Abstract
Nutritionally-induced growth faltering in the perinatal period has been associated with reduced adult skeletal muscle mass; however, the mechanisms responsible for this are unclear. To identify the factors that determine the recuperative capacity of muscle mass, we studied offspring of FVB mouse dams fed a protein-restricted diet during gestation (GLP) or pups suckled from postnatal day 1 (PN1) to PN11 (E-UN), or PN11 to PN22 (L-UN) on protein-restricted or control dams. All pups were refed under control conditions following the episode of undernutrition. Before refeeding, and 2, 7 and 21 days later, muscle protein synthesis was measured in vivo. There were no long-term deficits in protein mass in GLP and E-UN offspring, but in L-UN offspring muscle protein mass remained significantly smaller even after 18 months (P < 0.001). E-UN differed from L-UN offspring by their capacity to upregulate postprandial muscle protein synthesis when refed (P < 0.001), a difference that was attributable to a transient increase in ribosomal abundance, i.e. translational capacity, in E-UN offspring (P < 0.05); translational efficiency was similar across dietary treatments. The postprandial phosphorylation of Akt and extracellular signal-regulated protein kinases were similar among treatments. However, activation of the ribosomal S6 kinase 1 via mTOR (P < 0.02), and total upstream binding factor abundance were significantly greater in E-UN than L-UN offspring (P < 0.02). The results indicate that the capacity of muscles to recover following perinatal undernutrition depends on developmental age as this establishes whether ribosome abundance can be enhanced sufficiently to promote the protein synthesis rates required to accelerate protein deposition for catch-up growth.
Introduction
The organism's environment during pre- and postnatal life is determined primarily by maternal health and the adequacy of its nutrient supply. During critical windows of development when there is still considerable plasticity in the differentiation processes, the interplay between an organism's genes and its environment serves to establish the developmental trajectory of its organs and tissues so that its phenotype at maturity will be appropriately adapted for its environment. While this phenomenon of ‘developmental programming’ is advantageous in a stable environment, the adaptations can be detrimental for the organism's health when developmental and adult environments are mismatched (Bateson et al. 2004).
It is now recognized that skeletal muscle mass is susceptible to developmental programming in response to the nutritional environment present during development (reviewed by Sayer & Cooper, 2005; Brown, 2014). When nutrient supply is insufficient, muscle growth is impaired, and the deficit is not always recuperated following nutritional rehabilitation. The resulting deficit in skeletal muscle mass has widespread ramifications beyond its function in locomotion (Wolfe, 2006) including exacerbation of the risk for and severity of the chronic diseases frequently present in adults who were of low birth weight (Osmond & Barker, 2000). Despite this documented effect of early life nutrition on adult muscle mass in both humans and animal models (primates, sheep, pigs and rodents), we still do not understand the mechanisms responsible. The investigations reported here were performed in mice to fill this gap in our knowledge.
Animal studies have identified two developmental stages when exposure to insufficient nutrients can produce permanent changes in muscle mass. The first occurs during fibre formation in early fetal life when a severe deficit in fetal nutrient supply permanently reduces myofibre number (reviewed by Sayer & Cooper, 2005; Brown, 2014). The second stage occurs after fibres have formed and are undergoing maturation and hypertrophy (Ontell & Kozeka, 1984; Desai et al. 1996); this second stage spans the perinatal period (depending on the degree of maturity at birth of the species) when muscle growth is most vulnerable to the adequacy of its nutrient supply (Davis & Fiorotto, 2009). In rodents, the late fetal and suckling periods represent the latter period of vulnerability.
Two general mechanisms can be hypothesized to explain the later period of vulnerability. First, alterations in nutrient supply and the resulting metabolic and endocrine responses could produce stable changes in gene expression that result in lifelong consequences for structure and function, including the responsiveness to environmental signals (Burdge et al. 2007). Thus, the muscle's capacity to recuperate its normal growth potential would be permanently compromised. Such epigenetic programming is more likely to occur in the context of a permissive epigenetic landscape as might be present in newly differentiated fibres in which extensive modifications in gene activity are driving maturation of the tissue. Due to the variable timing of differentiation among muscles, in the mouse this period spans the end of gestation and the first half of the suckling period. The processes occurring over this time include the organization and expansion of the sarcoplasmic reticulum and transverse-tubular systems (Luff & Atwood, 1971), the reorganization of the innervation (Fladby & Jansen, 1990), and the preferential synthesis and accretion of myofibrillar proteins (Fiorotto et al. 2000; Gokhin et al. 2008) followed by the switch in expression of sarcomeric proteins from immature to mature isoforms (Agbulut et al. 2003; Gokhin et al. 2008). These combined changes in morphology and composition are evident in the acquisition of functional competence towards the end of the second week of life (Gokhin et al. 2008). A second explanation for the inability to recuperate muscle mass following postnatal undernutrition is based on the premise that the growth of immature fibres is dependent on the balance of positive and negative signals that cue the muscles’ anabolic pathways, and that this balance becomes progressively less positive as muscles mature. This premise is supported by several lines of evidence. When dietary protein and energy intake are adequate, the immature muscle can sustain elevated rates of protein accretion as a result of the heightened responsiveness to insulin and amino acids of the pathways that regulate translation initiation, as well as its large protein synthetic capacity (Davis & Fiorotto, 2009). This anabolic drive diminishes with maturation (Davis et al. 1993). Following differentiation, expression of the insulin-like growth factors (IGFs) in muscle is high and their autocrine signalling (Fiorotto et al. 2003 and references therein) promotes muscle hypertrophy; however, their expression decreases exponentially as muscle maturation advances. The hypothalamic–pituitary–adrenal axis is hypo-responsive to stress during the first 10–12 days of life and plasma glucocorticoid levels, which are inhibitory for protein anabolism, are very low rising to adult levels during the third week of life (Schmidt et al. 2003). The transforming growth factor-β superfamily of proteins and their receptors, such as myostatin, follistatin and activin IIB receptor, undergo marked changes in expression with a balance towards greater inhibition of anabolic pathways as muscles mature (Suryawan et al. 2006). Thus, if recuperation from a nutritional growth restriction is implemented when the balance of anabolic cues is not sufficiently positive, the rates of protein accretion necessary for catch-up growth may not be supported. Both mechanisms may also participate in the programming of muscle mass.
To determine the contribution of these two mechanisms, we restricted the nutrient intake of mouse pups from postnatal (PN) day 1 to PN11 (Early Undernutrition, E-UN), and then nutritionally rehabilitated them with the anticipation that their muscles would not undergo catch-up growth if stable changes in gene expression resulted in an anabolic resistance despite the continued presence of anabolic signals during the latter part of the suckling period. A second group of pups was undernourished from PN11 (Late Undernutrition, L-UN), and their muscles’ growth response to nutritional rehabilitation from PN22 was assessed. We hypothesized that if grow capacity is dependent on the presence of a positive balance of anabolic cues, catch-up growth would not occur in this group, even though the early period of muscle maturation occurred unperturbed by dietary factors. Pups were undernourished postnatally by suckling them on dams fed on a protein-restricted diet ad libitum. Protein-restricted dams produce less milk with a slightly lower protein content and a higher fat concentration. Thus, pups experience a global nutrient deficit with a slightly greater deficit in protein intake (Sampson et al. 1986; Grimble & Mansaray, 1987; Pine et al. 1994; Derrickson & Lowas, 2007). To isolate prenatal effects, we studied pups born to dams fed a protein-restricted diet (GLP) from 1 week before conception to the end of gestation, but suckled from birth by well-nourished dams. To identify the regulatory mechanisms responsible for differences in muscle growth among treatment groups, we measured rates of muscle protein synthesis, protein synthetic capacity and efficiency, and the phosphorylation of key signalling intermediaries as a measure of the activation of the main pathways that transduce anabolic signals to the protein synthetic machinery of the muscle. These measurements were performed in the undernourished condition and at different times during nutritional rehabilitation.
Methods
Ethical approval
Experiments were carried out according to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council and approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Animals, diet and experimental design
Second and third parity FVB (FVB/N; Charles River Laboratories, Wilmington, MA, USA) mouse dams were fed either a semi-purified control diet (20% protein; Research Diets, New Brunswick, NJ, USA) based on AIN93G, or a low-protein (LP) diet (8% protein) beginning 1 week before mating (Table1). Mating was timed by introducing males for a 24 h period; only pups born within the same 24 h period (PN0) were studied. On PN1 all pups born to dams on the same diet were pooled, and redistributed to one of four experimental groups: (i) Control (CON; n = 16 litters), pups born to control dams and suckled on control dams; (ii) E-UN (n = 7), pups born to control dams, suckled on LP dams from PN1 to PN11, and refed by cross-fostering to control dams from PN11 to PN 22; (iii) L-UN (n = 8 litters), pups born to and suckled on control dams from PN1 to PN11, and cross-fostered to LP dams from PN11 to PN 22; and (iv) GLP (n = 9 litters), pups born to LP dams but suckled on control dams from PN1 to PN22. Each dam received seven pups (4–5 males and 2–3 females) and individual pups within a litter were identified with a unique tattoo. Pups were allocated so that on PN1, average pup weight for E-UN, L-UN, and CON litters was the same; for GLP litters, the average weight of each sex on PN1 was determined, and litters were reformed so that average litter weights were similar. Litter size was maintained constant through lactation by introducing ‘donor’ pups of similar body weight and age to replace any deaths or pups removed for study; donor pups were not studied. At PN22 pups were weaned to the control diet; diet and water were available ad libitum. Body weights were measured every 3–4 days to PN43 and every 4 weeks thereafter. Mice were housed on wood-chip bedding in a single room maintained at 23°C with a 12 h light/dark cycle. The experimental protocol was repeated on four separate occasions to achieve the necessary number of replicates; however, analyses were performed only after all tissues had been collected.
Table 1.
Composition of control and low-protein diets*
| Control | Low-Protein | |
|---|---|---|
| Ingredients (g kg−1) | ||
| Casein | 219 | 91 |
| Methionine | 3 | 1.36 |
| Corn starch | 304 | 369 |
| Maltodextrin | 101 | 123 |
| Sucrose | 203 | 242 |
| Cellulose | 50 | 50 |
| Soybean oil | 70 | 71 |
| tert-Butylhydroquinone | 0.014 | 0.014 |
| Mineral mix (AIN-93 G)b | 35 | 35 |
| Potassium phosphate | 4.6 | |
| Potassium citrate | 3.65 | |
| Vitamin mix (AIN-93)b | 10 | 10 |
| Choline bitartarate | 2.5 | 2.5 |
| Macronutrient composition (g kg−1) | ||
| Protein | 195 | 80 |
| Carbohydrate | 608 | 734 |
| Fat | 70 | 71 |
| Fibre | 50 | 50 |
| Metabolizable energy (kJ g−1) | 16.1 | 16.3 |
| Protein (% energy) | 20.3 | 8.2 |
aDiets prepared by Research Diets (New Brunswick, NJ, USA).
Mineral mix product no. S10022G; vitamin mix product no. V10037.
Muscles were collected for analyses from E-UN and L-UN pups before refeeding and 2, 7 and 21 days after refeeding; GLP pups were studied only on PN22 and at 18 months. Age-matched CON were also analysed. One male pup from each litter was studied at each time point. For litters with five male pups, the remaining pup was aged to 18 months when a subset of measurements was performed (CON, n = 8; E-UN, n = 6; L-UN, n = 7; GLP, n = 6). On tissue collection days, dams were removed from the home cage for 2–3 h (for suckling pups), or food was removed from the cage for 4–6 h (for weaned pups). The mice were then refed by returning the dams or diet to the cage. Exactly 60 min after the start of refeeding the fractional synthesis rate (FSR) of muscle protein was measured in vivo. After pups were killed, individual muscles (quadriceps, gastrocnemius and tibialis anterior) from both hind limbs were dissected quantitatively and weighed. Femur and tibias were removed and lengths measured with Vernier callipers. The stomach was dissected, and its contents removed and weighed. Blood was collected and serum isolated for measurement of plasma insulin; tissue collections were all performed between 08.00 and 12.00 h. Tissues were stored in liquid nitrogen vapour, and plasma was stored at −80°C.
Skeletal muscle fractional protein synthesis rate
Measurements were made using a flooding dose of l-[4-3H]-phenylalanine (American Radiolabelled Chemicals, St. Louis, MO, USA) as previously described (Fiorotto et al. 2012; Radley-Crabb et al. 2014). Briefly, l-[4-3H]-phenylalanine (20 mL kg body weight−1, 1.5 mmol phenylalanine kg body weight−1 and 150 μCi per mouse) was injected through a lateral tail vein or I.P. in PN11 and PN13 pups. After 15 min, mice were killed by decapitation, blood was collected and a portion was acidified to 0.2 m perchloric acid for estimation of blood free phenylalanine specific radioactivity. The hind limbs were chilled after which muscles were dissected on ice and frozen in liquid nitrogen.
For analysis, all muscles from one hind limb of each mouse were pooled and powdered at liquid nitrogen temperature. A muscle aliquot was homogenized in water; a sample was retained for determining protein concentration and the remainder was processed for measurement of the muscle-free phenylalanine precursor pool-specific radioactivity, total RNA and l-[4-3H] phenylalanine incorporated into the muscle proteins. The protein concentration of muscle homogenates was determined using the bicinchoninic acid (BCA) protein assay (Smith et al. 1985) (Thermo Fisher Scientific, Rockford, IL, USA) after solubilizing in 0.1 m NaOH.
Calculations
The FSR, i.e. the percentage of the protein mass synthesized in a day, was calculated as:
where SB and SA are the specific radioactivities of the protein-bound and free precursor pool l-[4-3H] phenylalanine, respectively, and t is the labelling time in minutes. Blood and tissue free phenylalanine specific radioactivity values were compared to verify equilibration of the tracer. Hind limb muscle protein mass was calculated as the product of the pooled quadriceps, tibialis anterior and gastrocnemius muscle weights and protein concentration.
RNA concentrations
Total RNA was measured quantitatively in the total protein perchloric acid-insoluble precipitate using a modified Schmidt-Thannhauser procedure (Munro & Fleck, 1966).
The absolute abundance of 18S rRNA, IGF-I, IGF-II and IGF-1R, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNAs were determined using TaqMan quantitative (q) RT-PCR. Briefly, powdered muscles were extracted with TRIzol (Life Technologies, Carlsbad, CA, USA), total RNA concentration determined colorimetrically using the RiboGreen reagent (Life Technologies) and integrity evaluated by fractionation on the Experion automated electrophoresis system (Bio-Rad Laboratories, Hercules, CA, USA). After DNase treatment, total RNA was reverse transcribed using the SuperScript III First-Strand Synthesis System (Life Technologies). qPCR was performed on triplicates of each cDNA using an Applied Biosystems 7900HT cycler (Life Technologies), and the Platinum Quantitative PCR SuperMix-UDG reagents (Life Technologies). Primers and 5′-FAM/3′-TAMRA probes (sequences available upon request) were designed using Primer Express Software (Applied Biosystems). For quantification, standard curves were generated using the primers to amplify mouse cDNA, followed by gel purification and quantification of the purified amplicon. GAPDH mRNA abundance in individual samples was used to correct for sampling error.
Serum insulin concentration
Blood and serum were maintained on ice from collection until frozen for storage. Prior to thawing for the assay, 4-(chloromercuri) benzenesulfonic acid was added to each tube (1 vol.: 25 vol. serum) to a final concentration of 0.4 mm to block insulin-degrading enzyme activity (Chevenne et al. 1998). Insulin was measured by radioimmunoassay using reagents provided in kit form and specific for rodent insulin (RI-13K; Millipore, Billerica, MA, USA). Measurements were performed in duplicate.
Western blot analyses
Aliquots of the powdered hind limb muscles were sonicated (3 × 10 s at 20% amplitude on ice) in chilled buffer (20 mm Hepes, pH 7.9, 10 mm KCl, 1.5 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 1% NP40, 5 mm Na4P2O7, 25 mm α-sodium glycerophosphate, 10 mm NaF, 0.5% deoxycholate and 10% glycerol) containing phosphatase and protease inhibitors (PhosSTOP and cOmplete protease-inhibitor cocktail; Roche, Mannheim, Germany). Samples were microfuged at 4°C for 20 min, and the supernatant was stored at −80°C; protein concentration was determined by the BCA method (Smith et al. 1985).
Protein extracts were solubilized in Laemmli sample buffer (2% SDS, 60 mm Tris pH 6.8, 5 mg mL−1 bromophenol blue, 10% glycerol, 4% β-mercaptoethanol) and 10 μg protein (for Akt and ERK1/2 detection) or 50 μg protein (for ribosomal protein S6 kinase 1 (S6K1) and upstream binding factor (UBF)) of each sample were resolved on triple-wide SDS-PAGE gels (CBS Scientific, Del Mar, CA, USA) using the Laemmli buffer system followed by wet transfer to FluoroTrans W PVDF (Pall Corp., Port Washington, NY, USA) membranes in 0.5 Towbin buffer (12.5 mm Tris; 81 mm glycine; 10% methanol, pH 8.8). Membranes were blotted for: phospho-Akt (Ser473 and Thr308) and total panAkt; phospho-ERK1/2 (Thr202/Tyr204) and total ERK1/2; phospho-S6K1 (Thr389) and total S6K1 (with antibodies from Cell Signaling Technologies, Danvers, MA, USA), and total UBF (Santa Cruz Biotechnologies, Santa Cruz, CA, USA). The loading controls used were α-tubulin (Cell Signaling) for Akt and S6K1, GAPDH for ERK1/2 and β-actin for UBF. All primary antibodies were diluted in 5% IgG-free bovine serum albumin (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Primary antibody binding was detected using goat anti-rabbit, HRP-conjugated secondary antibodies (Bio-Rad; diluted in 5% non-fat dried milk) and visualized with ECL Plus (GE Lifesciences, Pittsburgh, PA, USA). Chemifluorescence was quantified using the 450 nm laser of the STORM Blot Imaging system (GE Lifesciences,) and the output was quantified with ImageQuant TL (GE Lifesciences) software. As not all samples from the study could be analysed on one gel, several measures were taken to control for blot-to-blot variability: (i) for each protein analysed all PVDF membranes were probed and visualized concurrently (Akt and ERK1/2 were analysed on the same blot concurrently); (ii) for each antibody (primary and secondary), a single dilution was prepared that was sufficient for all concurrent membrane incubations; and (iii) a control muscle extract was loaded in duplicate on every blot. If the control value for any blot differed from the mean value for all blots by > 5%, a correction factor was derived and all values were corrected accordingly. For each protein analysed, separate membranes were prepared for determining the abundances of the total and phosphorylated forms.
Statistics and calculations
Data were analysed by ANOVA using a general linear model (MINITAB, release 14, State College, PA, USA) with age and diet as the grouping variables. As pups within a litter are not statistically independent, we considered the litter as the statistical unit. Thus, pups at different ages within a litter were treated as a repeated measure, and litter was nested within dietary treatment. Results for E-UN, L-UN and GLP groups of offspring were analysed separately. All possible interactions were evaluated and, when significant (P < 0.1), differences between means within treatment or age were tested post-hoc by F-test using Tukey's method for multiple comparisons. Differences with P values < 0.05 were considered significantly different. Values are expressed as least square means ± SEM.
To estimate the accretion rate of muscle protein, a muscle protein growth curve was generated for each litter from the muscle protein mass of pups within the litter at 0, 2, 7 and 21 days of refeeding. The values were fitted by a polynomial equation that best described the curve (quadratic for CON; cubic for E-UN and L-UN); regression coefficients were all > 0.98. Instantaneous growth rates were determined from the first derivative of the equation at each age. Fractional protein growth rate (FGR) was calculated by dividing the growth rate by the muscle mass at each time point. Fractional degradation rate (FDR) was calculated from the difference between FSR and FGR.
Results
Body weight
Body weights over the entire experiment are illustrated in Fig. 1; weights to PN43 are shown on an expanded scale in the inset. Up to PN43, group means were calculated using the mean weight of male offspring within a litter; thereafter weights are means of individual male mice. Average body weight of E-UN offspring on PN11 was 25% (P < 0.001) less than those of age-matched CON pups. At PN22 and thereafter average body weight of E-UN and CON male offspring did not differ. At 18 months their body weights were 39.2 ± 2.0 and 44.6 ± 1.7 g, respectively (n.s.). L-UN offspring weighed 16% less than CON at PN22 (P < 0.001). After 3 weeks of refeeding (PN43) they weighed 12% less than CON offspring (P < 0.001). These differences persisted at 7 months of age when weights of CON and L-UN offspring were 38.9 ± 1.2 g (n = 8) and 33.0 ± 1.0 g (n = 7), respectively (P < 0.001). Subsequently, CON offspring continued to gain weight, but not L-UN pups; at 18 months L-UN body weights were 30.5 ± 1.9 g (CON vs. L-UN, P < 0.001; E-UN vs. L-UN, P < 0.05). GLP offspring were similar in body weight to the CON group at PN1 (GLP (n = 10): 1.35 ± 0.03 g; CON: 1.36 ± 0.01 g), weaning (GLP: 11.4 ± 0.3 g; CON: 12.3 ± 0.4) and 18 months of age (GLP: 43.2 ± 1.7 g; CON: 44.8 ± 1.5 g).
Figure 1. Body weights of mice from birth to 18 months of age.
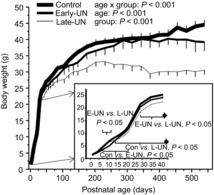
Control offspring were suckled on control dams; E-UN offspring were suckled on protein-restricted dams from PN1 to PN11 and control dams from PN11 to PN22; L-UN offspring were suckled on control dams from PN1 to PN11 and on protein-restricted dams from PN11 to PN22. All offspring were born to dams fed a control diet and were weaned to the same diet at PN22. Inset shows data from PN1 to PN43 on an expanded scale. Brackets encompass ages where body weights were significantly different as indicated. Values are least square means ± SEM; n = 7–8 litters per treatment group.
Bone length
Tibia and femur lengths provide an index of linear growth (data not shown). Both bones were approximately 6% shorter at PN11 in E-UN pups compared with CON pups (P < 0.001), but had returned to CON lengths after 1 week of refeeding. For L-UN pups, both bones were approximately 5% shorter at the end of the period of undernutrition (PN22). The tibia remained significantly shorter until the end of the study, whereas femur lengths were no longer different from CON at 18 months of age. Bone lengths were not different between GLP and CON offspring at any age.
Muscle growth
During early postnatal life, muscle growth includes changes in muscle composition and length. Due to changes in hydration, protein concentration increased from 98 mg protein g−1 muscle at PN11 to 158 mg g−1 muscle at PN43 and was not affected by dietary treatment. Thus, muscle weight significantly under-represents the gain in muscle protein mass over this time. Muscle growth (Fig. 2) is presented by the combined protein masses of the gastrocnemius, tibialis anterior and quadriceps muscles corrected for bone length.
Figure 2. Protein mass and fractional growth rates.
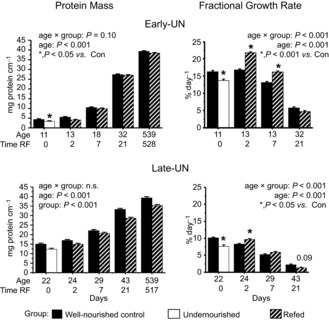
Protein mass and fractional growth rates of pooled gastrocnemius, tibialis anterior and quadriceps muscle from one hind limb of Control mice, mice undernourished from PN1 to PN11 (Early-UN) or PN11 to PN22 (Late-UN), or refed (RF) for 2 days, 7 days, 21 days or 18 months (protein mass only). Protein mass is normalized for bone length. Fractional growth rates were calculated using the first derivative of a polynomial that described the change of muscle protein mass with age for each litter. Values are least square means ± SEM; n = 7–8 litters per treatment group.*Significant difference between age-matched groups where age×group is significant.
The protein mass of E-UN muscles at PN11 was 76% of CON values (P < 0.001); this deficit also was evident in the FGR at this age (Fig. 2). Upon refeeding, there was an immediate acceleration in growth velocity and after 2 days it was 30% higher than in age-matched CON (P < 0.001). At PN18, FGR was still significantly higher than in CON offspring and muscle protein mass of the E-UN pups was restored to normal. Thereafter, FGR and protein masses of E-UN and CON muscles were indistinguishable.
The L-UN muscle protein mass at PN22 was 82% of CON values (P < 0.001); this deficit also was evident in the FGR at this age (P < 0.05). With refeeding, FGR accelerated briefly (P < 0.05) and some of the deficit in protein mass was recuperated so that after 7 days of refeeding the muscle protein mass was only 7% less than in CON (P < 0.001). However, unlike the E-UN group, this acceleration was not sustained for 7 days of refeeding and by PN43 the deficit had returned to approximately 13% and remained at this level until 18 months of age (P < 0.001).
There was no significant difference in the muscle protein masses of GLP and CON offspring at PN22 (13.8 ± 0.5 and 13.5 ± 0.15 mg protein cm−1 bone length for GLP and CON muscles, respectively) and 18 months of age (40.3 ± 1.0 and 38.0 ± 0.7 mg protein cm−1 bone length for GLP and CON muscles, respectively).
Protein synthesis and degradation rates
In CON pups, postprandial FSR declined by approximately 75% from PN11 to PN43 (Fig. 3); this developmental decline continued so that by18 months, values were approximately 60% of PN43 values. FDR in CON offspring remained relatively unchanged to 4 weeks of age and decreased thereafter. In 18-month-old mice, when growth has ceased, FDR and FSR were equivalent by definition.
Figure 3. Fractional rates of muscle protein synthesis and protein degradation.
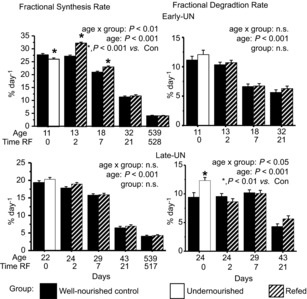
Fractional rates of muscle protein synthesis and protein degradation for pooled hind limb muscles from Control mice, mice undernourished from PN1 to PN11 (Early-UN) or PN11 to PN22 (Late-UN), or refed (RF) for 2 days, 7 days, 21 days or 18 months (synthesis rates only). Protein synthesis rate was measured in vivo using a flooding dose of l-[4-3H]-phenylalanine; protein degradation rate was calculated as the difference between fractional protein synthesis and growth rates. Values are least square means ± SEM; n = 7–8 litters per treatment group.*Significant difference between age-matched groups where age×group is significant.
A reduction in FSR was largely responsible for the reduced muscle protein mass in E-UN progeny at PN11 (P < 0.001; Fig. 3); FDR was the same as in CON. After 2 days of refeeding, FSR accelerated above CON values, and remained higher for at least a week (P < 0.001). As there was no change in FDR, the responses in FSR were responsible for the acceleration in E-UN muscle protein accretion. The reduced muscle protein mass in L-UN mice at PN22 was attributable to an increase in FDR (P < 0.01). With refeeding, FDR immediately returned to normal which, together with a slightly greater FSR (albeit not statistically significant), resulted in the brief increase in FGR (P < 0.05). These small changes cumulatively supported some catch-up growth over the first week, but thereafter this was insufficient to keep up with the CON progeny.
Translational efficiency (KRNA) and capacity
Changes in protein synthesis occur as a result of an increase in either the efficiency with which ribosomes translate mRNA and/or the abundance of ribosomes, i.e. translational capacity. To verify that variation in the total RNA reflects ribosomal abundance and is not influenced by age or dietary treatment, we quantified the amount of 18S rRNA per unit of total RNA by qRT-PCR. There was no age × treatment (P = 1), age (P = 0.82) or dietary treatment (P = 0.97) effect; mean values for CON, undernourished and rehabilitated muscles (regardless of age) were 206, 204 and 208 (± 20 pooled SE) fmol 18S rRNA μg−1 RNA reverse transcribed, respectively; mean values at PN11 and PN43 (regardless of treatment) were both 204 (± 20 pooled SE) fmol 18S rRNA. Thus, the ratio of RNA to total protein (RNA/TP) provides an accurate reflection of ribosomal abundance.
KRNA (Fig. 4) increased slightly until weaning and then remained relatively unchanged to PN43. At 18 months of age, however, KRNA was significantly lower than at any other age. Maternal dietary treatment or nutritional rehabilitation had no effect on KRNA at any age.
Figure 4. Protein synthetic efficiency and protein synthetic capacity.
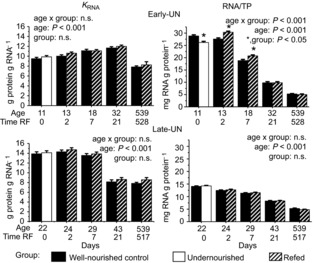
Protein synthetic efficiency (KRNA) and protein synthetic capacity (RNA/TP) for pooled hind limb muscles from Control mice, mice undernourished from PN1 to PN11 (Early-UN) or PN11 to PN22 (Late-UN), or refed (RF) for 2 days, 7 days, 21 days, or 18 months. KRNA was calculated from the fractional synthesis rate and RNA content (RNA/TP). Values are least square means ± SEM; n = 7–8 litters per treatment group.*Significant difference between age-matched groups where age×group is significant.
In CON muscles, RNA/TP declined steadily with age and was at its lowest at 18 months of age. In the muscles of the undernourished E-UN offspring, RNA/TP was significantly lower than CON (P < 0.05), but with refeeding values increased above the levels in the age-matched CON (P < 0.05). The increase was sustained for 1 week, and then values returned to CON levels. Thus, the changes in FSR with undernutrition and refeeding in the E-UN mice were largely explained by changes in translational capacity. Like FSR, RNA/TP values for L-UN muscles were not different from those of age-matched CON at all ages regardless of nutritional status.
Insulin and IGF
To assess if the observed differences in the growth response between E-UN and L-UN could be due to differences in nutrient availability or endocrine stimulation, we measured plasma insulin concentrations, and muscle IGF-I, IGF-II and IGF-1R expression.
In CON mice plasma insulin increased from PN11 to PN32 with no further change between PN32 and PN43 (Fig. 5). In both undernourished E-UN and L-UN mice plasma insulin was significantly lower than in age-matched CON (P < 0.05). In E-UN offspring, levels increased upon refeeding, and at PN13 they were approximately double CON values (P = 0.02). At subsequent times, values were not different from CON. In L-UN mice, insulin increased with age, but at all ages they were significantly lower than in CON mice (P < 0.001). At both PN29 and PN43 the insulin concentration values for L-UN offspring were significantly lower than for E-UN mice at PN32 (P < 0.01). By 18 months plasma insulin had risen in all groups (CON: 105 ± 24 μU ml−1; E-UN: 128 ± 25 μU ml−1; L-UN: 105 ± 24 μU ml−1, n.s.).
Figure 5. Serum insulin concentrations and weight of food in the stomach.
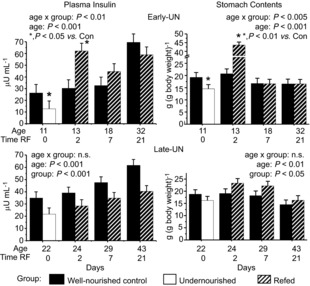
Serum insulin concentrations and weight of food in the stomach immediately following death for Control mice, mice undernourished from PN1 to PN11 (Early-UN) or PN11 to PN22 (Late-UN), or refed (RF) for 2, 7 or 21 days. Values are least square means ± SEM; n = 7–8 litters per treatment group.*Significant difference between age-matched groups where age×group is significant.
To determine if the trends in insulin concentrations could be explained by the amount of food eaten immediately preceding the tissue sampling, we measured the weight of food in the pups’ stomach (Fig. 5). When normalized for body weight, stomach contents were similar across all ages in CON pups (age effect, P = 0.19). Both undernourished E-UN (PN11) and L-UN (PN22) pups had reduced stomach contents compared to age-matched CON, consistent with their lower insulin values (P < 0.05). After refeeding for 2 days, stomach fill was significantly higher in both E-UN (P < 0.01) and L-UN (P < 0.05) pups than in age-matched CON. This difference was much greater for E-UN pups, matching their higher plasma insulin values. Thereafter, values for stomach fill were similar for E-UN and CON offspring. In L-UN offspring, stomach fill remained consistently higher than in age-matched CON (P < 0.05).
Local production of IGFs was measured quantitatively from tissue mRNA expression patterns (Fig. 6). The mRNA abundances are expressed in absolute molar abundances and can be compared across all ages. In muscles from CON mice, IGF-I mRNA abundance decreased by > 50% between PN11 and PN18 and then remained relatively unchanged (age effect n.s., from PN18). At PN11, IGF-II mRNA was approximately 10-fold more abundant than IGF-I mRNA but had decreased by 88% at PN22, after which there was no further change; at PN43 the abundance was approximately half that of IGF-I mRNA. IGF-1R decreased by 40% between PN11 and PN18, and then remained unchanged. In both E-UN and L-UN groups, undernutrition was associated with a reduction in the expression of IGF-I mRNA (P < 0.05). On refeeding, there was an immediate up-regulation of its expression, in both groups, and after 2 days values were 25% higher than in age-matched CON (P < 0.05). The increase was not sustained 7 days after refeeding. IGF-II mRNA levels were not affected by the dietary treatments. IGF-1R mRNA expression was up-regulated in the muscle of the undernourished E-UN offspring (P < 0.01), and returned to normal upon re-feeding. IGF-1R mRNA expression for L-UN and age-matched CON was similar regardless of dietary treatment.
Figure 6. Expression of IGF-I, IGF-II and IGF-IR.
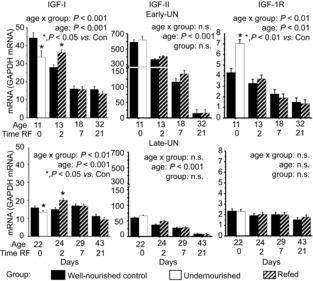
Expression of IGF-I, IGF-II and IGF-IR mRNAs mRNAs relative to GAPDH mRNA in pooled hind limb muscles from Control mice, mice undernourished from PN1 to PN11 (Early-UN) or PN11 to PN22 (Late-UN), or refed (RF) for 2, 7 or 21 days. mRNA was quantified by TaqMan qRT-PCR. Values are least square means ± SEM; n = 7–8 litters per treatment group.*Significant difference between age-matched groups where age×group is significant.
Intramuscular signalling pathways
In the growing muscle, key cellular signalling pathways that transduce the nutrient and hormone signals to regulate cell growth include the phosphoinositide 3-kinase/Akt pathway, the mitogen-activated protein kinase/ERK signalling cascade, and the mammalian target of rapamycin (mTOR) signalling pathways. Activation of these signalling cascades was assessed from the phosphorylation of Akt, ERK1/2 and S6K1, a downstream target of mTORC1. The total abundance of both Akt and ERK1/2 decreased with age (P < 0.001) with the most significant changes occurring over the first 3 weeks of age; undernutrition or refeeding had no effect on their total abundance (data not shown). For Akt, there was a reciprocal increase with age in the ratio of Ser473 (P < 0.001) or Thr308 (P < 0.02) phosphorylated Akt to total Akt so that total phosphorylation of either residue did not change with age. There was no effect of preweaning nutrition on Akt phosphorylation, suggesting that the activity of the upstream kinases in the postprandial state was unaffected by the preweaning diet (data not shown). The Thr202/Tyr204 phosphorylation of ERK1/2 also was not influenced by dietary treatment (data not shown). The combination of changes in total and relative phosphorylation were such that total phosphorylation remained relatively constant except at PN43 when levels were significantly lower than at all other ages.
Total S6K1 abundance decreased continuously with age and was not altered by the preweaning diet (Fig. 7). In CON muscles, relative phosphorylation of S6K1 increased until approximately PN22 so that total phosphorylation remained relatively unchanged (P = 0.17). Subsequently, relative phosphorylation remained unchanged; thus, given the reduction in total S6K1, total phosphorylation of S6K1 decreased with age (P < 0.05). An effect of preweaning nutrient intake on S6K1 was evident only in E-UN pups. In the undernourished pups, S6K1 phosphorylation was significantly lower than in CON offspring (P < 0.05). Upon refeeding, both relative and total phosphorylation almost doubled and were significantly higher than in age-matched CON for 2 days (P < 0.05). In the L-UN offspring, S6K1 phosphorylation was similar to CON regardless of dietary treatment.
Figure 7. Abundance of ribosomal S6 kinase 1 (S6K1) and S6K1 phosphorylated on Thr389.
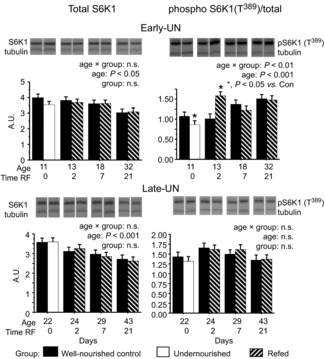
Abundance of ribosomal S6K1 and S6K1 phosphorylated on Thr389 (relative to total S6K1) in pooled hind limb muscles from Control mice, mice undernourished from PN1 to PN11 (Early-UN) or PN11 to PN22 (Late-UN), or refed (RF) for 2, 7 or 21 days. Insets are representative blots showing total and phospho-S6K1 and α-tubulin (loading control) for an individual mouse from the corresponding group identified below the blot. Values are least square means ± SEM; n = 7–8 litters per treatment group.*Significant difference between age-matched groups where age×group is significant.
Total UBF
Total UBF decreased exponentially over the studied age range (Fig. 8). Undernutrition in E-UN pups resulted in a significant decrease in expression (P < 0.05). With refeeding there was a rapid increase in abundance that was sustained to PN18 (P < 0.05); by PN32 values were similar to those for age-matched CON. For L-UN pups, values were not different from those for age-matched CON regardless of nutritional status.
Figure 8. Abundance of total UBF1 and 2.
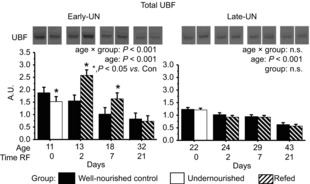
Total UBF1 and 2 in pooled hind limb muscles from Control mice, mice undernourished from PN1 to PN11 (Early-UN) or PN11 to PN22 (Late-UN), or refed (RF) for 2, 7 or 21 days. Insets are representative blots showing total UBF for an individual mouse from the corresponding group identified below the blot. Values are least square means ± SEM; n = 7–8 litters per treatment group.*Significant difference between age-matched groups where age × group is significant.
Discussion
Our data emphasize the significant impact that even a brief perturbation in the nutritional environment at a critical period during early life has on adult size and skeletal muscle mass. This phenomenon has been observed in most mammalian species, indicating that the effects of the undernutrition are exerted on mechanisms that are fundamental to the regulation of anabolic processes that govern growth. Experiments in animal models, as well as observations in human populations, indicate that the magnitude of the effect is largely influenced by four variables: the nature of the nutritional insult, the developmental age when it is experienced, its severity and the duration. Our data demonstrate that with regard to the skeletal musculature, the developmental window during which the nutritionally-induced growth deficit is experienced is a fundamental determinant of the muscles’ capacity to recover and of adult muscle mass.
The experiment was designed to induce growth faltering over distinct developmental stages. Several studies using the rat model have compared the effects of prenatal vs. postnatal malnutrition on skeletal muscle (Glore & Layman, 1983; Desai et al. 1996). As we did not measure muscle weight at birth, we cannot discern if maternal protein restriction during gestation impaired intrauterine muscle growth. Although there was no deficit in body weight at PN1, body weight does not necessarily mirror muscle weight or metabolic mass (Kim et al. 1981; Gokulakrishnan et al. 2012). Regardless, our data for the GLP offspring indicate that in the mouse, like the rat, maternal dietary protein restriction from preconception and throughout gestation did not produce any long-term deficit in muscle mass. Thus, it is unlikely that fibre number is impacted in this nutritional model, confirming observations in the rat (Mallinson et al. 2007). Combined with observations, including our own (Desai et al. 1996; Balasa et al. 2011), that muscle mass in the offspring both born to and suckled by protein-restricted dams is compromised over the long term, we infer that it is the postnatal not the prenatal period of muscle development that is the critical determinant of adult muscle mass unless the degree of restriction is severe, or abruptly altered during critical periods (as discussed by Mallinson et al. 2007). Thus, the majority of our measurements were restricted to the evaluation of the two postnatal treatment groups, E-UN and L-UN.
The comparison between the two postnatal groups was designed to maintain the duration and severity of the growth restriction constant, and only alter its timing. All measurements relating to overall growth are consistent in demonstrating that following nutrition-induced growth retardation over the first 11 days of life, catch-up growth was able to restore the pups to the growth trajectory of CON pups. Upon refeeding, the ability of E-UN offspring to sustain higher rates of muscle protein accretion than CON pups enabled the deficit in muscle mass to be recuperated. The deficit in bone lengths also was restored after 1 week, whereas body weight took up to 2 weeks to return to normal. Data on recovery of body and organ weights for the rat following 8–10 days of neonatal undernutrition using the expanded litter model (Winick et al. 1968; Jou et al. 2013) have shown more rapid recovery of body weight when pups were refed on a higher plane of nutrition than in our study. This rapid refeeding, however, was associated with greater adipose tissue accretion; skeletal muscle was not evaluated. Pups subjected to a more moderate rate of refeeding after 10 days of undernutrition (Jou et al. 2013) were not followed beyond weaning. In our study, the recovery of muscle mass and body size in E-UN offspring was sustained to 18 months of age. Thus, the combined observations from GLP and E-UN offspring do not favour the possibility that a nutrient deficit during the most active stage of muscle maturation incurs permanent changes to the anabolic pathways that regulate muscle growth.
In contrast, undernutrition for 11 days in the second half of the suckling period, i.e. L-UN, produced a smaller deficit in muscle protein and body weight (when compared to age-matched CON) than the same length of undernutrition in E-UN offspring. Refeeding, however, was associated with a smaller acceleration in protein accretion that was sustained for less than 1 week. Indeed, after 3 weeks of refeeding there was a tendency (P < 0.09) for a deceleration in the protein FGR in L-UN offspring compared to CON. Thus, over the first week of refeeding there was a slight narrowing of the protein mass deficit, but the difference widened again after 3 weeks and remained as such to 18 months of age. Overall, despite the smaller growth deficit incurred, L-UN offspring did not regain their muscle mass. This comparison demonstrates that the long-term consequences of a nutritional deficit and consequent growth retardation for adult skeletal muscle mass was primarily dependent on the developmental window during which the insult was incurred and recovery initiated, and less dependent on the severity of the growth insult.
Previously, we have demonstrated that the primary driving force for the elevated rate of protein accretion in the immature muscle is the capacity to attain high rates of protein synthesis (Davis & Fiorotto, 2009). Thus, we evaluated the extent to which the contrasting growth responses in E-UN and L-UN offspring could be explained by differences in the muscles’ ability to accelerate protein synthesis upon refeeding. All the measurements were performed with the offspring in the postprandial state, as verified by the presence of food in the stomach, and therefore reflect the maximum rates of protein synthesis. In young rodents that suckle or eat with high frequency to support their rapid growth rates, the diurnal variations in muscle FSR are relatively small (Hall et al. 1979; Reeds et al. 1986). Thus, our estimates for FSR and the derived FDR provide a reasonably accurate measure of the most prevalent condition at this age. The lower FSR in the undernourished E-UN offspring was responsible for the reduced muscle growth rates. The capacity of E-UN offspring to attain higher FSR than CON up to 7 days following the start of refeeding enabled catch-up growth to occur. Changes in FDR did not appear to contribute to the enhanced growth. In contrast, the lower FGR of undernourished L-UN offspring can be attributed to their higher FDR. This difference in the response to undernutrition compared to E-UN pups may be attributable to the disparity in the maturity of the hypothalamic–pituitary–adrenal axis at these two ages. Until PN12, basal corticosterone levels are low, and the response to a stress is markedly blunted (Schmidt et al. 2003). By the third week of life, inadequacies in both protein and energy intakes are associated with high plasma corticosterone levels (Monk et al. 2006) which stimulate protein degradation. With higher glucocorticoid levels, however, lower FSRs might have been anticipated in undernourished L-UN muscles compared to CON offspring (Odedra et al. 1983; Gokulakrishnan et al. 2012). This discrepancy is probably explained by the different time scale over which the FGR and FSR measurements were made, and that the FSR measurement was made only in the postprandial state. Predictably, refeeding the higher protein CON diet normalized FDR in L-UN pups (Nagasawa et al. 1998) which, together with a small increase, albeit non-statistically significant, in FSR at PN24 probably accounted for the brief acceleration in protein deposition in L-UN offspring. Additionally, we cannot exclude the possibility that FSR increased acutely sometime between the start of refeeding and 2 days later. Subsequently, neither FSR nor FDR differed between CON and L-UN offspring and thus the acceleration in growth rate necessary to achieve catch-up growth did not occur. Thus, age-dependent differences in the extent to which FSR could be raised with refeeding determine whether skeletal muscle protein mass was recuperated.
The primary determinants of FSR are the muscles’ ribosome abundance, which determines maximum translational capacity, and the rate at which ribosomes are stimulated to initiate translation, i.e. KRNA. In the E-UN progeny, translational capacity before refeeding at PN11 was significantly lower than in age-matched CON. The increase in translational capacity was sustained for at least 1 week upon refeeding and enabled the recovering E-UN offspring to increase maximum FSR relative to CON, and thereby accelerate the rate of protein deposition. As protein deposition outpaced the increase in RNA, the ratio of RNA to protein returned to CON levels after 3 weeks of refeeding. This response in RNA levels was completely absent when the L-UN pups were refed, and thus any acceleration in FSR in this group could only occur by means of an increase in the synthetic efficiency.
Although translational capacity may be elevated, the rate at which proteins are synthesized also depends on the ribosomes’ translational efficiency. As we observed previously for suckling rats (Davis et al. 1993), KRNA increased over the first month of life, decreased in the pubertal period, and at PN43 and 18 months values were significantly lower than in the younger mice. No effect of nutrient status on KRNA was observed in either E-UN or L-UN offspring. Thus, in the E-UN offspring, the increase in FSR with refeeding can probably be ascribed to the higher ribosomal abundance. We cannot exclude entirely the possibility that there was no increase in FSR, and hence KRNA, over the first 2 days of refeeding. However, this is unlikely as it would have entailed an increase above the values for the CON offspring, a possibility for which there is little support in the literature. The gains in either KRNA or translational capacity that are necessary to accelerate FSR and protein accretion did not occur in L-UN offspring and consequently catch-up growth was not supported.
Given the difference in the response to nutritional rehabilitation at the two ages, we considered if there were differences between groups in the extracellular cues and/or the activation of the signalling pathways that transmit these cues to the muscle's translational machinery. In immature animals plasma insulin and amino acids are strong promoters of muscle anabolism and both are determined primarily by food intake (Davis & Fiorotto, 2009). Estimates of acute food intake can be obtained by measuring the amount of food present in the stomach after a brief fast (Henning et al. 1979). The stomach content of both undernourished groups of pups was significantly smaller than in the age-matched CON, consistent with the effect of dietary protein restriction on reducing milk production. The higher stomach contents of pups in both groups compared to CON after 2 days of refeeding (PN13 and PN24) suggest that at both ages there was a compensatory increase in food intake. In the E-UN group the difference in contents relative to age-matched CON was substantially greater than in the L-UN group and may be explained by the immaturity of satiety control mechanisms that do not mature until the third week of life (Hall & Rosenblatt, 1977; Henning et al. 1979). Indeed, stomach contents at PN18 and PN32 in the E-UN group were similar to those of the age-matched CON. In contrast, stomach contents of L-UN offspring were significantly higher than CON at all ages. This response is consistent with the higher expression levels of hypothalamic orexigenic peptides observed in the weaned offspring of dams fed protein-restricted diets during lactation (Cripps et al. 2009; Coupe et al. 2009). Thus, nutrient availability during the undernutrition period was limiting, but it cannot explain the contrasting growth responses of L-UN and E-UN offspring upon nutritional rehabilitation.
Plasma insulin concentrations in E-UN pups were commensurate with food intake: values were lower compared to CON while undernourished, increased above CON values 2 days after re-feeding, but were similar thereafter. In L-UN offspring, undernutrition also resulted in lower serum insulin concentrations. However, unlike the E-UN group, values remained lower than CON at all ages following refeeding, despite the larger amount of food present in the stomach. Although this response could be attributed to a reduction in insulin production, it could also reflect a reduced need for insulin due to increased end organ sensitivity, especially the sensitivity of skeletal muscle. Indeed, a heightened sensitivity of skeletal muscle to insulin following perinatal undernutrition has been observed repeatedly provided diets do not have a high fat content (Ozanne et al. 1996; Gavete et al. 2005; Muhlhausler et al. 2009). In support of this, we found equivalent phosphorylation (absolute or relative to total) of Akt and ERK1/2 in L-UN and CON pups despite the lower plasma insulin levels. In vivo, Akt and ERK1/2 are both phosphorylated following insulin receptor activation and, through parallel mechanisms, they stimulate mTORC1 signalling to activate translation (Winter et al. 2011). The absence of differences in Akt and ERK1/2 phosphorylation despite the higher insulin levels at PN13 in E-UN offspring is probably due to the attainment of maximal activation at lower insulin levels as observed for CON pups. Together, these data indicate that the activation and responsiveness of the muscle insulin signalling pathways that regulate protein translation in muscle are not compromised by undernutrition or following nutritional rehabilitation during either the early or the later parts of the sucking period and therefore do not contribute to the difference in growth responses of E-UN vs. L-UN muscles.
Activation of protein synthesis with feeding is also mediated in part by the resulting increase in circulating amino acids (Davis et al. 1993). Amino acids, primarily leucine, mediate this effect by activating the mTORC1 complex independently of Akt and ERK1/2 (Davis & Fiorotto, 2009; Winter et al. 2011). When FSR is below maximum, the stimulatory effects of leucine and insulin are additive (O'Connor et al. 2003). To assess mTORC1 activity, we measured the phosphorylation of S6K1 on the Thr389 residue, a target of mTORC1. In undernourished E-UN muscles, phosphorylation of S6K1 was significantly reduced compared to age-matched CON. As activation of Akt and ERK1/2 were not different from CON values, this response probably reflects reduced amino acid-induced mTORC1 activation as a result of the pups’ reduced protein intake. However, as KRNA was not different from CON values, it suggests that the reduction in mTORC1 signalling was not of a magnitude sufficient to impact translation initiation. After 2 days of refeeding, mTORC1 phosphorylation of S6K1 was significantly higher than in CON, consistent with the higher values for stomach food content, plasma insulin and FSR; but again this response did not impact translational efficiency because this was already maximal. In the L-UN pups, neither the undernourished condition nor refeeding at any time altered the phosphorylation of S6K1 compared to age-matched CON muscles. Thus, the difference in mTORC1 activity towards S6K1, together with the differences in total RNA, distinguish the protein synthetic responses of E-UN and L-UN offspring to undernutrition and rehabilitation. Moreover, these responses do not entail differences in KRNA, and hence the processes of translation initiation, elongation and termination, but are the result of the muscles’ capacity for protein synthesis.
Ribosomal DNA transcription by RNA polymerase 1 is the primary limiting factor for ribosome biogenesis (Hannan et al. 2003b). Studies in cardiomyocytes and skeletal muscle myotubes have demonstrated that ribosome biogenesis to increase the cells’ protein synthetic capacity underlies the hypertrophic growth in post-mitotic, differentiated cells (Hannan et al. 2003b; Nader et al. 2005). The nucleolar transcription factor UBF is a key regulator of RNA polymerase1 activity and rRNA transcription in both cardiomyocyte and skeletal muscle hypertrophy. Up-regulation of UBF protein abundance, its phosphorylation and/or its availability following a hypertrophic stimulus are essential for the resulting increases in rRNA synthesis, protein synthesis, and cell size (Hannan et al. 1996, 2003b; von Walden et al. 2012). Our measurements demonstrated a decline in UBF abundance as the muscle matured, similar to the pattern observed during the differentiation of cultured myoblasts (Larson et al. 1993). These changes parallel the decrease in RNA concentration with age. In the younger E-UN offspring, the expression of UBF was reduced when pups were undernourished, and upon re-feeding its abundance doubled relative to CON levels within 2 days and remained greater than CON for at least 7 days. These values mirror the differences in RNA abundance that occurred at the same time, and are commensurate with accelerated rDNA transcription and ribosomal production. The absence of differences in UBF abundance in L-UN pups compared to CON is also consistent with the lack of acceleration in their muscle growth.
There are a number of independent ways in which UBF activity is regulated. Importantly, it has been well-documented that inhibition of mTORC1 activity by rapamycin also inhibits UBF activity by either reducing its availability (Nader et al. 2005) or impairing key phosphorylation events (Hannan et al. 2003a). Of pertinence to our study is the demonstration that in both proliferating NIH 3T3 and non-proliferating cardiac myocytes, rDNA transcription requires the activation of S6K1 by mTOR (Hannan et al. 2003a). In addition, the translation of mRNAs that contain a 5′-terminal oligopyrimidine tract, including the mRNAs that encode all ribosomal proteins, is highly sensitive to mTORC1 activity (Thoreen et al. 2012). The increased production of these proteins, in conjunction with the increase in rDNA transcription, will promote ribosome production. Thus, the ability of the E-UN muscles, in contrast to the L-UN muscles, to enhance mTORC1 activity and S6K1 phosphorylation in the early stages of nutritional rehabilitation may represent one critical difference in the disparate responses in muscle growth observed at the two ages. The factors responsible for the transiently greater phosphorylation of the mTORC1 signalling pathway in the E-UN pups at PN13 (but not Akt or ERK) are unclear. It is possible that the high milk intake of the pups at this time results in greater amino acid availability and increased activation of mTORC1 independently of the insulin signalling pathway (Davis & Fiorotto, 2009).
The expression of IGF-I, IGF-II and IGF-1R over the two age ranges when rehabilitation occurred also could be of pertinence in explaining the divergent responses of E-UN and L-UN muscles. Signalling via the IGF-1R increases the phosphorylation and transcriptional activation of UBF, and in some cell types it also increases UBF abundance by stabilizing the protein (Wu et al. 2005; Sun et al. 2007). Thus, the substantially higher expression levels of IGFs and IGF-1R at the younger ages, especially at PN13, may serve to further enhance the production and activity of UBF in the recovering E-UN pups.
Perspectives/conclusions
The design of our experiment enabled us to establish that developmental age at the time of nutritional rehabilitation is the crucial determinant of the skeletal muscle's capacity to regain its normal growth trajectory following an episode of nutritionally-induced growth retardation. As shown previously for other compositional aspects of limb muscles (Fiorotto & Davis, 1997), the maturation of the musculature, unlike growth of its protein mass, is relatively resilient to the effects of a reduction in food intake, provided that it is not extreme. Should the preweaning food supply be suboptimal, this response will enhance the chance of survival by favouring the functional development of the muscle over its growth. However, in so doing the heightened responsiveness of the muscles’ anabolic processes to growth stimuli, including ribosomal production, as well as the stimuli themselves will diminish and thereby the capacity to accelerate growth becomes blunted. In addition our results suggest that, provided the muscle is still responsive to anabolic stimuli, the provision of adequate protein to maximally stimulate activation of the mTORC1 signalling pathway may be a crucial determinant of the rate of catch-up growth. However, this intervention is also likely to be of limited benefit once the muscles’ critical window of anabolic responsiveness has closed.
Translational perspective
Both historical cohort studies of human populations and prospective studies in humans and experimental animals observe that skeletal muscle mass is permanently compromised by events during development that impair normal growth, including suboptimal nutrition. Reduced adult muscle mass is correlated with shorter lifespan, increased morbidities and reduced quality of life. Despite numerous investigations, the relative importance of the duration, severity, and developmental timing of the nutritional insult on the programming of muscle mass are unclear. The goals of this study were to differentiate between these possibilities and identify the mechanism responsible. We studied the recovery of muscle mass in offspring of mouse dams fed a protein-restricted diet during gestation, or undernourished from birth to 11 days of age, or 11 to 22 days of age; distinct processes are responsible for muscle growth at these ages. We determined that a growth deficit incurred before 11 days of age could be recuperated, but not if recovery was delayed to 22 days, despite a similar duration and severity of growth restriction. The recovery of muscle protein mass at the younger ages was enabled by the capacity to accelerate protein synthesis due to increased ribosome production; the response was absent in older offspring. Identification of the factors responsible for this age-dependent response, as well as clarification of the role of specific nutrients in modulating the responses, will facilitate translation of the findings to human infants. The results emphasize the importanceof considering developmental age in the management of infants for whom standard feeding is precluded.
Acknowledgments
The authors acknowledge E.O. Smith for assistance with statistical analysis and A. Suryawan for technical input.
Glossary
- BCA
bicinchoninic acid
- CON
control
- ERK1/2
extracellular signal-regulated protein kinases 1 and 2
- E-UN
early undernutrition treatment
- FDR
fractional degradation rate
- FGR
fractional growth rate
- FSR
fractional synthesis rate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GLP
gestation low protein diet treatment
- IGF-I
insulin-like growth factor-I
- IGF-II
insulin-like growth factor-II
- IGF-1R
type 1 IGF receptor
- KRNA
ribosomal translational efficiency
- LP
low protein
- L-UN
late undernutrition treatment
- mTOR
mammalian target of rapamycin
- PN
postnatal
- S6K1
ribosomal protein S6 kinase 1
- TP
total protein
- UBF
upstream binding factor
Key points
Inadequate nutrient intake during early life can programme a low adult muscle mass. We have used a mouse model to identify the developmental window when the skeletal musculature is vulnerable to programming and to identify factors that limit the muscle's ability to respond when normal nutrition is restored.
We established that the developmental age when nutritional rehabilitation occurs following an episode of poor nutrition, rather than the duration or severity of the nutrient restriction, is the critical factor that determines if muscle mass can be recuperated.
The ability to recover depends on whether the muscles’ translational capacity, i.e. ribosomal abundance, can increase sufficiently to raise protein synthesis rates sufficiently to accelerate protein deposition.
We show that the ability to increase ribosomal abundance was associated with increased expression of the nucleolar transcription factor UBF (upstream binding factor), which regulates RNA polymerase 1 activity and rRNA transcription, the limiting factor for ribosomal production.
Additional information
Competing interests
The authors have no competing interests.
Funding
The research was funded by NIH grant no. AR46308 and USDA CRIS 6250-51000-054 to M.L.F.
Author contributions
M.L.F. and T.A.D. were responsible for conception and design of the experiments, interpretation of data and writing of the manuscript; M.L.F., H.A.S., C.V-M., I.E. and R.F. contributed to the collection and analysis, and interpretation of data. All authors approved the final version of the manuscript. All experiments were performed at the Children's Nutrition Research Center at Baylor College of Medicine, Houston, Texas, USA.
References
- Agbulut O, Noirez P, Beaumont F. Butler-Browne G. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell. 2003;95:399–406. doi: 10.1016/s0248-4900(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Balasa A, Sanchez-Valle A, Sadikovic B, Sangi-Haghpeykar H, Bravo J, Chen L, Liu W, Wen S, Fiorotto ML. Veyver IB. Chronic maternal protein deprivation in mice is associated with overexpression of the cohesin–mediator complex in liver of their offspring. J Nutr. 2011;141:2106–2112. doi: 10.3945/jn.111.146597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG. Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Brown LD. Endocrine regulation of fetal skeletal muscle growth: impact on future metabolic health. J Endocrinol. 2014;221:R13–R29. doi: 10.1530/JOE-13-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdge GC, Hanson MA, Slater-Jefferies JL. Lillycrop KA. Epigenetic regulation of transcription: a mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br J Nutr. 2007;97:1036–1046. doi: 10.1017/S0007114507682920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevenne D, Letailleur A, Trivin F. Porquet D. Effect of hemolysis on the concentration of insulin in serum determined by RIA and IRMA. Clin Chem. 1998;44:354–356. [PubMed] [Google Scholar]
- Coupe B, Grit I, Darmaun D. Parnet P. The timing of “catch-up growth” affects metabolism and appetite regulation in male rats born with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2009;297:R813–R824. doi: 10.1152/ajpregu.00201.2009. [DOI] [PubMed] [Google Scholar]
- Cripps RL, Martin-Gronert MS, Archer ZA, Hales CN, Mercer JG. Ozanne SE. Programming of hypothalamic neuropeptide gene expression in rats by maternal dietary protein content during pregnancy and lactation. Clin Sci. 2009;117:85–93. doi: 10.1042/CS20080393. [DOI] [PubMed] [Google Scholar]
- Davis TA. Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care. 2009;12:78–85. doi: 10.1097/MCO.0b013e32831cef9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Nguyen HV. Reeds PJ. Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol. 1993;265:R334–R340. doi: 10.1152/ajpregu.1993.265.2.R334. [DOI] [PubMed] [Google Scholar]
- Derrickson EM. Lowas SR. The effect of dietary protein levels on milk protein levels and postnatal growth in laboratory mice (Musmusculus. J Mammol. 2007;88:1475–1481. [Google Scholar]
- Desai M, Crowther NJ, Lucas A. Hales CN. Organ-selective growth in the offspring of protein-restricted mothers. Br J Nutr. 1996;76:591–603. doi: 10.1079/bjn19960065. [DOI] [PubMed] [Google Scholar]
- Fiorotto ML. Davis TA. Food intake alters muscle protein gain with little effect on Na+-K+-ATPase and myosin isoforms in suckled rats. Am J Physiol. 1997;272:R1461–R1471. doi: 10.1152/ajpregu.1997.272.5.R1461. [DOI] [PubMed] [Google Scholar]
- Fiorotto ML, Davis TA. Reeds PJ. Regulation of myofibrillar protein turnover during maturation in normal and undernourished rat pups. Am J Physiol Regul Integr Comp Physiol. 2000;278:R845–R854. doi: 10.1152/ajpregu.2000.278.4.R845. [DOI] [PubMed] [Google Scholar]
- Fiorotto ML, Schwartz RJ. Delaughter MC. Persistent IGF-I overexpression in skeletal muscle transiently enhances DNA accretion and growth. FASEB J. 2003;17:59–60. doi: 10.1096/fj.02-0289fje. [DOI] [PubMed] [Google Scholar]
- Fiorotto ML, Sosa HA., Jr Davis TA. In vivo measurement of muscle protein synthesis rate using the flooding dose technique. Methods Mol Biol. 2012;798:245–264. doi: 10.1007/978-1-61779-343-1_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fladby T. Jansen JK. Development of homogeneous fast and slow motor units in the neonatal mouse soleus muscle. Development. 1990;109:723–732. doi: 10.1242/dev.109.3.723. [DOI] [PubMed] [Google Scholar]
- Gavete ML, Martin MA, Alvarez C. Escriva F. Maternal food restriction enhances insulin-induced glut-4 translocation and insulin signaling pathway in skeletal muscle from suckling rats. Endocrinology. 2005;146:3368–3378. doi: 10.1210/en.2004-1658. [DOI] [PubMed] [Google Scholar]
- Glore SR. Layman DK. Cellular development of skeletal muscle during early periods of nutritional restriction and subsequent rehabilitation. Pediatr Res. 1983;17:602–605. doi: 10.1203/00006450-198307000-00017. [DOI] [PubMed] [Google Scholar]
- Gokhin DS, Ward SR, Bremner SN. Lieber RL. Quantitative analysis of neonatal skeletal muscle functional improvement in the mouse. J Exp Biol. 2008;211:837–843. doi: 10.1242/jeb.014340. [DOI] [PubMed] [Google Scholar]
- Gokulakrishnan G, Estrada IJ, Sosa HA. Fiorotto ML. In utero glucocorticoid exposure reduces fetal skeletal muscle mass in rats independent of effects on maternal nutrition. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1143–R1152. doi: 10.1152/ajpregu.00466.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimble RF. Mansaray YK. Effects in rats of dietary protein inadequacy on lactose production, milk volume and components of the lactose synthetase complex (EC 2.4.1.22) Ann Nutr Metab. 1987;31:179–184. doi: 10.1159/000177266. [DOI] [PubMed] [Google Scholar]
- Hall RD, Leahy JP. Robertson WM. The effects of protein malnutrition on the behavior of rats during the suckling period. Dev Psychobiol. 1979;12:455–466. doi: 10.1002/dev.420120505. [DOI] [PubMed] [Google Scholar]
- Hall WG. Rosenblatt JS. Suckling behavior and intake control in the developing rat pup. J Comp Physiol Psychol. 1977;91:1232–1247. [Google Scholar]
- Hannan KM, Brandenburger Y, Jenkins A, Sharkey K, Cavanaugh A, Rothblum L, Moss T, Poortinga G, McArthur GA, Pearson RB. Hannan RD. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol. 2003a;23:8862–8877. doi: 10.1128/MCB.23.23.8862-8877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan RD, Jenkins A, Jenkins AK. Brandenburger Y. Cardiac hypertrophy: a matter of translation. Clin Exp Pharmacol Physiol. 2003b;30:517–527. doi: 10.1046/j.1440-1681.2003.03873.x. [DOI] [PubMed] [Google Scholar]
- Hannan RD, Stefanovsky V, Taylor L, Moss T. Rothblum LI. Overexpression of the transcription factor UBF1 is sufficient to increase ribosomal DNA transcription in neonatal cardiomyocytes: implications for cardiac hypertrophy. Proc Natl Acad Sci U S A. 1996;93:8750–8755. doi: 10.1073/pnas.93.16.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning SJ, Chang SS. Gisel EG. Ontogeny of feeding controls in suckling and weanling rats. Am J Physiol. 1979;237:R187–R191. doi: 10.1152/ajpregu.1979.237.3.R187. [DOI] [PubMed] [Google Scholar]
- Jou MY, Lonnerdal B. Griffin IJ. Effects of early postnatal growth restriction and subsequent catch-up growth on body composition, insulin sensitivity, and behavior in neonatal rats. Pediatr Res. 2013;73:596–601. doi: 10.1038/pr.2013.27. [DOI] [PubMed] [Google Scholar]
- Kim HL, Picciano MF. O'Brien W. Influence of maternal dietary protein and fat levels on fetal growth in mice. Growth. 1981;45:8–18. [PubMed] [Google Scholar]
- Larson DE, Xie W, Glibetic M, O'Mahony D, Sells BH. Rothblum LI. Coordinated decreases in rRNA gene transcription factors and rRNA synthesis during muscle cell differentiation. Proc Natl Acad Sci USA. 1993;90:7933–7936. doi: 10.1073/pnas.90.17.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff AR. Atwood HL. Changes in the sarcoplasmic reticulum and transverse tubular system of fast and slow skeletal muscles of the mouse during postnatal development. J Cell Biol. 1971;51:369–383. doi: 10.1083/jcb.51.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallinson JE, Sculley DV, Craigon J, Plant R, Langley-Evans SC. Brameld JM. Fetal exposure to a maternal low-protein diet during mid-gestation results in muscle-specific effects on fibre type composition in young rats. Br J Nutr. 2007;98:292–299. doi: 10.1017/S0007114507701678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk JM, Makinen K, Shrum B. Woodward B. Blood corticosterone concentration reaches critical illness levels early during acute malnutrition in the weanling mouse. Exp Biol Med. 2006;231:264–268. doi: 10.1177/153537020623100304. [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, Duffield JA, Ozanne SE, Pilgrim C, Turner N, Morrison JL. McMillen IC. The transition from fetal growth restriction to accelerated postnatal growth: a potential role for insulin signalling in skeletal muscle. J Physiol. 2009;587:4199–4211. doi: 10.1113/jphysiol.2009.173161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro HN. Fleck A. Recent developments in the measurement of nucleic acids in biological materials. Analyst. 1966;91:78–88. doi: 10.1039/an9669100078. [DOI] [PubMed] [Google Scholar]
- Nader GA, McLoughlin TJ. Esser KA. mTOR function in skeletal muscle hypertrophy: increased ribosomal RNA via cell cycle regulators. Am J Physiol Cell Physiol. 2005;289:C1457–C1465. doi: 10.1152/ajpcell.00165.2005. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Hirano J, Yoshizawa F. Nishizawa N. Myofibrillar protein catabolism is rapidly suppressed following protein feeding. Biosci Biotechnol Biochem. 1998;62:1932–1937. doi: 10.1271/bbb.62.1932. [DOI] [PubMed] [Google Scholar]
- O'Connor PM, Bush JA, Suryawan A, Nguyen HV. Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E110–E119. doi: 10.1152/ajpendo.00326.2002. [DOI] [PubMed] [Google Scholar]
- Odedra BR, Bates PC. Millward DJ. Time course of the effect of catabolic doses of corticosterone on protein turnover in rat skeletal muscle and liver. Biochem J. 1983;214:617–627. doi: 10.1042/bj2140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontell M. Kozeka K. The organogenesis of murine striated muscle: a cytoarchitectural study. Am J Anat. 1984;171:133–148. doi: 10.1002/aja.1001710202. [DOI] [PubMed] [Google Scholar]
- Osmond C. Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. 2000;108(Suppl 3):545–553. doi: 10.1289/ehp.00108s3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne SE, Wang CL, Coleman N. Smith GD. Altered muscle insulin sensitivity in the male offspring of protein-malnourished rats. Am J Physiol. 1996;271:E1128–E1134. doi: 10.1152/ajpendo.1996.271.6.E1128. [DOI] [PubMed] [Google Scholar]
- Pine AP, Jessop NS. Oldham JD. Maternal protein reserves and their influence on lactational performance in rats. 3. The effects of dietary protein restriction and stage of lactation on milk composition. Br J Nutr. 1994;72:815–830. doi: 10.1079/bjn19940087. [DOI] [PubMed] [Google Scholar]
- Radley-Crabb HG, Marini JC, Sosa HA, Castillo LI, Grounds MD. Fiorotto ML. Dystropathology increases energy expenditure and protein turnover in the mdx mouse model of Duchenne Muscular Dystrophy. PLoS One. 2014;19:e89277. doi: 10.1371/journal.pone.0089277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeds PJ, Palmer RM, Hay SM. McMillan DN. Protein synthesis in skeletal muscle measured at different times during a 24 hour period. Biosci Rep. 1986;6:209–213. doi: 10.1007/BF01115008. [DOI] [PubMed] [Google Scholar]
- Sampson DA, Hunsaker HA. Jansen GR. Dietary protein quality, protein quantity and food intake: effects on lactation and on protein synthesis and tissue composition in mammary tissue and liver in rats. J Nutr. 1986;116:365–375. doi: 10.1093/jn/116.3.365. [DOI] [PubMed] [Google Scholar]
- Sayer AA. Cooper C. Fetal programming of body composition and musculoskeletal development. Early Hum Dev. 2005;81:735–744. doi: 10.1016/j.earlhumdev.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Enthoven L, van der Mark M, Levine S, de Kloet ER. Oitzl MS. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci. 2003;21:125–132. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ. Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sun H, Tu X, Liu M. Baserga R. Dual regulation of upstream binding factor 1 levels by IRS-1 and ERKs in IGF-1-receptor signaling. J Cell Physiol. 2007;212:780–786. doi: 10.1002/jcp.21072. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Frank JA, Nguyen HV. Davis TA. Expression of the TGF-β family of ligands is developmentally regulated in skeletal muscle of neonatal rats. Pediatr Res. 2006;59:175–179. doi: 10.1203/01.pdr.0000196718.47935.6e. [DOI] [PubMed] [Google Scholar]
- Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS. Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Walden F, Casagrande V, OstlundFarrants AK. Nader GA. Mechanical loading induces the expression of a Pol I regulon at the onset of skeletal muscle hypertrophy. Am J Physiol Cell Physiol. 2012;302:C1523–C1530. doi: 10.1152/ajpcell.00460.2011. [DOI] [PubMed] [Google Scholar]
- Winick M, Fish I. Rosso P. Cellular recovery in rat tissue after a brief period of malnutrition. J Nutr. 1968;95:623–626. doi: 10.1093/jn/95.4.623. [DOI] [PubMed] [Google Scholar]
- Winter JN, Jefferson LS. Kimball SR. ERK and Aktsignaling pathways function through parallel mechanisms to promote mTORC1 signaling. Am J Physiol Cell Physiol. 2011;300:C1172–C1180. doi: 10.1152/ajpcell.00504.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- Wu A, Tu X, Prisco M. Baserga R. Regulation of upstream binding factor 1 activity by insulin-like growth factor I receptor signaling. J Biol Chem. 2005;280:2863–2872. doi: 10.1074/jbc.M406138200. [DOI] [PubMed] [Google Scholar]


