing the pressure (mmHg) at which SD (n = 7, 7 and 9) and WKY (n = 10, 9 and 11) rats display pain behaviours in response to colorectal distension when treated with saline, xIL-6R or xIL-6R and antalarmin. C, schematic illustration of potential intracellular signalling mechanisms activated by CRF and IL-6 binding to their receptors. Asterisks indicate *P < 0.05, **P < 0.01 and ***P < 0.001.
xIL-6R and antalarmin improve visceral pain sensitivity
CRD (0–80 mmHg over 8 min) evoked pain behaviours at lower pressures in control WKY as compared with SD rats (n = 10, P < 0.001, Fig.6B). However, neutralizing IL-6Rs increased the pain threshold to CRD in WKY rats (n = 9, P < 0.05, Fig.6B) and this was further improved by co-application of antalarmin with xIL-6R (n = 12, P < 0.01), such that visceral sensitivity in WKY rats was equivalent to SD controls (n = 10, P > 0.05). Interestingly, pain thresholds in SD rats following treatment with xIL-6R (n = 8, P < 0.01) and xIL-6R plus antalarmin (n = 8, P < 0.01) were also increased (Fig.6B).
ELISA analysis of plasma IL-6 concentrations revealed no differences between saline-treated control WKY and SD rats (P > 0.05) and no changes following treatment with xIL-6R (P > 0.05) or xIL-6R plus antalarmin (P > 0.05, data not shown).
Colonic mucosal protein expression is altered by xIL-6R and antalarmin treatment
Underlying the beneficial effects of treatment of GI function were changes in colonic protein expression. Depletion of the tight junction protein occludin is linked with increased intestinal barrier permeability (Al-Sadi et al. 2011), and thus the observed increase in occludin expression in WKY control tissues (P < 0.05) suggests tighter barrier control. This was unchanged by combined treatment with xIL-6R plus antalarmin, although xIL-6R treatment alone reduced occludin expression levels (P < 0.05, n = 5 rats, Fig.7A). In contrast, increased expression of another tight junction protein, claudin 2, is thought to reduce barrier tightness (Amasheh et al. 2002) and expression of this protein was also elevated in WKY colons (P < 0.05) but reduced following treatment with xIL-6R alone or xIL-6R and antalarmin (P < 0.05, n = 5 rats, Fig.7B). Given that we have previously found that transepithelial resistance, as an indicator of colonic permeability, is comparable between SD and WKY rats (O'Malley et al. 2012) it may be that the sum effect of increased expression of claudin 2 and occludin is no net change in permeability. Consistent with the viscerally sensitive phenotype of the WKY rat, Cav3.2, T-type calcium channels linked to visceral pain sensitivity (Marger et al. 2011), were also elevated in WKY tissue (P < 0.01). Moreover, consistent with amelioration of the visceral sensitivity following treatment with xIL-6R alone and xIL-6R plus antalarmin, expression of Cav3.2 was also reduced (P < 0.01, n = 5 rats, Fig.7C).
Figure 7. IL-6R neutralization and antalarmin alter colonic mucosal protein expression.
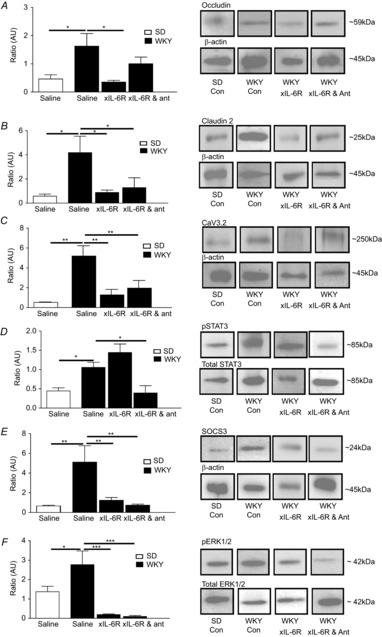
Bar charts illustrating the ratios of expression of occludin (A, n = 5), claudin 2 (B, n = 5) and Cav3.2 (C, n = 5) over the β actin loading control, phosphoSTAT3 as a fraction of total STAT3 (D, n = 5), SOCS3 as a fraction of the β actin loading control (E, n = 5) and phosphoERK1/2 over total ERK1/2 (F, n = 5) in mucosal samples from the distal colon of SD and WKY rats treated with saline, anti-IL-6 receptor antibody (xIL-6R) and xIL-6R with antalarmin (ant). Asterisks indicate *P < 0.05, **P < 0.01 and ***P < 0.001.
IL-6 activates the intracellular janus tyrosine kinase and signal transducers and activators of transcription (JAK-STAT) signalling cascade and indeed phosphorylation of STAT3 was elevated in WKY rats which have elevated levels of IL-6 as compared with SD colons (P < 0.05). Treatment with xIL-6R had no effect on pSTAT3 expression (P > 0.05), whereas co-administration of xIL-6R and antalarmin suppressed pSTAT3 expression (P < 0.05, n = 5 rats, Fig.7D). SOCS3, a suppressor of cytokine signalling, was also elevated in WKY controls (P < 0.01), and was reduced to levels equivalent to SD controls following xIL-6R (P > 0.05) and xIL-6R plus antalarmin (P > 0.05, n = 5 rats, Fig.7E) treatment. IL-6 binding also activates the intracellular signalling molecule pERK1/2 and this was greater in control WKY compared with SD colons (P < 0.05), but was markedly reduced by xIL-6R (P < 0.001) and xIL-6R plus antalarmin (P < 0.001, n = 5 rats, Fig.7F) treatment.
Discussion
Neuroimmune and neuroendocrine interactions contribute to IBS pathophysiology
The importance of immune factors such as IL-6 (Liebregts et al. 2007), IL-8 (Dinan et al. 2006,2008; McKernan et al. 2011b) and the stress hormone CRF (Mayer, 2000; Bohmelt et al. 2005; Dinan et al. 2006; Chang et al. 2009) in the aetiology of human IBS has been reported. Moreover, we have previously reported the effects of these factors on the excitability of submucosal neurons leading to functional changes in secretory activity (O'Malley et al. 2011c, 2013). The studies reported here have identified that the pro-inflammatory cytokines IL-6 and IL-8 are acting as neuromodulators, which is consistent with the work of Kelles et al. (2000), who described the stimulation of guinea-pig ileum myenteric neurons by IL-1β and IL-6. However, we have also determined that the stress factor CRF activates myenteric neurons and that interaction between the immune and stress factors may occur, thereby contributing to bowel symptom flares. Myenteric neurons are associated with GI motility and in terms of pathophysiology, GI spasm and abdominal pain (Sarna, 2007). Indeed, consistent with recent reports describing how recombinant IL-6 (Zhang et al. 2013) and an acute stressor (Liang et al. 2012) increased contractile activity, we found that IL-6, IL-8 and CRF induced colonic contractions. IL-8, which had minimal effects on submucosal excitability (O'Malley et al. 2011c), evoked robust responses in myenteric neurons and stimulated large colonic contractions. The amplitude of contractions evoked by IL-6 and IL-8 was greatly reduced by TTX, whereas the inhibitory effect was less for CRF-evoked contractions, which may be acting directly on smooth muscle cells or interstitial cells of Cajal. The observed increase in the frequency of GI smooth muscle basal contractility in the presence of TTX may due to endogenous inhibitory neural tone (Huizinga et al. 1990). Furthermore, the stimulatory effects of the immune and stress factors were additive, with the largest neuronal and contractile response being evoked by the combined presence of all three factors. These findings are consistent with our proposal that hyperactivity of the stress response, in conjunction with low-grade immune activation, may underlie symptom flares in functional bowel disorders (O'Malley et al. 2011d).
To test this hypothesis further, an LMMP preparation was exposed to clinical plasma samples from IBS patients and healthy controls. The much larger neuronal responses to IBS plasma indicates that certain soluble mediators present in this plasma have neuroexcitatory effects. Neutralizing IL-6 and IL-8 and the CRFR1 antagonist antalarmin attenuated the IBS plasma-evoked neuronal responses, thereby demonstrating the importance of these factors to the neuroexcitatory actions of IBS plasma. Indeed, IL-6 has previously been shown to act as a neuromodulator in myenteric S and AH neurons (Kelles et al. 2000). Although others have described the neuroexcitatory effects of other mucosally secreted soluble factors, such as serotonin, histamine and proteases in submucosal neurons (Buhner et al. 2009,2012), our findings demonstrate that blood-borne IL-6, CRF, IL-8 and, to a lesser extent, CRP activate the myenteric neurons.
IBS plasma also activated JAK-STAT and ERK-MAPK intracellular signalling cascades. Interestingly, inhibiting the NFκB signalling pathway, which normally induces transcription of immune and inflammatory factors, potentiated the neuroexcitatory actions of IBS plasma, contrasting with its inhibitory effects on recombinant IL-6 in submucosal neurons (O'Malley et al. 2011c). This may be due to an interaction with one of the other neuroexcitatory mediators present in the plasma.
Inhibiting both IL-6 and CRF1 receptors improves IBS-like symptoms in WKY rats
Consistent with the variable phenotype and multi-factorial nature of IBS, our studies have identified several elements which contribute to altered bowel motility and visceral pain sensitivity in the WKY rats. As previously reported (Martinez et al. 2007; O'Mahony et al. 2010; O'Malley et al. 2010b), WKY rats defecated more in the anxiogenic OF arena and have lower pain thresholds to CRD, thereby mimicking symptoms of altered bowel habit and visceral pain in humans. However, these IBS-like symptoms were ameliorated by blocking IL-6R signalling and normalized to control levels when both xIL-6R and antalarmin were co-administered. Moreover, consistent with evidence that psychological stress elevates IL-6 in humans, our findings illustrate the intricate relationship between immune and stress factors (O'Malley et al. 2011d, 2013). Perhaps overlooking the importance of pro-inflammatory cytokines in symptom flares may account for the disappointing outcomes of clinical trials of CRFR1 antagonists in IBS patients (Tache et al. 2009).
Underlying the improvements in GI function caused by xIL-6R and antalarmin co-treatment, were dramatic changes in mucosal protein expression. Figure6C summarizes a potential signalling cascade underlying the IBS-like symptoms exhibited by WKY rats. Downstream of IL-6Rs, which comprise α-chains and the signal transduction gp130 subunit, is the JAK-STAT signalling cascade (Hemmann et al. 1996). STAT3 phosphorylation was elevated in WKY colonic tissue, perhaps due to raised mucosal levels of IL-6 (O'Malley et al. 2011a). However, other cytokines or growth factors may also activate this transcription molecule as STAT3 phosphorylation was not altered following blockade of IL-6 signalling. Interestingly, given that CRFR1 activation is not associated with JAK-STAT signalling, co-treatment with antalarmin normalized pSTAT3 levels, demonstrating a complex interaction between the immune and stress factor. SOCS3, which terminates JAK/STAT signalling via negative feedback following STAT3 activation (Croker et al. 2003), was elevated in control WKY colons, and expression of this downstream regulatory molecule was inhibited by treatment with xIL-6R or xIL-6R plus antalarmin. Activation of the ERK 1/2 signalling cascade by either CRF (Stengel & Tache, 2009) or IL-6 (Hoffmann et al. 2002) was evident in the WKY colonic mucosa and this was reduced following treatment with xIL-6R or xIL-6R plus antalarmin. This is consistent with previous studies in submucosal neurons which demonstrated that CRF tends to drive IL-6-mediated signalling via the ERK/MAPK cascade (O'Malley et al. 2013), possibly due to ERK activation antagonizing JAK-STAT signalling (Bonni et al. 1997).
Both JAK-STAT and ERK/MAPK signalling evoke transcription and de novo protein synthesis. In WKY colonic mucosa, expression of two tight junction proteins, occludin and claudin 2, which are crucial to GI barrier function and permeability, were both increased. Functionally, transepithelial resistance (TER) is equivalent between WKY and SD rats (O'Malley et al. 2012) but given that increased expression of occludin is thought to induce tighter barrier control and claudin 2 is thought to reduce barrier tightness (Amasheh et al. 2002) the sum effect of elevated mucosal levels of both proteins may be no net change in permeability, although with the presence of many additional tight junction proteins, this requires further research. Expression of claudin 1 and 2 is also increased in IBS (Martinez et al. 2013). Conversely, others have demonstrated decreased expression of occludin in bowel inflammation (Poritz et al. 2011) and, in caco cells, exposure to IL-6 decreased claudin 2 expression resulting in decreased TER (Suzuki et al. 2011). We have previously demonstrated that acute administration of IL-6 increases TER in WKY colons (O'Malley et al. 2012) and others have shown that chronic exposure to IL-6 increases gut permeability (Natale et al. 2003). Our study has provided evidence that blocking IL-6Rs decreased expression of both occludin and claudin 2, and the addition of antalarmin reduced expression of claudin 2. The decreases in tight junction protein expression indicate a possible change in TER, although functional studies will be required to certify this.
Finally, expression of the T-type calcium channel Cav3.2, which is linked to visceral pain in a rodent model of IBS (Marger et al. 2011), was increased in WKY colons. Consistent with improvements in pain threshold to CRD, treatment with xIL-6R and antalarmin resulted in reduced expression of Cav3.2. CRFR1 antagonists alleviate visceral sensitivity in the WKY rat and CRFR1 has been shown to functionally couple to Cav3.2 in a cell line, inhibiting the calcium current (Tao et al. 2008). Thus, as has recently been proposed (Beyder et al. 2014), specific ion channelopathies may contribute to visceral pain in some IBS patients. However, to our knowledge, this is the first study to link IL-6 signalling with Cav3.2 expression and visceral pain sensitivity.
Thus, in a multifactorial disorder such as IBS, where the stress system is chronically activated and cytokine levels of IL-6 and IL-8 are elevated, interaction and crosstalk between these biologically active factors results in increased stimulation of myenteric neurons, which subsequently affects contractile activity. Indeed, the demonstrated effectiveness in ameliorating IBS-like pathophysiology, such as defecation patterns and visceral pain sensitivity, in the WKY rat by targeting IL-6 and CRF1 and possibly also IL-8 receptors establishes that these immune and stress molecules do indeed contribute to these symptoms. Moreover, we have determined that the neuroexcitatory effects of human IBS plasma are evoked primarily by the immune mediators IL-6 and IL-8 and the stress hormone CRF. These studies in the WKY rat, which mimics symptoms of IBS, offers proof of principle that combined targeting of both immune and stress factors may be a viable therapeutic strategy for the treatment of IBS.
Acknowledgments
We thank Drs Declan McKernan, Gabor Gaszner, Sinead Heuston and Rachel Moloney, Colette Manley and Patrick Fitzgerald for assistance with various parts of this study.
Glossary
- CRD
colorectal distension
- CRF
corticotropin-releasing factor
- CRFR
CRF receptor
- CRP
C-reactive protein
- ELISA
enzyme-linked immunosorbent assay
- ERK-MAPK
extracellular signal-regulated kinase-mitogen activated protein kinase
- GI
gastrointestinal
- HPA
hypothalamic-pituitary-adrenal
- IBS
irritable bowel syndrome
- IKK2
IkappaB kinase2
- IL
interleukin
- IL-6R
interleukin-6 receptor
- JAK-STAT
janus tyrosine kinase and signal transducers and activators of transcription
- LMMP
longitudinal muscle myenteric plexus
- NFκB
nuclear factor κ-light-chain-enhancer of activated B cells
- OF
open field
- SD
Sprague Dawley
- SOCS
suppressor of cytokine signalling
- TER
trans-epithelial resistance
- WKY
Wistar Kyoto
- xIL-6R
anti-IL-6R
Key points
Hyperactivity of the stress system and low-grade immune activation characterize the functional bowel disorder irritable bowel syndrome (IBS).
These studies show that interleukin (IL)-6 and IL-8 and the stress hormone corticotropin-releasing factor (CRF), present in IBS plasma, have functional effects on gastrointestinal activity by stimulating myenteric neurons and colonic contractions.
Moreover, in the Wistar Kyoto rat model of IBS, which exhibits altered gastrointestinal motility and visceral pain sensitivity, blocking IL-6 and/or CRF1 receptors alleviates these IBS-like symptoms.
Underlying these effects are altered colonic protein expression of tight junction proteins which regulate gut barrier function and the T-type calcium channel CaV3.2, which has been linked to visceral pain.
These findings demonstrate the importance of the enteric nervous system and intestinal physiology in bowel dysfunction.
Additional information
Competing interests
The authors declare that there are no competing interests regarding this publication.
Author contributions
M.M.B.: acquisition of data; analysis and interpretation of data; drafting of the manuscript; K.D.O'H.: critical revision of the manuscript for important intellectual content; M.G.R.: critical revision of the manuscript for important intellectual content; T.G.D.: critical revision of the manuscript for important intellectual content; D.O'M.: study concept and design, critical revision of the manuscript for important intellectual content; obtained funding, study supervision.
Funding
This publication emanated from research supported by the Health Research Board (HRA_POR/2010/52) and by a research grant from the Science Foundation Ireland (SFI) under Grant Number 07/CE/B1368 in the Alimentary Pharmabiotic Centre. M.M.B. was part funded by the Department of Physiology, UCC.
References
- Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M. Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1054–1064. doi: 10.1152/ajpgi.00055.2011. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD. Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. &. [DOI] [PubMed] [Google Scholar]
- Beyder A, Mazzone A, Strege PR, Tester DJ, Saito YA, Bernard CE, Enders FT, Ek WE, Schmidt PT, Dlugosz A, Lindberg G, Karling P, Ohlsson B, Gazouli M, Nardone G, Cuomo R, Usai-Satta P, Galeazzi F, Neri M, Portincasa P, Bellini M, Barbara G, Camilleri M, Locke GR, 3rd, Talley NJ, D'Amato M, Ackerman MJ. Farrugia G. Loss-of-function of the voltage-gated sodium channel NaV1.5 (Channelopathies) in patients with irritable bowel syndrome. Gastroenterology. 2014;146:1659–1668. doi: 10.1053/j.gastro.2014.02.054. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmelt AH, Nater UM, Franke S, Hellhammer DH. Ehlert U. Basal and stimulated hypothalamic–pituitary–adrenal axis activity in patients with functional gastrointestinal disorders and healthy controls. Psychosom Med. 2005;67:288–294. doi: 10.1097/01.psy.0000157064.72831.ba. &. [DOI] [PubMed] [Google Scholar]
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD. Greenberg ME. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science (New York, NY) 1997;278:477–483. doi: 10.1126/science.278.5337.477. &. [DOI] [PubMed] [Google Scholar]
- Buhner S, Li Q, Berger T, Vignali S, Barbara G, De Giorgio R, Stanghellini V. Schemann M. Submucous rather than myenteric neurons are activated by mucosal biopsy supernatants from irritable bowel syndrome patients. Neurogastroenterol Motil. 2012;24:1134–e572. doi: 10.1111/nmo.12011. &. [DOI] [PubMed] [Google Scholar]
- Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H, Michel K. Schemann M. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425–1434. doi: 10.1053/j.gastro.2009.07.005. &. [DOI] [PubMed] [Google Scholar]
- Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A. Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. &. [DOI] [PubMed] [Google Scholar]
- Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, Ameen VZ. Mayer EA. Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Quigley EM, Cryan JF. Dinan TG. Irritable bowel syndrome: towards biomarker identification. Trends Mol Med. 2009;15:478–489. doi: 10.1016/j.molmed.2009.08.001. &. [DOI] [PubMed] [Google Scholar]
- Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW. Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nature Immunol. 2003;4:540–545. doi: 10.1038/ni931. &. [DOI] [PubMed] [Google Scholar]
- Cryan JF. O'Mahony SM. The microbiome–gut–brain axis: from bowel to behavior. Neurogastroent Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. &. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Clarke G, Quigley EM, Scott LV, Shanahan F, Cryan J, Cooney J. Keeling PW. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570–2576. doi: 10.1111/j.1572-0241.2008.01871.x. &. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Quigley EM, Ahmed SM, Scully P, O'Brien S, O'Mahony L, O'Mahony S, Shanahan F. Keeling PW. Hypothalamic–pituitary–gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. &. [DOI] [PubMed] [Google Scholar]
- Gunter WD, Shepard JD, Foreman RD, Myers DA. Greenwood-Van Meerveld B. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol Behav. 2000;69:379–382. doi: 10.1016/s0031-9384(99)00254-1. &. [DOI] [PubMed] [Google Scholar]
- Heitkemper M, Jarrett M, Cain K, Shaver J, Bond E, Woods NF. Walker E. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. Am J Gastroenterol. 1996;91:906–913. &. [PubMed] [Google Scholar]
- Hemmann U, Gerhartz C, Heesel B, Sasse J, Kurapkat G, Grotzinger J, Wollmer A, Zhong Z, Darnell JE, Jr, Graeve L, Heinrich PC. Horn F. Differential activation of acute phase response factor/Stat3 and Stat1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. II. Src homology SH2 domains define the specificity of stat factor activation. J Biol Chem. 1996;271:12999–13007. doi: 10.1074/jbc.271.22.12999. &. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H. Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. &. [PubMed] [Google Scholar]
- Huizinga JD, Berezin I, Daniel EE. Chow E. Inhibitory innervation of colonic smooth muscle cells and interstitial cells of Cajal. Can J Physiol Pharmacol. 1990;68:447–454. doi: 10.1139/y90-063. &. [DOI] [PubMed] [Google Scholar]
- Kelles A, Janssens J. Tack J. IL-1β and IL-6 excite neurones and suppress cholinergic neurotransmission in the myenteric plexus of the guinea pig. Neurogastroenterol Motil. 2000;12:531–538. doi: 10.1046/j.1365-2982.2000.00228.x. &. [DOI] [PubMed] [Google Scholar]
- Kindt S, Vanden Berghe P, Boesmans W, Roosen L. Tack J. Prolonged IL-1β exposure alters neurotransmitter and electrically induced Ca2+ responses in the myenteric plexus. Neurogastroenterol Motil. 2010;22:321–e85. doi: 10.1111/j.1365-2982.2009.01414.x. &. [DOI] [PubMed] [Google Scholar]
- Liang C, Luo H, Liu Y, Cao J. Xia H. Plasma hormones facilitated the hypermotility of the colon in a chronic stress rat model. PloS One. 2012;7:e31774. doi: 10.1371/journal.pone.0031774. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebregts T, Adam B, Bredack C, Roth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ. Holtmann G. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. &. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F. Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. &. [DOI] [PubMed] [Google Scholar]
- Lovell RM. Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. &. [DOI] [PubMed] [Google Scholar]
- Marger F, Gelot A, Alloui A, Matricon J, Ferrer JF, Barrere C, Pizzoccaro A, Muller E, Nargeot J, Snutch TP, Eschalier A, Bourinet E. Ardid D. T-type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci U S A. 2011;108:11268–11273. doi: 10.1073/pnas.1100869108. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Lobo B, Pigrau M, Ramos L, Gonzalez-Castro AM, Alonso C, Guilarte M, Guila M, de Torres I, Azpiroz F, Santos J. Vicario M. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160–1168. doi: 10.1136/gutjnl-2012-302093. &. [DOI] [PubMed] [Google Scholar]
- Martinez V, Ryttinger M, Kjerling M. Astin-Nielsen M. Characterisation of colonic accommodation in Wistar Kyoto rats with impaired gastric accommodation. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:205–216. doi: 10.1007/s00210-007-0195-1. &. [DOI] [PubMed] [Google Scholar]
- Mawdsley JE. Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. doi: 10.1136/gut.2005.064261. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan DP, Dennison U, Gaszner G, Cryan JF. Dinan TG. Enhanced peripheral toll-like receptor responses in psychosis: further evidence of a pro-inflammatory phenotype. Transl Psychiatry. 2011a;1:e36. doi: 10.1038/tp.2011.37. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan DP, Gaszner G, Quigley EM, Cryan JF. Dinan TG. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther. 2011b;33:1045–1052. doi: 10.1111/j.1365-2036.2011.04624.x. &. [DOI] [PubMed] [Google Scholar]
- Natale L, Piepoli AL, De Salvia MA, De Salvatore G, Mitolo CI, Marzullo A, Portincasa P, Moschetta A, Palasciano G. Mitolo-Chieppa D. Interleukins 1 beta and 6 induce functional alteration of rat colonic motility: an in vitro study. Eur J Clin Invest. 2003;33:704–712. doi: 10.1046/j.1365-2362.2003.01200.x. &. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Bulmer DC, Coelho AM, Fitzgerald P, Bongiovanni C, Lee K, Winchester W, Dinan TG. Cryan JF. 5-HT2B receptors modulate visceral hypersensitivity in a stress-sensitive animal model of brain-gut axis dysfunction. Neurogastroenterol Motil. 2010;22:573–578. doi: 10.1111/j.1365-2982.2009.01432.x. &. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF. Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. &. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Cryan JF. Dinan TG. Crosstalk between interleukin-6 and corticotropin-releasing factor modulate submucosal plexus activity and colonic secretion. Brain Behav Immun. 2013;30:115–124. doi: 10.1016/j.bbi.2013.01.078. &. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Dinan TG. Cryan JF. Altered expression and secretion of colonic interleukin-6 in a stress-sensitive animal model of brain–gut axis dysfunction. J Neuroimmunol. 2011a;235:48–55. doi: 10.1016/j.jneuroim.2011.04.003. &. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Dinan TG. Cryan JF. Neonatal maternal separation in the rat impacts on the stress responsivity of central corticotropin-releasing factor receptors in adulthood. Psychopharmacology. 2011b;214:221–229. doi: 10.1007/s00213-010-1885-9. &. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Dinan TG. Cryan JF. Interleukin-6 modulates colonic transepithelial ion transport in the stress-sensitive Wistar Kyoto rat. Front Pharmacol. 2012;3:190. doi: 10.3389/fphar.2012.00190. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley D, Julio-Pieper M, Gibney SM, Dinan TG. Cryan JF. Distinct alterations in colonic morphology and physiology in two rat models of enhanced stress-induced anxiety and depression-like behaviour. Stress. 2010a;13:114–122. doi: 10.3109/10253890903067418. &. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Julio-Pieper M, Gibney SM, Gosselin RD, Dinan TG. Cryan JF. Differential stress-induced alterations of colonic corticotropin-releasing factor receptors in the Wistar Kyoto rat. Neurogastroenterol Motil. 2010b;22:301–311. doi: 10.1111/j.1365-2982.2009.01412.x. &. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Liston M, Hyland NP, Dinan TG. Cryan JF. Colonic soluble mediators from the maternal separation model of irritable bowel syndrome activate submucosal neurons via an interleukin-6-dependent mechanism. Am J Physiol. 2011c;300:G241–252. doi: 10.1152/ajpgi.00385.2010. &. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Quigley EM, Dinan TG. Cryan JF. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain Behav Immun. 2011d;25:1333–1341. doi: 10.1016/j.bbi.2011.04.009. &. [DOI] [PubMed] [Google Scholar]
- Poritz LS, Harris LR, 3rd, Kelly AA. Koltun WA. Increase in the tight junction protein claudin-1 in intestinal inflammation. Digest Dis Sci. 2011;56:2802–2809. doi: 10.1007/s10620-011-1688-9. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna SK. Enteric descending and afferent neural signaling stimulated by giant migrating contractions: essential contributing factors to visceral pain. Am J Physiol. 2007;292:G572–581. doi: 10.1152/ajpgi.00332.2006. [DOI] [PubMed] [Google Scholar]
- Shanahan F. Anton P. Neuroendocrine modulation of the immune system. Possible implications for inflammatory bowel disease. Digest Dis Sci. 1988;33:41S–49S. doi: 10.1007/BF01538130. &. [DOI] [PubMed] [Google Scholar]
- Spiller R. Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. &. [DOI] [PubMed] [Google Scholar]
- Stengel A. Tache Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol. 2009;71:219–239. doi: 10.1146/annurev.physiol.010908.163221. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Yoshinaga N. Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y. Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y, Kiank C. Stengel A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr Gastroenterol Rep. 2009;11:270–277. doi: 10.1007/s11894-009-0040-4. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Hildebrand ME, Liao P, Liang MC, Tan G, Li S, Snutch TP. Soong TW. Activation of corticotropin-releasing factor receptor 1 selectively inhibits CaV3.2 T-type calcium channels. Mol Pharmacol. 2008;73:1596–1609. doi: 10.1124/mol.107.043612. &. [DOI] [PubMed] [Google Scholar]
- Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ. Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–47. doi: 10.1136/gut.45.2008.ii43. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Hu HZ, Liu S, Ren J, Zafirov DH. Wood JD. IL-1β and IL-6 excite neurons and suppress nicotinic and noradrenergic neurotransmission in guinea pig enteric nervous system. J Clin Invest. 1999;103:1309–1316. doi: 10.1172/JCI5823. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hu L, Chen M. Yu B. Exogenous interleukin-6 facilitated the contraction of the colon in a depression rat model. Dig Dis Sci. 2013;58:2187–2196. doi: 10.1007/s10620-013-2656-3. &. [DOI] [PubMed] [Google Scholar]


