Abstract
During constant-power high-intensity exercise, the expected increase in oxygen uptake (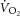 ) is supplemented by a
) is supplemented by a 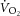 slow component (
slow component (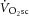 ), reflecting reduced work efficiency, predominantly within the locomotor muscles. The intracellular source of inefficiency is postulated to be an increase in the ATP cost of power production (an increase in P/W). To test this hypothesis, we measured intramuscular ATP turnover with 31P magnetic resonance spectroscopy (MRS) and whole-body
), reflecting reduced work efficiency, predominantly within the locomotor muscles. The intracellular source of inefficiency is postulated to be an increase in the ATP cost of power production (an increase in P/W). To test this hypothesis, we measured intramuscular ATP turnover with 31P magnetic resonance spectroscopy (MRS) and whole-body 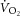 during moderate (MOD) and heavy (HVY) bilateral knee-extension exercise in healthy participants (n = 14). Unlocalized 31P spectra were collected from the quadriceps throughout using a dual-tuned (1H and 31P) surface coil with a simple pulse-and-acquire sequence. Total ATP turnover rate (ATPtot) was estimated at exercise cessation from direct measurements of the dynamics of phosphocreatine (PCr) and proton handling. Between 3 and 8 min during MOD, there was no discernable
during moderate (MOD) and heavy (HVY) bilateral knee-extension exercise in healthy participants (n = 14). Unlocalized 31P spectra were collected from the quadriceps throughout using a dual-tuned (1H and 31P) surface coil with a simple pulse-and-acquire sequence. Total ATP turnover rate (ATPtot) was estimated at exercise cessation from direct measurements of the dynamics of phosphocreatine (PCr) and proton handling. Between 3 and 8 min during MOD, there was no discernable 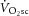 (mean ± SD, 0.06 ± 0.12 l min−1) or change in [PCr] (30 ± 8 vs. 32 ± 7 mm) or ATPtot (24 ± 14 vs. 17 ± 14 mm min−1; each P = n.s.). During HVY, the
(mean ± SD, 0.06 ± 0.12 l min−1) or change in [PCr] (30 ± 8 vs. 32 ± 7 mm) or ATPtot (24 ± 14 vs. 17 ± 14 mm min−1; each P = n.s.). During HVY, the 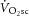 was 0.37 ± 0.16 l min−1 (22 ± 8%), [PCr] decreased (19 ± 7 vs. 18 ± 7 mm, or 12 ± 15%; P < 0.05) and ATPtot increased (38 ± 16 vs. 44 ± 14 mm min−1, or 26 ± 30%; P < 0.05) between 3 and 8 min. However, the increase in ATPtot (ΔATPtot) was not correlated with the
was 0.37 ± 0.16 l min−1 (22 ± 8%), [PCr] decreased (19 ± 7 vs. 18 ± 7 mm, or 12 ± 15%; P < 0.05) and ATPtot increased (38 ± 16 vs. 44 ± 14 mm min−1, or 26 ± 30%; P < 0.05) between 3 and 8 min. However, the increase in ATPtot (ΔATPtot) was not correlated with the 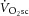 during HVY (r2 = 0.06; P = n.s.). This lack of relationship between ΔATPtot and
during HVY (r2 = 0.06; P = n.s.). This lack of relationship between ΔATPtot and 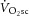 , together with a steepening of the [PCr]–
, together with a steepening of the [PCr]–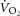 relationship in HVY, suggests that reduced work efficiency during heavy exercise arises from both contractile (P/W) and mitochondrial sources (the O2 cost of ATP resynthesis; P/O).
relationship in HVY, suggests that reduced work efficiency during heavy exercise arises from both contractile (P/W) and mitochondrial sources (the O2 cost of ATP resynthesis; P/O).
Introduction
During constant-power exercise below the lactate threshold (LT; moderate intensity), the rate of pulmonary oxygen uptake (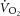 ) increases exponentially, reaching a steady state within 2–3 min. A steady-state
) increases exponentially, reaching a steady state within 2–3 min. A steady-state 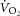 indicates that the exercise-related energy transfer is accounted for by oxidative phosphorylation. However, above the LT (heavy intensity), the dynamics of
indicates that the exercise-related energy transfer is accounted for by oxidative phosphorylation. However, above the LT (heavy intensity), the dynamics of 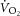 become complicated by an additional, slow component (
become complicated by an additional, slow component (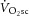 ; Poole et al. 1994). This becomes especially important at power outputs above critical power, where the
; Poole et al. 1994). This becomes especially important at power outputs above critical power, where the 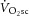 will draw
will draw 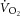 inexorably towards its physiological maximum. In this intensity domain, the limit of tolerance is reached rapidly, and the exercise cannot continue unless the power output is reduced below critical power (Coats et al. 2003). Although the
inexorably towards its physiological maximum. In this intensity domain, the limit of tolerance is reached rapidly, and the exercise cannot continue unless the power output is reduced below critical power (Coats et al. 2003). Although the 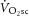 is intimately related to exercise intolerance (Murgatroyd et al. 2011), the aetiology of the
is intimately related to exercise intolerance (Murgatroyd et al. 2011), the aetiology of the 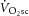 remains poorly understood.
remains poorly understood.
The 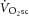 represents an impairment of exercise economy and is predominantly (∼85%) due to increased O2 consumption in the muscles engaged in the locomotor work (Poole et al. 1991; Rossiter et al. 2002; Krustrup et al. 2009). However, the intracellular source of this inefficiency is uncertain. It has been postulated that the
represents an impairment of exercise economy and is predominantly (∼85%) due to increased O2 consumption in the muscles engaged in the locomotor work (Poole et al. 1991; Rossiter et al. 2002; Krustrup et al. 2009). However, the intracellular source of this inefficiency is uncertain. It has been postulated that the 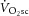 is related to an increased phosphate cost of force or power production; that is, an increase in the rate of ATP consumption per unit power output (or P/W) is met instantaneously by phosphocreatine (PCr; via the Lohmann reaction), the breakdown of which signals an increase in the rate of oxidative phosphorylation (Rossiter et al. 2002). However, distinguishing between this and the alternative hypothesis, that supra-LT exercise is associated with reductions in mitochondrial coupling (Krustrup et al. 2003), i.e. the ratio of the ATP resynthesized per oxygen converted to water (P/O), is technically challenging in humans.
is related to an increased phosphate cost of force or power production; that is, an increase in the rate of ATP consumption per unit power output (or P/W) is met instantaneously by phosphocreatine (PCr; via the Lohmann reaction), the breakdown of which signals an increase in the rate of oxidative phosphorylation (Rossiter et al. 2002). However, distinguishing between this and the alternative hypothesis, that supra-LT exercise is associated with reductions in mitochondrial coupling (Krustrup et al. 2003), i.e. the ratio of the ATP resynthesized per oxygen converted to water (P/O), is technically challenging in humans.
To test these two hypotheses requires knowledge of dynamic changes in total ATP turnover rate (ATPtot) in concert with power output and 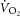 . Specifically, were the intramuscular source of the
. Specifically, were the intramuscular source of the 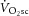 to be caused by an increase in P/W (in line with current views; Rossiter, 2011; Poole & Jones, 2012), then the magnitude of the
to be caused by an increase in P/W (in line with current views; Rossiter, 2011; Poole & Jones, 2012), then the magnitude of the 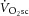 during heavy exercise would be strongly related to the magnitude of the change in ATPtot. Alternatively, if no proportionality between the
during heavy exercise would be strongly related to the magnitude of the change in ATPtot. Alternatively, if no proportionality between the 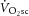 and the change in ATPtot were evident, then changes in P/W could not be the sole source of the
and the change in ATPtot were evident, then changes in P/W could not be the sole source of the 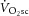 .
.
The technical challenge thus becomes, how best to establish ATPtot during heavy-intensity exercise that elicits a 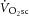 ? One approach uses 31P magnetic resonance spectroscopy (MRS; Kemp et al. 2001; Layec et al. 2009a) to partition ATP delivery from oxidative phosphorylation, PCr breakdown and glycogenolysis. 31P MRS provides direct measurement of [PCr] and allows the glycogenolytic rate (a relatively minor component of ATPtot in exercise of this kind) to be estimated using reasonable assumptions about muscle H+ buffering (Kemp et al. 2001, 2014). Several methods have been proposed to calculate oxidative ATP yield using 31P MRS, but these show poor agreement (Layec et al. 2011). Previous studies to estimate ATPtot during supra-LT exercise have assumed a linear
? One approach uses 31P magnetic resonance spectroscopy (MRS; Kemp et al. 2001; Layec et al. 2009a) to partition ATP delivery from oxidative phosphorylation, PCr breakdown and glycogenolysis. 31P MRS provides direct measurement of [PCr] and allows the glycogenolytic rate (a relatively minor component of ATPtot in exercise of this kind) to be estimated using reasonable assumptions about muscle H+ buffering (Kemp et al. 2001, 2014). Several methods have been proposed to calculate oxidative ATP yield using 31P MRS, but these show poor agreement (Layec et al. 2011). Previous studies to estimate ATPtot during supra-LT exercise have assumed a linear 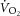 –[PCr] relationship and a fixed time constant (τ) of PCr breakdown and resynthesis (Meyer, 1988; Walter et al. 1999; Lanza et al. 2005; Faraut et al. 2007) or first-order [ADP]–
–[PCr] relationship and a fixed time constant (τ) of PCr breakdown and resynthesis (Meyer, 1988; Walter et al. 1999; Lanza et al. 2005; Faraut et al. 2007) or first-order [ADP]–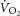 relationship in order to transform [PCr] into a rate of oxidative ATP turnover (Layec et al. 2009a). However, it is clear that the
relationship in order to transform [PCr] into a rate of oxidative ATP turnover (Layec et al. 2009a). However, it is clear that the 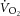 –[PCr] relationship is not linear through the intensity domains (Kemp, 2008; Wüst et al. 2011; Kemp et al. 2014), and accordingly, τPCr is not invariant across exercise intensities (Yoshida & Watari, 1993, 1994; Rossiter et al. 2002; Jones et al. 2008), making this an unreliable assumption on which to base estimation of ATPtot. Assuming τPCr to be invariant is equivalent to assuming that any change in [PCr] is directly proportional to change in ATPtot; when τPCr changes across exercise intensity and/or duration, this proportionality is lost (Kemp et al. 2014). These new findings mean that the close coherence between [PCr] and
–[PCr] relationship is not linear through the intensity domains (Kemp, 2008; Wüst et al. 2011; Kemp et al. 2014), and accordingly, τPCr is not invariant across exercise intensities (Yoshida & Watari, 1993, 1994; Rossiter et al. 2002; Jones et al. 2008), making this an unreliable assumption on which to base estimation of ATPtot. Assuming τPCr to be invariant is equivalent to assuming that any change in [PCr] is directly proportional to change in ATPtot; when τPCr changes across exercise intensity and/or duration, this proportionality is lost (Kemp et al. 2014). These new findings mean that the close coherence between [PCr] and 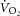 during the slow component phase (Rossiter et al. 2002; Layec et al. 2009a) is no longer sufficient evidence to imply that an increase in P/W alone is the responsible mechanism. Consequently, a direct measurement of oxidative ATP yield during supra-LT exercise that does not rely on these assumptions is required to distinguish whether change in P/W is the dominant mechanism for the
during the slow component phase (Rossiter et al. 2002; Layec et al. 2009a) is no longer sufficient evidence to imply that an increase in P/W alone is the responsible mechanism. Consequently, a direct measurement of oxidative ATP yield during supra-LT exercise that does not rely on these assumptions is required to distinguish whether change in P/W is the dominant mechanism for the 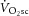 .
.
Oxidative ATP turnover (the dominant proportion of ATPtot) at exercise cessation may be assessed directly from the initial rate of postexercise PCr resynthesis (Vi[PCr]), easily measured by 31P MRS; the only assumptions required (the evidence for which is reviewed elsewhere; Kemp et al. 2014) are that PCr recovery is driven overwhelmingly by oxidative ATP synthesis and that any basal component of ATP turnover (i.e. ATP production not available for use by myosin ATPase, sarco(endo)plasmic reticulum Ca2+-ATPase or Na+–K+-ATPase during exercise or PCr resynthesis during recovery) is small and reasonably constant. Therefore, temporal characterization of oxidative energy yield during dynamic exercise can be made simply by halting the exercise and measuring Vi[PCr]. Although this method has inherently poor temporal resolution (it is valid only at the instant of exercise cessation), it provides the accuracy necessary to isolate the intracellular source of inefficiency during high-intensity exercise. The other, much smaller, components of ATPtot can be estimated at the end of exercise by 31P MRS in ways that are relatively robust against uncertainty or changes in the underpinning assumptions.
The purpose of this study, therefore, was to characterize the rate of ATP turnover during sub- and supra-LT exercise in human quadriceps during bilateral, prone, knee-extension exercise using 31P MRS. The rate of pulmonary oxygen uptake was measured in the same conditions to quantify the 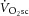 . We hypothesized that the close association between the dynamics of the [PCr] and
. We hypothesized that the close association between the dynamics of the [PCr] and 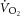 slow components during supra-LT exercise would be reflected in the dynamics of ATPtot (measured independently), thereby confirming the hypothesis that increased P/W during heavy-intensity exercise is the predominant mechanism of the
slow components during supra-LT exercise would be reflected in the dynamics of ATPtot (measured independently), thereby confirming the hypothesis that increased P/W during heavy-intensity exercise is the predominant mechanism of the 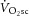 .
.
Methods
Ethical approval
The Biological Sciences Faculty Research Ethics Committee, University of Leeds, and the University of Liverpool Committee on Research Ethics approved this study, and all procedures complied with the latest revision of the Declaration of Helsinki. Written informed consent was obtained from all volunteers prior to their participation in the study.
Participants
Fourteen healthy volunteers (one female, 13 male) agreed to participate in this study [mean ± SD: age 27 ± 8 years; height 177 ± 8 cm; mass 75 ± 12 kg; bilateral knee-extension peak 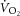 (
(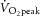 ) 2.0 ± 0.5 l min−1]. All participants were undertaking a regular exercise regimen, ranging from recreational fitness to amateur competitive sport. Volunteers were screened for cardiovascular disease risk with a resting ECG and a health history questionnaire.
) 2.0 ± 0.5 l min−1]. All participants were undertaking a regular exercise regimen, ranging from recreational fitness to amateur competitive sport. Volunteers were screened for cardiovascular disease risk with a resting ECG and a health history questionnaire.
Exercise protocols
All exercise tests were undertaken on an MR-compatible computer-controlled electromagnetically braked knee-extension ergometer (MRI Ergometer Up/Down, Lode BV, Groningen, The Netherlands) customized for use at 3 T by the addition of extended carbon-fibre lever arms. The participants lay prone, with their feet strapped into moulded plastic stirrups, which were attached to carbon-fibre/aluminium arms, linking to the ergometer crank arms. The participants’ hips were secured to the patient bed with nylon and Velcro straps in order to isolate power production to the quadriceps and minimize movement from hip flexion/extension. Knee movements were constrained by the scanner bore, allowing for ∼35 deg of bilateral knee extension (Whipp et al. 1999; Cannon et al. 2013). No resistance was applied during knee flexion, other than the constant work required to lift the mass of the lower leg.
The testing protocol began with a rigorous familiarization phase that took place in a temperature-controlled laboratory with pulmonary gas exchange measurements. Ramp incremental (RI) and constant-power protocols were completed until reproducible physiological measurements were obtained across two consecutive visits for each condition. The second phase of the study took place within the bore of an MR scanner for measurement of muscle phosphates. The same MRI ergometer was used for both phases of the protocol.
Initially, participants completed an RI exercise test to the limit of tolerance. For this, participants lay at rest for ∼3–4 min, followed by a low-power exercise (5 W) for ∼2–4 min. The power was then increased as a function of time at 2–5 W min−1 (the rate of increase was dependent on the volunteer's size and strength) until the limit of tolerance was reached. Ramp rates were adjusted using ‘trial and error’ to determine a ramp rate that resulted in a ramp duration of ∼10–12 min. The frequency of knee extension was constrained at 90 min−1 with the use of a metronome. This cadence was chosen to allow synchronization with the MR scanner acquisitions (one pulse per two knee extensions) and also acted to ensure that the ergometer flywheel was maintained above its minimal operating speed. The RI was terminated upon the participant being unable to maintain the required cadence, despite strong verbal encouragement. The results of the RI were used to determine the 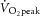 and to calculate power for subsequent tests. There is a substantial learning effect with the exercise model (large gains in peak power were achieved with consecutive tests), and therefore typically, more than three RI tests were completed by each participant until reproducible performances were achieved.
and to calculate power for subsequent tests. There is a substantial learning effect with the exercise model (large gains in peak power were achieved with consecutive tests), and therefore typically, more than three RI tests were completed by each participant until reproducible performances were achieved.
A series of constant-power exercise tests were then undertaken. These consisted of an 8 min moderate-intensity bout, followed by a 6 min rest and an 8 min heavy-intensity exercise bout. During moderate-intensity exercise, the target power was 80% of estimated LT (LT was ∼60–70% 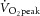 ), and during heavy-intensity bouts the target power was halfway between estimated LT and
), and during heavy-intensity bouts the target power was halfway between estimated LT and 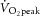 . These intensity domains were confirmed post hoc from the profile of
. These intensity domains were confirmed post hoc from the profile of 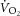 during constant-power bouts (Whipp, 1996). If necessary, power was adjusted in subsequent familiarization tests to ensure the absence (moderate) or presence (heavy) of the
during constant-power bouts (Whipp, 1996). If necessary, power was adjusted in subsequent familiarization tests to ensure the absence (moderate) or presence (heavy) of the 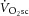 . Once familiarized, participants repeated this protocol three times on separate days to combine respired gas exchange data and improve the signal-to-noise ratio.
. Once familiarized, participants repeated this protocol three times on separate days to combine respired gas exchange data and improve the signal-to-noise ratio.
During the second phase of experiments, participants completed constant-power bouts within the bore of the superconducting magnet for 31P MRS. Two trials of constant-power tests were completed in a random order consisting of the following sequences: (i) 4 min of rest, followed by 3 min of moderate exercise, 6 min of rest and 3 min of heavy exercise; and (ii) 4 min of rest, followed by 8 min of moderate exercise, 6 min of rest and 8 min of heavy exercise. Each protocol was preceded by ∼10 min of magnet shimming to optimize the MRS signal, and separated by at least 30 min outside of the MR scanner. Therefore, ∼60–90 min elapsed between the two exercise trials.
Pulmonary gas exchange
Participants breathed through a low-resistance (<0.1 kPa l−1 s−1 up to 15 l s−1), low-dead-space (90 ml) mouthpiece for the measurement of respired gases. Flow rates and volumes were measured with an infrared turbine flow sensor (Interface Associates, Laguna Niguel, CA, USA), while a quadrupole mass spectrometer was used to measure respired gas concentrations after sampling air at 0.5 ml s−1 from the mouthpiece (MSX; nSpire Health Ltd, Hertford, UK). Gas concentration signals were time aligned with the flow sensor signal using proprietary software for the calculation of breath-by-breath gas exchange. These algorithms identified the end of each breath with the flow sensor and time aligned the changes in respired gases.
Prior to each experiment, the flow sensor and gas analysers were calibrated according to the manufacturers’ instructions. The turbine volume transducers were calibrated with a 3 l syringe (Hans Rudolph Inc., Shawnee, KS, USA). The calibration was completed with flow rates ranging from 0.2 to 6 l s−1, mimicking flow rates expected for humans at rest and during exercise. After the completion of the flow sensor calibration, the flow volumes were verified over 10 syringe strokes of varying flow rates and accepted when the means were within ±0.01 l, with an SD and coefficient of variation of 0.02 l and 1%, respectively. Additionally, the mass spectrometer was calibrated with atmospheric air and precision-verified gases with concentrations of O2, CO2 and N2 spanning the physiological range. Following each experiment, mass spectrometer calibration factor drift was verified as negligible by sampling the calibration gases.
Data analyses for pulmonary measures
Breath-by-breath 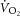 was filtered for errant breaths (i.e. values resulting after sighs, swallows, coughs etc., defined as residing outside of 99% prediction limits; Lamarra et al. 1987). Responses from like transitions were combined to improve the signal-to-noise ratio using an averaging technique that preserves the breath-by-breath density measured during the exercise transition. This method requires time aligning and sorting of all
was filtered for errant breaths (i.e. values resulting after sighs, swallows, coughs etc., defined as residing outside of 99% prediction limits; Lamarra et al. 1987). Responses from like transitions were combined to improve the signal-to-noise ratio using an averaging technique that preserves the breath-by-breath density measured during the exercise transition. This method requires time aligning and sorting of all 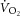 data from exercise transitions in the time domain. Time and
data from exercise transitions in the time domain. Time and 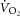 are then averaged into bins of n breaths, where n is the number of exercise transitions completed (Murgatroyd et al. 2011). The magnitude of the
are then averaged into bins of n breaths, where n is the number of exercise transitions completed (Murgatroyd et al. 2011). The magnitude of the 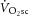 was expressed as the difference in
was expressed as the difference in 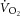 between 3 and 8 min of exercise.
between 3 and 8 min of exercise.
Power output and flywheel speed from the ergometer were sampled continuously and digitized by a data-recording system and stored on a PC (PowerLab 8/30 with LabChart Pro; ADInstruments Pty Ltd, Bella Vista, NSW, Australia).
31P Magnetic resonance spectroscopy
Muscle phosphorus-containing metabolites were measured with a 3 T superconducting magnet (Magnetom Trio; Siemens AG, Erlangen, Germany). A one-pulse MRS acquisition was employed using a dual-tuned (1H and 31P) 15- and 18-cm-diameter surface RF coil (RAPID Biomedical GmbH, Rimpar, Germany), which was placed under the knee extensors, halfway between the hip and knee. The concave RF coil was stabilized with sandbags and was secured to the table once the participants’ hips were strapped to the scanner table. A series of axial, sagittal and coronal gradient-recalled echo images of the thigh were acquired to confirm the placement of the RF coil relative to the knee-extensor muscles and to prescribe the volume over which shimming was achieved. Subsequently, a standard 1H shimming protocol was used to optimize the homogeneity of the magnetic field (β0). A fully relaxed spectrum (repetition time of 10 s; number of scans = 4) was initially obtained to provide a high-resolution unsaturated resting spectrum along with a 32 scan spectrum with a repetition time of 2 s. Following this, free induction decays for 31P spectra were collected every 2 s with a spectral width of 3200 Hz and 1024 data points throughout the rest-to-exercise-to-rest transitions. The 31P data were averaged over four acquisitions, yielding a datum every 8 s.
Kinetic analysis of 31P MRS data
Signal intensities, frequencies and line widths of inorganic phosphate (Pi), PCr, γ-ATP, α-ATP and β-ATP were determined using Java-based Magnetic Resonance User Interface (jMRUI; Naressi et al. 2001) in order to transform the raw data into a time series for each of the phosphates of interest. Intramuscular pH (pHi) was estimated from the chemical shift of Pi (Moon & Richards, 1973), as follows:
| 1 |
where δ is the chemical shift of the Pi peak, relative to PCr.
Phosphocreatine kinetics were modelled using non-linear least-squares regression (OriginPro 7.5; OriginLab Corp., Northampton, MA, USA). The 31P MRS data were filtered for errant values resulting from artefacts (Rossiter et al. 2000) prior to characterization with the following function:
| 2 |
where τ is a time constant and [PCr](t), [PCr]0, and A are the time-variant form, baseline and fundamental amplitude, respectively. The fitting window was determined from an iterative process (Rossiter et al. 2001) to ensure the exclusion of phase III (steady state or slow component, depending on the intensity domain). The magnitude of the PCr slow component ([PCr]sc) was expressed as the difference in [PCr] between the third and eighth minute of exercise.
The ATPtot was estimated from the contributions from oxidative phosphorylation (Q), PCr breakdown (D) and glycogenolysis (L), which were determined from the 31P MRS data acquired during exercise and recovery, using methods described elsewhere (Kemp et al. 2001, 2007, 2014; Layec et al. 2011) and outlined below.
Production of ATP from PCr breakdown (D)
The rate of PCr breakdown by creatine kinase (D) yields one component of ATP production (millimolar per minute) and was determined over 32 s (four spectra) immediately prior to exercise cessation, according to the following equation:
| 3 |
In the present experiments, where [PCr] is either close to steady state or changing only slowly by the end of exercise, D is a very small component of end-exercise ATPtot.
Production of ATP from oxidative phosphorylation (Q)
The rate of oxidative ATP yield (Q) is reflected in the rate of [PCr] recovery at the instant of exercise cessation (Vi[PCr]) and was calculated (millimolar per minute) as follows:
| 4 |
where A is the amplitude of [PCr] change (millimolar). The rate constant (k) was estimated by fitting the PCr recovery kinetics with the following function:
| 5 |
where [PCr](t) is the time-dependent variant of [PCr], and [PCr]end is the concentration of PCr measured at the end of exercise. We make the well-evidenced assumption (Kemp et al. 2014) that the rate of suprabasal oxidative synthesis at the start of recovery [Vi[PCr] from eqn (4)] is a good estimate of the suprabasal rate of oxidative synthesis at the end of exercise (Qend).
Production of ATP from anaerobic glycolysis (L)
During exercise, glycogenolysis and the resulting lactate and H+ production cause disturbances in pHi. These changes in pHi are readily measured by 31P MRS data and can therefore be used to estimate ATP production from glycogenolysis; 1 mol of H+ resulting in 1.5 mol of ATP. This requires estimation of the flux rates as follows: H+ production accompanying changes in PCr concentration via the creatine kinase reaction (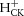 , which is positive, i.e. H+ ‘consumption’, when [PCr] is falling in exercise, and negative, i.e. H+ generation, when [PCr] is rising in recovery); by the buffers of the muscle cytosol (
, which is positive, i.e. H+ ‘consumption’, when [PCr] is falling in exercise, and negative, i.e. H+ generation, when [PCr] is rising in recovery); by the buffers of the muscle cytosol (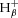 , which is positive, i.e. H+ ‘buffering’ when pHi is falling in exercise and negative, i.e. H+ ‘unbuffering’ when pHi is rising in recovery); and proton efflux from the cells (
, which is positive, i.e. H+ ‘buffering’ when pHi is falling in exercise and negative, i.e. H+ ‘unbuffering’ when pHi is rising in recovery); and proton efflux from the cells (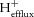 ). Together, these sum to the total proton yield (P) during exercise:
). Together, these sum to the total proton yield (P) during exercise:
| 6 |
From which:
| 7 |
The number of protons consumed at the creatine kinase reaction was calculated from the time-dependent changes in [PCr] using the proton stoichiometric coefficient, γ (Kushmerick, 1997), as follows:
| 8 |
Protons buffered (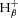 , millimolar per minute) was calculated from the apparent buffering capacity, βtotal (in millimoles of acid added per unit change in pHi) and from the (smoothed) rate of pH change during exercise, as follows:
, millimolar per minute) was calculated from the apparent buffering capacity, βtotal (in millimoles of acid added per unit change in pHi) and from the (smoothed) rate of pH change during exercise, as follows:
| 9 |
where
| 10 |
The intrinsic cytosolic buffering capacity ( ) is calculated from initial-exercise data:
) is calculated from initial-exercise data:
| 11 |
where the apparent β(βa) is obtained from the initial rate of change in [PCr] (ΔPCri) and alkalinization of pH (ΔpHi):
| 12 |
The value of 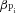 was calculated as follows:
was calculated as follows:
| 13 |
where K = 1.77 × 10−7 (Conley et al. 1998). The βbicarbonate was neglected, which assumes that muscle is a closed system during short-duration exercise in vivo (Kemp et al. 1993). Proton efflux (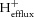 , millimolar per minute) was estimated for each time point of exercise assuming a linear pH-dependence constant, λ, as follows:
, millimolar per minute) was estimated for each time point of exercise assuming a linear pH-dependence constant, λ, as follows:
| 14 |
This proportionality constant, λ (millimolar per minute per pH unit) was estimated from initial recovery after exercise cessation, as follows:
| 15 |
At the cessation of exercise, the PCr resynthesized in the creatine kinase reaction is essentially a consequence solely of oxidative ATP production (Kemp et al. 2014). Therefore, 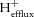 can be calculated from the rate of proton production from creatine kinase (
can be calculated from the rate of proton production from creatine kinase (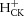 ) and the rate of pH change on the other side, as follows:
) and the rate of pH change on the other side, as follows:
| 16 |
Where ΔpHi is very low, eqn (14) becomes unreliable, and the end-exercise rate of 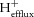 is simply assumed to be equal to
is simply assumed to be equal to 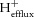 calculated in from the initial recovery data by eqn (16).
calculated in from the initial recovery data by eqn (16).
In the present experiments, where pHi is close to steady state or changing only slowly by the end of exercise, L is a very small component of ATPtot.
Statistical analyses
Relationships between variables were assessed with a Pearson correlation coefficient, where appropriate. The differences between 31P measures at discrete time points and across exercise intensities were compared with a two-factor (time × intensity domain) repeated-measures ANOVA. Bonferroni-corrected Student's paired t tests were used post hoc to identify simple effects in the case of a significant main effect. For all tests, α = 0.05. Analyses were completed using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA).
Results
During RI exercise, participants attained a peak power output of 47 ± 11 W and a 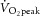 of 2.00 ± 0.48 l min−1. Based on peak power output and estimated LT (∼60–70%
of 2.00 ± 0.48 l min−1. Based on peak power output and estimated LT (∼60–70% 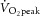 ), moderate (sub-LT; 19 ± 4 W) and heavy (supra-LT; 46 ± 11 W) constant-power exercise bouts were assigned. The dynamics of
), moderate (sub-LT; 19 ± 4 W) and heavy (supra-LT; 46 ± 11 W) constant-power exercise bouts were assigned. The dynamics of 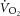 were examined post hoc to confirm the appropriate intensity assignment (Whipp, 1996; Rossiter, 2011).
were examined post hoc to confirm the appropriate intensity assignment (Whipp, 1996; Rossiter, 2011).
During moderate exercise, there was no discernable pulmonary 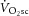 (0.06 ± 0.12 l min−1). However, during heavy exercise the
(0.06 ± 0.12 l min−1). However, during heavy exercise the 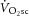 was 0.37 ± 0.16 l min−1 (Fig.1A), or a 22 ± 8% increase. The [PCr] did not change between 3 and 8 min of moderate-intensity exercise (30 ± 8 vs. 32 ± 7 mm; n.s.). Conversely, during heavy exercise [PCr] fell from 3 to 8 min (19 ± 7 vs. 18 ± 7 mm or 12 ± 15% fall; P < 0.05; Fig.1B).
was 0.37 ± 0.16 l min−1 (Fig.1A), or a 22 ± 8% increase. The [PCr] did not change between 3 and 8 min of moderate-intensity exercise (30 ± 8 vs. 32 ± 7 mm; n.s.). Conversely, during heavy exercise [PCr] fell from 3 to 8 min (19 ± 7 vs. 18 ± 7 mm or 12 ± 15% fall; P < 0.05; Fig.1B).
Figure 1. Rate of whole-body O2 uptake (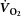 ; A), phosphocreatine concentration ([PCr]; B) and pH (C) plotted as a function of time for moderate-intensity (filled circles) and heavy-intensity prone bilateral knee-extension exercise (open circles).
; A), phosphocreatine concentration ([PCr]; B) and pH (C) plotted as a function of time for moderate-intensity (filled circles) and heavy-intensity prone bilateral knee-extension exercise (open circles).
Black bar denotes exercise bout from time 0 to 8 min. Data points are 8 s means, with error bars representing SD.
The ATP yield during moderate and heavy exercise from oxidative phosphorylation (Q), PCr hydrolysis (D), lactate production (L) and, consequently, ATPtot are presented in Table1. The Vi[PCr], calculated as described in eqn (4) is shown, along with the rate constant of PCr resynthesis (k) and the amplitude of PCr recovery (A), in Figs2 (moderate) and 3 (heavy).
Table 1.
Rates of ATP turnover from oxidative phosphorylation (Q), phosphocreatine hydrolysis (D), lactate production (L) and the sum (ATPtot) during moderate and heavy constant-power exercise at two time points
| Moderate exercise |
Heavy exercise |
|||
|---|---|---|---|---|
| Parameter | 3 min | 8 min | 3 min | 8 min |
| Q (mm min−1)† | 23 (14) | 17 (13) | 35 (17) | 42 (13)* |
| D (mm min−1) | 0.6 (1.2) | 0.2 (1.0) | 1.1 (2.6) | 0.7 (0.9) |
| L (mm min−1) | 1.0 (1.3) | 0.3 (0.6) | 1.5 (1.3) | 1.3 (1.7) |
| ATPtot (mm min−1)‡ | 24 (14) | 17 (14) | 38 (16) | 44 (14)* |
Values are presented in millimolar per minute as means (SD).
Different from 3 min; P < 0.05.
Time × intensity interaction; P < 0.01; F(1,13) = 17.2; η2 = 0.57.
Time × intensity interaction; P < 0.01; F(1,13) = 17.2; η2 = 0.57
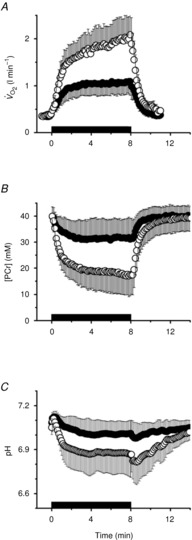
Figure 2. Moderate-intensity exercise recovery rate constant (k; A), amplitude of PCr resynthesis (termed ‘A’; B), initial rate of PCr resynthesis (Vi[PCr]; C) and total ATP turnover rate (ATPtot; D) at 8 min of exercise, plotted as a function of 3 min of exercise.
Dashed line is y = x.
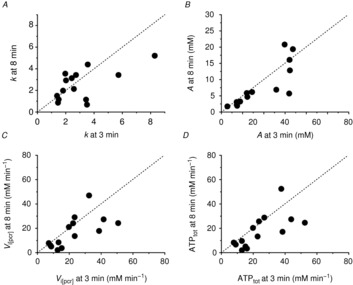
Figure 3. Heavy-intensity exercise recovery rate constant (k; A), amplitude of PCr resynthesis (termed ‘A’; B), Vi[PCr] (C) and ATPtot (D) at 8 min of exercise, plotted as a function of 3 min of exercise.
Dashed line is y = x.
Comparisons of ATPtot revealed a significant interaction (time × intensity domain; F(1,13) = 17.2; P < 0.01; η2 = 0.57). The ATPtot was not different between 3 and 8 min of moderate exercise (n.s.; Fig.2 and Table1), but ATPtot increased (ΔATPtot) during heavy exercise from 3 to 8 min (CI95 of the difference; CIDifference 1.9, 12.6 mm min−1; P < 0.05; Fig.3 and Table1), equating to a 26 ± 30% increase in ATPtot from 3 to 8 min (Fig.4A). This percentage change in ATPtot was not different to that measured in both the [PCr]sc and the 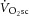 (F(2,26) = 2.4; n.s.; η2 = 0.16; Fig.4A). However, among participants the individual values of ΔATPtot during heavy exercise were
(F(2,26) = 2.4; n.s.; η2 = 0.16; Fig.4A). However, among participants the individual values of ΔATPtot during heavy exercise were
Figure 4. Magnitudes and relationship between  slow component and muscle [PCr] and ATPtot slow components.
slow component and muscle [PCr] and ATPtot slow components.
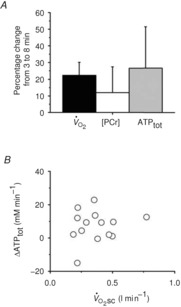
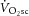 and [PCr]sc and ΔATPtot from minute 3 to 8 of heavy exercise expressed as a percentage change (A). B shows ΔATPtot during heavy exercise plotted as a function of the
and [PCr]sc and ΔATPtot from minute 3 to 8 of heavy exercise expressed as a percentage change (A). B shows ΔATPtot during heavy exercise plotted as a function of the 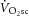 .
.
not significantly correlated with the magnitude of the 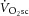 (Fig.4B).
(Fig.4B).
To examine the relationship between 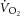 and [PCr], a correction for the transit delay from muscle to lung was applied. The
and [PCr], a correction for the transit delay from muscle to lung was applied. The 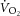 data were time corrected using 12 s difference with respect to 31P measures (Rossiter et al. 1999; Krustrup et al. 2009). The relationship between
data were time corrected using 12 s difference with respect to 31P measures (Rossiter et al. 1999; Krustrup et al. 2009). The relationship between  and [PCr] was linear during moderate exercise and the first 3 min of heavy exercise (r2 = 0.94; Fig.5). However, the slope of the [PCr]–
and [PCr] was linear during moderate exercise and the first 3 min of heavy exercise (r2 = 0.94; Fig.5). However, the slope of the [PCr]– relationship was significantly steeper when data from 8 min of heavy exercise were included (−67 ± 25 vs. −61 ± 25 ml min mm−1; P < 0.05).
relationship was significantly steeper when data from 8 min of heavy exercise were included (−67 ± 25 vs. −61 ± 25 ml min mm−1; P < 0.05).
Figure 5. Relationship between pulmonary  and [PCr] during moderate (filled circles) and heavy exercise (open circles).
and [PCr] during moderate (filled circles) and heavy exercise (open circles).
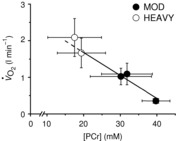
The regression shown (continuous line) was fitted to data from moderate exercise and from the first 3 min of heavy exercise and extrapolated (dashed line) to 8 min of heavy exercise. Error bars represent SD. The 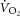 data were phase aligned with respect to [PCr] measurements.
data were phase aligned with respect to [PCr] measurements.
Discussion
The [PCr] slow component ([PCr]sc), like the 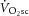 , is present only during exercise above the LT. The finding that the [PCr]sc and
, is present only during exercise above the LT. The finding that the [PCr]sc and 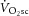 are of similar magnitude (Rossiter et al. 2002) led to the argument that the
are of similar magnitude (Rossiter et al. 2002) led to the argument that the 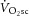 is caused by an increased phosphate cost of power production (P/W) during heavy-intensity exercise. However, this is at odds with the observed dissociation between the [PCr]sc and
is caused by an increased phosphate cost of power production (P/W) during heavy-intensity exercise. However, this is at odds with the observed dissociation between the [PCr]sc and 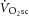 in endurance-trained individuals (Layec et al. 2009b, 2012), and both observations relied upon equivocal assumptions about the dynamic relationships between [ADP] and
in endurance-trained individuals (Layec et al. 2009b, 2012), and both observations relied upon equivocal assumptions about the dynamic relationships between [ADP] and 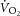 or
or 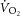 and [PCr] (Yoshida & Watari, 1993, 1994; Rossiter et al. 2002; Jones et al. 2008; Kemp, 2008; Wüst et al. 2011). Our present data agree with previous reports that mean [PCr]sc and
and [PCr] (Yoshida & Watari, 1993, 1994; Rossiter et al. 2002; Jones et al. 2008; Kemp, 2008; Wüst et al. 2011). Our present data agree with previous reports that mean [PCr]sc and 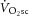 magnitudes were not statistically different. Crucially, however, the data add that, among individuals, the increase in the
magnitudes were not statistically different. Crucially, however, the data add that, among individuals, the increase in the 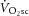 during heavy-intensity exercise (averaging ∼22%) is not correlated with the increase in the phosphate cost of power production, ATPtot (average ∼26%). Thus, while the exercising limb is likely to remain the major source of the
during heavy-intensity exercise (averaging ∼22%) is not correlated with the increase in the phosphate cost of power production, ATPtot (average ∼26%). Thus, while the exercising limb is likely to remain the major source of the 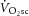 (Poole et al. 1991; Rossiter et al. 2002; Bailey et al. 2010; Dimenna et al. 2010), the observed dissociation between
(Poole et al. 1991; Rossiter et al. 2002; Bailey et al. 2010; Dimenna et al. 2010), the observed dissociation between 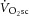 and ΔATPtot (Fig.4B) strongly suggests that the progressive increase in
and ΔATPtot (Fig.4B) strongly suggests that the progressive increase in 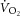 during heavy exercise is not solely due to contractile inefficiency (P/W). Thus, other explanations, such as a reduction in mitochondrial efficiency (P/O), should also be considered.
during heavy exercise is not solely due to contractile inefficiency (P/W). Thus, other explanations, such as a reduction in mitochondrial efficiency (P/O), should also be considered.
ATP turnover during moderate and heavy constant-power exercise
The primary aim of this investigation was to estimate the ATP turnover rate for exercise below and above the LT and over time without assumptions about the [ADP]–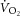 or
or 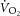 –[PCr] relationships. By using the most robust estimations of ATPtot (Kemp et al. 1995; Walter et al. 1999; Lanza et al. 2005; Faraut et al. 2007), we provided 31P MRS-derived estimates of ATP yield from oxidative phosphorylation, lactate production and PCr hydrolysis at 3 and 8 min of exercise that were unencumbered by the recently challenged assumptions about the [ADP]–
–[PCr] relationships. By using the most robust estimations of ATPtot (Kemp et al. 1995; Walter et al. 1999; Lanza et al. 2005; Faraut et al. 2007), we provided 31P MRS-derived estimates of ATP yield from oxidative phosphorylation, lactate production and PCr hydrolysis at 3 and 8 min of exercise that were unencumbered by the recently challenged assumptions about the [ADP]–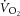 relationship (Kemp, 2008; Wüst et al. 2011; Glancy & Balaban, 2012; Kemp et al. 2014).
relationship (Kemp, 2008; Wüst et al. 2011; Glancy & Balaban, 2012; Kemp et al. 2014).
Unsurprisingly, there were no changes in ATPtot during exercise below the lactate threshold, where negligible muscle fatigue is expected (Sargeant & Dolan, 1987; Yano et al. 2001), reflecting steady-state conditions. Conversely, during heavy exercise in which the 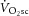 and [PCr]sc were present, ATPtot was increased between 3 and 8 min of exercise. This is consistent with the suggestions that the
and [PCr]sc were present, ATPtot was increased between 3 and 8 min of exercise. This is consistent with the suggestions that the 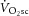 is consequent to increased P/W in the large locomotor muscles during supra-LT exercise (Rossiter et al. 2002), perhaps associated with muscle fatigue and a reduction in contractile efficiency. However, the lack of relationship between ΔATPtot and
is consequent to increased P/W in the large locomotor muscles during supra-LT exercise (Rossiter et al. 2002), perhaps associated with muscle fatigue and a reduction in contractile efficiency. However, the lack of relationship between ΔATPtot and 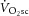 is in contrast to this postulate and challenges the current understanding of the aetiology of
is in contrast to this postulate and challenges the current understanding of the aetiology of 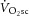 (Rossiter, 2011; Poole & Jones, 2012).
(Rossiter, 2011; Poole & Jones, 2012).
Dissociation of the 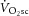 and changes in the phosphate cost of exercise may have a few explanations. It may indicate an increase in
and changes in the phosphate cost of exercise may have a few explanations. It may indicate an increase in 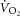 originating from regions within the knee extensors that are not interrogated by the surface coil. While we can only speculate on this, a similar finding has been reported where the
originating from regions within the knee extensors that are not interrogated by the surface coil. While we can only speculate on this, a similar finding has been reported where the 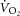 and [PCr] slow components were dissociated in endurance-trained participants but not in sedentary control subjects, despite increasing EMG activity in both participant groups during the
and [PCr] slow components were dissociated in endurance-trained participants but not in sedentary control subjects, despite increasing EMG activity in both participant groups during the 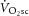 (Layec et al. 2009b, 2012). It was hypothesized that the exercise-trained volunteers may be better able to optimize motor unit recruitment patterns to maintain high-intensity exercise (e.g. compared with active but untrained subjects; Rossiter et al. 2002), thereby recruiting motor unit pools that reside outside of the muscle volume being interrogated by MRS. It should be noted, however, that our surface coil interrogated a large muscle volume (∼300 g) compared with alternative techniques, e.g. biopsy (∼200 mg). Additionally, controversy exists about whether progressive recruitment itself is even responsible for the slow component (Zoladz et al. 2008; Cannon et al. 2011; Vanhatalo et al. 2011), in which case recruitment of muscle outside the surface coil view would seem to be an unlikely explanation if the recruitment pattern is stable.
(Layec et al. 2009b, 2012). It was hypothesized that the exercise-trained volunteers may be better able to optimize motor unit recruitment patterns to maintain high-intensity exercise (e.g. compared with active but untrained subjects; Rossiter et al. 2002), thereby recruiting motor unit pools that reside outside of the muscle volume being interrogated by MRS. It should be noted, however, that our surface coil interrogated a large muscle volume (∼300 g) compared with alternative techniques, e.g. biopsy (∼200 mg). Additionally, controversy exists about whether progressive recruitment itself is even responsible for the slow component (Zoladz et al. 2008; Cannon et al. 2011; Vanhatalo et al. 2011), in which case recruitment of muscle outside the surface coil view would seem to be an unlikely explanation if the recruitment pattern is stable.
The source of the 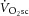 may even reside outside of the locomotor muscles. Progressive increases in respiratory (Wasserman et al. 1995; Żołądź & Korzeniewski, 2001) or cardiac work, or even work from non-power-producing musculature, such as stabilizing effort during cycling (Billat et al. 1998), may contribute to a reduction in exercise efficiency during the slow component. It is unlikely that the stabilizing effort would contribute to prone knee extension, where the work of stabilizing the torso is minimized by the body position, the ergometer and the heavy strapping used to isolate quadriceps activity. Nonetheless, the work of ventilation during prone knee extension may still contribute a meaningful proportion, particularly as the locomotor muscle mass in our study is relatively small in comparison to cycling or running.
may even reside outside of the locomotor muscles. Progressive increases in respiratory (Wasserman et al. 1995; Żołądź & Korzeniewski, 2001) or cardiac work, or even work from non-power-producing musculature, such as stabilizing effort during cycling (Billat et al. 1998), may contribute to a reduction in exercise efficiency during the slow component. It is unlikely that the stabilizing effort would contribute to prone knee extension, where the work of stabilizing the torso is minimized by the body position, the ergometer and the heavy strapping used to isolate quadriceps activity. Nonetheless, the work of ventilation during prone knee extension may still contribute a meaningful proportion, particularly as the locomotor muscle mass in our study is relatively small in comparison to cycling or running.
Finally, dissociation of the 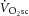 and ΔATPtot could result from mitochondrial uncoupling (reduced P/O; Fig. 5). In this scenario, an increased O2 cost of ATP resynthesis may contribute to driving the increase in
and ΔATPtot could result from mitochondrial uncoupling (reduced P/O; Fig. 5). In this scenario, an increased O2 cost of ATP resynthesis may contribute to driving the increase in 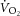 during heavy exercise, rather than it coming exclusively from an increased ATP cost of muscle power generation.
during heavy exercise, rather than it coming exclusively from an increased ATP cost of muscle power generation.
The 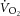 –[PCr] relationship and mitochondrial coupling during heavy-intensity exercise
–[PCr] relationship and mitochondrial coupling during heavy-intensity exercise
Without an invasive measure of 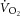 across the volume of tissue interrogated by MRS, the relationship between whole-body
across the volume of tissue interrogated by MRS, the relationship between whole-body 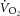 and localized [PCr] is the next best estimate for coupling of O2 uptake and ATP turnover. Our data show that the mean
and localized [PCr] is the next best estimate for coupling of O2 uptake and ATP turnover. Our data show that the mean 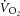 –[PCr] relationship was linear over the moderate intensity and during the first minutes of heavy exercise (r2 = 0.94; Fig. 5). Importantly, this relationship became steeper (P < 0.05) with the inclusion of data from the final minutes of heavy exercise. With some important assumptions, these data suggest a reduced P/O between 3 and 8 min of heavy exercise, implicating mitochondrial uncoupling as an additional mechanism of the
–[PCr] relationship was linear over the moderate intensity and during the first minutes of heavy exercise (r2 = 0.94; Fig. 5). Importantly, this relationship became steeper (P < 0.05) with the inclusion of data from the final minutes of heavy exercise. With some important assumptions, these data suggest a reduced P/O between 3 and 8 min of heavy exercise, implicating mitochondrial uncoupling as an additional mechanism of the 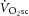 .
.
It is important to recognize that the slope of the 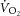 –[PCr] relationship reflects the combined influence of mitochondrial density, the rate constant (k) of [PCr] breakdown relative to k of
–[PCr] relationship reflects the combined influence of mitochondrial density, the rate constant (k) of [PCr] breakdown relative to k of 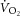 , the total [creatine] and the P/O (Meyer, 1988; Kemp et al. 2014). Mitochondrial density and total [creatine] are constant during acute exercise, and therefore any divergence in
, the total [creatine] and the P/O (Meyer, 1988; Kemp et al. 2014). Mitochondrial density and total [creatine] are constant during acute exercise, and therefore any divergence in 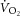 –[PCr] slope would result from changes in k[PCr] and/or P/O over the exercise intensities. While the k[PCr] was not different between 3 and 8 min of heavy-intensity exercise (p = n.s.), there was variance among individuals (Fig.3A). Therefore, while we base our interpretation on the group mean, we cannot rule out the influence of variance in the individual changes in k[PCr] in interpreting the
–[PCr] slope would result from changes in k[PCr] and/or P/O over the exercise intensities. While the k[PCr] was not different between 3 and 8 min of heavy-intensity exercise (p = n.s.), there was variance among individuals (Fig.3A). Therefore, while we base our interpretation on the group mean, we cannot rule out the influence of variance in the individual changes in k[PCr] in interpreting the 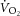 –[PCr] slope. In addition, we used a fixed transit delay to phase align the
–[PCr] slope. In addition, we used a fixed transit delay to phase align the 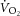 and [PCr] measurements in the time domain. This correction provided the best fit to the kinetics that we could make, but it is a limitation for interpreting the
and [PCr] measurements in the time domain. This correction provided the best fit to the kinetics that we could make, but it is a limitation for interpreting the 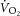 –[PCr] relationship. Specifically, small errors in transit delay adjustment result in non-linear distortion when plotting single participant data, although this influence is greater during the early kinetics (first 2 min) than between 3 and 8 min of exercise, when the kinetics are slower. Finally, the progressive intramuscular acidification during exercise would be expected to dissociate the dynamics of
–[PCr] relationship. Specifically, small errors in transit delay adjustment result in non-linear distortion when plotting single participant data, although this influence is greater during the early kinetics (first 2 min) than between 3 and 8 min of exercise, when the kinetics are slower. Finally, the progressive intramuscular acidification during exercise would be expected to dissociate the dynamics of 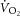 and [PCr], speeding the former and slowing the latter (Iotti et al. 1993; Gerbino et al. 1996; Layec et al. 2013). Therefore, while substantial assumptions necessarily underlie the interpretation of the
and [PCr], speeding the former and slowing the latter (Iotti et al. 1993; Gerbino et al. 1996; Layec et al. 2013). Therefore, while substantial assumptions necessarily underlie the interpretation of the 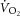 –[PCr] relationship, it is currently the only way to examine change in P/O as a potential mechanism explaining the lack of relationship between the magnitude of the
–[PCr] relationship, it is currently the only way to examine change in P/O as a potential mechanism explaining the lack of relationship between the magnitude of the 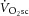 and ΔATPtot. These data suggest that P/O is stable during moderate-intensity exercise and the first 3 min of heavy-intensity exercise, in agreement with the other 31P MRS studies (e.g. where the
and ΔATPtot. These data suggest that P/O is stable during moderate-intensity exercise and the first 3 min of heavy-intensity exercise, in agreement with the other 31P MRS studies (e.g. where the 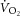 –[PCr] relationship is strikingly linear throughout the metabolic rate range; Bailey et al. 2010), but that sustained heavy-intensity exercise beyond 3 min may be accompanied by a reduction in P/O. Consequently, contrary to the prevailing hypothesis (Rossiter et al. 2002), the
–[PCr] relationship is strikingly linear throughout the metabolic rate range; Bailey et al. 2010), but that sustained heavy-intensity exercise beyond 3 min may be accompanied by a reduction in P/O. Consequently, contrary to the prevailing hypothesis (Rossiter et al. 2002), the 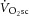 may be, at least in part, a result of mitochondrial uncoupling in the active muscle during acidifying exercise.
may be, at least in part, a result of mitochondrial uncoupling in the active muscle during acidifying exercise.
Potential mechanisms of mitochondrial uncoupling
There are various mechanisms that might cause the mitochondrial transmembrane proton gradient to dissipate during exercise. This proton ‘leak’ is regulated by uncoupling proteins and contributes to setting the resting P/O. If this process is augmented during exercise, the ATP yield per atomic oxygen consumed would fall. Others have shown upregulation of uncoupling proteins 2 and 3 (both expressed in skeletal muscle) with an acute bout of exercise, and these can induce mitochondrial uncoupling, which is likely to minimize production of, and damage from, reactive oxygen species (Brand et al. 2004; Bo et al. 2008; Jiang et al. 2009). This effect may be akin to the chronic uncoupling reported with ageing, posited as a protective mechanism against damage from reactive oxygen species (Brand et al. 2004; Amara et al. 2007), particularly as leak respiration comprises a large proportion of resting 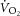 . However, the kinetics of upregulation of uncoupling proteins are relatively slow in comparison with the duration of exercise in our study; upregulation of uncoupling proteins is typically present ∼45–90 min after acute exercise. Additionally, investigations into mitochondrial uncoupling have relied on relatively long bouts of exercise (>30 min), and evidence from human muscle suggests that acute exercise may not be sufficient to elicit the same effect size for upregulation as seen in the rat (Fernström et al. 2004). Therefore, upregulation of uncoupling proteins seems less likely to explain fully the lack of relationship between
. However, the kinetics of upregulation of uncoupling proteins are relatively slow in comparison with the duration of exercise in our study; upregulation of uncoupling proteins is typically present ∼45–90 min after acute exercise. Additionally, investigations into mitochondrial uncoupling have relied on relatively long bouts of exercise (>30 min), and evidence from human muscle suggests that acute exercise may not be sufficient to elicit the same effect size for upregulation as seen in the rat (Fernström et al. 2004). Therefore, upregulation of uncoupling proteins seems less likely to explain fully the lack of relationship between 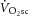 and ΔATPtot during heavy exercise.
and ΔATPtot during heavy exercise.
Alternatively, dissociation of the 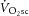 and ΔATPtot may result from high [H+] or [Pi] during exercise (Walsh et al. 2002). Low pH can reduce [ADP] from a shift in the creatine kinase equilibrium (Conley et al. 2001) and also serve to dissociate creatine kinase from the mitochondrial membrane, leading to a disruption in oxidative phosphorylation (Walsh et al. 2002). While evidence for a direct effect of acidosis is certainly not conclusive (Suleymanlar et al. 1992; Kemp et al. 2014), numerous studies show disturbances to oxidative phosphorylation through the inhibition of respiratory enzymes or reductions in the proton motive force (Hillered et al. 1984; Harkema & Meyer, 1997; Jubrias et al. 2003), but fail to result in change to P/O alone (Tonkonogi & Sahlin, 1999). Nevertheless, the variable relationships between the magnitude of the
and ΔATPtot may result from high [H+] or [Pi] during exercise (Walsh et al. 2002). Low pH can reduce [ADP] from a shift in the creatine kinase equilibrium (Conley et al. 2001) and also serve to dissociate creatine kinase from the mitochondrial membrane, leading to a disruption in oxidative phosphorylation (Walsh et al. 2002). While evidence for a direct effect of acidosis is certainly not conclusive (Suleymanlar et al. 1992; Kemp et al. 2014), numerous studies show disturbances to oxidative phosphorylation through the inhibition of respiratory enzymes or reductions in the proton motive force (Hillered et al. 1984; Harkema & Meyer, 1997; Jubrias et al. 2003), but fail to result in change to P/O alone (Tonkonogi & Sahlin, 1999). Nevertheless, the variable relationships between the magnitude of the 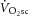 and ΔATPtot, together with a steepened
and ΔATPtot, together with a steepened 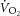 –[PCr] relationship, suggest P/O change as a possible scenario during heavy exercise.
–[PCr] relationship, suggest P/O change as a possible scenario during heavy exercise.
Technical considerations and study limitations
While limitations accompany the estimations, our study design provides an advantage over previous reports of ATP turnover rate in the literature. Prior estimations have relied on extrapolation of Vi[PCr], which is assumed to be affected only by the [PCr] recovery amplitude. This model constrains P/O with a linear 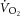 –[PCr] relationship, by definition (Layec et al. 2009a, which is in contrast with recent findings (Kemp, 2008; Wüst et al. 2011; Glancy & Balaban, 2012) and the observations in this study (Fig.5). Conversely, [PCr] recovery dynamics may be plastic during supra-LT exercise where intracellular acidification (Yoshida & Watari, 1993, 1994), fatigue-related metabolite accumulation (Jones et al. 2008) and muscle fatigue (Yano et al. 2001; Cannon et al. 2011) have been reported. While the group mean for k[PCr] resynthesis (or time constant, τ = 1/k) is not different following sub- and supra-LT exercise in this study and others (Rossiter et al. 2002), our data suggest that k[PCr] is not constant within an individual. Therefore, in our study, Vi[PCr] (and, thus, Q and ATPtot) were not constrained to increase in response only to changes in [PCr]. In other words, the augmented amplitude of [PCr] during the slow component did not result in an obligatorily faster initial rate of change following the cessation of exercise; our measurement was dependent on the recovery dynamics characterized and specific to that moment in time. Consequently, the estimations provided for oxidative ATP yield in our study are devoid of the assumptions about the
–[PCr] relationship, by definition (Layec et al. 2009a, which is in contrast with recent findings (Kemp, 2008; Wüst et al. 2011; Glancy & Balaban, 2012) and the observations in this study (Fig.5). Conversely, [PCr] recovery dynamics may be plastic during supra-LT exercise where intracellular acidification (Yoshida & Watari, 1993, 1994), fatigue-related metabolite accumulation (Jones et al. 2008) and muscle fatigue (Yano et al. 2001; Cannon et al. 2011) have been reported. While the group mean for k[PCr] resynthesis (or time constant, τ = 1/k) is not different following sub- and supra-LT exercise in this study and others (Rossiter et al. 2002), our data suggest that k[PCr] is not constant within an individual. Therefore, in our study, Vi[PCr] (and, thus, Q and ATPtot) were not constrained to increase in response only to changes in [PCr]. In other words, the augmented amplitude of [PCr] during the slow component did not result in an obligatorily faster initial rate of change following the cessation of exercise; our measurement was dependent on the recovery dynamics characterized and specific to that moment in time. Consequently, the estimations provided for oxidative ATP yield in our study are devoid of the assumptions about the 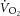 –[ADP] and
–[ADP] and 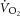 –[PCr] relationships.
–[PCr] relationships.
ATPtot is most heavily weighted on changes in Vi[PCr], a measure that is sensitive to noise in the MRS signal (e.g. Fig. 7 of Rossiter et al. 2000); this initial rate is derived from characterization of the kinetics of [PCr] recovery. The influence of noise in [PCr] recovery kinetics, particularly in the early transient, is likely to be the largest source of variability to resolve ATPtot. Conversely, the confidence in characterizing [PCr] off-kinetics is substantially greater than for pulmonary 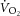 or even [PCr] during the on-transient. Any improvement in the characterization of 31P dynamics will take a considerable leap in signal-to-noise ratio and more rapid acquisition of spectra.
or even [PCr] during the on-transient. Any improvement in the characterization of 31P dynamics will take a considerable leap in signal-to-noise ratio and more rapid acquisition of spectra.
The heterogeneous nature of skeletal muscle metabolism (Koga et al. 2007; Damon et al. 2008; Saitoh et al. 2009; Cannon et al. 2013) may have obscured the characterization of [PCr] dynamics, and therefore ATPtot. Using 31P MRS, we measured a volume of tissue (∼300 g) that may not be representative of the entire knee extensor group responsible for the power output or the diversity of metabolic strain within this group. Finally, the unmeasured work of knee flexion is not accounted for with our ergometer. Therefore, the work of knee flexion (to lift the leg) is assumed to be constant in our experiments, but does contribute to the pulmonary 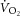 signal.
signal.
Conclusions
Similar to previous studies, the mean magnitudes of the 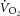 and [PCr] slow components were not different during heavy exercise, consistent with the prevailing hypothesis for the intramuscular source of the
and [PCr] slow components were not different during heavy exercise, consistent with the prevailing hypothesis for the intramuscular source of the 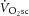 , i.e. an increase in the phosphate cost of force production. Although the magnitude of the
, i.e. an increase in the phosphate cost of force production. Although the magnitude of the 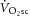 (∼22%) was similar to the increase in ATPtot (∼26%) from 3 to 8 min during heavy exercise, there was no relationship detected between these measures among individuals. Therefore, our data suggest that the pulmonary
(∼22%) was similar to the increase in ATPtot (∼26%) from 3 to 8 min during heavy exercise, there was no relationship detected between these measures among individuals. Therefore, our data suggest that the pulmonary 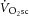 does not originate solely from increases in the phosphate cost of power production (increased P/W). Other mechanisms, such as an increased O2 cost of ATP resynthesis (reduced P/O) during acidifying exercise, may also contribute to generating the
does not originate solely from increases in the phosphate cost of power production (increased P/W). Other mechanisms, such as an increased O2 cost of ATP resynthesis (reduced P/O) during acidifying exercise, may also contribute to generating the 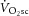 .
.
Acknowledgments
We are very grateful to Dr Peter Brooks and Emma Routeledge from the Department of Mechanical Engineering at the University of Leeds, for the design and construction of the customized carbon-fibre lever arms of the MR ergometer. We also wish to thank all volunteers for their time and dedication.
Glossary
- A
amplitude
- ATPtot
total ATP turnover rate
- D
ATP production from phosphocreatine breakdown
- k
rate constant
- L
ATP production from glycogenolysis
- LT
lactate threshold
- MRS
magnetic resonance spectroscopy
- PCr
phosphocreatine
- PCrsc
phosphocreatine slow component
- pHi
intramuscular pH
- Pi
inorganic phosphate
- P/O
ATP yield per O → H2O
- P/W
ATP cost per unit power output
- Q
ATP production from oxidative phosphorylation
- RI
ramp incremental
arterial oxygenation
- τ
time constant
- Vi[PCr]
initial rate of phosphocreatine resynthesis
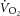
rate of whole-body O2 uptake
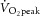
peak rate of O2 uptake
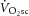
slow component of O2 uptake
Key points
Heavy-intensity exercise causes a progressive increase in energy demand that contributes to exercise limitation.
This inefficiency arises within the locomotor muscles and is thought to be due to an increase in the ATP cost of power production; however, the responsible mechanism is unresolved.
We measured whole-body O2 uptake and skeletal muscle ATP turnover by combined pulmonary gas exchange and magnetic resonance spectroscopy during moderate and heavy exercise in humans.
Muscle ATP synthesis rate increased throughout constant-power heavy exercise, but this increase was unrelated to the progression of whole-body inefficiency.
Our data indicate that the increased ATP requirement is not the sole cause of inefficiency during heavy exercise, and other mechanisms, such as increased O2 cost of ATP resynthesis, may contribute.
Additional information
Competing interests
None declared.
Author contributions
D.T.C., G.J.K. and H.B.R. conceived and designed experiments and analysed data. All authors performed experiments and interpreted data. D.T.C. prepared the figures. D.T.C. and H.B.R. wrote the manuscript. All authors critically reviewed and approved the final version of the manuscript.
Funding
This research was supported by Biotechnology and Biological Sciences Research Council (BBSRC) UK BB/I001174/1 and BB/I00162X/1.
References
- Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ. Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci U S A. 2007;104:1057–1062. doi: 10.1073/pnas.0610131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N. Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
-
Billat VL, Richard R, Binsse VM, Koralsztein JP. Haouzi P. The
 o2 slow component for severe exercise depends on type of exercise and is not correlated with time to fatigue. J Appl Physiol. 1998;85:2118–2124. doi: 10.1152/jappl.1998.85.6.2118. [DOI] [PubMed] [Google Scholar]
o2 slow component for severe exercise depends on type of exercise and is not correlated with time to fatigue. J Appl Physiol. 1998;85:2118–2124. doi: 10.1152/jappl.1998.85.6.2118. [DOI] [PubMed] [Google Scholar] - Bo H, Jiang N, Ma G, Qu J, Zhang G, Cao D, Wen L, Liu S, Ji LL. Zhang Y. Regulation of mitochondrial uncoupling respiration during exercise in rat heart: role of reactive oxygen species (ROS) and uncoupling protein 2. Free Radic Biol Med. 2008;44:1373–1381. doi: 10.1016/j.freeradbiomed.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Brand MD, Buckingham JA, Esteves TC, Green K, Lambert AJ, Miwa S, Murphy MP, Pakay JL, Talbot DA. Echtay KS. Mitochondrial superoxide and aging: uncoupling-protein activity and superoxide production. Biochem Soc Symp. 2004:203–213. doi: 10.1042/bss0710203. [DOI] [PubMed] [Google Scholar]
- Cannon DT, Howe FA, Whipp BJ, Ward SA, McIntyre DJ, Ladroue C, Griffiths JR, Kemp GJ. Rossiter HB. Muscle metabolism and activation heterogeneity by combined 31P chemical shift and T2 imaging, and pulmonary O2 uptake during incremental knee-extensor exercise. J Appl Physiol. 2013;115:839–849. doi: 10.1152/japplphysiol.00510.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DT, White AC, Andriano MF, Kolkhorst FW. Rossiter HB. Skeletal muscle fatigue precedes the slow component of oxygen uptake kinetics during exercise in humans. J Physiol. 2011;589:727–739. doi: 10.1113/jphysiol.2010.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Coats EM, Rossiter HB, Day JR, Miura A, Fukuba Y. Whipp BJ. Intensity-dependent tolerance to exercise after attaining
 o2 max in humans. J Appl Physiol. 2003;95:483–490. doi: 10.1152/japplphysiol.01142.2002. [DOI] [PubMed] [Google Scholar]
o2 max in humans. J Appl Physiol. 2003;95:483–490. doi: 10.1152/japplphysiol.01142.2002. [DOI] [PubMed] [Google Scholar] - Conley KE, Kemper WF. Crowther GJ. Limits to sustainable muscle performance: interaction between glycolysis and oxidative phosphorylation. J Exp Biol. 2001;204:3189–3194. doi: 10.1242/jeb.204.18.3189. [DOI] [PubMed] [Google Scholar]
- Conley KE, Kushmerick MJ. Jubrias SA. Glycolysis is independent of oxygenation state in stimulated human skeletal muscle in vivo. J Physiol. 1998;511:935–945. doi: 10.1111/j.1469-7793.1998.935bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damon BM, Wadington MC, Lansdown DA. Hornberger JL. Spatial heterogeneity in the muscle functional MRI signal intensity time course: effect of exercise intensity. Magn Reson Imaging. 2008;26:1114–1121. doi: 10.1016/j.mri.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimenna FJ, Fulford J, Bailey SJ, Vanhatalo A, Wilkerson DP. Jones AM. Influence of priming exercise on muscle [PCr] and pulmonary O2 uptake dynamics during ‘work-to-work’ knee-extension exercise. Respir Physiol Neurobiol. 2010;172:15–23. doi: 10.1016/j.resp.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Faraut B, Giannesini B, Matarazzo V, Marqueste T, Dalmasso C, Rougon G, Cozzone PJ. Bendahan D. Downregulation of uncoupling protein-3 in vivo is linked to changes in muscle mitochondrial energy metabolism as a result of capsiate administration. Am J Physiol Endocrinol Metab. 2007;292:E1474–E1482. doi: 10.1152/ajpendo.00292.2006. [DOI] [PubMed] [Google Scholar]
- Fernström M, Tonkonogi M. Sahlin K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J Physiol. 2004;554:755–763. doi: 10.1113/jphysiol.2003.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbino A, Ward SA. Whipp BJ. Effects of prior exercise on pulmonary gas-exchange kinetics during high-intensity exercise in humans. J Appl Physiol. 1996;80:99–107. doi: 10.1152/jappl.1996.80.1.99. [DOI] [PubMed] [Google Scholar]
- Glancy B. Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012;51:2959–2973. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema SJ. Meyer RA. Effect of acidosis on control of respiration in skeletal muscle. Am J Physiol Cell Physiol. 1997;272:C491–C500. doi: 10.1152/ajpcell.1997.272.2.C491. [DOI] [PubMed] [Google Scholar]
- Hillered L, Ernster L. Siesjö BK. Influence of in vitro lactic acidosis and hypercapnia on respiratory activity of isolated rat brain mitochondria. J Cereb Blood Flow Metab. 1984;4:430–437. doi: 10.1038/jcbfm.1984.62. [DOI] [PubMed] [Google Scholar]
- Iotti S, Lodi R, Frassineti C, Zaniol P. Barbiroli B. In vivo assessment of mitochondrial functionality in human gastrocnemius muscle by 31P MRS. The role of pH in the evaluation of phosphocreatine and inorganic phosphate recoveries from exercise. NMR Biomed. 1993;6:248–253. doi: 10.1002/nbm.1940060404. [DOI] [PubMed] [Google Scholar]
- Jiang N, Zhang G, Bo H, Qu J, Ma G, Cao D, Wen L, Liu S, Ji LL. Zhang Y. Upregulation of uncoupling protein-3 in skeletal muscle during exercise: a potential antioxidant function. Free Radic Biol Med. 2009;46:138–145. doi: 10.1016/j.freeradbiomed.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, DiMenna F, Fulford J. Poole DC. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol. 2008;294:R585–R593. doi: 10.1152/ajpregu.00731.2007. [DOI] [PubMed] [Google Scholar]
- Jubrias SA, Crowther GJ, Shankland EG, Gronka RK. Conley KE. Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J Physiol. 2003;553:589–599. doi: 10.1113/jphysiol.2003.045872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp G. Physiological implications of linear kinetics of mitochondrial respiration in vitro. Am J Physiol Cell Physiol. 2008;295:C844–C846. doi: 10.1152/ajpcell.00264.2008. author reply C847–C848. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Ahmad RE, Nicolay K. Prompers JJ. Quantification of skeletal muscle mitochondrial function by 31P magnetic resonance spectroscopy techniques: a quantitative review. Acta Physiol (Oxf) 2014 doi: 10.1111/apha.12307. in press, doi: 10.1111/apha.12307. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Meyerspeer M. Moser E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review. NMR Biomed. 2007;20:555–565. doi: 10.1002/nbm.1192. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Roussel M, Bendahan D, Le Fur Y. Cozzone PJ. Interrelations of ATP synthesis and proton handling in ischaemically exercising human forearm muscle studied by 31P magnetic resonance spectroscopy. J Physiol. 2001;535:901–928. doi: 10.1111/j.1469-7793.2001.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp GJ, Taylor DJ, Styles P. Radda GK. The production, buffering and efflux of protons in human skeletal muscle during exercise and recovery. NMR Biomed. 1993;6:73–83. doi: 10.1002/nbm.1940060112. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Thompson CH, Taylor DJ. Radda GK. ATP production and mechanical work in exercising skeletal muscle: a theoretical analysis applied to 31P magnetic resonance spectroscopic studies of dialyzed uremic patients. Magn Reson Med. 1995;33:601–609. doi: 10.1002/mrm.1910330504. [DOI] [PubMed] [Google Scholar]
- Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E. Barstow TJ. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol. 2007;103:2049–2056. doi: 10.1152/japplphysiol.00627.2007. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Ferguson RA, Kjær M. Bangsbo J. ATP and heat production in human skeletal muscle during dynamic exercise: higher efficiency of anaerobic than aerobic ATP resynthesis. J Physiol. 2003;549:255–269. doi: 10.1113/jphysiol.2002.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Jones AM, Wilkerson DP, Calbet JA. Bangsbo J. Muscular and pulmonary O2 uptake kinetics during moderate- and high-intensity sub-maximal knee-extensor exercise in humans. J Physiol. 2009;587:1843–1856. doi: 10.1113/jphysiol.2008.166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick MJ. Multiple equilibria of cations with metabolites in muscle bioenergetics. Am J Physiol Cell Physiol. 1997;272:C1739–C1747. doi: 10.1152/ajpcell.1997.272.5.C1739. [DOI] [PubMed] [Google Scholar]
- Lamarra N, Whipp BJ, Ward SA. Wasserman K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol. 1987;62:2003–2012. doi: 10.1152/jappl.1987.62.5.2003. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Befroy DE. Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol. 2005;99:1736–1744. doi: 10.1152/japplphysiol.00566.2005. [DOI] [PubMed] [Google Scholar]
- Layec G, Bringard A, Le Fur Y, Vilmen C, Micallef JP, Perrey S, Cozzone PJ. Bendahan D. Effects of a prior high-intensity knee-extension exercise on muscle recruitment and energy cost: a combined local and global investigation in humans. Exp Physiol. 2009a;94:704–719. doi: 10.1113/expphysiol.2008.044651. [DOI] [PubMed] [Google Scholar]
- Layec G, Bringard A, Le Fur Y, Vilmen C, Micallef JP, Perrey S, Cozzone PJ. Bendahan D. Comparative determination of energy production rates and mitochondrial function using different 31P MRS quantitative methods in sedentary and trained subjects. NMR Biomed. 2011;24:425–438. doi: 10.1002/nbm.1607. [DOI] [PubMed] [Google Scholar]
- Layec G, Bringard A, Vilmen C, Micallef JP, Le Fur Y, Perrey S, Cozzone PJ. Bendahan D. Does oxidative capacity affect energy cost? An in vivo MR investigation of skeletal muscle energetics. Eur J Appl Physiol. 2009b;106:229–242. doi: 10.1007/s00421-009-1012-y. [DOI] [PubMed] [Google Scholar]
- Layec G, Bringard A, Yashiro K, Le Fur Y, Vilmen C, Micallef JP, Perrey S, Cozzone PJ. Bendahan D. The slow components of phosphocreatine and pulmonary oxygen uptake can be dissociated during heavy exercise according to training status. Exp Physiol. 2012;97:955–969. doi: 10.1113/expphysiol.2011.062927. [DOI] [PubMed] [Google Scholar]
- Layec G, Malucelli E, Le Fur Y, Manners D, Yashiro K, Testa C, Cozzone PJ, Iotti S. Bendahan D. Effects of exercise-induced intracellular acidosis on the phosphocreatine recovery kinetics: a 31P MRS study in three muscle groups in humans. NMR Biomed. 2013;26:1403–1411. doi: 10.1002/nbm.2966. [DOI] [PubMed] [Google Scholar]
- Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol Cell Physiol. 1988;254:C548–C553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- Moon RB. Richards JH. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973;248:7276–7278. [PubMed] [Google Scholar]
- Murgatroyd SR, Ferguson C, Ward SA, Whipp BJ. Rossiter HB. Pulmonary O2 uptake kinetics as a determinant of high-intensity exercise tolerance in humans. J Appl Physiol. 2011;110:1598–1606. doi: 10.1152/japplphysiol.01092.2010. [DOI] [PubMed] [Google Scholar]
- Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R. Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- Poole DC, Barstow TJ, Gaesser GA, Willis WT. Whipp BJ. VO2 slow component: physiological and functional significance. Med Sci Sports Exerc. 1994;26:1354–1358. [PubMed] [Google Scholar]
- Poole DC. Jones AM. Oxygen uptake kinetics. Compr Physiol. 2012;2:933–996. doi: 10.1002/cphy.c100072. [DOI] [PubMed] [Google Scholar]
- Poole DC, Schaffartzik W, Knight DR, Derion T, Kennedy B, Guy HJ, Prediletto R. Wagner PD. Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans. J Appl Physiol. 1991;71:1245–1260. doi: 10.1152/jappl.1991.71.4.1245. [DOI] [PubMed] [Google Scholar]
- Rossiter HB. Exercise: kinetic considerations for gas exchange. Compr Physiol. 2011;1:203–244. doi: 10.1002/cphy.c090010. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Howe FA, Ward SA, Kowalchuk JM, Griffiths JR. Whipp BJ. Intersample fluctuations in phosphocreatine concentration determined by 31P-magnetic resonance spectroscopy and parameter estimation of metabolic responses to exercise in humans. J Physiol. 2000;528:359–369. doi: 10.1111/j.1469-7793.2000.t01-1-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR. Whipp BJ. Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol. 1999;518:921–932. doi: 10.1111/j.1469-7793.1999.0921p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR. Whipp BJ. Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol. 2001;537:291–303. doi: 10.1111/j.1469-7793.2001.0291k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR. Whipp BJ. Dynamic asymmetry of phosphocreatine concentration and O2 uptake between the on- and off-transients of moderate- and high-intensity exercise in humans. J Physiol. 2002;541:991–1002. doi: 10.1113/jphysiol.2001.012910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Ferreira LF, Barstow TJ, Poole DC, Ooue A, Kondo N. Koga S. Effects of prior heavy exercise on heterogeneity of muscle deoxygenation kinetics during subsequent heavy exercise. Am J Physiol Regul Integr Comp Physiol. 2009;297:R615–R621. doi: 10.1152/ajpregu.00048.2009. [DOI] [PubMed] [Google Scholar]
- Sargeant AJ. Dolan P. Effect of prior exercise on maximal short-term power output in humans. J Appl Physiol. 1987;63:1475–1480. doi: 10.1152/jappl.1987.63.4.1475. [DOI] [PubMed] [Google Scholar]
- Suleymanlar G, Zhou HZ, McCormack M, Elkins N, Kucera R, Reiss OK. Shapiro JI. Mechanism of impaired energy metabolism during acidosis: role of oxidative metabolism. Am J Physiol Heart Circ Physiol. 1992;262:H1818–H1822. doi: 10.1152/ajpheart.1992.262.6.H1818. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M. Sahlin K. Actively phosphorylating mitochondria are more resistant to lactic acidosis than inactive mitochondria. Am J Physiol Cell Physiol. 1999;277:C288–C293. doi: 10.1152/ajpcell.1999.277.2.C288. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Poole DC, DiMenna FJ, Bailey SJ. Jones AM. Muscle fiber recruitment and the slow component of O2 uptake: constant work rate vs. all-out sprint exercise. Am J Physiol Regul Integr Comp Physiol. 2011;300:R700–R707. doi: 10.1152/ajpregu.00761.2010. [DOI] [PubMed] [Google Scholar]
- Walsh B, Tiivel T, Tonkonogi M. Sahlin K. Increased concentrations of Pi and lactic acid reduce creatine-stimulated respiration in muscle fibers. J Appl Physiol. 2002;92:2273–2276. doi: 10.1152/japplphysiol.01132.2001. [DOI] [PubMed] [Google Scholar]
- Walter G, Vandenborne K, Elliott M. Leigh JS. In vivo ATP synthesis rates in single human muscles during high intensity exercise. J Physiol. 1999;519:901–910. doi: 10.1111/j.1469-7793.1999.0901n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman K, Stringer WW. Casaburi R. Is the slow component of exercise VO2 a respiratory adaptation to anaerobiosis. Adv Exp Med Biol. 1995;393:187–194. [PubMed] [Google Scholar]
- Whipp BJ. Domains of aerobic function and their limiting parameters. In: Ward SA, Steinacker JM, editors. The Physiology and Pathophysiology of Exercise Tolerance. New York: Plenum; 1996. pp. 83–89. [Google Scholar]
- Whipp BJ, Rossiter HB, Ward SA, Avery D, Doyle VL, Howe FA. Griffiths JR. Simultaneous determination of muscle 31P and O2 uptake kinetics during whole body NMR spectroscopy. J Appl Physiol. 1999;86:742–747. doi: 10.1152/jappl.1999.86.2.742. [DOI] [PubMed] [Google Scholar]
- Wüst RC, Grassi B, Hogan MC, Howlett RA, Gladden LB. Rossiter HB. Kinetic control of oxygen consumption during contractions in self-perfused skeletal muscle. J Physiol. 2011;589:3995–4009. doi: 10.1113/jphysiol.2010.203422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Yunoki T. Ogata H. Relationship between the slow component of oxygen uptake and the potential reduction in maximal power output during constant-load exercise. J Sports Med Phys Fitness. 2001;41:165–169. [PubMed] [Google Scholar]
- Yoshida T. Watari H. Changes in intracellular pH during repeated exercise. Eur J Appl Physiol Occup Physiol. 1993;67:274–278. doi: 10.1007/BF00864228. [DOI] [PubMed] [Google Scholar]
- Yoshida T. Watari H. Exercise-induced splitting of the inorganic phosphate peak: investigation by time-resolved 31P-nuclear magnetic resonance spectroscopy. Eur J Appl Physiol Occup Physiol. 1994;69:465–473. doi: 10.1007/BF00239861. [DOI] [PubMed] [Google Scholar]
-
Zoladz JA, Gladden LB, Hogan MC, Nieckarz Z. Grassi B. Progressive recruitment of muscle fibers is not necessary for the slow component of
 o2 kinetics. J Appl Physiol. 2008;105:575–580. doi: 10.1152/japplphysiol.01129.2007. [DOI] [PubMed] [Google Scholar]
o2 kinetics. J Appl Physiol. 2008;105:575–580. doi: 10.1152/japplphysiol.01129.2007. [DOI] [PubMed] [Google Scholar] -
Żołądź JA. Korzeniewski B. Physiological background of the change point in
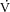 O2 and the slow component of oxygen uptake kinetics. J Physiol Pharmacol. 2001;52:167–184. [PubMed] [Google Scholar]
O2 and the slow component of oxygen uptake kinetics. J Physiol Pharmacol. 2001;52:167–184. [PubMed] [Google Scholar]


