Abstract
Physical and psychological trauma which results in mood disorders and the disruption of complex behaviours is associated with reductions in hippocampal volume. Clinical evaluation of neuropathic pain reveals mood and behavioural change in a significant number of patients. A rat model of neuropathic injury results in complex behavioural changes in a subpopulation (∼30%) of injured rats; these changes are co-morbid with a range of other ‘disabilities’. The specific objective of this study was to determine in rats the morphology of the hippocampus and dentate gyrus in individuals with and without complex behavioural disruptions following a constriction injury of the sciatic nerve, and to determine whether rats that develop disabilities following nerve injury have a reduced hippocampal volume compared with injured rats with no disabilities. The social behaviours of nerve-injured rats were evaluated before and after nerve injury. The morphology of the hippocampus of rats with and without behavioural disruptions was compared in serial histological sections. Single-housing and repeated social-interaction testing had no effect on the morphology of either the hippocampus or the dentate gyrus. Rats with transient or ongoing disability identified by behavioural disruption following sciatic nerve injury, show bilateral reductions in hippocampal volume, and lateralised reduction in the dentate gyrus (left side). Disabled rats display a combination of behavioural and physiological changes, which resemble many of the criteria used clinically to diagnose mood disorders. They also show reductions in the volume of the hippocampus similar to people with clinically diagnosed mood disorders. The sciatic nerve injury model reveals a similarity to the human neuropathic pain presentation presenting an anatomically specific focus for the investigation of the neural mechanisms underpinning the co-morbidity of chronic pain and mood disorder.
Keywords: depression, emotional coping, neuropathic pain, rat, sciatic nerve
Introduction
The co-morbidity of mood disorders with chronic pain is well documented (Bair et al. 2003; Lépine & Briley, 2004; Réthelyi et al. 2004; Argoff, 2007; Tsang et al. 2008; Miller & Cano, 2009). The incidence of mood disorder in chronic neuropathic pain is around 30–35%. Diagnostic indicators of altered mood include: disturbed sleep; disrupted social relations; poor attention and memory; and hypothalamo-pituitary adrenal (HPA) axis dysfunction (Affleck et al. 1997, 1999; Lentjes et al. 1997; Malick et al. 2001; Bosley et al. 2004; Lépine & Briley, 2004; Réthelyi et al. 2004; Tsuno et al. 2005; Argoff, 2007; Tsang et al. 2008; Gormsen et al. 2010; Turk et al. 2010). In this subgroup of neuropathic pain patients, the presence of altered mood is not predicted by pain intensity (Chapman, 1953; Chapman & Turner, 1953; Fordyce, 1953; Jacobson & Mariano, 1953; Turner & Romano, 1953; Sternbach, 1974; Timmermans & Sternbach, 1974; Procacci et al. 1979; Reuler et al. 1980; Harding et al. 1994; Galer & Jensen, 1997; Bennett, 1999; Menefee et al. 2000; Jensen et al. 2001; Meyer-Rosberg et al. 2001; Zimmerman, 2001).
In rodent models of neuropathic pain, indications of altered mood have been sought by quantifying ‘depressive-like’ behaviours, a search which has resulted in a number of inconsistent observations (Kontinen et al. 1999; Suzuki et al. 2007; Roeska et al. 2008, 2009; Hu et al. 2009; Wang et al. 2011). Some studies suggest that nerve-injured rodents show no evidence of ‘depressive-like’ behaviours (Kontinen et al. 1999), whereas others report that all nerve-injured rodents show evidence of ‘depressive-like’ behaviours (Suzuki et al. 2007; Roeska et al. 2008, 2009; Hu et al. 2009; Seminowicz et al. 2009). The inconsistencies between studies have been attributed to: time of testing post injury (Wang et al. 2011); habituation following repeated testing (Kontinen et al. 1999); strain and species differences (Roeska et al. 2009); and differences according to the type of nerve injury (Roeska et al. 2008). A principle feature of these studies has been an intrinsic assumption that all nerve-injured animals will show behavioural change indicative of altered mood, an hypothesis at odds with the clinical picture described above. A second feature of these studies has been a focus on using tests of ‘anxiety’ (i.e. elevated plus maze, open field, dark-light exploration) rather than measuring the behavioural signs of mood disorder most frequently reported by neuropathic pain patients (i.e. disturbed sleep, disrupted social relations, poor attention and memory, and poor endocrine dysfunction) (Affleck et al. 1997; Lentjes et al. 1997; Malick et al. 2001; Bosley et al. 2004; Lépine & Briley, 2004; Réthelyi et al. 2004; Argoff, 2007; Tsang et al. 2008; Gormsen et al. 2010; Turk et al. 2010). The third feature has been the evaluation of animals during the light phase, the time at which laboratory rodents are least active and/or asleep (Roeska et al. 2008, 2009). The adequacy and usefulness of animal models for any pathological state depends significantly on the extent to which these models incorporate and evaluate the major features of the human condition which they are being used to investigate. The sciatic nerve constriction injury model (Bennett & Xie, 1988) has been used extensively as a model of chronic neuropathic pain due to its ability to reliably produce sensory changes characteristic of human neuropathic pain states (Bennett, 1993; Kim et al. 1997). Recent work from our laboratory has, however, provided strong evidence that this model reflects, at a more fundamental level, the human neuropathic pain state because of its ability to trigger changes in complex behaviours and endocrine function in a subpopulation of injured individuals. These include sleep disturbances, altered social behaviours, dysregulation of the HPA and HPT axes, and anhedonia (Monassi et al. 2003; Keay et al. 2004; Kilburn-Watt et al. 2010; Hakim & Keay, 2011). These changes reflect closely the commonly reported problems of chronic neuropathic pain patients with co-morbid altered mood. We have termed this constellation of behavioural and physiological dysfunction, ‘disability’. Thus the CCI model reflects the human experience in that some individuals cope with injury at its onset, or after a short period of disability, whereas a failure to cope persists in others. Critically, as in people, the trajectory into the disabled state in rats is not related to the intensity of the sensory consequences of the nerve injury (cf. Monassi et al. 2003 with Chapman, 1953; Chapman & Turner, 1953; Fordyce, 1953; Jacobson & Mariano, 1953; Turner & Romano, 1953; Sternbach, 1974; Timmermans & Sternbach, 1974; Procacci et al. 1979; Reuler et al. 1980; Harding et al. 1994; Galer & Jensen, 1997; Bennett, 1999; Menefee et al. 2000; Jensen et al. 2001; Meyer-Rosberg et al. 2001; Zimmerman, 2001).
A growing body of literature places the hippocampus in a pivotal position in the development of the range of conditions broadly described as ‘mood disorders’, which includes major depressive disorder (with or without anxiety) (MDD). A critical involvement of the hippocampus in MDD has been argued for, based on changes in volume in affected individuals when compared with healthy controls. However, close analysis of the literature reveals contradictory findings. For example, claims that reduced hippocampal volume is associated with MDD (Sheline et al. 2003; Saylam et al. 2006; Colla et al. 2007; MacMaster & Kusumakar, 2008; MacMaster et al. 2008; Malykhin et al. 2010) are not supported by others (Vakili et al. 2000; Rusch et al. 2001; Ahdidan et al. 2011). Furthermore, when detected, the locations of these morphological differences differ, i.e. in the posterior (tail) segment (Neumeister et al. 2005; Maller et al. 2007) vs. anterior (body) subregion (Zhao et al. 2008; Malykhin et al. 2010). Gender differences as well as the use of prescribed and non-prescribed medications further complicate this picture (Malykhin et al. 2010 cf. Woon & Hedges, 2011). Although these differences can be controlled for, using multilevel covariate and factor analyses, conclusions are often drawn from quite small sample sizes (Videbech & Ravnkilde, 2004; McKinnon et al. 2009). Notwithstanding these issues, meta-analyses conclude that adults with MDD have smaller hippocampi than found in appropriate control adults (Campbell & Macqueen, 2004; Videbech & Ravnkilde, 2004; McKinnon et al. 2009). The susceptibility of the hippocampus to changes in gross morphology following traumatic physical and psychological events (Kitayama et al. 2005; Woon & Hedges, 2008; Woon et al. 2010) and the relationship of altered mood with reductions in hippocampal size led to the hypothesis that nerve-injured rats with altered complex behaviours following nerve injury, will show reduced hippocampal volumes, whereas rats without these behavioural alterations following neuropathic injury will not. Our suggestion is further supported by evidence in mice that spared nerve injury (SNI) alters the rate of hippocampal neurogenesis, which could result in gross morphological changes (Mutso et al. 2012). That the hippocampus is affected by nerve injury has also been shown by the following: altered neuronal function (Mutso et al. 2012); elevations of hippocampal tumour necrosis factor-alpha (TNF-α), which impairs both working and short-term memory (Ren et al. 2011); and altered hippocampal long-term potentiation (Kodama et al. 2007). Both SNI and CCI procedures in rats elevate hippocampal cytokine levels (Del Rey et al. 2011); elevate hippocampal TNF-α impairing both working and short-term memory (Ren et al. 2011) and alter CA1 neuronal activity (Cardoso-Cruz et al. 2013).
The aims of this study were to determine the effects of constriction injury of the sciatic nerve on the morphology of the hippocampus and dentate gyrus, and to determine whether distinct patterns of morphological changes were associated with the presence or absence of disabilities following nerve injury.
Materials and methods
All experimental procedures were carried out following the guidelines of the NHMRC ‘Code for Care and Use of Animals in Research in Australia’ and the ‘Ethical Guidelines for Investigation Association for the Study of Pain’ (Zimmerman, 1993). All procedures were approved by the University of Sydney Animal Care and Ethics Committee (AEC numbers K03/3920 and K03/4852).
Animal groups
All experiments were performed on male Sprague-Dawley rats, (∼180 g, ARC, Perth, WA, Australia). Three experimental groups were compared: (i) single-housed, social-interaction tested rats (n = 11); (ii) single-housed, sham-injured and social-interaction tested rats (n = 7); and; (iii) single-housed, nerve-injured, social-interaction tested animals. The rats in group (iii) were divided post-hoc into Pain alone (n = 6), Pain & Disability (n = 7), and Pain & Transient Disability (n = 5). The testing protocol is summarised in Fig. 1.
Fig. 1.
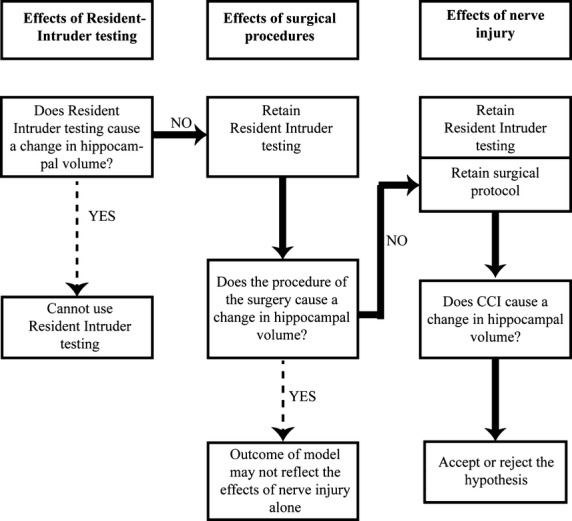
Flowchart summarising the experimental protocol used in this study. The systematic evaluation of the effects of resident–intruder, social interaction testing and sham surgical procedures on the volume of the hippocampus and dentate gyrus were established prior to evaluation of the effects of nerve injury on these dependent measures.
In addition, the hippocampal morphologies of these experimental groups were compared with a group of standard laboratory group housed rats (n = 12).
Rats in each of the experimental groups were behaviourally tested. The animals were housed individually on arrival in Perspex cages (40 × 36 × 24 cm) and allowed to habituate for 2 weeks, to a reverse dark–light cycle (12 : 12 h); all experiments were conducted during the dark phase. These rats are termed resident rats in the social interaction test.
Social interaction testing
The resident–intruder, social interaction testing procedures were significantly modified from those originally reported by Koolhaas et al. (2013) and have been described in detail earlier (Monassi et al. 2003). Following the introduction of an age-, weight- and sex-matched intruder into the cage of a resident rat, social interactions were recorded for 6 min using an infrared camera (DCRA-C155; Sony). The same intruder did not meet a resident rat more than two times, and never on consecutive days. Intruders were also housed under the same reverse dark–light cycle (12 : 12 h). The resident rat's behaviour was analysed for 6 min. The behaviour of the animal was assigned to one of four mutually exclusive categories. Dominance behaviour: Standing on top of the supine intruder, biting, chasing, aggressive grooming, boxing and sideway lateral pushing. Social behaviour: Sniffing and exploration of the intruder specifically focused around the anogenital region. Non-social: Exploration of the cage and self-grooming. Submissive: Defensive alerting, fleeing behaviour and supine postures.
Rats were tested on five consecutive days prior to nerve or sham injuries (days 1–5) to ensure the stability of each resident's behaviour towards an intruder, as described in multiple cohorts in previous studies (Monassi et al. 2003; Austin et al. 2010; Kilburn-Watt et al. 2010; Mor et al. 2010). On day 6, rats received a nerve injury, a sham injury or were not behaviourally tested. On days 7–12, rats underwent a further 6 days of social interaction testing.
The behaviour of each rat following nerve injury was analysed to determine the animals in which dominance behaviours changed, which we have previously shown to reflect a number of physiological and behavioural changes that we have termed ‘disability’. As shown by Monassi and colleagues and replicated in several studies (Monassi et al. 2003; Austin et al. 2010; Kilburn-Watt et al. 2010; Mor et al. 2010, 2011), rats could be categorised in the following way: Pain alone (No disability) – no change between pre- and post-CCI behaviours; Pain & Transient Disability – a decrease of at least 30% in dominance behaviours on the first 2 or 3 post-CCI test days returning to pre-CCI levels for the last 3 days of testing; Pain & Disability: decrease in the duration of dominance behaviours by at least 30%, for at least 75% of the post-CCI period, including the last 3 days of testing.
Chronic constriction injury of the sciatic nerve
The surgical procedure was performed as described by Bennett & Xie (1988). Under halothane anesthesia (5% in 100% oxygen, Laser Animal Health, Australia) an incision was made in the right thigh, the muscle was gently parted by blunt dissection, and the sciatic nerve was identified at its trifurcation. Four chromic gut ligatures (5–0 Ethicon; Johnson & Johnson Medical) were loosely tied around the isolated nerve just medial to the trifurcation. The skin was sutured and the animal allowed to recover under close observation. Surgical anaesthesia and procedures lasted no longer than 20 min. An identical procedure, without the nerve constriction, was performed on sham-injured rats.
Tissue preparation
Twenty-four hours after the final social interaction test (day 12), all rats were deeply anaesthetised with barbituate (Lethabarb 130 mg kg−1 i.p.) and perfused transcardially with heparinised saline (500 mL of 0.9% NaCl at 4 °C containing 10 IU heparin) followed by 4% paraformaldehyde in acetate-borate buffer (500 mL, pH 9.6 at 4 °C). The brains were removed immediately and placed in cold fixative for 48 h, and then in 10% sucrose in phosphate buffer (0.1 m pH 7.6) until the brains sank (∼3 days).
Each brain was assigned a number by investigators not involved in these experiments, and from this point, all processing and analysis was performed blind. Only when the study was completed and the final statistical analyses were performed, was this blinding reversed and each experimental and control group identified.
A brain block containing the entire hippocampus was isolated, frozen in mounting medium and sectioned coronally at 40 μm on a freezing microtome (Leica SM2000R). A one in five series of sections was taken, with every 5th section immediately mounted onto gelatinised glass slides and air-dried for 72 h. The neuronal cell bodies in the sections on each slide were then stained using a standard nissl body counterstaining protocol (1% Thionin). The slides were placed overnight in 50% ethanol, 50% chloroform. The slides were then immersed in a descending alcohol series (100, 90, 70 and 50%) for 1 min each. They were then placed in acetic acid/ethanol solution (1 : 4 ratio) for 20 s followed by immersion in 1% Thionin stain for 10 s. The sections were washed in distilled water and placed into an ascending alcohol series of 50% and then 70% followed by immersion into acetic acid/ethanol solution (1 : 4 ratio) for 10 s, then 90 and 100% alcohol solutions for 1 min each. All slides were cleared overnight in Histoclear and coverslipped using DPX mounting medium.
Morphological assessment
Photomicrographs were taken of every section of the 1 : 5 series at 40× magnification. Volumetric analyses were conducted with the use of image analysis software (uthscsa imagetool version 3.0). The boundaries of the hippocampus and dentate gyrus were traced onto each photomicrograph (see Fig. 2). The original sections were cross-referred to a higher magnification when needed. The anatomical boundaries of the hippocampus and the dentate gyrus were defined with reference to the stereotaxic atlas of the rat brain as per Paxinos & Watson (2005).
Fig. 2.
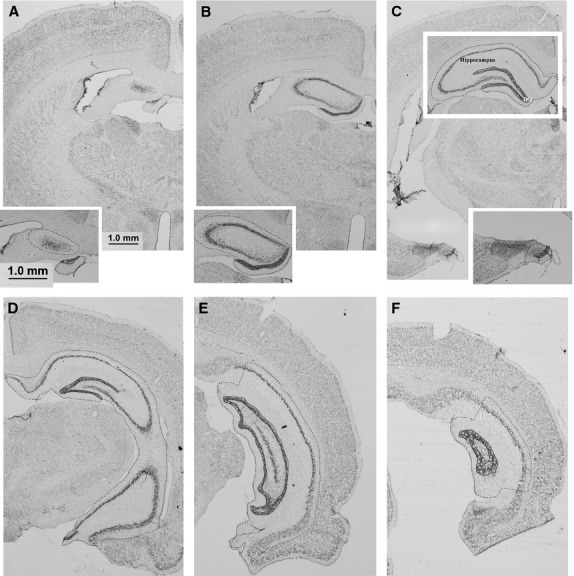
Photomicrographs of select coronal Thionin-stained sections through the hippocampus. Dashed lines indicate the boundaries used to define the hippocampus and solid lines those used to define the dentate gyrus. (A,B) Dorsal (septal pole) hippocampus. (C,D) Dorsal and ventral hippocampus. (E,F) Posterior/intermediate hippocampus. Scale bar: 1.0 mm.
Hippocampal boundaries
The rostral/septal pole was identified by the presence of CA3 cells at approximately –1.72 mm bregma (Fig. 2A). The caudal pole of the hippocampus was identified by the disappearance of the molecular layer of the dentate gyrus and the CA1 regions, at approximately −6.8 mm caudal to bregma (Fig. 2F). The medial border was defined by the disappearance of the densely labelled pyramidal cells, defining the CA1 region, at approximately −5.16 mm from bregma (Fig. 2D,E). In the region of CA1, the border was defined by a perpendicular line drawn from the hippocampal fissure to the alveolus (Fig. 2E). The lateral border was established by the change in staining between oreins of the hippocampus and the alveolus. At the point where the CA2 region changes into the ventral subiculum a line was drawn between the end of the pyramidal layer and the hippocampal fissure.
It is staightforward to delineate the dorsal and ventral hippocampus in regions where they are spatially separate (Fig. 2C) and to distinguish them on sections where the CA3 regions of the dorsal and ventral subregions are separated by the oriens layer of the hippocampus (Fig. 2D). This distinction becomes less reliable and somewhat arbitrary when the oriens layer, rather than separating the two regions, runs parallel with them (Fig. 2E). To avoid arbitrary boundary divisions (i.e. dorsal vs. ventral, medial vs. lateral) and to maintain a systematic and unbiased approach to volume analyses, total hippocampal volumes were calculated ( Fig. 5).
Fig. 5.
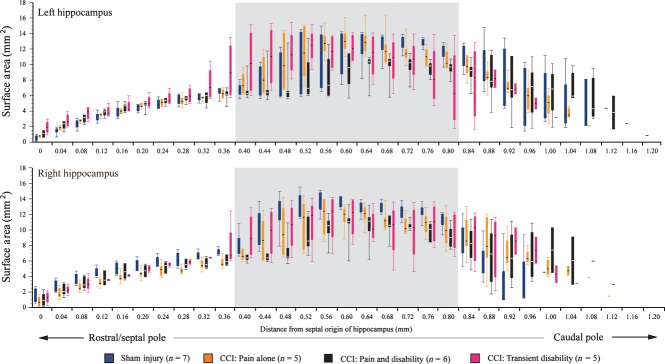
Box plots illustrating the means and range of the surface areas of the hippocampus in the following groups: sham-injured control rats (n = 7, blue); Pain alone following CCI (n = 5, yellow); Pain and Disability following CCI (n = 6, black); and Pain and Transient Disability following CCI (n = 5, pink). Serial sections were analysed and plotted from the septal pole (dorsal hippocampus) to the posterior/intermediate pole of the hippocampus. The grey shading highlights the coronal sections where both the dorsal and ventral subregions of the hippocampus are found, which we suggest are not anatomically separable (see text).
Dentate gyrus boundaries
The molecular layer borders were easily distinguished on Thionin-stained sections. The dorsal and ventral subregions were defined in accordance with Paxinos & Watson (2005) and the posterior subregion was defined from the point where the dorsal and ventral subregions join, and the point at which the molecular layer is no longer present.
To ensure that there were no differences in tissue shrinkage between animals, sections from randomly selected rats, perfused on different days, were used to compare cortical thickness, the width of the diencephalon, and the distance from the dorsal surface to superior tip of the third ventricle at the same antero-posterior level. This procedure did not reveal any variability in the effects of fixation on these tissues.
Statistical analysis
A two-way between-group anova evaluated the effects of time and post-injury behavioural group on dominant, social, non-social and submissive behaviours. Significant main effects were probed further using Tukey's post-hoc comparisons between each behavioural group (PA, PD, TD, BS, BSC). A repeated measure anova and Fisher's protected least significance post-hoc analyses were used to determine significant changes in dominance, social, non-social and submissive behaviours pre- and post-CCI. Morphological comparisons were made between the five groups (BC, BS, PA, PD and TD) using a one-way anova, followed where permissable by Tukey HSD post-hoc testing.
Results
Comparison of hippocampal and denatate gyrus morphology between experimental groups
Significant differences were detected between the experimental groups in the volume of the hippocampus [anova, left: (F5,40 = 5.660, P < 0.001), right (F5,40 = 6.977, P < 0.001)]; the volume of the whole dentate gyrus [anova, left: (F5,42 = 6.433, P < 0.001), right (F5,42 = 3.530, P = 0.009)]; in the dorsal dentate gyrus [anova, dorsal left: (F5,42 = 2.557, P = 0.042), dorsal right (F5,42 = 3.658, P = 0.008)]; in the left but not right posterior (intermediate) dentate gyrus [anova, posterior/intermediate left: (F5,42 = 4.320, P = 0.003), posterior/intermediate right (F5,42 = 1.004, P = 0.427)], but not in the ventral dentate gyrus [anova, ventral left: (F5,42 = 1.989, P = 0.1), ventral right (F5,42 = 1.095, P = 0.377)].
Effects of resident–intruder testing
Behaviour
In behavioural control rats, social interaction testing over a period of 12 days produced stable levels of dominant and submissive behaviours. During the last 3 days of testing, rats showed a modest, statistically significant reduction in social behaviours (Friedman's 2-way anova, P = 0.038; pairwise comparison post-CCI days 1–3 vs. post-CCI days 4–6, P = 0.03) (Fig. 3). During the test period (6 min), rats spent the majority of their time (∼50%) in non-social behaviours, which included investigation of the cage and self-grooming. They spent approximately 30% of the time in dominance behaviours and the remaining ∼20% investigating the intruder animal (social behaviours). Submissive behaviours were seldom seen.
Fig. 3.
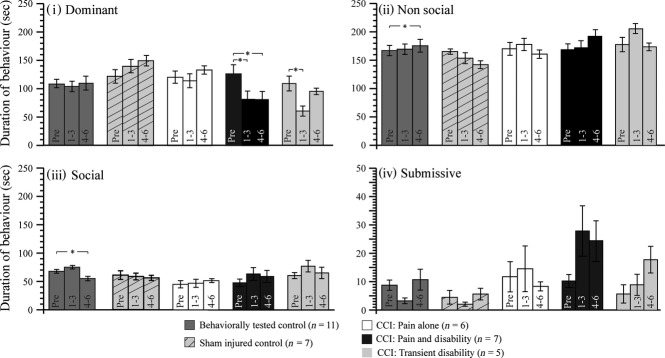
Bargraphs showing the mean (± SEM) durations of (i) Dominant, (ii) Non-social, (iii) Social and (iv) Submissive behaviours on pre-injury days 3–5 and post-injury days 1–3 and 4–6, in: (1) behaviorally tested control rats (n = 11); (2) sham-injured control rats (n = 7); (3) Pain alone following CCI (n = 6); (4) Pain and Disability following CCI (n = 7); and (5) Pain and Transient Disability following CCI (n = 5). Significant difference between pre- and post-injury days (Fisher's least significant difference) are shown: *P < 0.05.
Morphology of hippocampus and dentate gyrus
Post-hoc testing (Tukey HSD) did not reveal any differences in the size of the hippocampus between rats that had undergone social interaction testing for 12 days (BC) and the group of standard laboratory-housed rats (n = 12, data not illustrated).
Effects of surgical procedures: sham injury and social interaction testing
Behaviour
Sham injury triggered a small but non-significant increase in dominance behaviours which was mirrored by a small but significant reduction in non-social behaviour (Friedman's 2-way anova, P = 0.036: pairwise comparison pre-CCI days 1–3 vs. post-CCI days 4–6; P = 0.04) (Fig. 3).
Morphology of hippocampus and dentate gyrus
When compared with behaviourally tested control rats, sham injury and behavioural testing had no significant effect on the overall volumes of the hippocampus, the dentate gyrus or any of their subregions (Fig. 4).
Fig. 4.
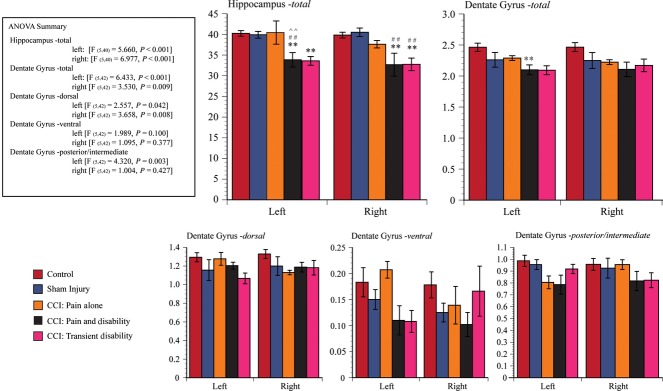
Bargraphs illustrate the mean (± SEM) volumes of (i) Hippocampus-total, (ii) Dentate Gyrus-total, (iii) Dentate Gyrus – dorsal, (iv) Dentate Gyrus – ventral and (v) Dentate Gyrus – posterior/intermediate for the following groups: behaviorally tested control rats (n = 11, red column); sham-injured control rats (n = 7, blue); Pain alone following CCI (hippocampus: n = 5; dentate gyrus: n = 6, yellow); Pain and Disability following CCI (hippocampus: n = 6; dentate gyrus: n = 7, black); and Pain and Transient Disability following CCI (n = 5, pink). Significant differences (post-hoc Tukey LSD) between groups are indicated by **P < 0.01 when compared with behaviourally tested control rats, by ##P < 0.01 when compared with sham-injured control rats, and by ∧∧P < 0.01 when compared with Pain alone following CCI.
Effects of sciatic nerve injury: pain alone group
Behaviour
In ∼50% of rats, sciatic nerve constriction injury has no effect on social interations in the resident intruder test (Monassi et al. 2003). In previous publications, these rats have been termed ‘Pain alone’. Identical to our earlier reports, this subgroup of rats showed no change from pre-injury levels in any of the behavioural categories measured after sciatic nerve CCI.
Morphology of hippocampus and the dentate gyrus
There were no differences in the volume of the hippocampus, dentate gyrus or its subregions between Pain alone (CCI) rats, sham-injured and behaviourally tested rats, and behaviourally tested rats (Fig. 4).
Effects of sciatic nerve injury: pain and disability group
Behaviour
Persistent reductions in dominance behaviours characterises approximately 25–30% of sciatic nerve-injured rats, defining a subgroup we have called in earlier publications ‘Pain and Disability’ rats (Monassi et al. 2003; Austin et al. 2010; Kilburn-Watt et al. 2010; Mor et al. 2010). In these rats, the significant decrease in dominance behaviour on days 1–3 and 4–6 post-CCI (Friedman's 2-way anova, P = 0.005: pairwise comparison pre-CCI vs. post-CCI days 1–3; P = 0.023; pre-CCI vs. post-CCI days 4–6; P = 0.01) is accounted for by an increase in non-social behaviours (primarily exploration and cage investigation), social behaviours and occasional submissive behaviours. The dominance behaviours expressed shifted to an approach–avoid style with the rat increasing approaches toward the partner but reducing dominance interactions. This pattern is described in detail in Monassi et al. (2003). Figure 3 illustrates the persistent reduction in dominance of these Pain and Disability rats.
Morphology of hippocampus and the dentate gyrus
Tukey HSD post-hoc testing revealed that the left hippocampus was smaller in Pain and Disability rats than in behaviourally tested controls (P < 0.02), sham-injured and behaviorally tested controls (P < 0.05) and Pain alone rats (P < 0.05). The right hippocampus was smaller in Pain and Disability rats than in behaviourally tested controls (P < 0.01), sham-injured and behaviorally tested controls (P < 0.01). Rats with Pain and Disability following CCI also have a smaller left (contralateral to the CCI) dentate gyrus (P < 0.03). The subregions of the dentate gyrus did not differ between the groups.
Effects of sciatic nerve injury: pain and transient disability group
Behaviour
Post-CCI, around 20–25% of rats show initial disability (2–3 days), followed by recovery; this subgroup of rats has been called the ‘Pain and Transient Disability’ group (Monassi et al. 2003). Figure 3 shows the behavioural profiles of rats classified in this group. The transient and significant reduction in dominance behaviours of the first three post-CCI days (Friedman's 2-way anova, P = 0.02: pairwise comparison pre-CCI vs. post-CCI days 1–3; P < 0.01) recovers to levels, which are not significantly different to the pre-CCI baseline (pairwise comparison pre-CCI vs. post-CCI days 4–6; P = 1.0).
Morphology of hippocampus and the dentate gyrus
Tukey H post-hoc testing revealed that the left hippocampus was smaller in Pain and Transient Disability rats than in behaviourally tested controls (P < 0.02), and the right hippocampus was smaller compared with behaviourally tested controls (P < 0.01), sham-injured and behaviorally tested controls (P < 0.01) (Fig. 4).
Morphological observations
Hippocampus
At the septal (rostral) pole of the hippocampus, which constitutes primarily the dorsal subregion, the surface area of the hippocampus when sectioned coronally, is similar in each of the experimental groups (Fig. 5). In Fig. 4, above we showed that rats with either Pain and Disability or Pain and Transient Disability each have reduced hippocampal volumes. Morphological observations revealed that the Pain and Transient Disability rats had a shorter hippocampus with overall larger cross-sectional areas when contrasted with the other experimental groups (Fig. 5). Further, the rats with Pain and Disability had a longer hippocampus with an overall smaller cross-sectional area when contrasted with the other experimental groups, although the most caudal sections contained the greatest cross-sectional areas of hippocampus (Fig. 5).
Dentate gyrus
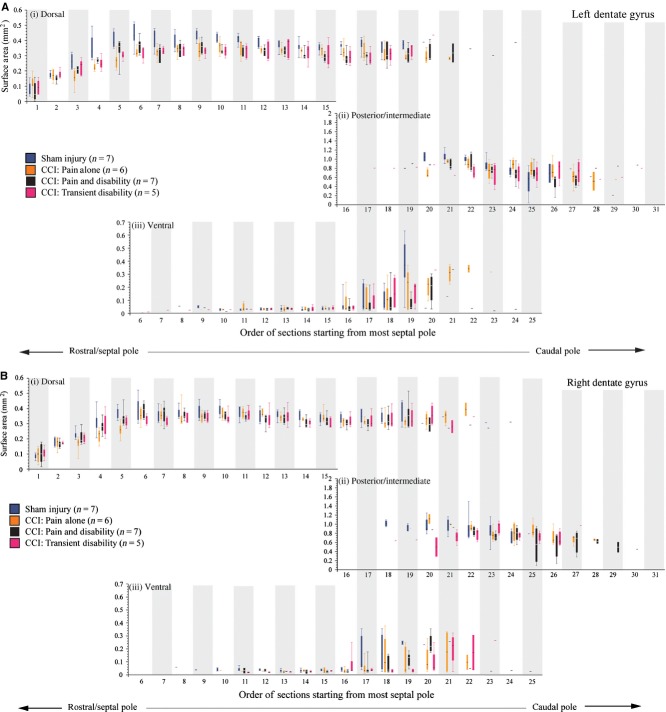
The reduction in volume of the dentate gyrus contralateral to the CCI (left) shown in Fig. 4 was not accounted for by select reductions in the volume of the dorsal, ventral or posterior regions (Fig. 6A,B).
Fig. 6.
Box plots illustrating the means and range of the surface areas of the left (A) and right (B) dentate gyrus in the following groups: sham-injured control rats (n = 7, blue); Pain alone following CCI (n = 5, yellow); Pain and Disability following CCI (n = 6, black); and Pain and Transient Disability following CCI (n = 5, pink). Serial sections were analysed and plotted from the septal pole (dorsal hippocampus) to the posterior/intermediate pole of the dentate gyrus. Surface area sizes for the dorsal, ventral and posterior/intermediate subregions of the dentate gyrus are shown in panels (i–iii).
Discussion
The aims of this study were to determine the effects of constriction injury of the sciatic nerve on the morphology of the hippocampus and dentate gyrus. In particular, we wanted to determine whether specific morphological changes were associated with the presence of complex behavioural disabilities triggered by the injury. To summarise, we identified that: single-housing and repeated social interaction testing had no effect on the morphology of either the hippocampus or the dentate gyrus; rats with nerve injury-evoked transient or persistent changes in social interactions (i.e. complex behavioural disabilities) had bilaterally reduced hippocampal volumes;rats with nerve injury-evoked persistent changes in social interactions had reduced dentate gyrus volumes, contralateral to the nerve injury (left side).
The discussion will deal with the following issues raised by our findings: (i) the effects of nerve injury on hippocampal and dentate gyrus morphology; (ii) possible mechanisms by which nerve injury could trigger morphological changes; (iii) what the morphological changes might represent, and (iv) the potential relationship of morphological change with the expression of disability.
Control rats: morphological observations
The size of the hippocampus and dentate gyrus is affected by many stressors, ranging from the relatively minor and acute, to the chronic (Sousa & Almeida, 1998; Lee & Son, 2009; Golub et al. 2011). The sensitivity of hippocampal and dentate gyrus size to context and environment compelled us to evaluate the impact of both the resident–intruder social interaction test and the sham surgical procedures. The volumes of the hippocampus and the dentate gyrus were unaffected by resident–intruder social interaction testing or sham surgical procedures.
Effects of nerve injury on the hippocampus
Our earlier observations have shown that in a subset of rats (∼30%), social interactions in the resident–intruder test are disrupted. The rats that show this altered behavioural response also show decreased slow-wave sleep and increased wakefulness (Monassi et al. 2003), and they have elevated plasma corticosterone and decreased plasma levels of ACTH, thyroxine and tri-iodothyronine (T3) (Kilburn-Watt et al. 2010). A subset of injured rats are also reported to show anhedonia (Hakim & Keay, 2011). It is this subset of rats, identified by their changes in social behaviours, that were shown in this study to have bilateral reductions in hippocampal volume.
This is the first report of morphological changes in the hippocampus following peripheral nerve injury in an animal model of neuropathic pain. Most importantly, the reductions in size are found only in rats that show complex behavioural and physiological changes in response to the injury. Changes in the microanatomy of the hippocampus have been reported following chronic but not acute hindpaw inflammatory pain in the rat, and spared nerve injury in the mouse. A bilateral reduction in neurogenesis in the dentate gyrus (both the hilus and the subgranular zones) was reported in all experimental animals (Duric & McCarson, 2006; Mutso et al. 2012). Whether the reduction in neurogenesis resulted in morphological change, was not evaluated in either study. However, hippocampus-dependent learning and anxiety measures were disrupted in the mice with spared nerve injuries (Mutso et al. 2012); whether the chronic inflammatory pain in the rats was associated with behavioural and physiological changes was not evaluated. Further, in a brief report in mice, it has been reported that a partial sciatic nerve injury can reverse the enhanced neurogenesis in the dentate gyrus driven by environmental enrichment, although this nerve injury had no effect on the level of neurogenesis under standard housing conditions (Terada et al. 2008). Zimmerman et al. (2009) have reported smaller hippocampal volumes in people with chronic pain of unspecified origin. Similarly, Mutso et al. (2012) have reported smaller hippocampal volumes in people with chronic regional pain syndrome, osteoarthritis and chronic backpain (Mutso et al. 2012). As yet, there are no reports of altered hippocampal volumes in neuropathic pain patient populations.
Mechanisms of nerve injury triggered morphological change
A critical question is, of course, how sciatic nerve injury could trigger changes in hippocampal morphology. The likely answer includes a combination of: (i) reduced neurogenesis, (ii) increased apoptosis and (iii) atrophy and remodelling of neurons.
In rats, reductions in neurogenesis in the hippocampus are triggered by glucocorticoids (Sapolsky, 1990; Cameron & Gould, 1994; Kim et al. 2004), social stress (Mitra et al. 2006; Lagace et al. 2010), sleep disturbances (Roman et al. 2005; Guzman-Marin et al. 2007; Lucassen et al. 2010), chronic inflammatory pain (Duric & McCarson, 2006; Lucassen et al. 2010), and chronic restraint (Pham et al. 2003). Injured rats with reduced hippocampal volumes certainly have elevated corticosterone, disturbed sleep and a localised inflammatory reaction (at the site of the CCI), each of which has proved sufficient to reduce neurogenesis in other studies.
Increased rates of apoptosis in the hippocampus may be triggered by the same stimuli that reduce rates of neurogenesis; the difficulties of detecting apoptotic cell death in histological samples has resulted in only a few anatomical analyses of the significance of this process for structural alterations of the hippocampus. Some investigators argue that apoptotic cell death increases in response to glucocorticoids and social stress and that following significant durations of exposure to these stimuli, the rates of cell death are sufficiently high to cause detectable changes in size and volume, whereas others argue that apoptosis is negligible in response to glucocorticoids and a range of stressors, and that regulating rates of neurogenesis is the key to regulation of hippocampal size (Czéh & Lucassen, 2007). There is evidence that pro-apoptotic ratios of Bax/Bcl2 as well as the presence of the marker of apoptosis-activated caspase-3 are triggered in the hippocampus by inflammation in the hindpaw. Similarly (Jalalvand et al. 2008), a single prolonged stress triggers a pro-apoptotic Bax/Bcl2 and caspase-3, -9 profile, and there is evidence of apoptosis defined by TUNEL-positive profiles (Li et al. 2010).
Atrophy and remodelling of hippocampal neurons has been demonstrated following restraint stress and social stress (Buwalda et al. 2005); this physical remodelling is argued to be glucocorticoid-dependent, although specific evidence for this is not available. On balance, it is likely that the significant alterations in hippocampal size and volume reported in this study are likely explained by each of these processes; defining the contribution of each process is the next important experimental step.
Morphological changes and the expression of disability
The dorsal (septal pole), ventral and posterior/intermediate subregions of the hippocampus each have distinct functional roles. The dorsal (septal pole) hippocampus is critical for spatial memory formation (Moser et al. 1995; Pothuizen et al. 2004), and the ventral and posterior/intermediate subregions play significant roles in regulating affective behaviours and modulating unconditioned responses to stressful or threatening stimuli (McHugh et al. 2004; Moser & Moser, 1998; Pentkowski et al. 2006; Fanselow & Dong, 2010) . The ventral and posterior/intermediate hippocampus is also reported to regulate the activity of the hypothalamo-pituitary-adrenal axis, controlling the level of adaptation of the system in the face of ongoing stressors (Moser & Moser, 1998). It has been usual to interpret reductions in hippocampal size and inferred reductions in neural density in order to approximate to some degree the functional effects of hippocampal lesions. Although this stance perhaps overestimates the functional impact of the size and volume changes reported, there is general agreement that learning and memory, as well as affect and HPA axis regulation, will be altered by structural changes in the dorsal (septal pole), ventral and posterior/intermediate subregions, respectively.
Conclusions
In a subpopulation of rats, sciatic nerve injury triggers a combination of behavioural and physiological changes that resemble many of the criteria used clinically to characterise mood disorder. These rats showed a reduction in the volume of the hippocampus similar to the changes defined in people with clinically diagnosed mood disorders, i.e. major depressive disorder. The sciatic nerve CCI model again reveals similarities to the human neuropathic pain presentation that hitherto have not been appreciated.
Conflicts of interest
The authors declare they have no conflicts of interest.
Funding
NHMRC (Australia) and NG Macintosh Memorial Fund.
References
- Affleck G, Urrows S, Tennen H, et al. A dual pathway model of daily stressor effects on rheumatoid arthritis. Ann Behav Med. 1997;19:161–170. doi: 10.1007/BF02883333. [DOI] [PubMed] [Google Scholar]
- Affleck G, Tennen H, Keefe FJ, et al. Everyday life with osteoarthritis or rheumatoid arthritis: independent effects of disease and gender on daily pain, mood, and coping. Pain. 1999;83:601–609. doi: 10.1016/S0304-3959(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Ahdidan J, Hviid LB, Chakravarty MM, et al. Longitudinal MR study of brain structure and hippocampus volume in major depressive disorder. Acta Psychiatr Scand. 2011;123:211–219. doi: 10.1111/j.1600-0447.2010.01644.x. [DOI] [PubMed] [Google Scholar]
- Argoff CE. The coexistence of neuropathic pain, sleep, and psychiatric disorders: a novel treatment approach. Clin J Pain. 2007;23:15–22. doi: 10.1097/01.ajp.0000210945.27052.b3. [DOI] [PubMed] [Google Scholar]
- Austin PJ, Beyer K, Bembrick AL, et al. Peripheral nerve injury differentially regulates dopaminergic pathways in the nucleus accumbens of rats with either ‘pain alone’ or ‘pain and disability’. Neuroscience. 2010;171:329–343. doi: 10.1016/j.neuroscience.2010.08.040. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, et al. Depression and pain co-morbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Bennett GJ. An animal model of neuropathic pain: a review. Muscle Nerve. 1993;16:1040–1048. doi: 10.1002/mus.880161007. [DOI] [PubMed] [Google Scholar]
- Bennett GJ. New frontiers in mechanisms and therapy of painful peripheral neuropathies. Acta Anaesthesiol Sinica. 1999;37:197–203. [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bosley BN, Weiner DK, Rudy TE, et al. Is chronic nonmalignant pain associated with decreased appetite in older adults? Preliminary evidence. J Am Geriatr Soc. 2004;52:247–251. doi: 10.1111/j.1532-5415.2004.52063.x. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Kole MH, Veenema AH, et al. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Cardoso-Cruz H, Lima D, Galhardo V. Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus-prefrontal cortex connectivity. J Neurosci. 2013;33:2465–2480. doi: 10.1523/JNEUROSCI.5197-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CR. The psychophysiology of pain. In: Bonica JJ, editor. The Management of Pain. Philadelphia: Lea and Febiger; 1953. pp. 461–477. [Google Scholar]
- Chapman CR, Turner JA. Psychological aspects of pain. In: Bonica JJ, editor. The Management of Pain. Philadelphia: Lea and Febiger; 1953. pp. 478–482. [Google Scholar]
- Colla M, Kronenberg G, Deuschle M, et al. Hippocampal volume reduction and HPA-system activity in major depression. J Psychiatr Res. 2007;41:553–560. doi: 10.1016/j.jpsychires.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Czéh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- Del Rey A, Yau HJ, Randolf A, et al. Chronic neuropathic pain-like behavior correlates with IL-1β expression and disrupts cytokine interactions in the hippocampus. Pain. 2011;152:2827–2835. doi: 10.1016/j.pain.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, McCarson KE. Neurokinin-1 (NK-1) receptor and brain-derived neurotrophic factor (BDNF) gene expression is differentially modulated in the rat spinal dorsal horn and hippocampus during inflammatory pain. Mol Pain. 2006;3:32. doi: 10.1186/1744-8069-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce WE. Learned pain: Pain as behaviour. In: Bonica JJ, editor. The Management of Pain. Philadelphia: Lea and Febiger; 1953. pp. 478–482. [Google Scholar]
- Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the neuropathic pain scale. Neurology. 1997;48:332–338. doi: 10.1212/wnl.48.2.332. [DOI] [PubMed] [Google Scholar]
- Golub Y, Kaltwasser SF, Mauch CP, et al. Reduced hippocampus volume in the mouse model of Posttraumatic Stress Disorder. J Psychiatr Res. 2011;45:650–659. doi: 10.1016/j.jpsychires.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Gormsen L, Rosenberg R, Bach FW, et al. Depression, anxiety, health-related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur J Pain. 2010;14:127. doi: 10.1016/j.ejpain.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Bashir T, Suntsova N, et al. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neuroscience. 2007;148:325–333. doi: 10.1016/j.neuroscience.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim JD, Keay KA. Loss of pleasure and motivation in neuropathic pain correlates with down-regulation of mu-opioid and d2-receptor mRNA in the nucleus accumbens. Proc Aust Neurosci Soc. 2011;31:64. [Google Scholar]
- Harding VR, Williams AC, Richardson PH, et al. The development of a battery of measures for assessing physical functioning of chronic pain patients. Pain. 1994;58:367–375. doi: 10.1016/0304-3959(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Hu B, Doods H, Treede RD, et al. Depression-like behaviour in rats with mononeuropathy is reduced by the CB2-selective agonist GW405833. Pain. 2009;143:206–212. doi: 10.1016/j.pain.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Mariano AJ. General consideration of chronic pain. In: Bonica JJ, editor. The Management of Pain. Philadelphia: Lea and Febiger; 1953. pp. 241–254. [Google Scholar]
- Jalalvand E, Javan M, Haeri-Rohani A, et al. Stress- and non-stress-mediated mechanisms are involved in pain-induced apoptosis in hippocampus and dorsal lumbar spinal cord in rats. Neuroscience. 2008;157:446–452. doi: 10.1016/j.neuroscience.2008.08.052. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Gottrup H, Sindrup SH, et al. The clinical picture of neuropathic pain. Eur J Pharmacol. 2001;429:1–11. doi: 10.1016/s0014-2999(01)01302-4. [DOI] [PubMed] [Google Scholar]
- Keay KA, Monassi CR, Levison DB, et al. Peripheral nerve injury evokes disabilities and sensory dysfunction in a subpopulation of rats: a closer model to human chronic neuropathic pain? Neurosci Lett. 2004;361:188–191. doi: 10.1016/j.neulet.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Kilburn-Watt E, Banati RB, Keay KA. Altered thyroid hormones and behavioural change in a sub-population of rats following chronic constriction injury. J Neuroendocrinol. 2010;22:960–970. doi: 10.1111/j.1365-2826.2010.02038.x. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113:200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- Kim JB, Ju JY, Kim JH, et al. Dexamethasone inhibits proliferation of adult hippocampal neurogenesis in vivo and in vitro. Brain Res. 2004;1027:1–10. doi: 10.1016/j.brainres.2004.07.093. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, et al. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Kodama D, Ono H, Tanabe M. Altered hippocampal long-term potentiation after peripheral nerve injury in mice. Eur J Pharmacol. 2007;574:127–132. doi: 10.1016/j.ejphar.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Kontinen VK, Kauppila T, Paananen S, et al. Behavioural measures of depression and anxiety in rats with spinal nerve ligation-induced neuropathy. Pain. 1999;80:341–346. doi: 10.1016/s0304-3959(98)00230-9. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Coppens CM, de Boer SF, et al. The resident–intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp. 2013;77:e4367. doi: 10.3791/4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, et al. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Son H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep. 2009;42:239–244. doi: 10.5483/bmbrep.2009.42.5.239. [DOI] [PubMed] [Google Scholar]
- Lentjes EG, Griep EN, Boersma JW, et al. Glucocorticoid receptors, fibromyalgia and low back pain. Psychoneuroendocrinology. 1997;22:603–614. doi: 10.1016/s0306-4530(97)00061-9. [DOI] [PubMed] [Google Scholar]
- Lépine JP, Briley M. The epidemiology of pain in depression. Hum Psychopharmacol. 2004;19:S3–S7. doi: 10.1002/hup.618. [DOI] [PubMed] [Google Scholar]
- Li X, Han F, Liu D, et al. Changes of Bax, Bcl-2 and apoptosis in hippocampus in the rat model of post-traumatic stress disorder. Neurol Res. 2010;32:579–586. doi: 10.1179/016164110X12556180206194. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Meerlo P, Naylor AS, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Kusumakar V. Hippocampal volume in early onset depression. BMC Med. 2008;2:2. doi: 10.1186/1741-7015-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster FP, Mirza Y, Szeszko PR, et al. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol Psychiatry. 2008;63:385–390. doi: 10.1016/j.biopsych.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malick A, Jakubowski M, Elmquist JK, et al. A neurohistochemical blueprint for pain-induced loss of appetite. Proc Natl Acad Sci U S A. 2001;98:9930–9935. doi: 10.1073/pnas.171616898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JJ, Daskalakis ZJ, Fitzgerald PB. Hippocampal volumetrics in depression: the importance of the posterior tail. Hippocampus. 2007;17:1023–1027. doi: 10.1002/hipo.20339. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Carter R, Seres P, et al. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J Psychiatry Neurosci. 2010;35:337–343. doi: 10.1503/jpn.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, et al. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, et al. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- Menefee LA, Frank ED, Doghramji K, et al. Self-reported sleep quality and quality of life for individuals with chronic pain conditions. Clin J Pain. 2000;16:290–297. doi: 10.1097/00002508-200012000-00003. [DOI] [PubMed] [Google Scholar]
- Meyer-Rosberg K, Kvarnstrom A, Kinnman E. Peripheral neuropathic pain – a multidimensional burden for patients. Eur J Pain. 2001;5:379–389. doi: 10.1053/eujp.2001.0259. [DOI] [PubMed] [Google Scholar]
- Miller LR, Cano A. Co-morbid chronic pain and depression: who is at risk? J Pain. 2009;10:619–627. doi: 10.1016/j.jpain.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Mitra R, Sundlass K, Parker KJ, et al. Social stress-related behavior affects hippocampal cell proliferation in mice. Physiol Behav. 2006;89:123–127. doi: 10.1016/j.physbeh.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Monassi CR, Bandler R, Keay KA. A subpopulation of rats show social and sleep-waking changes typical of chronic neuropathic pain following peripheral nerve injury. Eur J Neurosci. 2003;17:1907–1920. doi: 10.1046/j.1460-9568.2003.02627.x. [DOI] [PubMed] [Google Scholar]
- Mor D, Bembrick AL, Austin PJ, et al. Anatomically specific patterns of glial activation in the periaqueductal gray of the sub-population of rats showing pain and disability following chronic constriction injury of the sciatic nerve. Neuroscience. 2010;166:1167–1184. doi: 10.1016/j.neuroscience.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Mor D, Bembrick AL, Austin PJ, et al. Evidence for cellular injury in the midbrain of rats following chronic constriction injury of the sciatic nerve. J Chem Neuroanat. 2011;41:158–169. doi: 10.1016/j.jchemneu.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, et al. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutso AA, Radzicki D, Baliki MN, et al. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, et al. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th edn. Boston, MA: Academic Press; 2005. [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, et al. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci. 2006;23:2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, et al. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Zhang WN, Jongen-Rêlo AL, et al. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within-subject, within-task comparison of reference and working spatial memory. Eur J Neurosci. 2004;19:705–712. doi: 10.1111/j.0953-816x.2004.03170.x. [DOI] [PubMed] [Google Scholar]
- Procacci P, Zoppi M, Maresca M. Experimental pain in man. Pain. 1979;6:123–140. doi: 10.1016/0304-3959(79)90120-9. [DOI] [PubMed] [Google Scholar]
- Ren WJ, Liu Y, Zhou LJ, et al. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology. 2011;36:979–992. doi: 10.1038/npp.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réthelyi JM, Berghammer R, Ittzés A, et al. Comorbidity of pain problems and depressive symptoms in young women: results from a cross-sectional survey among women aged 15–24 in Hungary. Eur J Pain. 2004;8:63–69. doi: 10.1016/S1090-3801(03)00074-0. [DOI] [PubMed] [Google Scholar]
- Reuler JB, Girard DE, Nardone DA. The chronic pain syndrome: misconceptions and management. Ann Int Med. 1980;93:588–596. doi: 10.7326/0003-4819-93-4-588. [DOI] [PubMed] [Google Scholar]
- Roeska K, Doods H, Arndt K, et al. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain. 2008;139:349–357. doi: 10.1016/j.pain.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Roeska K, Ceci A, Treede RD, et al. Effect of high trait anxiety on mechanical hypersensitivity in male rats. Neurosci Lett. 2009;464:160–164. doi: 10.1016/j.neulet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Roman V, Van der Borght K, Leemburg SA, et al. Sleep restriction by forced activity reduces hippocampal cell proliferation. Brain Res. 2005;1065:53–59. doi: 10.1016/j.brainres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Rusch BD, Abercrombie HC, Oakes TR, et al. Hippocampal morphometry in depressed patients and control subjects: relations to anxiety symptoms. Biol Psychiatry. 2001;50:960–964. doi: 10.1016/s0006-3223(01)01248-3. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, hippocampal damage and the glutamatergic synapse. Prog Brain Res. 1990;86:13–23. doi: 10.1016/s0079-6123(08)63163-5. [DOI] [PubMed] [Google Scholar]
- Saylam C, Uçerler H, Kiti O, et al. Reduced hippocampal volume in drug-free depressed patients. Surg Radiol Anat. 2006;28:82–87. doi: 10.1007/s00276-005-0050-3. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Laferriere AL, Millecamps M, et al. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage. 2009;47:1007–1014. doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;16:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sousa N, Almeida OF. Corticosteroids: sculptors of the hippocampal formation. Rev Neurosci. 1998;13:59–84. doi: 10.1515/revneuro.2002.13.1.59. [DOI] [PubMed] [Google Scholar]
- Sternbach RA. Psychological aspects of pain and the selection of patients. Clin Neurosurg. 1974;21:323–333. doi: 10.1093/neurosurgery/21.cn_suppl_1.323. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Amata M, Sakaue G, et al. Experimental neuropathy in mice is associated with delayed behavioral changes related to anxiety and depression. Anesth Analg. 2007;104:1570–1577. doi: 10.1213/01.ane.0000261514.19946.66. [DOI] [PubMed] [Google Scholar]
- Terada M, Kuzumaki N, Hareyama N, et al. Suppression of enriched environment-induced neurogenesis in a rodent model of neuropathic pain. Neurosci Lett. 2008;440:314–318. doi: 10.1016/j.neulet.2008.05.078. [DOI] [PubMed] [Google Scholar]
- Timmermans G, Sternbach RA. Factors of human chronic pain: an analysis of personality and pain reaction variables. Science. 1974;184:806–808. doi: 10.1126/science.184.4138.806. [DOI] [PubMed] [Google Scholar]
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9:883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66:1254–1269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- Turk DC, Audette J, Levy RM, et al. Assessment and treatment of psychosocial comorbidities in patients with neuropathic pain. Mayo Clin Proc. 2010;85:S42–S50. doi: 10.4065/mcp.2009.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA, Romano JM. Psychological and psychosocial evaluation. In: Bonica JJ, editor. The Management of Pain. Philadelphia: Lea and Febiger; 1953. pp. 229–341. [Google Scholar]
- Vakili K, Pillay SS, Lafer B, et al. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Wang J, Goffer Y, Xu D, et al. A single sub-anesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology. 2011;115:812–821. doi: 10.1097/ALN.0b013e31822f16ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18:729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Woon F, Hedges DW. Gender does not moderate hippocampal volume deficits in adults with posttraumatic stress disorder: a meta-analysis. Hippocampus. 2011;21:243–252. doi: 10.1002/hipo.20746. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Taylor WD, Styner M, et al. Hippocampus shape analysis and late-life depression. PLoS ONE. 2008;3:1837. doi: 10.1371/journal.pone.0001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1993;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zimmerman M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Pan JW, Hetherington HP, et al. Hippocampal correlates of pain in healthy elderly adults: a pilot study. Neurology. 2009;73:1567–1570. doi: 10.1212/WNL.0b013e3181c0d454. [DOI] [PMC free article] [PubMed] [Google Scholar]