Abstract
Thermotolerant Campylobacter spp. frequently cause bacterial gastroenteritis in humans commonly infected through the consumption of undercooked poultry meat. We examined Campylobacter jejuni heat-stress responses in vitro after exposure to 48°C and 55°C. The in vivo modulation of its pathogenicity was also investigated using BALB/c mice intravenously infected with stressed C. jejuni. Regardless of the bacterial growth phase, the culturability and viability of C. jejuni in vitro was reduced after exposure to 55°C. This correlated with the altered protein profile and decreased virulence properties observed in vivo. Heat stress at 48°C elicited the transition to more resistant bacterial forms, independent of morphological changes or the appearance of shorter spiral and coccoid cells. This treatment did not cause marked changes in bacterial virulence properties in vivo. These results indicated that the characteristics and pathogenicity of C. jejuni in response to heat stress are temperature dependent. Further studies on the responses of C. jejuni to stresses used during food processing, as well as the modulation of its virulence, are important for a better understanding of its contamination and infective cycle, and will, thus, contribute to improved safety in the food production chain.
Keywords: Campylobacter jejuni, heat-stress response, virulence, food safety
Campylobacter spp. have become one of the most frequent agents of common zoonotic food-borne diseases (6). The handling and consumption of contaminated poultry meat products have been identified as the most common sources of human Campylobacter spp. infection; however, raw vegetables and environmental water sources are also potential reservoirs (11).
Although Campylobacter spp. are generally regarded as being sensitive to the different environmental conditions exterior to animals or humans, they appear to be more resistant to stress than previously thought (12, 18). Thus, effective approaches to reduce human illnesses associated with these food-borne pathogens are needed in the food production and supply chains. The evolved mechanisms that allow Campylobacter spp. to deal with environmental stress conditions, and even develop resistance or potentially cross-protection to different stresses, need to be more fully elucidated. In addition to complex interaction, any single stress response may be regulated at different cellular levels; therefore, the relationships among the ecology, survival characteristics, and virulence properties of Campylobacter spp. need to be examined in more detail (7, 22, 24, 36).
Heat-shock regulation in C. jejuni has been shown to play an essential role in colonization of the intestinal tract (20). Campylobacter spp. are exposed to different thermal treatments during food processing, distribution, and storage that should be sufficient to either inhibit or inactivate them (34). To avoid the possible heat resistance of Campylobacter spp. in food, which may be harmful to public health, it is important to clarify changes in their cellular physiology as well as the activation of other global regulators as a consequence of inappropriate thermal treatments.
A proteomic approach can describe dynamic protein composition variations in a cell that constantly adjusts to meet the challenges of environmental changes, and can also be used to investigate cell responses to various stress conditions relevant to food processing and food safety (29, 38). Microbial adaptation during stress challenges is crucial not just for pathogen survival outside of the host, but also during host–pathogen interactions, and, thus, for bacterial pathogenicity (18, 19). A mouse model has proved to be an invaluable tool for understanding the pathogenesis of C. jejuni as well as the factors involved in host defense mechanisms (19, 37). Intravenous challenges in mice may be used to compare the virulence of bacterial strains exposed to various stress conditions. Differences in dissemination capabilities and tissue invasion can indicate changes in bacterial virulence potential.
However, the physiology of C. jejuni exposed to heat and after heat-shock regulation is poorly understood. The synthesis of a group of highly conserved heat-shock proteins (Hsps) is induced by heat stress (23). These proteins are known to have various roles in cell physiology, ribosome stability, stringent responses, temperature sensing, and the control of ribosomal function (1, 5, 16, 39). Dasti et al. (4) confirmed the role of Hsps in the thermotolerance and growth of C. jejuni in the chicken intestine. C. jejuni can use more than one strategy to simultaneously regulate sets of heat-shock-expressed genes and respond to temperature changes or other stresses, thereby enhancing its survival in the environment (4, 35). Incubation outside the normal temperature range of growth is one of the environmental inducers of the viable but non-culturable (VBNC) form (26, 27).
In the present study, we examined the stress responses of C. jejuni from different growth phases after heat treatments at 48°C and 55°C. These temperatures were chosen to mimic the temperatures applied in poultry processing plants: 48°C for locations close to the hot water in the defeathering environment and 55°C for scalding (15). We also explored the ability of this bacterium to acquire heat resistance after being previously exposed to another stress i.e. starvation. Changes in bacterial viability, culturability, morphology, and its protein profile were investigated. We also examined the influence of the stress response of C. jejuni on its virulence potential using a previously established experimental model of systemic murine campylobacteriosis (37).
Materials and Methods
Bacterial growth and stress conditions
C. jejuni K49/4 was isolated from poultry meat, identified phenotypically, and subcultured prior to the experimental test conditions. Microaerobic growth (5% O2, 10% CO2, 85% N2) in Preston broth (Oxoid, Hampshire, UK) containing 5% (v/v) defibrinated horse blood (Oxoid) at 42°C for 9 h induced the entry of these cultures into the exponential growth phase, and for 15 h, into the stationary growth phase (1). To produce heat-shock stress, the bacterial cells harvested from each growth phase were exposed to temperature shifts from 42°C to 48±1°C or 55±1°C using thermoblock (Eppendorf Thermomixer comfort, Eppendorf AG, Hamburg, Germany) for 3, 10, 20, or 30 min, and these cells were then shortly cooled on ice to achieve 42°C before being analyzed. Untreated C. jejuni from both growth-phase cultures were used as controls and kept at 42°C throughout the experiment. To evaluate resistance to the heat treatments, 5 μg mL−1 chloramphenicol was added as an inhibitor of protein synthesis before 5 h of starvation and the subsequent heat stress. C. jejuni cells were harvested by centrifugation (12,000×g for 5 min at 4°C), washed, resuspended in Ringer’s solution supplemented with 5 mM KH2PO4 (Kemika, Zagreb, Croatia), and incubated microaerobically for 5 h at 42°C for pre-starvation.
Culturability, viability, and morphology assays
Culturability was determined as colony-forming units per milliliter (CFU mL−1), and viability was determined using the LIVE/DEAD BacLight system (L-7012; Molecular Probes, Eugene, OR, USA) under the Eclipse TE300 microscope (Nikon, Tokyo, Japan), as previously described (1, 18). Viability was given as the percentage of viable cells in relation to the total number of cells obtained, as determined on 20 randomly chosen microscopic fields per filter for each evaluated sample. The numbers of coccoid and spiral cells were also determined. All experiments were independently repeated three times. The morphology of C. jejuni was assessed using transmission electron microscopy (Philips CM 100, Philips Electronics N.V. Eindhoven, the Netherlands). Cells were prepared as reported previously (17).
Protein extraction and two-dimensional gel electrophoresis
C. jejuni cells from the exponential growth phase exposed to 48°C or 55°C for 20 min were investigated in the two-dimensional (2-D) gel electrophoresis analysis. After the treatments, these cells were harvested by centrifugation (12,000×g for 5 min at 4°C), washed with Ringer’s solution, and resuspended in extraction solution (40 mM Tris-HCl, pH 7.5, 4% [w/v] 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate [CHAPS], 65 mM dithiothreitol, and protease inhibitor cocktail (1 tablet 10 mL−1 buffer) (Complete, Mini; Roche). To extract the protein, the cells were disrupted using three cycles of vortexing with zirconia/silica beads (BioSpec Products; diameter, 0.5 mm) for 1 min each, with 1-min intervals of cooling on ice. The homogenate was centrifuged (20,000×g for 20 min at 4°C), and the protein concentration in the supernatant (extract) was measured by the method of Bradford (2). The extract was then purified using 2-D Clean Up kits (GE Healthcare, Sweden).
Two-dimensional (2-D) gel electrophoresis was performed according to Görg (9), with minor modifications. The protein samples (100 μg) were resuspended in rehydration solution (9 M urea, 2% [w/v] CHAPS, 2% [v/v] immobilized pH gradient [IPG] buffer, 18 mM dithiothreitol, 0.002% [w/v] bromophenol blue), and loaded onto 13-cm IPG strips (4–7, GE Healthcare). After rehydration, isoelectric focusing was carried out using Multiphor II (GE Healthcare). The proteins were focused at: 300 V (gradient over 1 min), 3500 V (gradient over 1.5 h), and 3500 V (fixed for 4.33 h). After isoelectric focusing, the IPG strips were equilibrated for 15 min in SDS equilibration buffer (50 mM Tris-HCl, pH 8.8, 6 M urea, 30% [v/v] glycerol, 2% [w/v] SDS, 0.0002% [w/v] bromophenol blue) containing 1% dithiothreitol, and for an additional 15 min in SDS equilibration buffer containing 4.8% iodoacetamide. The second dimension (SDS–PAGE) was performed on 12% SDS-polyacrylamide gels on an SE 600 vertical discontinuous electrophoresis system (Hoeffer Scientific Instruments). The 2-D gels were stained using fluorescent Sypro Ruby (Invitrogen), and then recorded using an Artixscan 1800f scanner (Microtek). The protein profiles between control and treated cells were then compared using the 2-D Dymension software, version 2.02 (Syngene). 2-D electrophoresis was run at least twice for each sample.
In vivo experiments
Eight to twelve week old male BALB/c (H-2d) mice were used in all of the in vivo experiments. These mice were obtained from the Central Animal Facility of the School of Medicine, University of Rijeka, Croatia, and were given standard laboratory rodent food (Mucedola, Milan, Italy) and water ad libitum. Experiments were conducted in accordance with the guidelines of the International Guiding Principles for Biomedical Research Involving Animals (25). The Ethical Committee at the School of Medicine, University of Rijeka approved all of the animal experiments described here. Mice were infected intravenously via the lateral tail vein with a single dose (200 μL) of 0.5 to 1.0×109 CFU mL−1 unstressed (control) or heat-stressed C. jejuni cells, as determined by the turbidity of the bacterial suspension and confirmed retrospectively by plating the inoculum on blood agar and incubation microaerobically for 48 h at 42°C. At one, three, and eight d post-infection, the mice were sacrificed and their livers and spleens were removed aseptically and dissected from the surrounding tissue, with the C. jejuni CFU in the livers and spleens determined as previously described (19, 37). In bacterial organ burden experiments, two sets of mice were infected with each of the heat-treated or control C. jejuni, with at least three mice per group. These experiments were repeated three times, and the data from all of the replicate experiments were pooled and presented as means±standard deviations.
Statistical analysis
Differences in the bacterial counts among all of the experimental groups were calculated using the Kruskal–Wallis test, while the Mann–Whitney test was used to define differences between pairs of groups. All of the statistical values were considered significant at a p level <0.05. Statistical analyses were performed using SPSS 15.0 for Windows (Statsoft, Tulsa, OK, USA).
Results
Physiology and morphology of C. jejuni under heat stress
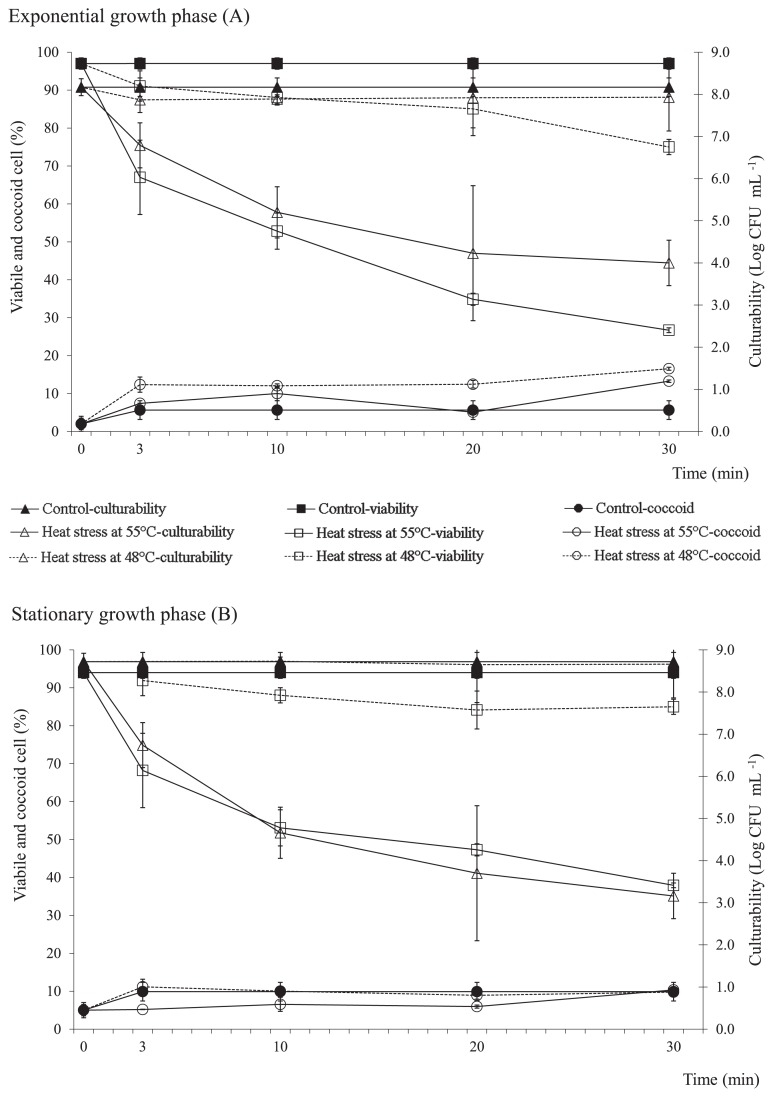
The viability of exponential growth phase cells exposed to 48°C was reduced by approximately 20% after 30 min, with the coccoid cell number increasing by 10% (Fig. 1A). This pattern was not observed in the stationary growth phase cultures, in which no significant differences were observed in culturability or coccoid cell numbers prior to and after the heat stress (Fig. 1B). The decline in viability in the stationary growth phase cultures was less pronounced than that in the exponential growth phase cultures (Fig. 1). Regardless of the growth phase, the heat treatment at 55°C, which lasted for over 3 min, resulted in a progressive decline in viability and culturability of the bacterial cells. Cell viability had already decreased by 50% after 10 min under the 55°C stress challenge, while culturability declined by at least 3.0 log units (Fig. 1). The proportion of coccoid cells was 20% (Fig. 1).
Fig. 1.
Viability, coccoid cell formation, and culturability in exponential (A) and stationary (B) growth phase C. jejuni exposed to 48°C or 55°C, as indicated. Data are expressed as means±standard deviations.
Transmission electron microscopy was used to examine morphological changes in C. jejuni and representative electron microscopy images were presented after evaluating at least 10% of randomly chosen copper grids for each evaluated sample (Fig. 2). Morphological changes were more visible among cells exposed to 55°C, which significantly changed their morphology from that in the control culture (Fig. 2). The temperature shift provoked morphological changes in C. jejuni from spiral to predominant shorter spiral and coccoid forms, indicating a possible transformation to the VBNC form.
Fig. 2.
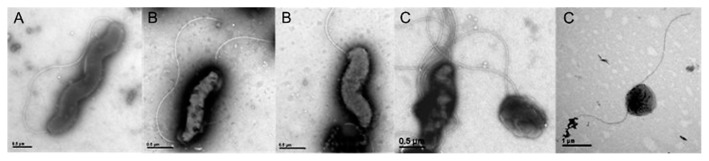
Representative electron microscopy showing control C. jejuni cells (A) and the effects of heat stress at 48°C (B) and 55°C (C) on bacterial morphology. Scale bars: as indicated.
Protein profile of C. jejuni under heat stress
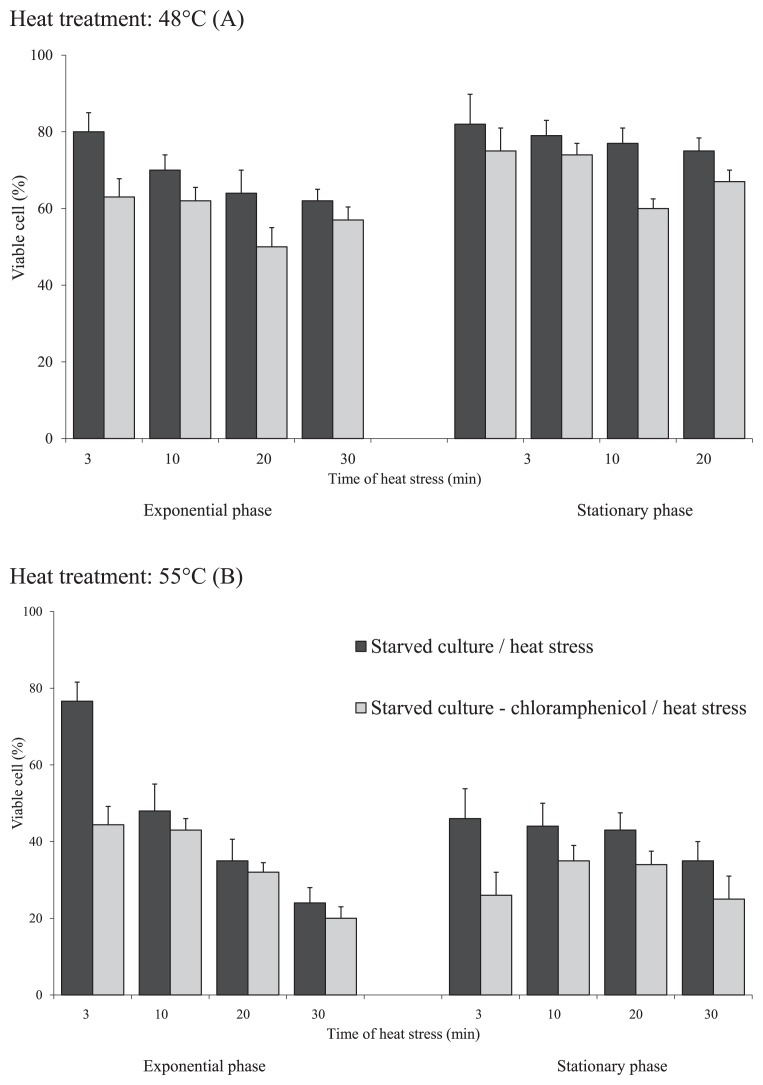
To determine the heat stress resistance of C. jejuni cells from both growth phases, we exposed the cultures to another stress, starvation, prior to the heat treatment. Acquired resistance to heat stress was more prominent in the exponential growth phase culture at 48°C (Fig. 3). When chloramphenicol was added before the exposure to 5 h starvation, the reduction observed in cell viability after heat stress at either 48°C or 55°C was more pronounced in treated cells than in non-treated cells, in which protein synthesis was maintained. We assumed that starvation induced protein synthesis in C. jejuni cells from the exponential growth phase, and may be important for the acquisition of heat resistance. Therefore, we further investigated the protein profile and virulence of C. jejuni under heat stress using cultures from the exponential growth phase.
Fig. 3.
Resistance to heat stress, evaluated according to the viability of exponential and stationary growth phase C. jejuni without or with chloramphenicol added for 5 h before pre-starvation and subsequent exposure to the heat treatment at 48°C (A) or 55°C (B), as indicated. Data are expressed as means±standard deviations.
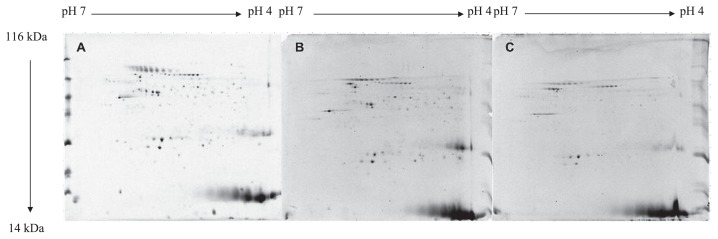
We compared the protein profiles of the untreated (control) and heat-treated (48°C, 55°C, for 20 min) cultures. The reduced numbers of proteins detected in the gels corresponded to decreases in both viability and culturability. Following the heat treatment at 48°C for 20 min, the expression of 36 proteins was lower and that of 4 proteins was higher than that of the control cells, while 3 proteins were absent. The exposure of C. jejuni to 55°C generally reduced the number of proteins detected, with 29 down-regulated signals being observed. The expression of only one protein was higher than that of the corresponding control (Fig. 4).
Fig. 4.
Representative protein profiles of the C. jejuni under the exponential growth phase, for the control cells (A) and the cells heat treated at 48°C (B) and 55°C (C), for 20 min.
Virulence of C. jejuni after heat stress
To define the effects of the stress response to heat treatment on the virulence of C. jejuni, we assessed systemic bacterial infections in a murine model. Bacterial numbers were assessed in the livers and spleens of mice 1, 3, and 8 d after an intravenous infection with untreated (control) or heat-treated C. jejuni (48°C for 3 or 20 min, 55°C for 3 min).
As expected, control bacteria efficiently established systemic infections with a lower bacterial burden being detected in the mouse spleen than in the liver for the whole experimental period (Fig. 5). Heat-stressed C. jejuni also quickly disseminated to the spleen and liver, except those exposed to 55°C for 20 min, which were not capable of inducing a systemic infection.
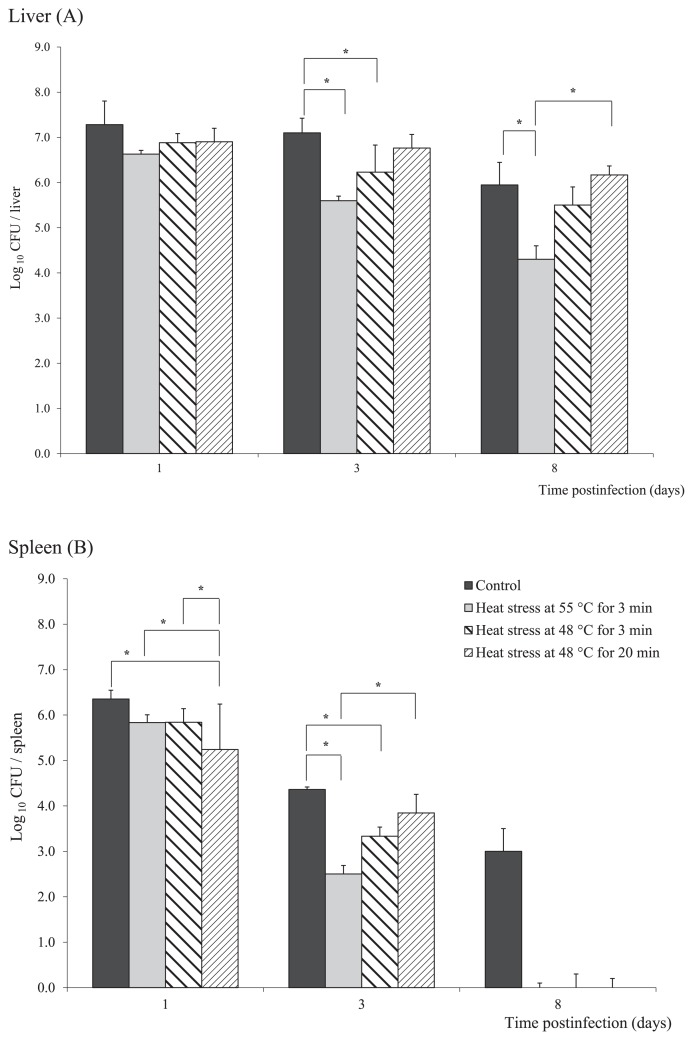
Fig. 5.
C. jejuni cell numbers in the liver (A) and spleen (B) of BALB/c mice intravenously infected with exponential growth phase C. jejuni cells, as untreated control cells, and cells exposed to heat treatments at 55°C for 3 min or 48°C for 3 min and 20 min, as indicated. Data are expressed as means±standard deviation of C. jejuni log10 CFU organ−1. (*p≤0.05).
The number of heat-stressed C. jejuni recovered from the livers and spleens was reduced 1 d post-infection (Fig. 5). The number of bacteria isolated from the livers 3 and 8 d post-infection was markedly reduced when bacteria treated with 55°C were used to infect the mice (a reduction of 2 log units after 8 d) (Fig. 5A). The same stresses caused marked decreases in bacterial numbers within the spleen, particularly 8 d post-infection, when heat-stressed C. jejuni cells could not be cultivated from the spleen regardless of the stress applied (Fig. 5B).
Discussion
Despite its particular growth requirements, C. jejuni has developed mechanisms for survival in diverse environments, in which it is subjected to various stresses, including high temperatures. The results of the present study clearly demonstrated that exposure to 48°C and to 55°C for a short time (3 min) changed the morphology and protein profile of C. jejuni, but did not affect its infectivity. However, a longer exposure to 55°C (20 min) completely abolished the virulence of C. jejuni, and generally reduced the number of proteins detected. Such responses and the adaptation of this foodborne pathogen to heat stress applied during food processing may constitute a microbial hazard.
The exposure of C. jejuni to 48°C as well as 55°C induced morphological changes and affected bacterial survival. The higher temperature resulted in marked changes in the morphology of C. jejuni cells, and reduced their culturability and viability in a manner that was dependent on the exposure time. C. jejuni has been shown to enter the VBNC state in response to starvation and oxidative stress, and, to a lesser extent, to temperature shifts (3, 14, 16–18, 27). When bacteria were exposed to 48°C, a moderate decrease was observed in C. jejuni CFU, which is consistent with the findings of Isohanni et al. (13). The altered cell morphology and decline in culturability support the possibility of the transformation of C. jejuni into the VBNC form as a consequence of a sublethal heat stress response. In the present study, the VBNC cells were mostly spiral shaped. Thus, we assumed that some VBNC populations may have consisted of both coccoid and spiral VBNC cells (8). It remains unknown whether the VBNC cells induced by the heat treatment maintained their cellular structure and biology, and also whether they retained their virulence with the ability to persist in the environment.
The mechanisms underlying stress regulation need to be elucidated in more detail in order to deepen our understanding of bacterial ecologies, as well as their survival in foods and human hosts. These risks become more apparent as we become more aware of non-culturable cells in potentially dormant forms. Based on the ability of a sub-lethal pre-stress to promote Campylobacter resistance, we confirmed the development of acquired resistance by C. jejuni cells from the exponential growth phase exposed to 48°C after 5 h starvation.
To survive in a permanently changing world, microorganisms must evolve mechanisms that adjust their biochemistry in response to different signals, and, thus, respond with appropriate alterations in their protein activities that lead to metabolic modifications. Considering the important relationship between physiological cell functions and the proteome of a cell (28), we compared the protein profiles of control and heat-stressed C. jejuni cells. The temperature shift to 55°C significantly reduced the number of proteins detected in the gels, corresponding to a reduction in both viability and culturability. Thus, damaged proteins are removed, while de-novo synthesis is not possible due to disrupted energy metabolism. As reported by Lazaro et al. (21), the small number of proteins detected in the gel in the pH range 4–7 could be attributed to a modification in the isoelectric points of the main protein components, which may result in protein alterations. Down-regulated expression was also detected in C. jejuni cells exposed to 48°C, whereas the expression of four proteins was significantly higher than that in the control cells. The latter could be related to the ability of these cells to cope with the sub-lethal heat stress, also resulting in higher viability and culturability (10, 30).
Information on the relationship between stress responses and virulence is limited, especially in vivo (14, 27). We previously demonstrated in cell-culture models that heat stress at 55°C strongly impaired bacterial adhesion and invasion in enterocytes (Caco-2, PSI) as well as in macrophages (J774) (31–33). To further define this in vitro modulation of the pathogenicity characteristics of C. jejuni in response to heat stress, we used an earlier established animal model to provide information on its virulence properties in vivo.
The exposure of C. jejuni to 48°C or 55°C for 20 min completely abolished its potential to cause systemic infections in a mouse. Despite the morphological changes induced, C. jejuni exposed to 55°C for 3 min remained capable of systemic spread; however, the number of bacteria recovered from the livers and spleens was lower than that of the control. These results confirmed that a 3-min exposure to 55°C did not affect the infectivity of C. jejuni, but reduced its virulence, which was consistent with our previous findings on starved Campylobacter spp. (18, 19).
In contrast, we previously showed that exposure to oxidative stress did not influence the infectivity or virulence of C. jejuni because no significant changes were observed in the bacterial load in the mouse liver (19). In the present study, we demonstrated that neither the 3-min nor the 20-min exposure of C. jejuni to 48°C affected the course of infection. The number of bacteria recovered from the liver was not significantly reduced 8 d post-infection. The different clearance pattern in the spleen and shorter duration of infection may be explained by different immune mechanisms controlling the infection. The short time exposure to 55°C, as well as to temperatures below this level, appeared to be insufficient to reduce the infectious potential of C. jejuni.
New findings relating to the modulation of C. jejuni virulence in response to environmental stress factors during food processing are important for providing a better understanding of contamination by and the infective cycle of C. jejuni. Elucidating the the different stress response mechanisms in more detail at the cellular level (proteins, metabolism, physiology, virulence) is needed to facilitate the development of appropriate intervention strategies to improve the safety of food supply and reduce the incidence of C. jejuni-associated diseases. Regardless of the growth phase, severe heat stress (at 55°C for 20 min) largely eliminated C. jejuni, and the adaptive stress response in the fraction of the population that survived did not provide the regulated protein expression that was crucial for their virulence properties in vivo. In contrast, the milder sub-lethal heat treatment (at 48°C) allowed for the survival of C. jejuni 48°C and its metabolic activity was maintained. The stress responses and cell adaptation induced were visible at the cellular level in terms of their resistance to heat stress as well as metabolic modifications in terms of protein expression. Using the in vivo mouse model of campylobacteriosis, we detected differences within the host, concerning the influence of the heat-stress response mechanisms on the reduction of virulence properties, though dependent of the observed organ.
Acknowledgements
This study was supported by the Ministry of Higher Education, Science and Technology of the Republic of Slovenia, by the Z1-2190 post-doctoral project of A.K., and through research projects financed by the Croatian Ministry of Science, Education and Sports (062-0621273-1235, 062-0621273-0949).
References
- 1.Bæk KT, Vegge CS, Skórko-Glonek J, Brøndsted L. Different contributions of HtrA protease and chaperone activities to Campylobacter jejuni stress tolerance and physiology. Appl Environ Microbiol. 2011;77:57–66. doi: 10.1128/AEM.01603-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 3.Cappelier JM, Rossero A, Federighi M. Demonstration of a protein synthesis in starved Campylobacter jejuni cells. Int J Food Microbiol. 2000;55:63–67. doi: 10.1016/s0168-1605(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 4.Dasti JI, Tareen AM, Lugert R, Zautner AE, Groß U. Campylobacter jejuni: A brief overview on pathogenicity—associated factors and disease-mediating mechanisms. Int J Med Microbiol. 2010;300:205–211. doi: 10.1016/j.ijmm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 5.De Angelis M, Di Cagno R, Huet C, Cercchio C, Fox PF, Gobbetti M. Heat shock response in Lactobacillus plantarum. Appl Environ Microbiol. 2004;70:1136–1346. doi: 10.1128/AEM.70.3.1336-1346.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Food Safety Authority. The Community summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in the European Union in 2008. EFSA J. 2010;1496:1–288. [Google Scholar]
- 7.European Food Safety Authority. Scientific opinion on Campylobacter in broiler meat production: control options and performance: objectives and/or targets at different stages of the food chain. EFSA J. 2011;9:2105–2246. [Google Scholar]
- 8.Federighi M, Tholozan JL, Cappelier JM, Tissier JP, Jouve JL. Evidence of non-coccoid viable but non-culturable Campylobacter jejuni cells in microcosm water by direct viable count, CTC-DAPI double staining, and scanning electron microscopy. Food Microbiol. 1998;15:539–550. [Google Scholar]
- 9.Görg A. Two-dimensional electrophoresis. Nature. 1991;349:545–546. [Google Scholar]
- 10.Hecker M, Völker U. General stress response of Bacillus subtilis and other bacteria. Adv Microb Physiol. 2001;44:35–91. doi: 10.1016/s0065-2911(01)44011-2. [DOI] [PubMed] [Google Scholar]
- 11.Horrocks SM, Anderson RC, Nisbet DJ, Ricke SC. Incidence and ecology of Campylobacter jejuni and coli in animals. Anaerobe. 2009;15:18–25. doi: 10.1016/j.anaerobe.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Humphrey T, O’Brien S, Madsen M. Campylobacters as zoonotic pathogens: a food production perspective. Int J Food Microbiol. 2007;117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Isohanni P, Huehn S, Aho T, Alter T, Lyhs U. Heat stress adaptation induces cross-protection against lethal acid stress conditions in Arcobacter butzleri but not in Campylobacter jejuni. Food Microbiol. 2013;34:431–435. doi: 10.1016/j.fm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Jackson DN, Davis B, Tirado SM, Duggal M, van Frankenhuyzen JK, Deaville D, Wijesinghe MA, Tessaro M, Trevors JT. Survival mechanisms and culturability of Campylobacter jejuni under stress conditions. Antonie van Leeuwenhoek. 2009;96:377–394. doi: 10.1007/s10482-009-9378-8. [DOI] [PubMed] [Google Scholar]
- 15.Keener KM, Bashor MP, Curtis PA, Sheldon BW, Kathariou S. Comprehensive review of Campylobacter and poultry processing. Compr Rev Food Sci Food Saf. 2004;3:105–116. doi: 10.1111/j.1541-4337.2004.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 16.Klančnik A, Botteldoorn N, Herman L, Smole Možina S. Survival and stress induced expression of groEL and rpoD of Campylobacter jejuni from different growth phases. Int J Food Microbiol. 2006;112:200–207. doi: 10.1016/j.ijfoodmicro.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Klančnik A, Zorman T, Smole Možina S. The effect of low temperature, starvation and oxidative stress on physiology of Campylobacter jejuni cells. Croatica Chemica Acta. 2008;81:41–46. [Google Scholar]
- 18.Klančnik A, Guzej B, Jamnik P, Vučković D, Abram M, Smole Možina S. Stress response and pathogenic potential of Campylobacter jejuni cells exposed to starvation. Res Microbiol. 2009;160:345–352. doi: 10.1016/j.resmic.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Klančnik A, Vučković D, Plankl M, Abram M, Smole Možina S. In vivo modulation of Campylobacter jejuni virulence in response to environmental stress. Foodborne Pathog Dis. 2013;10:566–572. doi: 10.1089/fpd.2012.1298. [DOI] [PubMed] [Google Scholar]
- 20.Konkel ME, Kim BJ, Klena JD, Young CR, Ziprin R. Characterization of the thermal stress response of Campylobacter jejuni. Infect Immun. 1998;66:3666–3672. doi: 10.1128/iai.66.8.3666-3672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazaro B, Carcamo J, Audicana A, Perales I, Fernandez Astorga A. Viability and DNA maintenance in nonculturable spiral Campylobacter jejuni cells after long-term exposure to low temperatures. Appl Environ Microbiol. 1999;65:4677–4681. doi: 10.1128/aem.65.10.4677-4681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin AE, Krastel K, Hobb RI, Thompson SQ, Cvitkovich DG, Gaynor EC. Atypical roles for Campylobacter jejuni amino acid ATP binding cassette transporter components PaqP and PaqQ in bacterial stress tolerance and pathogen-host cell dynamics. Infect Immun. 2009;77:4912–4924. doi: 10.1128/IAI.00571-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindquist S. The heat-shock response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 24.Moore JE, Madden RH. The effect of thermal stress on Campylobacter coli. J Appl Microbiol. 2000;89:892–899. doi: 10.1046/j.1365-2672.2000.01204.x. [DOI] [PubMed] [Google Scholar]
- 25.NCR, National Research Council (US) Institute for Laboratory Animal Research. Proceedings of the November 2003 International Workshop. Washington (DC): National Academies Press (US); 2004. The development of science-based guidelines for laboratory animal care. [PubMed] [Google Scholar]
- 26.Nowakowska J, Oliver JD. Resistance to environmental stresses by Vibrio vulnificus in the viable but nonculturable state. FEMS Microbiol Ecol. 2013;84:213–222. doi: 10.1111/1574-6941.12052. [DOI] [PubMed] [Google Scholar]
- 27.Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev. 2009;34:415–425. doi: 10.1111/j.1574-6976.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- 28.Pandey A, Mann M. Proteomics to study genes and genomes. Nature. 2000;405:837–846. doi: 10.1038/35015709. [DOI] [PubMed] [Google Scholar]
- 29.Renzone G, D’Ambrosio C, Arena S, Rullo R, Ledda L, Ferrara L, Scaloni A. Differential proteomic analysis in the study of prokaryotes stress resistance. Ann. Ist. Super Sanità. 2005;41:459–468. [PubMed] [Google Scholar]
- 30.Rosen R, Ron EZ. Proteome analysis in the study of the bacterial heat-shock response. Mass Spectrom Rev. 2002;21:244–265. doi: 10.1002/mas.10031. [DOI] [PubMed] [Google Scholar]
- 31.RubešaMihaljević R, Šikić M, Klančnik A, Brumini G, Smole Možina S, Abram M. Environmental stress factors affecting survival and virulence of Campylobacter jejuni. Microb Pathog. 2007;43:120–125. doi: 10.1016/j.micpath.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 32.ŠikićPogačar M, Rubeša Mihaljević R, Klančnik A, Brumini G, Abram M, Smole Možina S. Survival of stress exposed Campylobacter jejuni in the murine macrophage J774 cell line. Int J Food Microbiol. 2009;129:68–73. doi: 10.1016/j.ijfoodmicro.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 33.ŠikićPogačar M, Klančnik A, Smole Možina S, Cencič A. Attachment, invasion and translocation of Campylobacter jejuni in pig small-intestinal epithelial cells. Foodborne Pathog Dis. 2010;5:589–595. doi: 10.1089/fpd.2009.0301. [DOI] [PubMed] [Google Scholar]
- 34.Silva J, Leite D, Fernandes M, Mena C, Gibbs PA, Teixeira P. Campylobacter spp. as a foodborne pathogen: a review. Front Microbiol. 2011;2:200. doi: 10.3389/fmicb.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stintzi A. Gene expression profile of Campylobacter jejuni in response to growth temperature variation. J Bacteriol. 2003;185:2009–2016. doi: 10.1128/JB.185.6.2009-2016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Deun K, Haesebrouck F, Heyndrickx M, et al. Virulence properties of Campylobacter jejuni isolates of poultry and human origin. J Med Microbiol. 2007;56:1284–1289. doi: 10.1099/jmm.0.47342-0. [DOI] [PubMed] [Google Scholar]
- 37.Vučković D, Abram M, Dorić M. Primary Campylobacter jejuni infection in different mice strains. Microb Pathog. 1998;24:263–268. doi: 10.1006/mpat.1997.0194. [DOI] [PubMed] [Google Scholar]
- 38.Wells JM, Bennik MH. Genomics of food-borne bacterial pathogens. Nutr Res Rev. 2003;16:21–35. doi: 10.1079/NRR200358. [DOI] [PubMed] [Google Scholar]
- 39.Wu YL, Lee LH, Rollins DM, Ching WM. Heat shock and alkaline pH-induced proteins of Campylobacter jejuni: Characterization and Immunological properties. Infect Immun. 1994;62:4256–4260. doi: 10.1128/iai.62.10.4256-4260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]