Abstract
Rho proteins are a large family of GTPases involved in the control of actin cytoskeleton dynamics, proliferation and survival. Rnd1, Rnd2 and RhoE/Rnd3 form a subfamily of Rho proteins characterized by being constitutively active. The role of these proteins has been studied during the last years in several systems; however, little is known about their expression and functions in the reproductive organs. In this work we analysed the localization and the effect of RhoE deficiency in the testes using mice lacking RhoE expression (RhoE gt/gt), and our research shows some unexpected and relevant results. First, we have observed that RhoE is only expressed in Leydig cells within the testicular parenchyma and it is absent of seminiferous tubules. In addition, RhoE is expressed in the excurrent ducts of the testis, including the ductuli efferentes, epididymis and ductus deferens. Moreover, the testes of postnatal 15-day-old RhoE null mice are smaller, both in absolute values and in relation to the body weight. Furthermore, the dimensions of their seminiferous tubules are also reduced compared with wild-types. In order to study the role of RhoE in the adult, we analysed heterozygous animals as RhoE null mice die early postnatally. Our results show that the testes of adult RhoE heterozygous mice are also smaller than those of the wild-types, with a 17% decrease in the ratio testis weight/body weight. In addition, their seminiferous tubules have reduced tubular diameter (12%) and a thinner epithelial wall (33%) that appears disorganized and with a swollen lumen. Finally, and probably as a consequence of those alterations, the sperm concentration of heterozygous animals was found to be lower than in the wild-types. These results indicate that accurate levels of RhoE in the testes are necessary for a correct development and function of male gonads, and suggest novel and unexpected roles of Rnd GTPases in the reproductive physiology.
Keywords: epididymis, Leydig cells, Rho GTPase, Rnd, Rnd3, seminiferous tubules, testis
Introduction
Mammalian spermatogenesis is a highly complex process whose final consequence is the production of spermatozoa. Spermatogenesis takes place in the seminiferous tubules, and involves cell germ proliferation and migration (Lie et al. 2010). Many different signaling pathways are involved in spermatogenesis, but because of their function in controlling cytoskeletal dynamics and cell proliferation, Rho GTPases are candidates to play important roles in spermatogenesis regulation (Lui et al. 2003).
The mammalian Rho GTPases consist of more than 20 members, forming a subgroup of the Ras super family (Wennerberg & Der, 2004; Bustelo et al. 2007). Most Rho proteins have been shown to act as key molecular switches by cycling between an active GTP-bound state and an inactive GDP-bound state (Etienne-Manneville & Hall, 2002; Heasman & Ridley, 2008). They are involved in a variety of functions, including cell cycle progression, cell migration and morphogenesis, gene expression and cytoskeleton dynamics (Jaffe & Hall, 2005; Heasman & Ridley, 2008; Vega & Ridley, 2008). Rnd proteins are a subfamily formed by three members, Rnd1, Rnd2 and Rnd3/RhoE (hereafter referred to as RhoE), which are in a constitutively active state because they lack GTPase activity, and whose physiological role includes cytoskeleton dynamics regulation and cell cycle control (Chardin, 2006; Riou et al. 2010).
The role of these proteins in the testes remains unknown. Several Rho GTPases, including Cdc42, Rac1, Rac2, RhoB, RhoS and Rnd2 as well as some of their regulator proteins, have been identified in the testis (Togawa et al. 1999; Freeman et al. 2002; Naud et al. 2003; Lui et al. 2005; Sarkar et al. 2007; Wong & Cheng, 2009; Adly & Hussein, 2010; Zhang et al. 2010). Regarding Rnd proteins, Rnd2 is expressed at highest levels in the testes, being found in spermatocytes and spermatids (Nobes et al. 1998; Naud et al. 2003), whereas testicular levels of RhoE are considered low (Nobes et al. 1998; Ballester-Lurbe et al. 2009). Nevertheless, the precise distribution and role of the latter in male gonads has not been previously addressed.
To study the role of RhoE in vivo, we have previously generated mice lacking RhoE expression. Because RhoE null (gt/gt) mice die shortly after birth (Mocholi et al. 2011), they are not useful to study the effect in the adult gonads. However, RhoE heterozygous are viable and fertile, and do not show gross anomalies (Mocholi et al. 2011). In this work we use these mice to analyze the expression of RhoE in the testis, and to study the effect of the absence of RhoE and the eventual effect of RhoE dosage in the structure of adult testes. Our results show that RhoE is expressed in Leydig cells within the testes and its deficiency results in testicular anomalies.
Materials and methods
Animals
Mice deficient for RhoE expression (RhoE gt/gt) were generated at Lexicon by gene-trapping in embryonic stem (ES) cells, identification of trapped genes by using OmniBank™ Sequence Tags (OSTs), and characterization of retroviral gene-trap vector insertion points, as previously described (Zambrowicz et al. 1998, 2003; Mocholi et al. 2011). OmniBank ES cell clone OST364657 was used to generate RhoE gt/gt mice as described (Zambrowicz et al. 2003). The gene-trap vector VICTR 37 was inserted within intron 2 of the RhoE gene. This vector contained the β-GEO gene [a fusion gene formed from the β-galactosidase (β-gal) gene and the neomycine-resistance gene]. Mice were genotyped by polymerase chain reaction (PCR). The wild-type locus yields a PCR product of 600 bp using primers 5′-TTT ACA CAG TAG GCT GAC TC-3′ and 5′-TGA GCT AGG AAG ATG CGG ATG T-3′. The mutant locus yields a PCR product of 400 bp using primers 5′-AAA TGG CGT TAC TTA AGC TAG CTA GCT TGC-3′ and 5′-TGA GCT AGG AAG ATG CGG ATG T-3′ (not shown). Same sex littermates were group-housed under a 12-h light/dark schedule, at constant room temperature (22 °C), and with free access to water and standard mouse fodder. As RhoE gt/gt mice died shortly after weaning, they were kept with their mother until they died or were killed when their condition worsened. All animal procedures were approved by the local ethics committees (Ethics Committee for Animal Welfare of the Universidad CEU Cardenal Herrera, ID#CEBA-09/006), met the local guidelines (Spanish law 32/2007), European regulations (EU directive 86/609) and Standards for Use of Laboratory Animals nu A5388-01 (NIH). The experimenters held the official accreditation for animal work (Spanish law 32/2007). All efforts were made to minimize the number of animals used and their suffering. The mice were killed by an overdose of pentobarbital. The testes were obtained at postnatal day (P)15 and at 3 months (considered as adults). In P15 mice the testes were not separated from the epididymis. In the adults, the testes were isolated from the epididymis and weighted separately. All experimental protocols were approved by the Ethical Committee of the CEU-Cardenal Herrera University.
Tissue preparation and histological studies
After killing the animals, testes and epididymis were dissected and weighed, and the testes fixed overnight in 4% paraformaldehyde (PFA). After fixation, for histological studies the testes were dehydrated in increasing concentrations of ethanol, embedded in paraffin, serially sectioned (4 μm) in a HM310Microm microtome (Walldorf, Germany) and collected on polylysine-coated slides. Sections stained with hematoxylin and eosin were analyzed using a Leica DM2000 microscope (Wetzlar, Germany). To assess testicular damage, image analysis was performed using the cross-sectional ratio of the epithelial height (EH) and the seminiferous tubular diameter (TD) in the same tubule as described by Yue et al. (2011). The cross-sectional ratio of EH/TD was calculated for each tubule. One-hundred tubules taken from random fields were measured from each testis. To analyze β-gal expression and activity, tissues were postfixed with PFA 0.2%, 2 mm MgCl2 and permeabilized with detergent solution (0.02% NP40, 0.01% deoxycolate, 2 mm MgCl2, in 100 mm sodium phosphate buffer pH 7.3). For X-gal staining, the testes were incubated in the dark at 37 °C overnight in X-gal solution (1 mg mL−1 X-gal, 5 mm potassium ferricyanide and 5 mm potassium ferrocyanide, 0.02% NP40, 0.01% deoxycolate, 2 mm MgCl2, in 100 mm sodium phosphate buffer pH 7.3); then, sections were mounted and observed under light microscopy. For the immunofluorescence assays, fixed tissues were cryoprotected and frozen. Samples were then serially sectioned in a cryostat. Then, sections were washed with phosphate-buffered saline-tween, and antigen retrieval was performed by submerging the sections in citrate buffer (10 mm pH 8) at 100 °C during 10 min. Testes were stained overnight with anti-β-gal antibodies (70 508; ABD Serotec, 1 : 100), incubated for 1 h with rabbit-FITC (F9887; Sigma-Aldrich, 1 : 300), mounted with Vectashield with DAPI (H-1200; Vector Laboratories) and visualized with a fluorescence microscopy.
Western blot analysis
Testes were homogenized in lysis buffer (150 mm NaCl, 1% Triton X-100, 1 mm dithiothreitol, 50 mm Tris, pH 8.0, 10 mm NaF, 1 mm Na3VO4) and a cocktail of protease inhibitors (Complete Mini; Roche, Mannheim, Germany), and centrifuged at 20 800 g during 20 min, The supernatant was collected and quantified by the Bradford assay (Bio-Rad, Hercules, CA, USA). Fifty-microgram samples were resolved on 11% gels by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). They were then blocked with 5% non-fat milk for 20 min at room temperature and incubated at 4 °C overnight with a monoclonal mouse anti-RhoE from Upstate (Lake Placid, NY, USA) at 1 : 500, or peroxidase-conjugated mouse anti-actin from Sigma-Aldrich (St Louis, MO, USA) at 1 : 20 000. Membranes were then washed three times with 0.1 m phosphate buffer containing 0.1% Tween 20 and incubated with a peroxidase-conjugated secondary antibody. Finally, blots were developed using an enhanced chemiluminescence Western blotting detection kit from Amersham Pharmacia Biotech (Little Chalfont, UK).
Sperm concentration analysis
The abdominal cavity was opened, and the epididymis and the testicle were cut free. Testes and epididymis were weighed, and the epididymis transferred to a 35-mm Petri dish with 1 mL saline solution. Cauda epididymides were cut with fine scissors and mixed gently. After the incubation at 37 °C for 15 min, the sperm swim out and cell fragments sink to the bottom. The sperm cells were counted using a Burker camera (Marienfef, Germany).
Statistical analyses
The results are expressed as means ± SEM. Statistical comparisons were performed by the unpaired Student's t-test.
Results
RhoE localization in the testis
We first addressed the study of RhoE localization in the testes. For such purpose we used mice that have the lacZ gene knocked-into the RhoE locus, under the control of the RhoE gene regulatory region. The analysis of the blue staining resulting from the inserted β-gal activity, indicative of RhoE gene expression, in P15 RhoE +/gt testes showed that RhoE was only present in the interstitial tissue. Indeed, Leydig cells appeared moderately stained, while the seminiferous tubules were devoid of X-gal staining, both in Sertoli and in the germ cells (Fig. 1A). Strikingly, we detected β-gal activity in the epithelial cells of the ductuli efferentes and ductus epididymis. The labeling of epithelial cells of these ducts was strong and the highest intensity was localized apically, between the cell nucleus and the luminal surface of the cells (Fig. 1B,C). We also detected labeling in the epithelium of ductus deferens, in a similar manner to the epididymis with higher intensity in the apical part of the cells. The muscle layers surrounding the epithelium of the duct were not labeled (Fig. 1D). To study whether RhoE localization is developmentally regulated in the testes and it is eventually expressed in the more mature cells not yet present in immature testes, we analyzed β-gal activity in the testes of adult RhoE +/gt mice, which expressed reduced levels of RhoE protein compared with wild-types (Fig. 2A). We observed that adult RhoE localization did not differ from that of younger animals. As shown for P15 mice, the blue staining was evident in adult Leydig cells, whereas Sertoli and germ cells, including spermatids and spermatocytes, not present in the previous postnatal stage, lacked β-gal activity (Fig. 2B,C). The epididymis and ductus deferens were also intensely labeled (Fig. 2D–F). Moreover, the ductuli efferentes were also stained but less strongly than the neighbor epididymal cells (Fig. 2D,E). To confirm the specificity of the labeling, we performed the immunohistochemical detection of β-gal. β-gal immunoreactivity was observed in the same cells that showed β-gal activity, thus, confirming the specificity of the X-gal staining localization (Fig. 2G).
Fig. 1.
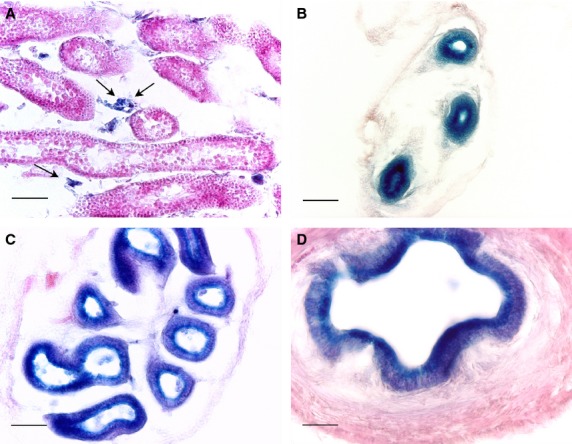
X-gal staining of 15-day-old RhoE +/gt mice male gonads. (A) Section of a testis where the blue staining indicative of RhoE expression (arrows) is observed in Leydig cells, whereas the seminiferous tubules are devoid of X-gal staining. The head (B) and tail (C) of the epididymis duct and the ductus deferens (D) show high levels of X-gal staining. Note that the labeling is especially strong in the apical part of the cytoplasm, between the nucleus and the ductal lumen. Scale bars: 50 μm (A–C); 25 μm (D).
Fig. 2.
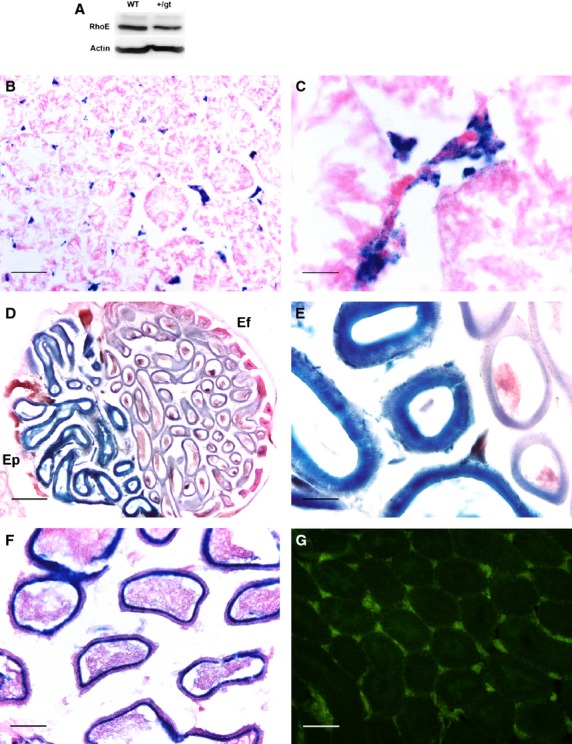
X-gal staining of adult testes of RhoE +/gt mice. (A) Western blot showing RhoE expression in the testis of wild-type (WT) and RhoE +/gt (+/gt) mice. (B) X-gal staining in the adult testes shows that RhoE expression is localized in Leydig cells, as in the P15 mice. (C) A higher magnification showing Leydig cell localization of X-gal staining. (D) Section of the capital pole of the testis. The ductuli efferentes (Ef) display a slight X-gal staining (right part of the section) compared with the high levels observed in the ductus epididymidis at the head of the epididymis (Ep; left part of the section). (E) A higher magnification showing the different intensity of the labeling. (F) Tail of an adult epididymis with high levels of X-gal staining in the epithelial cells. Observe that the sperm cells found in the lumen are not stained. (G) Immunodetection of β-gal in Leydig cells, matching X-gal staining. Scale bars: 100 μm (A, F); 25 μm (B); 200 μm (C); 50 μm (D, E).
Effect of RhoE deficiency in testicular dimensions and structure
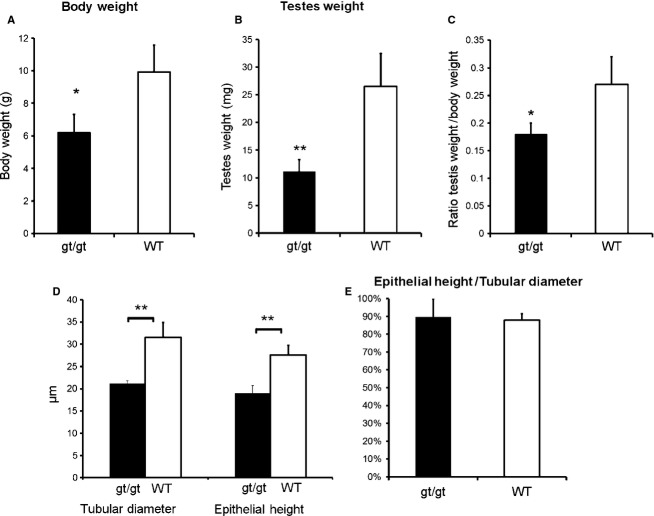
We next analyzed the dimensions and structure of postnatal RhoE null mice testes. As we have previously shown (Mocholi et al. 2011), the body weight of RhoE gt/gt mice was lower than the wild-types. Mice used in this study, which were 15 days old, showed a 38% reduction in body weight compared with wild-types (6.18 ± 1.12 g vs. 9.92 ± 1.65 g; Fig. 3A). Strikingly, testis weight of the mutants was also reduced, but this reduction was not proportional (Fig. 3B). Indeed, while the body weight of RhoE null mice was 38% lower, testis weight decreased 61% (11.15 ± 2.15 mg vs. 26.53 ± 5.93 mg). As a consequence, the ratio testis of weight to body weight was 33% lower in RhoE gt/gt mice compared with the wild-types (0.18 vs. 0.27, P < 0.05; Fig. 3C). Because the testes were smaller in RhoE null mice, we analyzed whether such a reduction in size was accompanied by a decrease in the size of the seminiferous tubules. The comparison of histological sections of wild-type and RhoE gt/gt mice showed that RhoE null mice tubules were significantly smaller than those of wild-type mice (Fig. 4 A,B). Quantification of the TD and the thickness of the epithelium or EH confirmed that both parameters were reduced in the null mutants more that 30% (21.17 ± 0.65 vs. 31.59 ± 3.25 μm for the TD and 18.95 ± 1.82 vs. 27.61 ± 2.17 μm for the EH; Fig. 3D,E).
Fig. 3.
Mice lacking RhoE expression have smaller testes. RhoE gt/gt (n = 3) and wild-type (WT; n = 5) P15 mice were compared in terms of body weight (A), testes weight (B), testis weight/body weight ratio (C), TD and EH (D), and EH/TD ratio (E). Differences were analyzed by an unpaired Student's t -test (*P < 0.05, **P < 0.01).
Fig. 4.
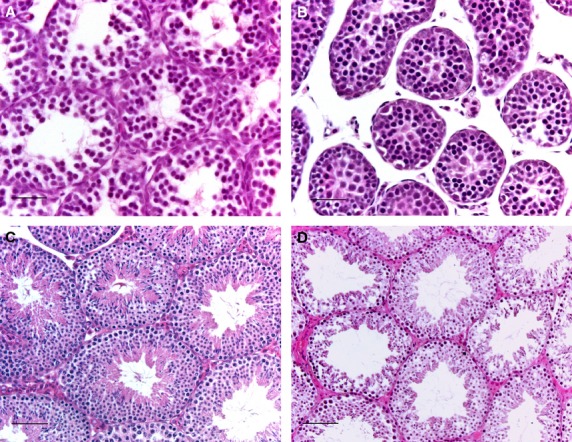
Histological sections of RhoE-deficient mice testes. Section of a P15 wild-type (A), a P15 RhoE gt/gt (B), an adult wild-type (C) and an adult RhoE +/gt (D). Seminiferous tubules of RhoE-deficient mice have smaller diameters and thinner walls. Scale bars: 25 μm (A, B); 50 μm (C, D).
As previously indicated, RhoE gt/gt mice do not reach adult life. To study RhoE localization and function in the adult, we analyzed RhoE +/gt mice. Whereas the body weight of RhoE +/gt was similar to the wild-type mice, the weight of the testes and epididymis was 16% and 19% lower, respectively, in the heterozygous (84.47 ± 7.49 g vs. 100.85 ± 12.65 g for the testes and 40.43 ± 7.54 mg vs. 49.77 ± 8.31 mg for the epididymis). Therefore, the testis weight/body weight ratio was 17% smaller in the heterozygous. Analysis of the seminiferous tubule walls showed that the diameter of the tubules was 12% smaller in RhoE +/gt mice (91.52 ± 5.15 vs. 103.48 ± 0.85) and that their wall was thinner in the mutants, as their EH was reduced in 33% compared with the wild-types (52.62 ± 4.93 μm vs. 78.93 ± 3.67 μm; Table 1; Fig. 4C,D). This implies that the reduction in the tubular size is mainly due to the decrease in the thickness of the seminiferous epithelium. This is confirmed when the EH/TD ratio (57% in the heterozygous and 76% in the wild-type) is calculated. Subsequently, the heterozygous mice displayed an increased tubular lumen diameter. In addition, RhoE +/gt mice seminiferous tubules showed a disorganized aspect with a lesser cell density and apparently the cells displayed reduced cytoplasm. This was evident in the elongating spermatids, whose cytoplasm appeared less voluminous. Moreover, enlarged spaces were also present between the cells and less densely packed sperm tails in the lumen were also observed (Fig. 4C,D).
Table 1.
Testicular dimensions in adult RhoE +/gt and wild-type mice
| RhoE +/gt | Wild-type | ||
|---|---|---|---|
| Body weight (g) | 28.05 ± 5.49 | 27.74 ± 4.01 | n.s. |
| Testes weight (mg) | 84.47 ± 7.49 | 100.85 ± 12.65 | P < 0.001 |
| Epididymis weight (mg) | 40.43 ± 7.54 | 49.77 ± 8.31 | P < 0.001 |
| Ratio testis weight/body weight | 0.31 ± 0.05 | 0.37 ± 0.07 | P < 0.01 |
| TD (μm) | 91.52 ± 5 | 103.48 ± 0.85 | P < 0.001 |
| EH (μm) | 52.62 ± 4.93 | 78.93 ± 3.67 | P < 0.01 |
| EH/TD % | 57 ± 4 | 76 ± 3 | P < 0.001 |
| Sperm concentration (106 cells per mL) | 22.00 ± 13.03 | 38.70 ± 13.66 | P < 0.05 |
RhoE +/gt: n = 14 (except sperm concentration, n = 10); wild-type: n = 17 (except sperm concentration, n = 8).
EH, epithelial height; TD, tubular diameter.
The alteration of TD and EH could be indicative of spermatogenic dysfunction. Therefore, our results would suggest that RhoE is important for testicular development and that even a reduction in the levels of RhoE can have deleterious results in the testes. To finally analyze whether these histological anomalies had a consequence in sperm production, we studied the concentration of spermatozoa obtained from the tail of the epididymis. Although a considerable degree of variability was found, our results show that the sperm concentration in RhoE +/gt mice was lower than in the wild-types (22.00 × 106 ± 13.03, n = 10, vs. 38.72 × 106 ± 13.66, n = 8; P < 0.05), indicating that RhoE deficiency results in a reduction in sperm production.
Discussion
Rho GTPases have been involved in different aspects of testicular organization mainly due to their known roles in the control of cytoskeleton and cell cycle (Lui et al. 2003). Rnd proteins form a Rho subfamily with atypical regulation, as they lack GTPase activity and therefore remain constitutively active (Chardin, 2006; Riou et al. 2010). They are involved in important functions in different systems, but their implication in testicular functions has not been previously addressed, except for the work of Naud et al. (2003), which reported Rnd2 as the main Rnd protein expressed in the testes. Concerning RhoE, during the last years several papers have shown diverse roles in different systems, but it seems especially important in the development of the nervous system (Mocholi et al. 2011; Pacary et al. 2011, 2013; Peris et al. 2012; Lin et al. 2013). Its levels in the testes were considered low (Nobes et al. 1998; Ballester-Lurbe et al. 2009), and perhaps for such circumstance a possible role in these organs has not deserved much attention.
In this work we studied the expression and function of RhoE in mouse testis, and our results show three striking and, to some extent, unexpected results: (i) RhoE is not expressed in the seminiferous tubules but in Leydig cells, ductuli efferentes, epididymis and ductus deferens; (ii) RhoE absence results postnatally in a decrease in testicular size and in seminiferous tubules dimensions; and (iii) a reduction of RhoE dosage also results in the adults in the reduction of testis and epididymis size, in important alterations of the seminiferous tubules wall and in a reduction of sperm concentration.
Spermatogenesis, which is developed in the seminiferous tubules, involves events such as changes in cell shape and size, Sertoli cells–germ cells interaction and germ cells movement, processes in which the cytoskeleton is involved (Lie et al. 2010). Therefore, the presence of Rho proteins in these cells could be expected, and several reports have shown the localization of different Rho proteins in the testes. Rac1, RhoA and cdc42 are detected in peritubular cells and in Sertoli cells (Naud et al. 2003). Concerning germ cells, spermatogonia also express Rac1 and cdc42 but not RhoA, and spermatocytes and spermatids produce the GTPase-activating protein for Rac, mgcRacGAP (Naud et al. 2003). The work by Naud and colleagues also shows that RhoB is present in sperm cells, although recently it has been shown that RhoB has a strong expression in the seminiferous epithelium (cytoplasm of Sertoli cells, spermatogonia and spermatocytes; Adly & Hussein, 2010). Finally, a new member, RhoS, has been found in the seminiferous tubules (Zhang et al. 2010). Regarding Rnd subfamily proteins, Rnd2, considered as the main Rnd member expressed in the testis, was located in spermatocytes and spermatids (Naud et al. 2003). Summarizing these results, it can be concluded that most Rho proteins are expressed in the tubules. Nevertheless, some Rho proteins, namely, RhoB (Adly & Hussein, 2010) and RhoA (Naud et al. 2003) have also been detected in Leydig cells. Therefore, the presence of RhoE in Leydig cells, while surprising to some extent, cannot be considered unforeseen. Interestingly, both proteins, RhoA and RhoB, have been found to be functionally related with RhoE. While it is well known that RhoE inhibits RhoA and ROCK activity (Riento et al. 2003), a relationship between RhoE and RhoB in endothelial cells was recently shown, where RhoE stimulates a strong increase in stress fibers by increasing RhoB expression (Gottesbuhren et al. 2013). The consequences of the coexistence of RhoE along with RhoA and RhoB in Leydig cells remain to be elucidated, but we can speculate that a balance between these three Rho proteins could control the cystoskeleton dynamics in these cells.
Our results also show that RhoE deficiency results in anomalies of the seminiferous tubules. Postnatal RhoE null mice have smaller testes than the wild-types, with a reduced TD and EH. Adult heterozygous testes (RhoE null mice do not reach the adulthood) are also reduced in size, with a lower TD and EH as well as a thinner wall of the seminiferous tubules and a greater luminal diameter. As a consequence, they have reduced sperm concentration. Some other Rho-related proteins are important for proper spermatogenesis. The Rho GDP dissociation inhibitor α null mice are infertile, lacking spermatids and spermatocytes, and showing vacuoles in the seminiferous epithelium (Togawa et al. 1999). Mice deficient in LIMK2, an enzyme involved in RhoE pathway (Peris et al. 2012), also have smaller testes with altered spermatogenesis (Takahashi et al. 2002). However, these proteins apparently are not expressed in Leydig cells. Interestingly, the levels of RhoB, which is normally present in Leydig cells, are decreased in some testicular alterations in humans, such as spermatogenic arrest and Sertoli cell only syndrome (Adly & Hussein, 2010).
RhoE is also present, at high levels, in the excretory ducts, including the epididymis and the ductus deferens. The aim of our work was not to investigate the role of RhoE in these ducts; however, the intensity of the labeling suggests that it could be involved in some important regulatory functions. Remarkably, two of the main proteins involved in RhoE pathways, RhoA and ROCK-II, are expressed in epithelial cells of the epididymis. There, the pharmacological inhibition of RhoA and ROCK-II induces actin depolymerization and, as a consequence, the proton pumping V-ATPase, which is involved in luminal acidification, a critical process for sperm maturation and storage, accumulates in the apical membrane of the cells (Shum et al. 2011). The presence of RhoE in the same cells suggests that it could be an upstream regulator of the RhoA–ROCK pathway in the control of V-ATPase. Deregulation of the proton pump has been associated with several pathological processes, including infertility, metastasis, renal tubular acidosis or osteoporosis (Wagner, 2011). Therefore, the knowledge of whether RhoE is involved in the pathways of proton pump regulation could help in the understanding of important pathological mechanisms affecting not only male reproductive processes but also in other different organs.
Concluding remarks
In summary, the results we show here indicate that RhoE is present in Leydig cells and in the excurrent ducts of the testis. Its presence is necessary for proper development of the testes as mice with reduced RhoE levels have smaller testes with smaller seminiferous tubules and lower sperm production. How the absence of RhoE in interstitial cells results in such alterations is an intriguing issue, and the mechanisms involved remain to be elucidated.
Acknowledgments
This work was supported by the Universidad CEU Cardenal Herrera (PRUCH and Santander-Copernicus) and MINECO SAF2013-49176-C2-1-R.
Conflict of interest
The authors declare that they have no conflict of interests.
References
- Adly MA, Hussein MR. Immunohistological profile of the Ras homologous B protein (RhoB) in human testes showing normal spermatogenesis, spermatogenic arrest and Sertoli cell only syndrome. Pathol Oncol Res. 2010;16:427–433. doi: 10.1007/s12253-009-9232-3. [DOI] [PubMed] [Google Scholar]
- Ballester-Lurbe B, Poch E, Mocholi E, et al. RhoE is spatiotemporally regulated in the postnatal mouse CNS. Neuroscience. 2009;163:586–593. doi: 10.1016/j.neuroscience.2009.06.062. [DOI] [PubMed] [Google Scholar]
- Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. BioEssays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol. 2006;7:54–62. doi: 10.1038/nrm1788. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Freeman EA, Jani P, Millette CE. Expression and potential function of Rho family small G proteins in cells of the mammalian seminiferous epithelium. Cell Commun Adhes. 2002;9:189–204. doi: 10.1080/15419060216016. [DOI] [PubMed] [Google Scholar]
- Gottesbuhren U, Garg R, Riou P, et al. Rnd3 induces stress fibres in endothelial cells through RhoB. Biol Open. 2013;2:210–216. doi: 10.1242/bio.20123574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Lie PP, Mruk DD, Lee WM, et al. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–1592. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Liu B, Yang X, et al. Genetic deletion of Rnd3 results in aqueductal stenosis leading to hydrocephalus through up-regulation of Notch signaling. Proc Natl Acad Sci USA. 2013;365:8236–8241. doi: 10.1073/pnas.1219995110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Rho GTPases and spermatogenesis. Biochim Biophys Acta. 2003;1593:121–129. doi: 10.1016/s0167-4889(02)00348-8. [DOI] [PubMed] [Google Scholar]
- Lui WY, Mruk DD, Cheng CY. Interactions among IQGAP1, Cdc42, and the cadherin/catenin protein complex regulate Sertoli-germ cell adherens junction dynamics in the testis. J Cell Physiol. 2005;202:49–66. doi: 10.1002/jcp.20098. [DOI] [PubMed] [Google Scholar]
- Mocholi E, Ballester-Lurbe B, Arque G, et al. RhoE deficiency produces postnatal lethality, profound motor deficits and neurodevelopmental delay in mice. PLoS ONE. 2011;6:e19236. doi: 10.1371/journal.pone.0019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naud N, Toure A, Liu J, et al. Rho family GTPase Rnd2 interacts and co-localizes with MgcRacGAP in male germ cells. Biochem J. 2003;372:105–112. doi: 10.1042/BJ20021652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Lauritzen I, Mattei MG, et al. A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J Cell Biol. 1998;141:187–197. doi: 10.1083/jcb.141.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacary E, Heng J, Azzarelli R, et al. Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-mediated inhibition of RhoA signaling. Neuron. 2011;69:1069–1084. doi: 10.1016/j.neuron.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacary E, Azzarelli R, Guillemot F. Rnd3 coordinates early steps of cortical neurogenesis through actin-dependent and -independent mechanisms. Nat Commun. 2013;4:1635. doi: 10.1038/ncomms2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris B, Gonzalez-Granero S, Ballester-Lurbe B, et al. Neuronal polarization is impaired in mice lacking RhoE expression. J Neurochem. 2012;121:903–914. doi: 10.1111/j.1471-4159.2012.07733.x. [DOI] [PubMed] [Google Scholar]
- Riento K, Guasch RM, Garg R, et al. RhoE binds to ROCK I and inhibits downstream signaling. Mol Cell Biol. 2003;23:4219–4229. doi: 10.1128/MCB.23.12.4219-4229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou P, Villalonga P, Ridley AJ. Rnd proteins: multifunctional regulators of the cytoskeleton and cell cycle progression. BioEssays. 2010;32:986–992. doi: 10.1002/bies.201000060. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Parikh N, Hearn SA, et al. Antagonistic roles of Rac and Rho in organizing the germ cell microenvironment. Curr Biol. 2007;17:1253–1258. doi: 10.1016/j.cub.2007.06.048. [DOI] [PubMed] [Google Scholar]
- Shum WW, Da Silva N, Belleannee C, et al. Regulation of V-ATPase recycling via a RhoA- and ROCKII-dependent pathway in epididymal clear cells. Am J Physiol Cell Physiol. 2011;301:C31–C43. doi: 10.1152/ajpcell.00198.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Koshimizu U, Miyazaki J, et al. Impaired spermatogenic ability of testicular germ cells in mice deficient in the LIM-kinase 2 gene. Dev Biol. 2002;241:259–272. doi: 10.1006/dbio.2001.0512. [DOI] [PubMed] [Google Scholar]
- Togawa A, Miyoshi J, Ishizaki H, et al. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene. 1999;18:5373–5380. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Wagner CA. Rho rocks H(+)-ATPases: focus on “regulation of V-ATPase recycling via a RhoA- and ROCKII-dependent pathway in epididymal clear cells”. Am J Physiol Cell Physiol. 2011;301:C18–C20. doi: 10.1152/ajpcell.00134.2011. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- Wong EW, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, She R, Bao H, et al. Exposure to 3-methyl-4-nitrophenol affects testicular morphology and induces spermatogenic cell apoptosis in immature male rats. Res Vet Sci. 2011;91:261–268. doi: 10.1016/j.rvsc.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Friedrich GA, Buxton EC, et al. Disruption and sequence identification of 2000 genes in mouse embryonic stem cells. Nature. 1998;392:608–611. doi: 10.1038/33423. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Abuin A, Ramirez-Solis R, et al. Wnk1 kinase deficiency lowers blood pressure in mice: a gene-trap screen to identify potential targets for therapeutic intervention. Proc Natl Acad Sci USA. 2003;100:14 109–14 114. doi: 10.1073/pnas.2336103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Liang J, Tian Y, et al. A novel testis-specific GTPase serves as a link to proteasome biogenesis: functional characterization of RhoS/RSA-14-44 in spermatogenesis. Mol Biol Cell. 2010;21:4312–4324. doi: 10.1091/mbc.E10-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]