Abstract
Crocodiles and their kin (Crocodylidae) use asymmetrical (bounding and galloping) gaits when moving rapidly. Despite being morphologically and ecologically similar, it seems alligators and their kin (Alligatoridae) do not. To investigate a possible anatomical basis for this apparent major difference in locomotor capabilities, we measured relative masses and internal architecture (fascicle lengths and physiological cross-sectional areas) of muscles of the pectoral and pelvic limbs of 40 individuals from six representative species of Crocodylidae and Alligatoridae. We found that, relative to body mass, Crocodylidae have significantly longer muscle fascicles (increased working range), particularly in the pectoral limb, and generally smaller muscle physiological cross-sectional areas (decreased force-exerting capability) than Alligatoridae. We therefore hypothesise that the ability of some crocodylians to use asymmetrical gaits may be limited more by the ability to make large, rapid limb motions (especially in the pectoral limb) than the ability to exert large limb forces. Furthermore, analysis of scaling patterns in muscle properties shows that limb anatomy in the two clades becomes more divergent during ontogeny. Limb muscle masses, fascicle lengths and physiological cross-sectional areas scale with significantly larger coefficients in Crocodylidae than Alligatoridae. This combination of factors suggests that inter-clade disparity in maximal limb power is highest in adult animals. Therefore, despite their apparent morphological similarities, both mean values and scaling patterns suggest that considerable diversity exists in the locomotor apparatus of extant Crocodylia.
Keywords: archosauria, biomechanics, locomotion, myology, ontogeny, scaling
Introduction
Smaller individuals of some species of extant Crocodylia use asymmetrical bounding and galloping gaits for rapid terrestrial locomotion. This remarkable behaviour has been reported in only two of the three extant crocodylian clades: Gavialoidea [the gharial, Gavialis gangeticus (Singh & Bustard, 1976)] and Crocodylidae [the Australian freshwater crocodile, Crocodylus johnstoni (Webb & Gans, 1982; Renous et al. 2002), the Australian saltwater crocodile, Crocodylus porosus (Zug 1974), the mugger crocodile Crocodylus palustris and the New Guinea crocodile Crocodylus novaeguineae (Whitaker & Andrews, 1988), and the West African dwarf crocodile Osteolamus tetraspis (Whitaker, 1981)].
Despite numerous analyses (e.g. Gatesy, 1991; Blob & Biewener, 1999; Blob, 2001; Willey et al. 2004), such asymmetrical gaits have yet to be observed in Alligatoridae [although Reilly & Elias (1998) describe one individual American alligator (Alligator mississippiensis) as ‘attempt(ing) to gallop’ for part of a stride]. Remarkably, this suggests that Alligatoridae, despite being morphologically more similar to Crocodylidae than either is to Gavialoidea (e.g. Brochu, 1997, 2012; Meers 1999), may lack a major locomotor trait found in both of those clades (Hutchinson, 2012). Additionally, anecdotal evidence (e.g. Cott 1960; Singh & Bustard, 1976) supports the inference that both Crocodylidae and Gavialoidea may lose the use of asymmetrical gaits past a certain size boundary (∼2 m total length). Such ontogenetic gait loss is rare among tetrapods, so analysis of crocodylian asymmetrical gaits is not only a source of comparative data on quadrupedal gaits in general (crocodylians represent the only non-mammalian vertebrates known to use asymmetrical quadrupedal gaits), it is also a useful case study in vertebrate locomotor ontogeny.
While the symmetrical gaits of Crocodylia (mainly A. mississippiensis) are reasonably well studied, equivalent systematic observations of asymmetrical (galloping and bounding) gaits unfortunately are scarce. To date, only two studies (Webb & Gans, 1982; Renous et al. 2002) have gathered sufficient data to quantify kinematic parameters of asymmetrical gaits, both in the Australian freshwater crocodile, C. johnstoni.
However, unlike behavioural data from living animals, descriptive anatomical data from crocodylian cadavers can be obtained relatively easily. These data can also be indirectly informative on comparative locomotor capabilities, inspiring hypotheses about locomotor function that future experimental or theoretical studies can test. Here, we investigate whether an anatomical basis for asymmetrical gait use can be found in the limbs of extant Crocodylia by comparing muscle architecture from representative Alligatoridae and Crocodylidae.
Muscle ‘architecture’ refers to anatomical properties of muscle (fascicle length, fascicle cross-section, and mass) directly linked to biomechanical principles of muscle function. Briefly stated (although see Calow & Alexander, 1973; Sacks & Roy, 1982; Alexander & Ker, 1990; Payne et al. 2005; Smith et al. 2006; and Allen et al. 2010 for more-in depth discussion), when comparing muscles of otherwise similar properties (muscle fibre type, internal and external tendon components and the size and geometry of the bony levers to which they attach), fascicle length determines the ‘working range’ over which a muscle may contract, whereas fascicle cross-sectional area (physiological cross-sectional area, PCSA) determines the force a muscle may generate. Muscle mass sets a constant, inverse relationship between fascicle length and area (a muscle may have either shorter fascicles or fewer long fascicles in a given volume), and so determines muscle work (force times distance) and, again, assuming equal fibre velocities, muscle power (force times distance/time). Analysis of significant differences and differential ontogenetic scaling patterns in muscle architecture among taxa with differing locomotor abilities may therefore be an informative proxy for functional differences underlying use/non-use of asymmetrical gaits.
In the footfall-based classification of Hildebrand (see Hildebrand, 1985 and references therein for full discussion of gait patterns), symmetrical gaits in Crocodylia studied to date can be generally be characterised as lateral sequence walking or a walking trot (Gatesy, 1991; Reilly & Elias 1998, Renous et al. 2002). Asymmetrical gaits were found to be highly variable, with four broad classes recognised (Renous et al. 2002). These include the bound (forelimbs then hindlimbs make successive ground contact as near-synchronous pairs), half-bound (individual forelimbs make contact in succession, followed by near-synchronous hindlimb contact) and two forms of gallop [individual forelimbs and hindlimbs make contact in a four-beat sequence, either with ipsilateral (transverse gallop) or contralateral (rotary gallop) fore-hind lead limbs].
In equivalently sized taxa [∼0.3 m snout-vent length (SVL)], maximum recorded relative velocities for trotting were generally far slower (∼1 SVL s−1, Reilly & Ellias 1998) than for bounding and galloping (∼9 SVL s−1, Webb & Gans, 1982; ∼15 SVL s−1, Renous et al. 2002, both using a bound). Minimum speeds recorded for asymmetrical gaits come close to, but do not overlap with, maximum symmetrical gait speeds (∼1.4 SVL s−1, Renous et al. 2002). Although duty factor (stance time/stride time) was in general less for asymmetrical gaits (∼0.5, Renous et al. 2002) than for symmetrical (∼0.7, Reilly & Elias 1998) gaits, no significant relationship between speed and duty factor was found for either gait class (Renous et al. 2002). Duty factors therefore remained high for an asymmetrical gait, and speed increases were achieved by increasing both stride length and frequency (Reilly & Elias 1998; Renous et al. 2002).
Asymmetrical gaits may therefore be tentatively associated with absolutely higher speeds and, because duty factor remains constant, larger and faster arcs of limb motion during stance as speed increases. Faster speeds (in addition to the presence of an aerial phase) require generally greater forces from limb extensor (antigravity) muscles (Weyand et al. 2000; Hutchinson, 2004a). Given the association of muscle architectural and functional properties, this suggests that crocodylian bounding/galloping may require generally longer muscle fascicles (to cycle the limbs through larger arcs), and larger extensor muscle PCSA (to provide greater support forces). Considering that these parameters cannot both be increased without increasing muscle mass, asymmetrical gaits may require larger extensor muscles.
Given the above points, we used our muscle architecture data to test the following hypotheses: first, that the apparent lack of asymmetrical gait usage ability in Alligatoridae is associated with significantly shorter limb muscle fascicles, smaller muscle PCSA, and less massive limb extensors (compared to Crocodylidae) and second, that the ontogenetic loss of asymmetrical gaits in Crocodylidae is associated with negatively allometric (compared with Alligatoridae) ontogenetic scaling of fascicle length, PCSA and muscle mass (i.e. scaling patterns will act to reduce differences in muscle architect-ure between Crocodylidae and Alligatoridae as ontogeny progresses).
Methods
We took the bulk of anatomical data from two species, Ameri-can alligators (A. mississippiensis, n = 15, body mass ∼0.5–57.7 kg), and Nile crocodiles (Crocodylus niloticus, n = 16, body mass ∼0.1–278 kg). We also obtained smaller anatomical datasets from the following species of Crocodylidae: Morelet's Crocodile (Crocodylus moreletii, n = 3, body masses ∼9.5–28 kg), Crocodylus johnstoni (n = 2, body masses ∼1.5–20 kg), and Osteolaemus tetraspis (n = 3, body masses ∼5.5–10 kg). Other than A. mississippiensis, the only other representative of Alligatoridae sampled was a single black caiman (Melanosuchus niger, body mass 90 kg). Our data on A. missisippiensis were taken from a previous publication (Allen et al. 2010) with minor modifications. We collected all other data from dissection of specimens obtained from La Ferme aux Crocodiles (Pierlatte, France), where they had died from natural causes unrelated to this study. All species other than A. mississippiensis were reared in captivity, which may influence body masses (see Results).
As the range in body masses for Alligatoridae (0.1–90 kg) differed by an order of magnitude from that for Crocodylidae (0.1–278 kg), we repeated our analyses restricting body masses for both clades to the same range (0.1–60 kg) to assess any associated bias in our results. The distribution of body masses in both samples is roughly similar (see Supporting Information Fig. S3 for a frequency scatter plot), although in the body mass range 10–20 kg Crocodylidae are better represented in our sample than Alligatoridae (10 individuals vs. 4).
We measured whole-body mass (Mbody) for each specimen using a hanging scale (Cely CR-200, accurate to 100 g) for animals larger than 1 kg, and electronic scales (Medler PM480 DeltaRange, accurate to 0.001 g) for smaller specimens, as well as for all individual muscle masses. We recorded muscle mass (Mmusc) after trimming all external tendon. The sum of all limb muscle masses was also calculated for the pectoral and pelvic limbs. We bisected muscle bellies parallel to fascicle orientation and recorded exposed fascicle lengths. Because fascicle lengths vary within individual muscles, we repeated bisection and measurement at multiple sites (approximately five per belly, varying with the complexity of the muscle) and calculated a mean value (Lfasc). We estimated fascicle pennation angle (angular difference between fascicles and internal tendons/aponeuroses) using a protractor. Again, multiple measurements were taken (also approximately five) and a mean value (θ, in degrees) calculated.
We estimated muscle volume (Vmusc) from Mmusc using a density value of 1.06 g cm−3 (typical vertebrate muscle, Mendez & Keys, 1960). PCSA was then estimated to be Vmusc divided by Lfasc, multiplied by the cosine of θ (Eq. 1, below):
| (1) |
To analyse differences in muscle architectural properties between Crocodylidae and Alligatoridae, we used a simple means-difference test on normalised muscle architectural datasets [Mmusc/Mbody, Lfasc/Mbody1/3 and PCSA/Mbody2/3, making the implicit assumption that these parameters do not stray too far from geometric similarity, i.e. isometry)] from both clades using r (v0.97, R Development Core Team 2008). Data for several species had significantly non-normal distributions (based on a Shapiro–Wilks test, α = 0.05); hence we used a bootstrap method (boot package for r set at 10 000 replications, http://cran.r-project.org/web/packages/boot/index.html) to derive confidence intervals for mean architectural data in each clade. The null hypothesis, zero difference in clade means, was rejected (at α = 0.05) if the 95% confidence intervals did not overlap.
To compare scaling patterns in muscle architectural properties between the two clades, we log-transformed muscle architecture datasets (Mmusc, Lfasc and PCSA) and regressed them against (also log-transformed) Mbody using a reduced major axis method (lmodel2 package for r, http://cran.r-project.org/web/packages/lmodel2/index.html). As above, due to non-normality in the input datasets, we tested hypotheses of significant inter-clade differences in architectural property scaling coefficients using a 10 000-replication bootstrap, generating 95% confidence intervals. The null hypothesis of no difference in scaling coefficient was rejected (again at α = 0.05) if the confidence intervals for each clade did not overlap. Additionally, we tested for positive or negative allometry in our architectural data by comparing our confidence intervals for regression coefficients against isometry, represented by a coefficient of 1 for muscle mass, 2/3 for PCSA, and 1/3 for fascicle length.
Results
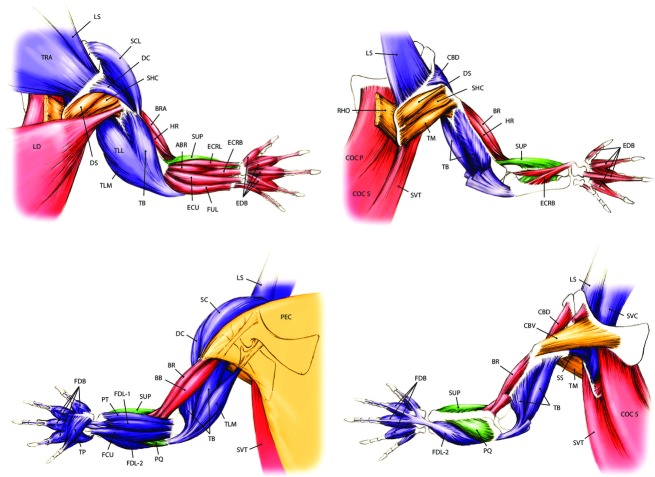
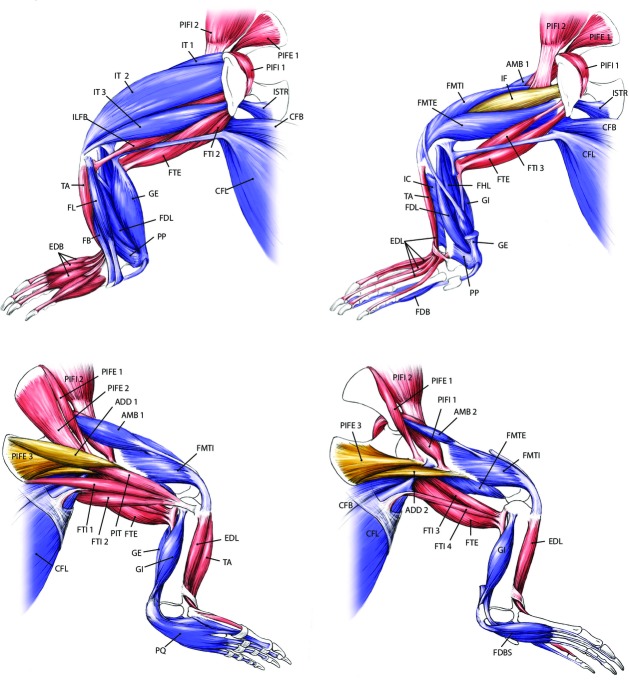
We obtained 27 225 measurements of 36 pectoral and 38 pelvic limb muscles (see Table 1 for names, estimated functions, and abbreviations, and Figs 1 and 2 for in situ anatomy of pectoral and pelvic limbs, respectively) from 40 individual crocodylians representing six species. Here we present our results for muscle architecture first for mean values (shown in Figs 5) and then for scaling relationships (shown in Figs 8). We found no significant differences in our results using all available specimens vs. restricting body mass to 0.1–60 kg for either clade. The results of both mean value comparisons and scaling analysis can be found, along with the raw data, in the Supporting Information Data S1 (fascicle length), S2 (muscle mass), S3 (PCSA), S4 (summed limb data) and S5 (raw data). Also included as Supporting Information are high-resolution versions of Figs 1 and 2 (Figs S1 and S2).
Table 1.
Crocodylian limb muscles, abbreviations and estimated primary functions
| Pectoral limb |
Pelvic limb |
||||
|---|---|---|---|---|---|
| Short name | Full name | Estimated function | Short name | Full name | Estimated function |
| SVT | Serratus ventralis thoracis | Pectoral girdle extensors | CFL | Caudofemoralis longus | Hip extensors/knee flexors |
| COCS | Costocoracoideus superficialis | Pectoral girdle extensors | CFB | Caudofemoralis brevis | Hip extensors/knee flexors |
| COCP | Costocoracoideus profundus | Pectoral girdle extensors | ISTR | Ischiotrochantericus | Hip extensors/knee flexors |
| LS | Levator scapulae | Pectoral girdle flexors | ILFB | Iliofibularis | Hip extensors/knee flexors |
| TRA | Trapezius | Pectoral girdle flexors | PIT | Pubo-ischio-tibialis | Hip extensors/knee flexors |
| SVC | Serratus ventralis cervicus | Pectoral girdle flexors | FTE | Flexor tibialis externus | Hip extensors/knee flexors |
| RHO | Rhomboideus | Pectoral girdle adductors | FTI 1 | Flexor tibialis internus 1 | Hip extensors/knee flexors |
| LD | Latissimus dorsi | Shoulder extensors | FTI 2 | Flexor tibialis internus 2 | Hip extensors/knee flexors |
| SS | Subscapularis | Shoulder extensors | FTI 3 | Flexor tibialis internus 3 | Hip extensors/knee flexors |
| SC | Supracoracoideus | Shoulder flexors | FTI 4 | Flexor tibialis internus 4 | Hip extensors/knee flexors |
| DC | Deltoideus clavicularis | Shoulder flexors | PIFE 1 | Pubo-ischio-femoralis externus 1 | Hip adductors |
| CBD | Coracobrachialis brevis dorsalis | Shoulder flexors | PIFI 2 | Pubo-ischio-femoralis internus 2 | Hip flexors |
| PEC | Pectoralis | Shoulder adductors | PIFI 1 | Pubo-ischio-femoralis internus 1 | Hip flexors |
| CBV | Coracobrachialis brevis ventralis | Shoulder adductors | ADD 1 | Adductor 1 | Hip adductors |
| DS | Deltoideus scapularis | Shoulder abductors | ADD 2 | Adductor 2 | Hip adductors |
| SHC | Scapulohumeralis caudalis | Shoulder abductors | PIFE 2 | Pubo-ischio-femoralis externus 2 | Hip adductors |
| TM | Teres major | Shoulder abductors | PIFE 3 | Pubo-ischio-femoralis externus 3 | Hip adductors |
| TB | Triceps brevis | Elbow extensors | IF | Iliofemoralis | Hip abductors |
| TLL | Triceps longus lateralis | Elbow extensors | IT 1 | Iliotibialis 1 | Knee extensors |
| TLM | Triceps longus medialis | Elbow extensors | IT 2 | Iliotibialis 2 | Knee extensors |
| BB | Biceps brachii | Elbow flexors | IT 3 | Iliotibialis 3 | Knee extensors |
| HR | Humeroradialis | Elbow flexors | FMTE | Femorotibialis externus | Knee extensors |
| BR | Brachialis | Elbow flexors | FMTI | Femorotibialis internus | Knee extensors |
| FUL | Flexor ulnaris | Elbow flexors | AMB 1 | Ambiens 1 | Knee extensors |
| ABR | Abductor radialis | Elbow flexors | AMB 2 | Ambiens 2 | Knee extensors |
| PT | Pronator teres | Elbow pronators | GE | Gastrocnemius externus | Ankle plantarflexors |
| PQ | Pronator quadratus | Elbow pronators | GI | Gastrocnemius internus | Ankle plantarflexors |
| SUP | Supinator | Elbow supinators | FDL | Flexor digitorum longus (pelvic) | Ankle plantarflexors |
| FCU | Flexor carpi ulnaris | Wrist plantarflexors | IC | Interosseus cruris | Ankle plantarflexors |
| FDL-1 | Flexor digitorum longus 1 (pectoral limb) | Wrist plantarflexors | FL | Fibularis longus | Ankle plantarflexors |
| FDL-2 | Flexor digitorum longus 2 (pectoral limb) | Wrist plantarflexors | FHL | Flexor hallucis longus | Ankle plantarflexors |
| ECRB | Extensor carpi radialis brevis | Wrist dorsiflexors | FB | Fibularis brevis | Ankle plantarflexors |
| ECRL | Extensor carpi radialis longus | Wrist dorsiflexors | PP | Pronator profundus | Ankle plantarflexors |
| ECUL | Extensor carpi ulnaris longus | Wrist dorsiflexors | TA | Tibialis anterior | Ankle dorsiflexors |
| FDBF | Flexor digitorum brevis (pectoralis) | Digital plantarflexors (pectoral) | EDL | Extensor digitorum longus | Ankle dorsiflexors |
| EDBF | Extensor digitorum brevis (pectoralis) | Digital dorsiflexors (pectoral) | FDBH | Flexor digitorum brevis (pelvic) | Digital plantarflexors (pelvic) |
| EDBH | Extensor digitorum brevis (pelvic) | Digital dorsiflexors (pelvic) | |||
| EHL | Extensor hallucis longus | Digital dorsiflexors (pelvic) | |||
Fig. 1.
Pectoral limb anatomy of a generalised crocodylian. See Table 1 for muscle abbreviations. Colour denotes hypothesised primary locomotor function: Blue/purple (extensors), red (flexors), gold (supinators), green (pronators).
Fig. 2.
Pelvic limb anatomy of a generalised crocodylian. See Table 1 for muscle abbreviations. Colour denotes hypothesised primary locomotor function: Blue/purple (extensors), red (flexors), gold (abductors/adductors).
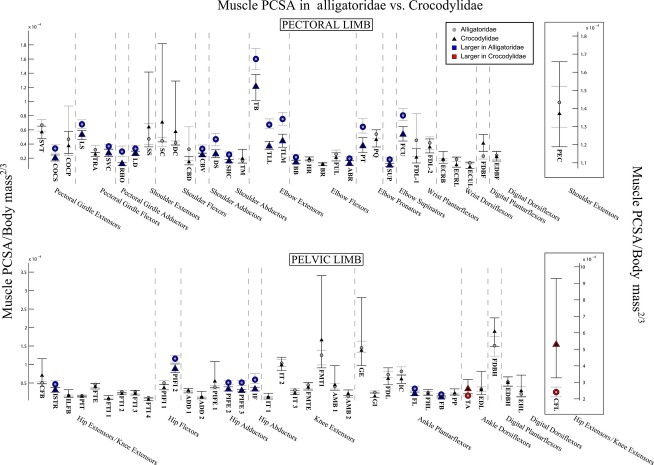
Fig. 5.
The 95% confidence intervals for mean limb muscle PCSA, normalised to body mass2/3, in Alligatoridae (grey circles) and Crocodylidae (black triangles). Muscles with significantly larger PCSA in Crocodylidae are highlighted in red; those with larger PCSA in Alligatoridae are highlighted in blue. See Table 1 for muscle abbreviations.
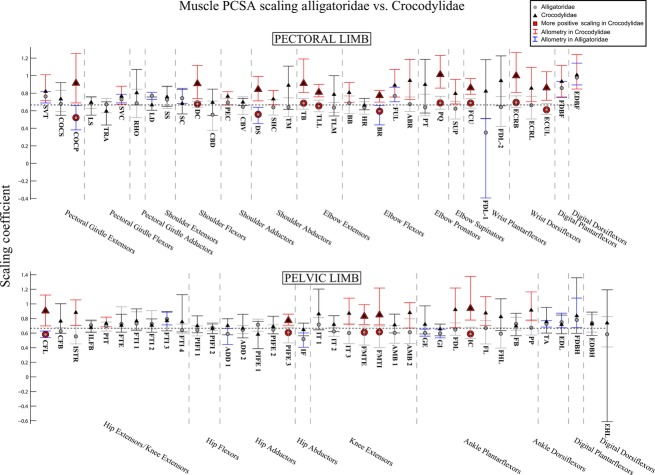
Fig. 8.
The 95% confidence intervals for scaling coefficients of limb muscle PCSA on body mass (log-log) in Alligatoridae (grey circles) and Crocodylidae (black triangles). Muscles with significantly higher coefficients in Crocodylidae are highlighted with red markers. Muscles of Crocodylidae that show allometry have their confidence intervals drawn in red. Those of Alligatoridae that show allometry have their confidence intervals drawn in blue. See Table 1 for muscle abbreviations.
Due to the potentially confounding effects of body mass in captive vs. wild animals (captive animals tend to be overweight), we also analysed a test subset of our data (muscle fascicle length) normalised to muscle belly length (i.e. muscle total length from origin to insertion, minus tendon length), rather than derivatives of body mass. We found no qualitative differences between these results and those obtained using body mass as a normaliser (see Fig. S4).
Mean values
Fascicle length
Both clades of Crocodylia showed proximal-distal decreases in fascicle length in both the pectoral and pelvic limbs (Fig. 3). Crocodylidae had significantly longer fascicles in most pectoral limb muscles (Fig. 3, red outlines), with the largest differences seen in pectoral girdle muscles. Exceptions to the trend were the digital muscles [M. flexor digitorum brevis (FDBF) and M. extensor digitorum brevis (EDBF), Fig. 3] and, interestingly, the largest single pectoral limb muscle [the shoulder adductor/extensor M. pectoralis (PEC)].
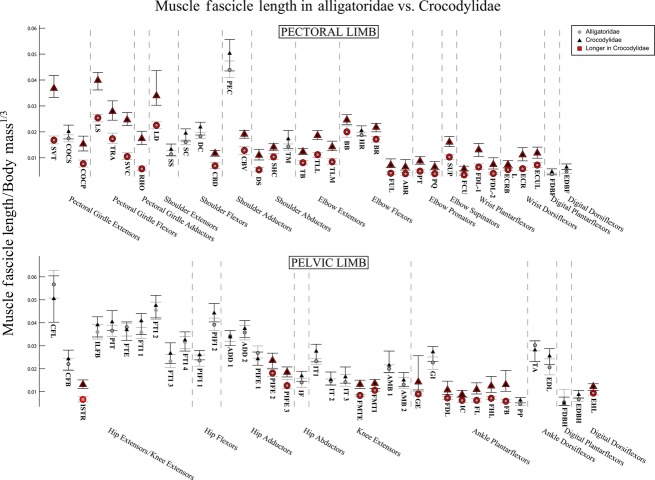
Fig. 3.
The 95% confidence intervals for mean limb muscle fascicle lengths, normalised to body mass1/3, in Alligatoridae (grey circles) and Crocodylidae (black triangles). Muscles with significantly longer fascicles in Crocodylidae are highlighted in red. See Table 1 for muscle abbreviations.
We found fewer significant differences in the pelvic limb. Fascicle lengths for the majority of hip and knee actuators were statistically indistinguishable between the two clades, although Crocodylidae had significantly longer fascicles in the more ventral heads of the M. puboischiofemoralis externus (PIFE 2 and 3) and the M. femorotibialis group (FMTI and FMTE, Fig. 3, red outlines). However, with the exception of the internal head of M. gastrocnemius (GI), we found Crocodylidae to have significantly longer fascicles in their ankle plantarflexors. We found no muscles in which Alligatoridae had significantly longer fascicles than Crocodylidae.
Muscle mass
Both clades also showed proximal-distal decreases in muscle masses within their limbs (Fig. 4). Unlike fascicle lengths, we found that masses for the majority of muscles in both limbs were statistically indistinguishable between Alligatoridae and Crocodylidae, and the remainder showed very small significant differences (Fig. 4). One notable exception was the M. serratus ventralis thoracis (SVT, Fig. 4, red outline), which was dramatically more massive in Crocodylidae. Interestingly, those significant differences that we did find indicated generally (slightly) more massive pectoral limb muscles in Crocodylidae (Fig. 4, red outlines), and (again, slightly) more massive pelvic limb muscles in Alligatoridae (Fig. 2, blue outlines).
Fig. 4.
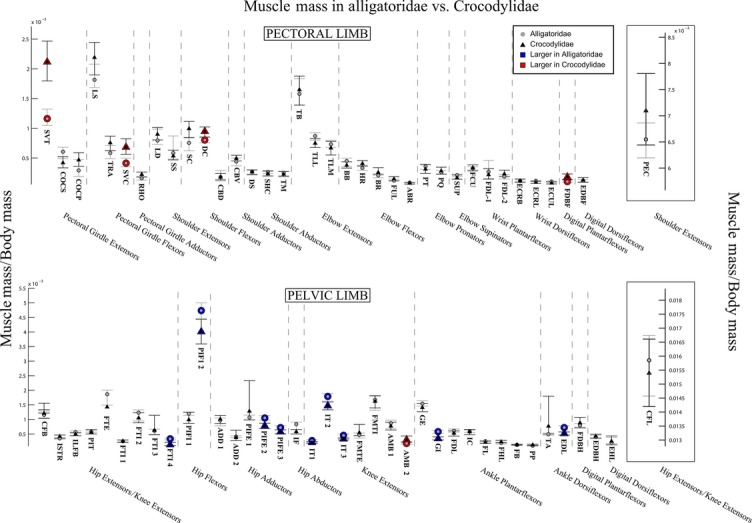
The 95% confidence intervals for mean limb muscle masses, normalised to body mass, in Alligatoridae (grey circles) and Crocodylidae (black triangles). Muscles with significantly larger masses in Crocodylidae are highlighted in red; those with larger masses in Alligatoridae are highlighted in blue. See Table 1 for muscle abbreviations. Boxes out on the right side shows the 95% confidence intervals for total limb muscle mass normalised to body mass (above), and for M. pectoralis (PEC) and M. caudofemoralis longus (CFL) (above and below).
Analysis of summed limb muscle masses (Fig. 4, boxout to right) indicates that while both Crocodylidae and Alligatoridae have a similar percentage of body mass dedicated to limb muscles (∼14% body mass, see Fig. 4, Data S4), the ratio of pectoral limb muscle mass: pelvic limb muscle mass differs significantly between clades – a ratio of 0.48 (Alligatoridae) vs. 0.62 (Crocodylidae – see Data S4). This is due to Crocodylidae having significantly more massive pectoral limbs (2.7% body mass vs. 2.2 in Alligatoridae see Fig. 4, Data S4).
Muscle PCSA
Unlike fascicle length and mass, we observed no particular proximal-distal trend in muscle PCSA (Fig. 5), which were (slightly) larger in the mid-limb (elbow and knee extensors) for both clades. We found that Alligatoridae had significantly larger PCSA for the majority of pectoral limb muscles (Fig. 5, blue outlines), and these differences were most pronounced for the elbow extensors, pronators and supinators.
We found fewer and generally smaller significant differences in the pelvic limb (Fig. 5), most of which also indicated (slightly) larger PCSA in Alligatoridae (Fig. 5, blue outlines). However, the single largest locomotor muscle, M. caudofemoralis longus (CFL, hip extensor and knee flexor, Fig. 5) had a dramatically larger PCSA in Crocodylidae (Fig. 5, red outline).
Scaling relationships
Fascicle length
In most muscles, scaling coefficients for fascicle lengths could not be statistically distinguished between the two clades (Fig. 6). There was only one exception to this pattern in the pectoral limb [the M. latissimus dorsi (LD), a shoulder extensor], and two in the pelvic limb [the third head of M. flexor tibialis internus (FTI 3), a hip extensor/knee flexor, and the M. tibialis anterior (TA), an ankle dorsiflexor], all of which showed more positive scaling coefficients in Crocodylidae (Fig. 6, red outlines).
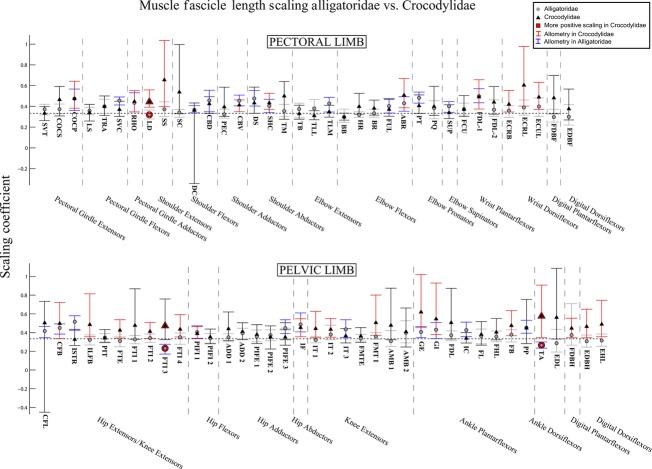
Fig. 6.
The 95% confidence intervals for scaling coefficients of limb muscle fascicle lengths on body mass (log-log) in Alligatoridae (grey circles) and Crocodylidae (black triangles). Muscles with significantly higher coefficients in Crocodylidae are highlighted with red markers. Muscles of Crocodylidae that show allometry have their confidence intervals drawn in red. Those of Alligatoridae that show allometry have their confidence intervals drawn in blue. See Table 1 for muscle abbreviations.
In general, scaling coefficients indicated slight positive allometry in muscle fascicle lengths for both limbs in both clades (Fig. 6, blue lines for Alligatoridae, red for Crocodylidae). However, we did find some differences in patterns of allometry vs. isometry in the two clades. We observed that Crocodylidae scaled shoulder extensor and wrist dorsiflexor fascicle lengths with mild positive allometry (Fig. 6, red lines), whereas Alligatoridae scaled them isometrically. We also identified (very slight) positive allometry of fascicle lengths in the shoulder flexors of Alligatoridae (vs. isometry in Crocodylidae). In the pectoral limb, Crocodylidae showed a slightly greater tendency to scale fascicle lengths for hip extensor/knee flexor and ankle/digital actuators (in general) positively.
Muscle mass
As we found for fascicle lengths, for most muscle masses, scaling coefficients could not be statistically distinguished between the two clades (Fig. 7), with some exceptions. In the pectoral limb, Crocodylidae scaled Mmusc significantly more positively (Fig. 7, red outlines) than Alligatoridae for the PEC, M. brachialis (BR, an elbow flexor), M. extensor carpi radialis brevis and M. extensor carpi ulnaris longus (ECRB & ECUL, both wrist dorsiflexors). In the pelvic limb, only the mass of M. interosseus cruris (IC, an ankle plantarflexor) was found to be significantly different, again scaling more positively in Crocodylidae.
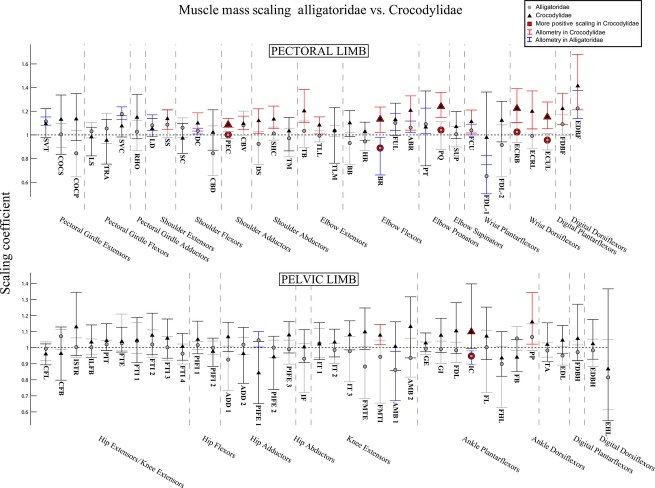
Fig. 7.
The 95% confidence intervals for scaling coefficients of limb muscle masses on body mass (log-log) in Alligatoridae (grey circles) and Crocodylidae (black triangles). Muscles with significantly higher coefficients in Crocodylidae are highlighted with red markers. Muscles of Crocodylidae that show allometry have their confidence intervals drawn in red. Those of Alligatoridae that show allometry have their confidence intervals drawn in blue. See Table 1 for muscle abbreviations.
Isometric vs. allometric scaling patterns did show marked differences between the two clades, however. Although positive allometry was more common in the pectoral than pelvic limbs of both clades (Fig. 7, blue lines for Alligatoridae, red for Crocodylidae), Crocodylidae scaled shoulder, wrist and interdigital muscle masses with positive allometry, whereas Alligatoridae scaled them either isometrically or with negative allometry (Fig. 7). There were few examples of allometry in the pelvic limb, showing no clear pattern.
Muscle PCSA
We found more statistically significant differences in scaling coefficients for muscle PCSA than for either fascicle length or mass (Fig. 8). In the pectoral limb, Crocodylidae scaled PCSA for elbow extensors and wrist dorsiflexors with significantly higher coefficients than Alligatoridae (Fig. 8, red outlines). In the pelvic limb, we found the same relationship for the CFL and the FMTE & FMTI.
Again, patterns of isometry vs. allometry differed between the two clades. In the pectoral limb and the distal pelvic limb, Crocodylidae scaled PCSA for most muscles with positive allometry (Fig. 8), whereas Alligatoridae showed mostly isometry (or mild negative allometry).
Discussion
Significant differences
Our analysis of mean architecture data indicates that significant differences exist between the limb musculature of Alligatoridae and Crocodylidae. Crocodylidae have longer muscle fascicles in both limbs, most markedly in the pectoral limb, and particularly the muscles of the pectoral girdle itself (Fig. 3). Crocodylian clades show less contrast in individual limb muscle masses, although analysis of summed limb muscle masses indicates that relative to Alligatoridae, Crocodylidae invest much more of body mass in the pectoral limb (Fig. 4, Data S4). This result suggests that Crocodylidae are able to perform more work (or have more available limb power) with their pectoral limbs, whereas Alligatoridae appear closer to parity between available power in the pectoral and pelvic limbs. PCSA for both limbs are in general significantly greater in Alligatoridae, particularly in the elbow extensor group. Strikingly, we observed the reverse relationship for the M. caudofemoralis longus (CFL), which has a markedly larger PCSA in Crocodylidae (Fig. 5).
How, then, do these data compare with our predictions for muscle architecture in crocodylian taxa that do and do not use asymmetrical gaits? Previous studies (e.g. Renous et al. 2002) inferred that faster-speed asymmetrical gaits involve larger arcs of limb motion than slower-speed symmetrical gaits. Muscle fascicle length, the architectural proxy for working range of a muscle, was noted to be generally lower in Alligatoridae (Fig. 3, although comparative muscle moment arms, which determine the transmission of muscle movement to limb movement, are not investigated here and could diminish or exaggerate this difference). If we provisionally accept (pending evidence to the contrary) that Alligatoridae lack the capacity to use asymmetrical gaits (Hutchinson, 2012), then their significantly shorter muscle fascicles may be a factor in this inability.
Furthermore, the larger difference observed in pectoral limb fascicle length suggests that the ability to perform large pectoral limb motions, and in particular motions of the pectoral girdle itself, may be important in crocodylian asymmetrical gaits (Fig. 3). This speculation could be tested by measurements of maximal limb joint ranges of motion in cadavers or in vivo. The significantly more massive pectoral limb muscles of Crocodylidae (Fig. 4) also indicate that generating (or absorbing) power with the pectoral limbs in particular may be important to asymmetrical gaits.
Available data support the inference that asymmetrical gaits are used at faster speeds, involve an aerial phase, and have lower duty factors than symmetrical gaits in Crocodylia (see Introduction). These gaits therefore must require greater (and more rapid) forces, particularly from extensor muscles. Muscle PCSA (the architectural proxy for muscle force) would consequently be expected to be larger, particularly for extensor muscles, in taxa that use asymmetrical gaits vs. those that do not. However, of the observed differences, only the larger PCSA (mean values differ by 2.21 × 10−4 body mass2/3, see Fig. 5, Fig. S3) of the CFL in Crocodylidae fits this prediction. All other extensors (and the majority of limb muscles in general) exhibit larger PCSA in Alligatoridae. Although differences in muscle moment arms, muscle fibre types, and the passive storage and release of energy in tendons (which we do not analyse) will all have significant effects on the forces and energies a limb can sustain, this difference in limb muscle PCSA leads us to speculate that active limb force (i.e. provided by muscular contraction) may not be a limiting factor in the use of asymmetrical gaits in Crocodylia.
However, it is interesting to note that the only muscle that does not follow this trend is M. caudofemoralis longus (CFL). The CFL is a massive hip extensor that is vital to crocodylian locomotion in general (Gatesy, 1991), with most of the belly housed in the ventral tail, attaching to the femur via its primary tendon, but also sending an accessory tendon to blend with the origin of the external head of M. gastrocnemius (GE, major plantarflexors of the ankle and pes). This anatomy places most of the muscle mass extrinsic to the limb, and allows the CFL to both (to some extent) flex the knee and plantarflex the ankle and distal limb, besides its hypothesised primary function of hip extension. As the CFL does not extend the knee, and has no way of actuating extension in the pectoral limb, a larger PCSA for the CFL therefore does not seem sufficient to compensate for the smaller PCSA of other pelvic limb extensors, or the generally smaller PCSA in the pectoral limb (Fig. 5), in providing limb supportive forces. However, without wishing to speculate further than our data allows (particularly as the large extrinsic pectoral limb extensor, PEC, does not also have a large PCSA in Crocodylidae – Fig. 5), a large PCSA for the CFL does fit with our prediction, based on muscle fascicle lengths, that rapid limb motion generated by muscular length changes is important to crocodylian asymmetrical gaits. Keeping limb muscle mass and hence inertia low would be advantageous for rapid movement because lighter limbs can be moved more quickly and (energetically) cheaply. If intrinsic muscles at multiple joints can be partially replaced by multi-articular extrinsic muscles (like the tail-based CFL), this would be an effective way of achieving this outcome. Quantitative data (EMG, ultrasound or sono-micrometry) on CFL activity during asymmetrical locomotion, and simulation-based analysis of its effect on distal limb joints, would be useful in further investigating its importance.
Scaling patterns
Our prediction that ontogenetic scaling would diminish differences between Alligatoridae and Crocodylidae appears to be false. We found very few statistically significant differences in scaling patterns for fascicle lengths (Fig. 6) and muscle mass (Fig. 7), suggesting that the relatively longer limb muscle fascicles of Crocodylidae remain so throughout ontogeny. In fact, what differences there are indicate Crocodylidae show a greater tendency to scale fascicle lengths and muscle masses with positive allometry, suggesting that in direct contradiction to our hypothesis, relative differences in fascicle length become more pronounced during ontogeny, not less.
The most consistent significant inter-clade differences in scaling patterns were for limb muscle PCSA (Fig. 8). With few exceptions, Alligatoridae scale limb muscle PCSA either isometrically or with mild negative allometry, whereas Crocodylidae PCSA generally scales with positive allometry (although less consistently in the pelvic limb). Considering the generally higher PCSA in Alligatoridae (Fig. 8), this scaling relationship would diminish relative inter-clade differences in at least some aspects of locomotor anatomy, bringing adult Crocodylidae closer to parity with adult Alligatoridae in the ability to exert limb forces.
However, lack of positive allometry in limb muscle PCSA (and hence available muscle force), particularly for extensor muscles, has been previously cited as evidence for an ontogenetic decline in general locomotor performance in Alligator mississippiensis (Blob, 2001; Allen et al. 2010). Furthermore, the tendency of Crocodylidae to scale both fascicle lengths and muscle masses positively (Figs 6 and 7) indicates a greater ability of adult Crocodylidae to generate locomotor work and power, not just force.
Conclusions
Our comparative analysis of muscle architecture demonstrates that significant differences in locomotor anatomy exist between Alligatoridae and Crocodylidae. Relative to body mass, Crocodylidae have generally longer muscle fascicles (especially in the pectoral limb) and smaller muscle PCSA than Alligatoridae. Large arcs of limb motion have been suggested to be a feature of crocodylian asymmetrical gaits (Renous et al. 2002), and fit with suggestions that crocodylians generally achieve faster speeds by increasing both stride length and frequency (Reilly & Ellias 1998). Use of asymmetrical gaits is common in Crocodylidae but has not been observed in Alligatoridae (Hutchinson, 2012). We therefore (tentatively) suggest that longer muscle fascicles, and hence the ability to cycle the limbs quickly through large arcs of motion, may be a limiting factor in crocodylian bounding and galloping. We also suggest that the more massive pectoral limb may be particularly important to the generation (and absorption) of locomotor power during the use of asymmetrical vs. symmetrical gaits. However, large muscle PCSA and the concomitant ability to exert large limb forces may be less important.
While direct measurement of locomotor dynamics in a broad sample of the two clades would be ideal, a more complete analysis of differences in locomotor anatomy between the two clades, including muscle fibre types, moment arms, and detailed tendon anatomy would shed further light on this interesting difference. Additionally, the axial skeleton may play a greater role than the limb skeleton in symmetrical vs. asymmetrical gaits (Salisbury & Frey, 2001; Molnar et al. 2014), but such questions remain under-explored.
Interestingly, our analysis of scaling patterns suggests that the divergence in locomotor anatomy becomes more pronounced during ontogeny, rather than being restricted to juveniles. While inter-clade differences in muscle scaling PCSA may act to equalise the abilities of adult Crocodylia to exert limb forces, the higher coefficients found for muscle fascicle lengths and masses suggest increasing inter-clade disparity in the maximal capacity to modulate limb power.
If these inter-clade differences in muscle architecture relate to differences in muscle and overall limb function, there may be appreciable diversity in crocodylian locomotion, beyond the differential abilities of individuals from different clades to use asymmetrical gaits. Mainly due to biogeographical convenience, the majority of studies of crocodylian locomotor dynamics involve the North American alligatorid, A. mississippiensis. However, as our results (and principles of comparative biology) suggest, study of this species alone is insufficient to describe Crocodylia as a whole.
Present knowledge holds that, unusually among terrestrially locomoting vertebrates, crocodylians make use of highly variable gaits with large speed overlaps (Renous et al. 2002), use both mediolateral and dorsoventral undulations of the vertebral column to increase stride length (e.g. Webb & Gans, 1982), and alter both stride length and frequency (rather than duty factor) to increase speed (Reilly & Ellias 1998). These features suggest important differences between crocodylian terrestrial quadrupedalism and more commonly studied mammalian and squamate forms (vide Molnar et al. 2014). If we are to understand terrestrial locomotion as a whole, and its evolution in this fascinating and unusual clade, more data on kinematics and kinetics in a broad range of Crocodylia are sorely needed.
Acknowledgments
We thank the Rockefeller Wildlife Refuge (Lake Charles, USA) and La Ferme aux Crocodiles (Pierlatte, France) for providing specimens for this study. We thank colleagues and students from the Structure and Motion Laboratory for assistance in dissection and discussion and suggestion of statistical methodology, in particular Nicola Jones, Jordon Wright and Simon Wilshin. For financial support we thank the Department of Comparative Biomedical Sciences of The Royal Veterinary College, and the Natural Environment Research Council for grant number NE/K004751/1; awarded to J.R.H. in 2009.
Author contributions
Conceived project: V.A., J.R.H.; provided specimens: J.R.H., V.A.; Collected data: V.A., J.M., W.P., A.P., G.N., J.R.H.; analysed and interpreted data: V.A., J.R.H.; wrote paper: V.A., J.R.H., J.M.; created figures: J.M., V.A.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. Analysis of muscle fascicle length.
Data S2. Analysis of muscle mass.
Data S3. Analysis of muscle PCSA.
Data S4. Analysis of summed limb muscle mass.
Data S5. Raw data (blank cells = missing data).
Fig. S1. High resolution version of figure 1.
Fig. S2. High resolution version of figure 2.
Fig. S3. Number of individual Alligatoridae (blue) and Crocodylidae (red) specimens used in study vs. specimen body mass (in 10Kg bins).
Fig. S4. 95% confidence intervals for mean limb muscle fascicle lengths, with alternative normalisation to individual muscle length, in Alligatoridae (grey circles) and Crocodylidae (black triangles). Muscles with significantly longer fasicles in Crocodylidae are highlighted in red. See Table 1 for muscle abbreviations. Overall pattern shown is similar to that when data are normalised to body mass1/3.
References
- Alexander R, Ker R. The architecture of leg muscles. In: Winters J, Woo S, editors. Multiple Muscle Systems: Biomechanics and Movement Organization. Berlin: Springer; 1990. pp. 568–577. [Google Scholar]
- Allen V, Elsey R, Jones N, et al. Functional specialization and ontogenetic scaling of limb anatomy in Alligator mississippiensis. J Anat. 2010;216:423–445. doi: 10.1111/j.1469-7580.2009.01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blob RW. Evolution of hindlimb posture in nonmammalian therapsids: biomechanical tests of paleontological hypotheses. Paleobiology. 2001;27:14–38. [Google Scholar]
- Blob R, Biewener A. In vivo locomotor strain in the hindlimb bones of Alligator mississippiensis and Iguana iguana: implications for the evolution of limb bone safety factor and non-sprawling limb posture. J Exp Biol. 1999;202:1023–1046. doi: 10.1242/jeb.202.9.1023. [DOI] [PubMed] [Google Scholar]
- Brochu CA. Morphology, fossils, divergence timing, and the phylogenetic relationships of Gavialis. Syst Biol. 1997;46:479–522. doi: 10.1093/sysbio/46.3.479. [DOI] [PubMed] [Google Scholar]
- Brochu CA. Phylogenetic approaches toward crocodylian history. Annu Rev Earth Planet Sci. 2003;31:357–397. [Google Scholar]
- Brochu CA. Phylogenetic relationships of Palaeogene ziphodont eusuchians and the status of Pristichampsus Gervais, 1853. Earth Environ Sci Trans R Soc Edinb. 2012;46:479–522. [Google Scholar]
- Calow L, Alexander R. A mechanical analysis of a hind leg of a frog (Rana temporaria. J Zool. 1973;171:293–321. [Google Scholar]
- Gatesy SM. Hind limb movements of the American alligator (Alligator mississippiensis) and postural grades. J Zool. 1991;224:577–588. [Google Scholar]
- Hildebrand M. Walking and running. In: Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Functional Vertebrate Morphology. Cambridge: Belknapp press; 1985. pp. 38–57. [Google Scholar]
- Hutchinson JR. Biomechanical modelling and sensitivity analysis of bipedal running ability. I. Extant taxa. J Morphol. 2004a;262:421–440. doi: 10.1002/jmor.10241. [DOI] [PubMed] [Google Scholar]
- Hutchinson JR. How did bounding and galloping gaits evolve in Crocodylomorpha? J Vert Paleontol. 2012;32(Suppl 2):114. [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metab, Clin Exp. 1960;9:184–188. [Google Scholar]
- Molnar JL, Pierce SE, Hutchinson R. An experimental and morphometric test of the relationship between vertebral morphology and joint stiffness in Nile crocodiles (Crocodylus niloticus. J Exp Biol. 2014;217:758–768. doi: 10.1242/jeb.089904. [DOI] [PubMed] [Google Scholar]
- Payne RC, Hutchinson JR, Robilliard JJ, et al. Functional specialisation of pelvic limb anatomy in horses (Equus caballus) J Anat. 2005;206:557–574. doi: 10.1111/j.1469-7580.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renous S, Gasc JP, Bels V, et al. Asymmetrical gaits of juvenile Crocodylus johnstoni, galloping Australian crocodiles. J Zool. 2002;256:311–325. [Google Scholar]
- Sacks RD, Roy RR. Architecture of the hind limb muscles of cats: functional significance. J Morphol. 1982;173:185–195. doi: 10.1002/jmor.1051730206. [DOI] [PubMed] [Google Scholar]
- Salisbury SW, Frey E. A biomechanical transformation model for the evolution of semi-spheroidal articulations between adjoining vertebral bodies in crocodilians. In: Grigg GC, Seebacher F, Franklin CE, editors. Crocodilian Biology and Evolution. Chipping Norton: Surrey Beatty & Sons; 2001. pp. 85–134. [Google Scholar]
- Singh L, Bustard HR. Locomotory behaviour during basking and spoor formation in the gharial (Gavialis gangeticus. Br J Herpetol. 1976;5:673–676. [Google Scholar]
- Smith NC, Wilson AM, Jespers KJ, et al. Muscle architecture and functional anatomy of the pelvic limb of the ostrich (Struthio camelus. J Anat. 2006;209:765–779. doi: 10.1111/j.1469-7580.2006.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb G, Gans C. Galloping in Crocodylus johnstoni – a reflection of terrestrial activity. Rec Aust Mus. 1982;34:607–618. [Google Scholar]
- Weyand PG, Sternlight DB, Bellizzi MJ, et al. Faster top running speeds are achieved with greater ground forces not more rapid leg movements. J Appl Physiol. 2000;89:1991–1999. doi: 10.1152/jappl.2000.89.5.1991. [DOI] [PubMed] [Google Scholar]
- Whitaker R. Breeding the African dwarf crocodile Osteolaemus tetraspis at Zoo Negara, Kuala Lumpur, with an observation on galloping. Hamadryad. 1981;62:14. [Google Scholar]
- Whitaker R, Andrews H. Notes on crocodylian locomotion. J Bombay Nat Hist Soc. 1988;853:621–622. [Google Scholar]
- Willey JS, Audrone RB, Reilly SM, et al. The tale of the tail: limb function and locomotor mechanics in Alligator mississippiensis. J Exp Biol. 2004;207:553–563. doi: 10.1242/jeb.00774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Analysis of muscle fascicle length.
Data S2. Analysis of muscle mass.
Data S3. Analysis of muscle PCSA.
Data S4. Analysis of summed limb muscle mass.
Data S5. Raw data (blank cells = missing data).
Fig. S1. High resolution version of figure 1.
Fig. S2. High resolution version of figure 2.
Fig. S3. Number of individual Alligatoridae (blue) and Crocodylidae (red) specimens used in study vs. specimen body mass (in 10Kg bins).
Fig. S4. 95% confidence intervals for mean limb muscle fascicle lengths, with alternative normalisation to individual muscle length, in Alligatoridae (grey circles) and Crocodylidae (black triangles). Muscles with significantly longer fasicles in Crocodylidae are highlighted in red. See Table 1 for muscle abbreviations. Overall pattern shown is similar to that when data are normalised to body mass1/3.