Abstract
Concern regarding household biofilms has grown due to their widespread existence and potential to threaten human health by serving as pathogen reservoirs. Previous studies identified Methylobacterium as one of the dominant genera found in household biofilms. In the present study, we examined the mechanisms underlying biofilm formation by using the bacterial consortium found in household pink slime. A clone library analysis revealed that Methylobacterium was the predominant genus in household pink slime. In addition, 16 out of 21 pink-pigmented bacterial isolates were assigned to the genus Methylobacterium. Although all of the Methylobacterium isolates formed low-level biofilms, the amount of the biofilms formed by Methylobacterium sp. P-1M and P-18S was significantly increased by co-culturing with other Methylobacterium strains that belonged to a specific phylogenetic group. The single-species biofilm was easily washed from the glass surface, whereas the dual-species biofilm strongly adhered after washing. A confocal laser scanning microscopy analysis showed that the dual-species biofilms were significantly thicker and tighter than the single-species biofilms.
Keywords: Methylobacterium, household biofilm, pink biofilm, intraspecies interaction
Many environments within the common household provide conditions in which microorganisms can thrive, often resulting in the formation of biofilms. Bacteria have been cultured from many environments in and around homes, particularly from moist settings such as those around water pipes, showerheads, toothbrushes, spas, and bathrooms (4–6, 11, 14, 15, 19). Cases of opportunistic infections in humans have steadily increased over the past decade, and the source of infection often remains unidentified (2, 8, 10, 12). The increasing number of opportunistic infections is thought to correspond to the growing number of immunocompromised patients, many of whom self-medicate (8, 10, 18, 25). The formation of potential or adventitious pathogens in households poses a particular threat to such patients (9).
The formation of a pink-pigmented biofilm is often observed in wet areas within the household and may become a hygiene concern. Previous studies identified Methylobacterium as one of the dominant genera found in pink-pigmented household biofilms (11, 27, 32). Members of the Methylobacterium species are widely distributed and play important roles in both natural and man-made habitats, including plants, soils, air, dust, freshwater, aquatic sediments, marine environments, water supplies, and masonry (21, 31). Methylobacterium have been described as beneficial bacteria owing to their function in toxic pollutant biodegradation, the stimulation of germination, and plant development (16, 31); however, they are also known to be opportunistic human pathogens (12). Methylobacterium have been shown to exhibit higher stress-tolerance than other species found in biofilms, particularly under conditions of extreme nutrient limitation (32). Thus, it is considered laborious to remove already established pink-pigmented biofilms. Previous studies investigated the diversity of and interactions between household biofilms (6, 29, 32). However, bacterial interactions among the Methylobacterium species in household biofilms have not yet been elucidated in detail. In order to develop methods to control pink-pigmented household biofilms, it is considered essential to analyze these bacterial interactions and understand the mechanisms underlying biofilm development for the bacterial consortium of pink-pigmented biofilms. In the present study, we isolated and identified pink-pigmented bacteria from household biofilms, investigated the bacterial interactions enhancing biofilm formation, and examined the structures of both single and dual species biofilms.
Materials and Methods
Clone library analysis
Samples of visually confirmable pink slimes, which formed on a bathroom floor of a house, house K in Utsunomiya, Tochigi, Japan, were collected using a cotton swab at two different time points (July and December 2011). The head of the cotton swab with the pink slime was cut into pieces, and total DNA was extracted using a Cica Geneus DNA Extraction Reagent (Kanto Kagaku, Tokyo, Japan). The 16S rRNA genes from total DNA were amplified by PCR with GoTaq DNA polymerase (Promega, Madison, WI, USA) and the previously described primers, 63f (5′-CAG GCC TAA CAC ATG CAA GTC-3′) and 1387r (5′-GGG CGG TGT GTA CAA GGC-3′) (17). PCR was performed using the following cycling parameters: 94°C for 30 s, 50°C for 30 s, and 74°C for 1 min for 27 cycles. The PCR products were cloned into a pGEM-T easy vector (Promega). The 16S rRNA regions were sequenced using a BigDye Terminator ver. 3.1 and Applied Biosystems 3500 Series Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Closest type-strain 16S rRNA gene relatives to each clone sequence were determined using the RDP II sequence match tool (http://rdp.cme.msu.edu/seqmatch).
Isolation and identification of pink-pigmented bacteria
Pink slimes were collected using cotton swabs from the bathrooms of five different houses, house M (collected in April 2011), house Y (May 2011), house T (July 2011), house S (August 2011), and house N (August 2011) in Utsunomiya, Tochigi, Japan (representative photos of the pink slimes are shown in Fig. S1). The isolation source of each isolate has been indicated with the last letter (M, Y, T, S or N) in the isolate name. The collected samples were serially diluted in sterilized water and spread onto MB medium (10 g L−1 peptone, 2 g L−1 yeast extract, 1 g L−1 MgSO4·7H2O, and 1% methanol) containing 1.5% (w/v) agar. After being incubated at 30°C for 1 week, the pink-pigmented colony that formed was collected and streaked onto new MB agar plates for single colony purification. To identify the bacterial species, the 16S rRNA gene fragments were amplified by PCR with GoTaq DNA polymerase and the primers 63f and 1387r. The resulting 16S rRNA sequences were aligned using the ClustalW program from DDBJ (30). A neighbor-joining tree was constructed using the NJplot software (23).
Biofilm formation assay
Biofilm formation was determined using the previously described method with modifications (24). Bacterial strains were inoculated into the MB medium and incubated for 24 h at 30°C. Bacterial cells were collected by centrifugation and resuspended in fresh MB medium to an OD600 of 0.2. To form a mixed-biofilm, the cell suspensions of two different strains were mixed at a 1:1 OD600 ratio. Each cell suspension was diluted to an OD600 of 0.01 in fresh MB medium. Flat-bottom 96-well polystyrene microtiter plates (Thermo Fisher Scientific Inc. IL, USA) were used to examine biofilm formation. An aliquot of 100 μL of the diluted cell suspension was added to each well. After being incubated at 30°C for 48 h, 25 μL of a 0.1% crystal violet solution was added to each well. The plates were incubated at room temperature for 15 min and rinsed twice with sterile water. Crystal violet was dissolved in 100 μL of 99.5% ethanol, and biofilm formation was analyzed at 595 nm using a Spectra Max 250 spectrophotometer (Molecular Devices, CA, USA).
Confocal laser scanning microscopy (CLSM) analysis
To prepare biofilm samples, bacterial strains were inoculated into the MB medium and incubated for 24 h at 30°C. Bacterial cells were collected by centrifugation and resuspended in fresh MB medium to an OD600 of 0.01. In order to form a mixed-biofilm, the prepared cell suspensions of two different strains were mixed at a 1:1 ratio. A volume of 3.5 mL of the cell suspensions was transferred to a 50-mL centrifuge tube. A sterile 18 × 18 mm glass cover slip was vertically dipped in the cell suspension and incubated statically for 3 d at 30°C to allow for biofilm formation. The biofilms that formed were stained using a FilmTracer LIVE/DEAD Biofilm Viability kit (Invitrogen, Carlsbad, CA, USA). Staining was conducted according to a standard protocol. Briefly, fluorescence-staining solutions were prepared by adding 3 μL of the SYTO9 stain for live organisms and 3 μL of the propidium iodide stain for dead organisms into 1 mL of sterilized water. A volume of 200 μL of the staining solution was then added on top of the biofilm, which was then incubated in the dark for 25 min. Samples were rinsed with sterilized water and examined using a FluoView FV10 laser scanning confocal microscope (Olympus, Tokyo, Japan). Simulated fluorescence projection images were generated using the FluoView application software package (Olympus). Nucleotide sequence accession numbers The nucleotide sequences reported in this study were deposited in the DDBJ, ENA, GenBank databases with the following accession numbers: AB900964–AB900979 (for Methylobacterium isolates), AB979860–AB979864 (for other pink-pigmented isolates) and LC002341–LC002519 (for clones).
Results and Discussion
Phylogenetic analysis of clone libraries
To determine the composition of the bacterial community in the pink slime, clone libraries of the 16S rRNA genes were prepared and sequenced from the pink slime that formed on the bathroom floor. A total of 87 and 92 clones were sequenced from the pink slime samples collected from the same position of house K in July and December 2011, respectively. We took relatedness clusters with 97% or higher sequence identity to correspond to a species-level relationship and clusters with 95% or higher sequence identity to correspond to a genus-level relationship (11). Methylobacterium and Sphingomonas were the predominant genera found in the pink slime at the two evaluated time points. Regarding the genus Methylobacterium, clone sequences that showed similarity to M. brachiatum B0021T (accession no. AB175649) were mainly found in the clone libraries of July and December 2011 (n = 20 and 47, respectively). The same number (n = 3) of clone sequences that showed similarity to other Methylobacterium species were also found in both clone libraries. Regarding the genus Sphingomonas, clone sequences that showed similarity to S. aquatilis JSS-7T (accession no. AF131295) were mainly found in the clone libraries (n = 9 and 15, respectively). Clone sequences that showed similarity to other Sphingomonas species were also found in both clone libraries (n = 7 and 4, respectively). The sequences of the other clones (n = 48 and 23, respectively) showed low identities (<95%) with the 16S rRNA sequences of the type strains. These 48 or 23 clones were divided into 10 and 14 groups having sequence identities above 97%, respectively. These results demonstrated that the pink slime that formed on the bathroom floor consisted of many kinds of bacteria, which primarily belonged to the genera of Methylobacterium or Sphingomonas. The genera of Methylobacterium and Sphingomonas were previously detected as the dominant members in biofilms that formed on vinyl shower curtains (11). This previous study also reported the co-existence of two or three species of Methylobacterium in the biofilms as shown here. However, M. brachiatum, which was mainly found in this study, was not detected in the previous study.
Isolation and identification of pink-pigmented bacteria
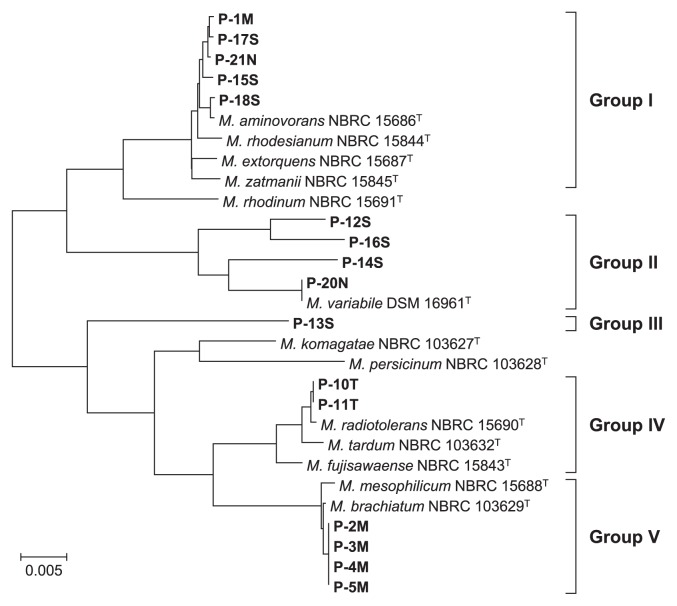
To analyze the cultivable bacterial composition of pink slime, samples of pink-pigmented bacteria were taken from the bathrooms of five different houses and screened. 16S rRNA gene fragments were amplified and sequenced to identify the pink-pigmented bacterial isolates. A total of 16 out of the 21 pink-pigmented bacterial isolates were assigned to the genus Methylobacterium. Previous studies identified the genus Methylobacterium as a major isolate in household biofilms. Our results confirmed that the genus Methylobacterium was one of the dominant groups of pink-pigmented bacteria. A BLAST search revealed that the 16S rRNA sequences of the other less-represented isolates (1 strain each) showed identities to that of Pedobacter suwonensis 15–52T (95.4%), Caulobacter henricii ATCC 15253T (79.7%), Terriglobus roseus KBS 63T (88.3%), Roseomonas rosea 173–96T (92.7%), and Hymenobacter rigui WPCB131T (95.6%), respectively. These bacterial species have been previously identified as pink-pigmented bacteria (1, 3, 7, 13, 26). Based on the results of the phylogenetic analysis with the 16S rRNA genes, we divided the sixteen Methylobacterium strains into five groups (Fig. 1). Group I showed over 96% identity to M. zatmanii NBRC 15845T, M. extorquens NBRC 15687T, M. rhodesianum NBRC 15844T, and M. aminovorans NBRC 15686T. Although one group II strain, P-20N, showed 100% identity to M. variabile DSM 16961T, other group II and III strains did not show similarity to the type strains belonging to the genus Methylobacterium. Group IV showed 98.8% identity to M. radiotolerans NBRC 15690T and group V showed 99.5% identity to M. brachiatum NBRC 103629T and M. mesophilicum NBRC 15688T.
Fig. 1.
Neighbor-joining trees of 16S rRNA gene sequences obtained for Methylobacterium strains. Thirteen type strains of the genus Methylobacterium and 16 isolates were used for the phylogenetic analysis with the 16S rRNA gene. The bacterial isolates used in this study are shown in bold. The scale bar represents 0.005 substitutions per nucleotide position. Bacteria were divided into 5 groups based on phylogenetic relationships.
Intraspecies interactions contributed to the formation of robust biofilms
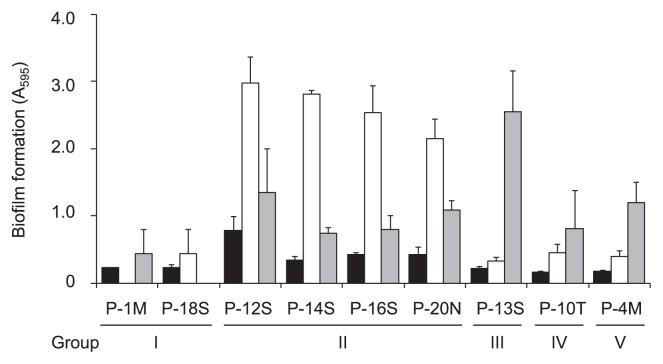
The formation of biofilms from the pink-pigmented bacterial isolates was evaluated on a polystyrene plastic surface. In the biofilm formation assay, Methylobacterium sp. P-1M and P-18S from group I, P-12S, P-14S, P-16S, and P-20N from group II, P-13S from group III, P-10T from group IV, and P-4M from group V, were selected. The selected strains were inoculated into MB medium in 96-well polystyrene plates and incubated for 48 h. The results of the biofilm assay revealed that all of the tested strains formed low-level biofilms (Fig. 2). Previous studies showed that mixed-species biofilms with interspecies interactions were often thicker and more physically and physiologically robust than single-species biofilms (27, 33). Therefore, we examined dual-species biofilm formation by co-incubating the selected Methylobacterium isolates as above along with the other pink-pigmented isolates, P. suwonensis, C. henricii, T. roseus, R. rosea, and H. rigui. However, none of the combinations demonstrated enhanced biofilm formation (data not shown). We then examined dual-species biofilm formation by mixing two of the nine selected Methylobacterium isolates with all possible combinations. The amount of the biofilms formed by the group I strain, P-1M, significantly increased when it was co-cultured with the four group II strains (Fig. 2). In addition, biofilm formation by another group I strain, P-18S, was also enhanced by co-culturing with a group III strain, P-13S (Fig. 2). However, mixed biofilm formation by two group I strains, P-1M and P-18S, was not enhanced over that by a single strain (Fig. 2). Furthermore, any other combination of the selected nine Methylobacterium isolates did not enhance biofilm formation (data not shown). Interspecies interactions within biofilm communities have been widely reported in previous studies (22, 27, 28, 33). To the best of our knowledge, this is the first study to demonstrate that intraspecies interactions in the genus Methylobacterium enhanced biofilm formation.
Fig. 2.
Biofilm formation by Methylobacterium strains on 96-well polystyrene plates. The overnight cultures of Methylobacterium strains were diluted and transferred to each well of a 96-well polypropylene microtiter dish. After being incubated at 30°C for 48 h, biofilms were stained with crystal violet and estimated by analyzing absorbance at 595 nm. Six wells of each sample were used to measure biofilm formation, and error bars indicate standard deviations. Single biofilms (black bars), mixed biofilms with P-1M (white bars), and mixed biofilms with P-18S (grey bars) are shown.
To investigate the phenotypes of enhanced biofilm formation, P-1M, P-12S, P-14S, P-16S, and P-20N were inoculated either solely or dually in 4 mL of MB medium in glass test tubes. After 3 d of static cultivation, the formation of a pellicle-like biofilm was observed at the liquid-gas interface of both the single- and dual-species cultures (Fig. 3). When the medium was removed and the biofilms were washed twice with the sterilized water, the single-species biofilm was easily washed from the glass surface, whereas the dual-species biofilm adhered strongly after washing (Fig. 3). These results showed that intraspecies interactions played an important role in the formation of the robust biofilms by the Methylobacterium strains.
Fig. 3.
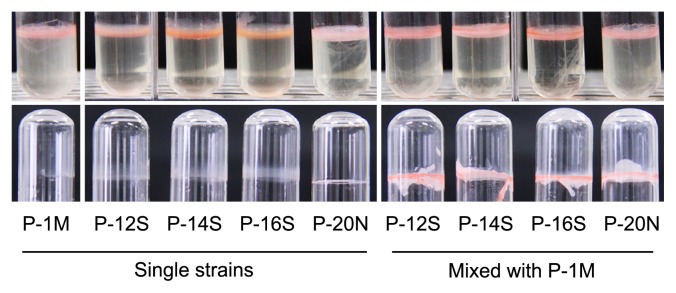
Single- and dual-species biofilms formed on the surface of glass tubes. Methylobacterium strains P-12S, P-14S, P-16S, and P-20N were inoculated with or without P-1M in 4 mL of MB medium in glass test tubes and statically incubated for 3 d at 30°C. The pellicle-like biofilms formed by the single- or dual-species are shown in the upper part. The remaining biofilms after washing are shown in the lower part.
CLSM analysis showed structural differences between single- and dual-species biofilms
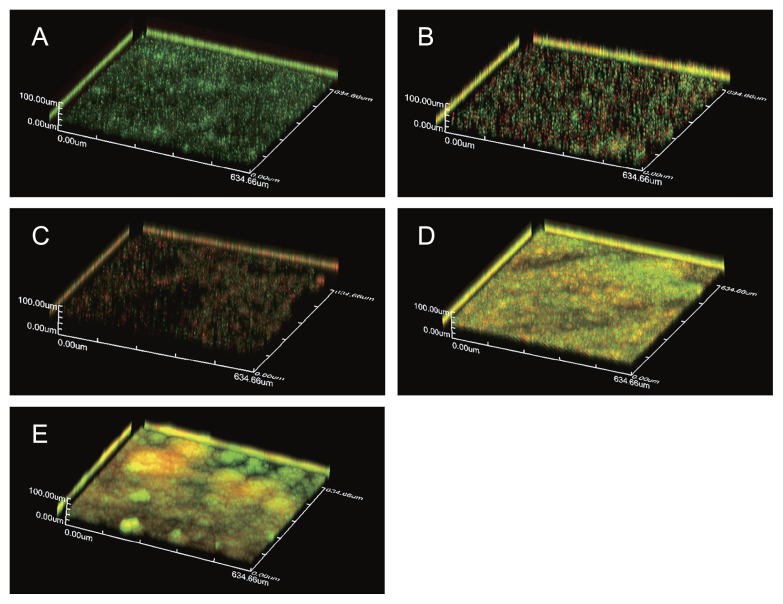
To examine the three-dimensional structures of single- and dual-species biofilms, we selected the three Methylobacterium strains, P-1M, P-14S, and P-20N. A LIVE/DEAD biofilm viability kit was employed to assay bacterial viability in the biofilms. The single-species biofilms of P-1M, P-14S, and P-20N were comparatively scattered with low cell density (Fig. 4A, B, C). However, the dual-species biofilms of P-1M with P-14S or P-20N were significantly tighter with a high cell density (Fig. 4D, E). Furthermore, several mushroom-shaped structures were observed in the dual-species biofilms of P-1M and P-20N (Fig. 4E). These results showed that intraspecies interactions affected the robustness of the biofilms and structure formation in Methylobacterium strains. Large amounts of dead cells were observed in the single species biofilm formed by P-14S or P-20N (Fig. 4B, C). It has been widely reported that extracellular DNA (eDNA), which is released from dead cells, plays an important role in biofilm formation (20). eDNA adsorbs to the cell surface, promoting adhesion to abiotic surfaces. In order to form a robust biofilm, a proper ratio of live/dead cells may be necessary to maintain a suitable amount of eDNA. Therefore, we assumed that the dead cells of P-14S and P-20N played the role of a supplier of eDNA in their mixed species biofilm.
Fig. 4.
CLSM analysis of a single P-1M biofilm (A), single P-14S biofilm (B), single P-20N biofilm (C), mixed P-1M and P-14S biofilm (D), and mixed P-1M and P-20N biofilm (E). Single- and dual-biofilms were formed on sterile glass cover slips and stained using a FilmTracer LIVE/DEAD Biofilm Viability kit. Samples were rinsed with sterilized water and examined using a FluoView FV10 laser scanning confocal microscope. Live cells are stained green and dead cells are stained red. The z-axis scale bar represents 100 μm.
Conclusion
Our results revealed that the genus Methylobacterium is one of the dominant bacteria found in the pink-pigmented slime that widely forms in bathrooms. Although most Methylobacterium strains independently formed poor biofilms, some specific Methylobacterium strains interacted with other phylogenetically different Methylobacterium strains to enhance biofilm formation. The results of the clone library analysis showed that M. brachiatum and the other Methylobacterium species co-existed in the same pink slime. It is highly possibility that intraspecies interactions between different Methylobacterium species contributed to the formation of the strong biofilms found in pink-pigmented household biofilms. Future studies to more clearly understand the molecular mechanisms underlying intraspecies interactions for the development of dual-species biofilms may provide useful information for the development of methods to control pink-pigmented household biofilms.
Supplementary Information
Acknowledgements
This work was supported by Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Agency (JST).
References
- 1.Baik KS, Seong CN, Moon EY, Park YD, Yi H, Chun J. Hymenobacter rigui sp. nov., isolated from wetland freshwater. Int J Syst Evol Microbiol. 2006;56:2189–2192. doi: 10.1099/ijs.0.64181-0. [DOI] [PubMed] [Google Scholar]
- 2.Casadevall A. Crisis in infectious diseases: time for a new paradigm? Clin Infect Dis. 1996;23:790–794. doi: 10.1093/clinids/23.4.790. [DOI] [PubMed] [Google Scholar]
- 3.Eichorst SA, Breznak JA, Schmidt TM. Isolation and characterization of soil bacteria that define Terriglobus gen. nov., in the phylum Acidobacteria. Appl Environ Microbiol. 2007;73:2708–2717. doi: 10.1128/AEM.02140-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Embil J, Warren P, Yakrus M, Stark R, Corne S, Forrest D, Hershfield E. Pulmonary illness associated with exposure to Mycobacterium-avium complex in hot tub water. Hypersensitivity pneumonitis or infection? Chest. 1997;111:813–816. doi: 10.1378/chest.111.3.813. [DOI] [PubMed] [Google Scholar]
- 5.Falkinham JO, 3rd, Norton CD, LeChevallier MW. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other Mycobacteria in drinking water distribution systems. Appl Environ Microbiol. 2001;67:1225–1231. doi: 10.1128/AEM.67.3.1225-1231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feazel LM, Baumgartner LK, Peterson KL, Frank DN, Harris JK, Pace NR. Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci USA. 2009;106:16393–16399. doi: 10.1073/pnas.0908446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodgson DA, Shaw P, Shapiro L. Isolation and genetic analysis of Caulobacter mutants defective in cell shape and membrane lipid synthesis. Genetics. 1984;108:809–826. doi: 10.1093/genetics/108.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hornei B, Luneberg E, Schmidt-Rotte H, Maass M, Weber K, Heits F, Frosch M, Solbach W. Systemic infection of an immunocompromised patient with Methylobacterium zatmanii. J Clin Microbiol. 1999;37:248–250. doi: 10.1128/jcm.37.1.248-250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahana LM, Kay JM, Yakrus MA, Waserman S. Mycobacterium avium complex infection in an immunocompetent young adult related to hot tub exposure. Chest. 1997;111:242–245. doi: 10.1378/chest.111.1.242. [DOI] [PubMed] [Google Scholar]
- 10.Kaye KM, Macone A, Kazanjian PH. Catheter infection caused by Methylobacterium in immunocompromised hosts: report of three cases and review of the literature. Clin Infect Dis. 1992;14:1010–1014. doi: 10.1093/clinids/14.5.1010. [DOI] [PubMed] [Google Scholar]
- 11.Kelley ST, Theisen U, Angenent LT, St Amand A, Pace NR. Molecular analysis of shower curtain biofilm microbes. Appl Environ Microbiol. 2004;70:4187–4192. doi: 10.1128/AEM.70.7.4187-4192.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korvick JA, Rihs JD, Gilardi GL, Yu VL. A pink-pigmented, oxidative, nonmotile bacterium as a cause of opportunistic infections. Arch Intern Med. 1989;149:1449–1451. [PubMed] [Google Scholar]
- 13.Kwon SW, Kim BY, Lee KH, Jang KY, Seok SJ, Kwon JS, Kim WG, Weon HY. Pedobacter suwonensis sp. nov., isolated from the rhizosphere of Chinese cabbage (Brassica campestris) Int J Syst Evol Microbiol. 2007;57:480–484. doi: 10.1099/ijs.0.64196-0. [DOI] [PubMed] [Google Scholar]
- 14.Le Dantec C, Duguet JP, Montiel A, Dumoutier N, Dubrou S, Vincent V. Occurrence of mycobacteria in water treatment lines and in water distribution systems. Appl Environ Microbiol. 2002;68:5318–5325. doi: 10.1128/AEM.68.11.5318-5325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee TC, Stout JE, Yu VL. Factors predisposing to Legionella pneumophila colonization in residential water systems. Arch. Environ Health. 1988;43:59–62. doi: 10.1080/00039896.1988.9934375. [DOI] [PubMed] [Google Scholar]
- 16.Lidstrom ME, Chistoserdova L. Plants in the pink: cytokinin production by Methylobacterium. J Bacteriol. 2002;184:1818. doi: 10.1128/JB.184.7.1818.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Dymock D, Wade WG. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol. 1998;64:795–799. doi: 10.1128/aem.64.2.795-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin DS, Oray-Schrom P, Amoateng-Adjepong Y. Emerging significance of Mycobacterium avium-complex infection in an inner-city hospital. Conn Med. 2002;66:323–330. [PubMed] [Google Scholar]
- 19.Nelson Filho P, Macari S, Faria G, Assed S, Ito IY. Microbial contamination of toothbrushes and their decontamination. Pediatr Dent. 2000;22:381–384. [PubMed] [Google Scholar]
- 20.Okshevsky M, Meyer RL. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol. 2013 doi: 10.3109/1040841X.2013.841639. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Omer ZS, Tombolini R, Gerhardson B. Plant colonization by pink-pigmented facultative methylotrophic bacteria (PPFMs) FEMS Microbiol Ecol. 2004;47:319–326. doi: 10.1016/S0168-6496(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 22.Pammi M, Liang R, Hicks J, Mistretta TA, Versalovic J. Biofilm extracellular DNA enhances mixed species biofilms of Staphylococcus epidermidis and Candida albicans. BMC Microbiol. 2013;13:257. doi: 10.1186/1471-2180-13-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perriere G, Gouy M. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie. 1996;78:364–369. doi: 10.1016/0300-9084(96)84768-7. [DOI] [PubMed] [Google Scholar]
- 24.Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 25.Rose AM, Sinka K, Watson JM, Mortimer JY, Charlett A. An estimate of the contribution of HIV infection to the recent rise in tuberculosis in England and Wales. Thorax. 2002;57:442–445. doi: 10.1136/thorax.57.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Porro C, Gallego V, Busse HJ, Kampfer P, Ventosa A. Transfer of Teichococcus ludipueritiae and Muricoccus roseus to the genus Roseomonas, as Roseomonas ludipueritiae comb. nov. and Roseomonas rosea comb. nov., respectively, and emended description of the genus Roseomonas. Int J Syst Evol Microbiol. 2009;59:1193–1198. doi: 10.1099/ijs.0.004820-0. [DOI] [PubMed] [Google Scholar]
- 27.Simoes LC, Simoes M, Vieira MJ. Biofilm interactions between distinct bacterial genera isolated from drinking water. Appl Environ Microbiol. 2007;73:6192–6200. doi: 10.1128/AEM.00837-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simoes LC, Simoes M, Vieira MJ. Intergeneric coaggregation among drinking water bacteria: evidence of a role for Acinetobacter calcoaceticus as a bridging bacterium. Appl Environ Microbiol. 2008;74:1259–1263. doi: 10.1128/AEM.01747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simoes LC, Simoes M, Vieira MJ. Influence of the diversity of bacterial isolates from drinking water on resistance of biofilms to disinfection. Appl Environ Microbiol. 2010;76:6673–6679. doi: 10.1128/AEM.00872-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Aken B, Yoon JM, Schnoor JL. Biodegradation of nitro-substituted explosives 2,4,6-trinitrotoluene, hexahydro-1,3,5-trinitro- 1,3,5-triazine, and octahydro-1,3,5,7-tetranitro-1,3,5-tetrazocine by a phytosymbiotic Methylobacterium sp. associated with poplar tissues (Populus deltoides x nigra DN34) Appl Environ Microbiol. 2004;70:508–517. doi: 10.1128/AEM.70.1.508-517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yano T, Kubota H, Hanai J, Hitomi J, Tokuda H. Stress tolerance of Methylobacterium biofilms in bathrooms. Microbes Environ. 2013;28:87–95. doi: 10.1264/jsme2.ME12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida S, Ogawa N, Fujii T, Tsushima S. Enhanced biofilm formation and 3-chlorobenzoate degrading activity by the bacterial consortium of Burkholderia sp. NK8 and Pseudomonas aeruginosa PAO1. J Appl Microbiol. 2009;106:790–800. doi: 10.1111/j.1365-2672.2008.04027.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.