Abstract
Microglia are resident mononuclear phagocytes that play a principal role in the maintenance of normal tissue homeostasis in the central nervous system (CNS). Microglia, rapidly activated in response to proinflammatory stimuli, are accumulated in brain lesions of neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease. The E26 transformation-specific (ETS) family transcription factor PU.1/Spi1 acts as a master regulator of myeloid and lymphoid development. PU.1-deficient mice show a complete loss of microglia, indicating that PU.1 plays a pivotal role in microgliogenesis. However, the comprehensive profile of PU.1/Spi1 target genes in microglia remains unknown. By analyzing a chromatin immunoprecipitation followed by deep sequencing (ChIP-Seq) dataset numbered SRP036026 with the Strand NGS program, we identified 5,264 Spi1 target protein-coding genes in BV2 mouse microglial cells. They included Spi1, Irf8, Runx1, Csf1r, Csf1, Il34, Aif1 (Iba1), Cx3cr1, Trem2, and Tyrobp. By motif analysis, we found that the PU-box consensus sequences were accumulated in the genomic regions surrounding ChIP-Seq peaks. By using pathway analysis tools of bioinformatics, we found that ChIP-Seq-based Spi1 target genes show a significant relationship with diverse pathways essential for normal function of monocytes/macrophages, such as endocytosis, Fc receptor-mediated phagocytosis, and lysosomal degradation. These results suggest that PU.1/Spi1 plays a crucial role in regulation of the genes relevant to specialized functions of microglia. Therefore, aberrant regulation of PU.1 target genes might contribute to the development of neurodegenerative diseases with accumulation of activated microglia.
Keywords: ChIP-Seq, GenomeJack, KeyMolnet, microglia, microgliopathy, Nasu–Hakola disease, PU.1, Spi1, Strand NGS
Introduction
Microglia are resident mononuclear phagocytes that play a principal role in the maintenance of normal tissue homeostasis in the central nervous system (CNS).1 They are derived from primitive c-kit+ erythromyeloid precursors (EMPs) in the yolk sac emerging as early as on day 8 post-conception during embryogenesis.2,3 EMPs develop into CD45+ c-kitlo CX3CR1− immature A1 cells that subsequently differentiate into CD45+ c-kit− CX3CR1+ A2 cells. Proliferating A2 cells enter into the developing CNS and are incorporated into the brain parenchyma as resident microglia. Microglia have a capacity to constantly scavenge invading pathogens, dying cells, and unwanted synapses by sensing them with a panel of pattern recognition receptors (PRRs).1 Microglia show a ramified morphology under physiological conditions. When exposed to infectious and traumatic stimuli, they rapidly adopt an amoeboid morphology, followed by secretion of various cytokines, chemokines, and reactive oxygen and nitrogen species. Depending on their microenvironment, microglia are activated to acquire two distinct priming states. Stimulation with lipopolysaccharide (LPS) or interferon-gamma (IFNγ) induces the “classically” activated (M1; proinflammatory) state relevant to defense against bacterial and viral infection, whereas exposure to interleukin (IL)-4 or IL-13 promotes the conversion to the “alternatively” activated (M2; anti-inflammatory) state involved in tissue repair and remodeling.1
Microglia play a central role in the pathophysiology of human neurodegenerative diseases that are characterized by chronic inflammation associated with accumulation of activated microglia in affected areas, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD).4,5 In AD, amyloid-beta (Aβ) activates microglia by signaling through Toll-like receptors (TLRs) and NOD-like receptors (NLRs), leading to production of proinflammatory mediators potentially toxic to neurons.6,7 In PD, alpha-synuclein (α-Syn), which serves as a danger-associated molecular pattern, directly activates microglia.8 In HD, mutant huntingtin promotes transcriptional activation of numerous proinflammatory genes in microglia.9 However, at present, the precise mechanism underlying gene regulation relevant to microglial activation in human neurodegenerative diseases remains largely unknown.
The E26 transformation-specific (ETS) family transcription factor PU.1, also named as Spi1 or Sfpi1 in mouse, acts as a master regulator of myeloid and lymphoid development, expressed chiefly in monocytes/macrophages, neutrophils, mast cells, B cells, and early erythroblasts.10 PU.1 comprises an N-terminal transactivation domain, a C-terminal DNA-binding domain, and an intervening PEST domain for protein–protein interactions. It activates expression of hundreds of downstream genes by binding to a purine-rich DNA sequence named the PU-box located on the targets. The expression levels of PU.1 target genes are highly variable in different cell types, owing to the difference in cellular concentration of PU.1, chromatin accessibility, motif-binding affinity, and cooperation with neighboring transcription factors.11 Importantly, PU.1-deficient mice show a complete loss of microglia, along with a lack of mature macrophages, mono-cytes, neutrophils, and B cells, indicating that PU.1 regulates key genes involved in differentiation and maturation of not only hematopoietic cells but also brain microglia.12,13 However, at present, the comprehensive profile of PU.1 target genes involved in microgliogenesis remains uncharacterized. In the adult human microglia, MCSF (CSF1) treatment elevates the expression levels of PU.1 and stimulates phagocytosis of Aβ, while knockdown of PU.1 reduces their viability and phago-cytic capability.14,15 Interferon regulatory factor 8 (Irf8) serves as an essential regulator of development of A2 microglial progenitor cells.3 Irf8-deficient microglia show fewer elaborated processes with decreased expression of Iba1 and reduced pro-liferative and phagocytic activities.16 Runt-related transcription factor 1 (Runx1), whose expression levels are elevated in amoeboid microglia, promotes reverse transition from amoeboid to ramified microglia.17
Recently, the rapid progress in the next-generation sequencing (NGS) technology has revolutionized the field of genome research. Chromatin immunoprecipitation followed by deep sequencing (ChIP-Seq) serves as one of NGS applications for genome-wide profiling of DNA-binding proteins, histone modifications, and nucleosomes.18 ChIP-Seq, with advantages of higher resolution, less noise, and greater coverage of the genome, compared with microarray-based ChIP-Chip, provides an innovative tool for studying gene regulatory networks on the whole genome scale. Furthermore, recent advances in systems biology help us to investigate the cell-wide map of the complex molecular interactions by using the literature-based knowledgebase of molecular pathways.19 Therefore, the integration of high dimensional ChIP-Seq NGS data with underlying molecular networks represents a rational approach to characterize the genome-wide network-based molecular mechanisms of gene regulation. To clarify the biological role of PU.1 in regulation of microglial functions, we attempted to characterize the comprehensive set of ChIP-Seq-based PU.1/Spi1 target genes in microglial cells by analyzing a dataset retrieved from public database.
Methods
ChIP-Seq dataset of microglial cells
A ChIP-Seq data-set of microglial cells was retrieved from DDBJ Sequence Read Archive (DRA) under the accession number SRP036026. The researchers in Dr. Christopher K. Glass’s Laboratory, University of California, San Diego, performed the original experiment to study the role of reactive microglia in HD.9 The raw data are open to public from March 2, 2014. Currently, no alternative datasets are publicly available for PU.1 ChIP-Seq of microglia. They cloned the N-terminus of wild-type (15Q) or mutant (128Q) human huntingtin in the pCDH-CMV-MCS-EF1-Puro vector (System Bioscience). Either the cloned vector or the empty vector was expressed in BV2 mouse microglial cells,20 by using the Lentiviral expression system (System Bioscience). Then, they were processed for ChIP-Seq analysis. We studied ChIP-Seq data derived from the cells transduced with the empty vector (no exogenous huntingtin). Following fixation with formaldehyde, sonicated nuclear lysates were immunoprecipitated with a rabbit polyclonal anti-PU.1 (Spi1) antibody (sc-352; Santa Cruz Biotechnology) (SRX451619) or a rabbit polyclonal anti-CCAAT-enhancer-binding protein alpha (C/EBPα, Cebpa) antibody (sc-61; Santa Cruz Biotechnology) (SRX451622). NGS libraries constructed from adapter-ligated ChIP DNA fragments were processed for deep sequencing on Genome Analyzer IIx (Illumina).
First, we evaluated the quality of NGS short reads by searching them on the FastQC program (www.bioinformatics.babraham.ac.uk/projects/fastqc). Then, we removed the reads of insufficient quality by filtering them out with the FASTX-toolkit (hannonlab.cshl.edu/fastx_toolkit). After cleaning the data, we mapped them on the mouse genome reference sequence version mm9 by a mapping tool named COBWeb of the Strand NGS2.0 program, formerly named Avadis NGS (Strand Genomics), or by the Bowtie2 version 2.1.0 program (bowtie-bio.sourceforge.net/bowtie2/index.shtml). Then, we identified the peaks of binding sites with fold enrichment (FE) ≥5 by using the Model-based Analysis of ChIP-Seq (MACS) program or the Probabilistic Inference for ChIP-Seq (PICS) program.21,22 We determined the genes corresponding to the peaks by a neighboring gene analysis tool of Strand NGS in the setting within a distance of 5,000 bp from peaks to genes. We characterized the genomic location of binding peaks by a peak-finding tool of Strand NGS that classifies the locations into the upstream region, 5′ untranslated region (5′UTR), exon, intron, and 3′UTR. We also imported the processed data into a genome viewer named GenomeJack v1.4 (Mit-subishi Space Software). We identified the consensus motif sequences in the genomic regions surrounding the peaks by using the GADEM program.23
Molecular network analysis
To identify molecular networks biologically relevant to ChIP-Seq-based Spi1 target genes, we imported the corresponding Entrez Gene IDs into the Functional Annotation tool of Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.7 (david.abcc.ncifcrf.gov).24 DAVID identifies relevant pathways constructed by Kyoto Encyclopedia of Genes and Genomes (KEGG), composed of the genes enriched in the given set, followed by statistical evaluation by a modified Fisher’s exact test corrected by Bonferroni multiple comparison test. KEGG (www.kegg.jp) is a publicly accessible knowledgebase that covers a wide range of pathway maps on metabolic, genetic, environmental, and cellular processes, and human diseases, currently composed of 332,680 pathways generated from 466 reference pathways.25
We also imported Entrez Gene IDs into Ingenuity Pathways Analysis (IPA) (Ingenuity Systems; www.ingenuity.com). IPA is a commercial knowledgebase that contains approximately 3,000,000 biological and chemical interactions and functional annotations with definite scientific evidence. Upon uploading the list of Gene IDs, the network-generation algorithm identifies focused genes integrated in global molecular pathways and networks. IPA calculates the score P-value that reflects the statistical significance of association between the genes and the pathways or networks by the Fisher’s exact test.
KeyMolnet (KM Data; www.km-data.jp), a different commercial knowledgebase, contains manually curated content on 164,000 relationships among human genes and proteins, small molecules, diseases, pathways, and drugs.26 They include the core content collected from selected review articles with the highest reliability. Upon importing the list of Gene IDs, KeyMolnet automatically provides corresponding molecules as nodes on the network. The neighboring network-search algorithm selected one or more molecules as starting points to generate the network of all kinds of molecular interactions around starting molecules, including direct activation/inactivation, transcriptional activation/repression, and the complex formation within one path from starting points. The generated network was compared side by side with 501 human canonical pathways of the KeyMolnet library. The algorithm counting the number of overlapping molecular relations between the extracted network and the canonical pathway makes it possible to identify the canonical pathway showing the most significant contribution to the extracted network.
Results
Identification of 5,264 ChIP-Seq-based Spi1 target genes in mouse microglia
First, we evaluated the quality of ChIP-Seq NGS data examined in the present study. After cleaning, the quality scores mostly exceeded 30 across the bases on FastQC, indicating an acceptable quality for downstream analysis (Supplementary Fig. 1, panels a, b). After mapping them on mm9 by COBWeb, we identified 56,278 Spi1-ChIP peaks detected by MACS and 15,141 Spi1-ChIP peaks detected by PICS. From these, we selected peaks located within a distance of 5,000 bp from protein coding genes, and then extracted 5,264 genes overlapping between data derived from two distinct peak-finding algorithms MACS and PICS termed as the most reliable Spi1 targets (Supplementary Table 1). The peaks were accumulated in the upstream (18.7%) and intronic (69.5%) regions. The motif analysis by GADEM revealed an existence of the PU-box consensus sequences defined as 5′-GAGGAA-3′ located within the genomic regions surrounding ChIP-Seq peaks (Fig. 1).
Figure 1.
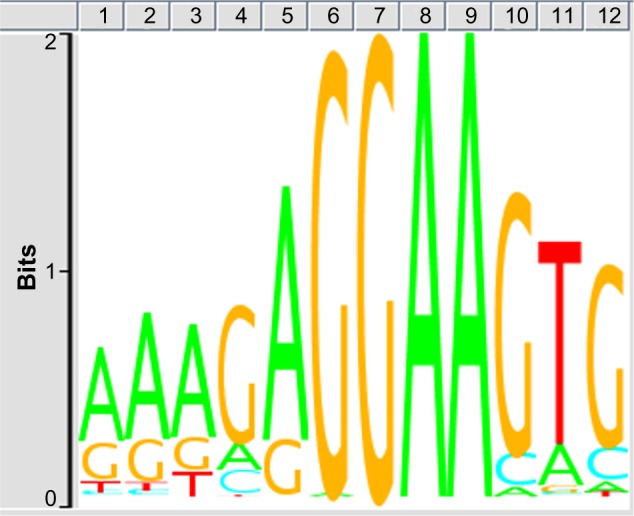
Spi1-binding consensus sequence motif. The consensus motif sequences surrounding Spi1 ChIP-Seq peaks were identified by the GADEM program. The PU-box consensus sequences defined as 5′-GAGGAA-3′ were located on 80.3% of the peaks detected by MACS.
We identified both Spi1 (FE = 19.3) and Irf8 (FE = 27.8) in the list of Spi1 target genes (Supplementary Table 1; Figs. 2 and 3). Both of them are known to serve as a crucial regulator of differentiation of microglia from EMPs during early embryogenesis.3 We also found Runx1 (FE = 26.7), a transcription factor acting to constitute a negative feedback loop of PU.1,27 along with Csf1r (FE = 10.7), Csf1 (FE = 17.8), and Il34 (FE = 31.5), acting as a key growth factor for differentiation of microglia,28 as Spi1 targets (Supplementary Table 1). Furthermore, we identified known cell type-specific markers for microglia, such as Aif1 (Iba1, FE = 32.9), Cx3cr1 (FE = 17.8), Cd68 (FE = 20.3), Trem2 (FE = 12.8), and Tyrobp (Dap12) (FE = 14.6) in the list of Spi1 target genes (Supplementary Table 1; Supplementary Figs. 2 and 3). Importantly, loss of function of either TREM2 or DAP12, components of a receptor/adapter complex on human microglia, plays a causative role in Nasu–Hakola disease (NHD).29 Furthermore, we found Syk (FE = 17.8), a downstream signal transducer of the Trem2/Dap12 pathway, as a Spi1 target gene.
Figure 2.
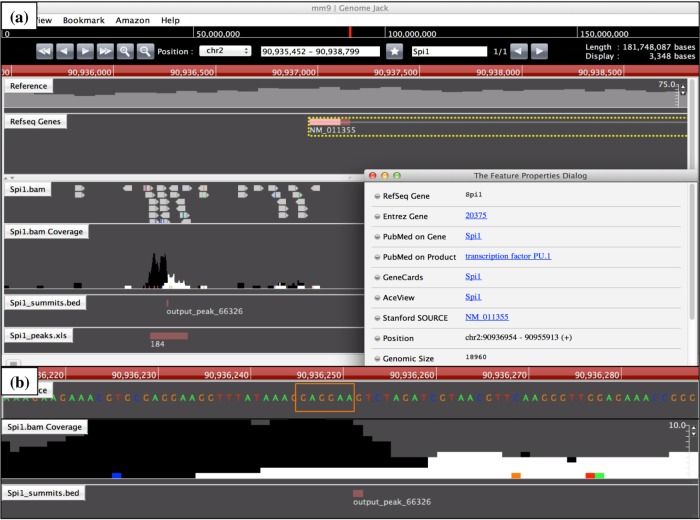
Genomic location of Spi1 ChIP-Seq peak on the Spi1 gene. The genomic location of Spi1 ChIP-Seq peaks was determined by importing the processed data into GenomeJack. An example of transcription factor PU.1 (Spi1; Entrez Gene ID 20375) is shown, where a MACS peak numbered 66326 in the Spi1.bam Coverage track is located in the promoter region of the Spi1 gene (panel a) with a Spi1-binding consensus sequence motif highlighted by orange square (panel b).
Figure 3.
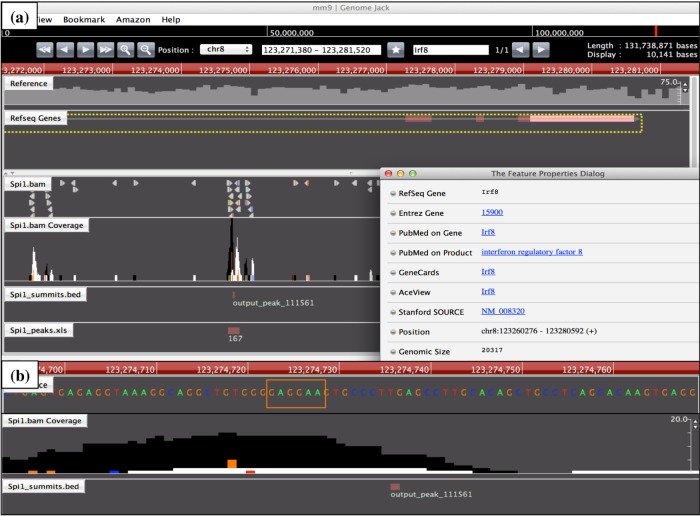
Genomic location of Spi1 ChIP-Seq peak on the Irf8 gene. The genomic location of Spi1 ChIP-Seq peaks was determined by importing the processed data into GenomeJack. An example of interferon regulatory factor 8 (Irf8; Entrez Gene ID 15900) is shown, where a MACS peak numbered 111561 in the Spi1.bam Coverage track is located in the intronic region of the Irf8 gene (panel a) with a Spi1-binding consensus sequence motif highlighted by orange square (panel b).
Next, we studied ChIP-Seq-based Cebpa target genes in BV2 microglial cells. We identified 12,685 Cebpa-ChIP peaks detected by MACS and 10,311 Cebpa-ChIP peaks detected by PICS. From these, we selected peaks located within a distance of 5,000 bp from protein coding genes, and then extracted 3,106 genes overlapping between data derived from MACS and PICS termed as the most reliable Cebpa targets. We found that 1,844 genes are shared between Spi1 targets and Cebpa targets, suggesting the possibility that Cebpa coregulates a substantial proportion (35%) of Spi1 target genes in microglial cells (Supplementary Table 1, underline).
A recent study by direct RNA sequencing of flow cytometry-sorted mouse brain microglia has characterized a set of 100 transcripts exclusively expressed in microglia.30 The study designated them as “the microglial sensome” (Supplementary Table 2). Importantly, we found that 63 out of 100 microglial sensome genes correspond to ChIP-Seq-based Spi1 target genes, indicating that Spi1 plays a pivotal role in regulation of the genes relevant to specialized functions of microglia (Table 1).
Table 1.
The set of 63 microglial sensome genes corresponding to ChIP-Seq-based Spi1 target genes.
| CHROMOSOME | START OF PEAKS | END OF PEAKS | FOLD ENRICHMENT | ENTREZ GENE ID | GENE SYMBOL | GENE NAME |
|---|---|---|---|---|---|---|
| chr7 | 132757191 | 132757492 | 38.659794 | 60504 | 1121r | interleukin 21 receptor |
| chr3 | 89709374 | 89709685 | 37.668518 | 16194 | 116ra | interleukin 6 receptor, alpha |
| chr6 | 122902489 | 122902814 | 37.181004 | 73149 | Clec4a3 | C-type lectin domain family 4, member a3 |
| chr11 | 60955751 | 60956142 | 35.720745 | 57916 | Tnfrsf13b | tumor necrosis factor receptor superfamily, member 13b |
| chrX | 13213539 | 13213888 | 33.61721 | 23890 | Gpr34 | G protein-coupled receptor 34 |
| chr4 | 66504343 | 66504608 | 33.29038 | 21898 | Tlr4 | toll-like receptor 4 |
| chr7 | 108112592 | 108113189 | 33.136967 | 233571 | P2ry6 | pyrimidinergic receptor P2Y, G-protein coupled, 6 |
| chr18 | 35879188 | 35879366 | 32.216496 | 68545 | Ecscr | endothelial cell-specific Chemotaxis regulator |
| chr4 | 149501529 | 149501774 | 30.927835 | 56485 | Slc2a5 | solute carrier family 2 (facilitated glucose transporter), member 5 |
| chr3 | 30763607 | 30763924 | 30.746971 | 71862 | Gpr160 | G protein-coupled receptor 160 |
| chr7 | 16829398 | 16829780 | 29.842648 | 319430 | C5ar2 | complement component 5a receptor 2 |
| chr11 | 120818969 | 120819371 | 29.78236 | 80879 | Slc16a3 | solute carrier family 16 (monocarboxylicacid transporters), member 3 |
| chr1 | 121972010 | 121972524 | 28.747026 | 170706 | Tmem37 | transmembrane protein 37 |
| chr9 | 110948641 | 110949068 | 27.061855 | 17002 | Ltf | lactotransferrin |
| chr9 | 116075399 | 116075615 | 26.661926 | 21813 | Tafbr2 | transforming growth factor, beta receptor II |
| chr18 | 60966814 | 60967338 | 26.09944 | 16149 | Cd74 | CD74 antigen (invariant polypeptide of major histocompatibility complex, class II antigen-associated) |
| chr5 | 114094521 | 114094878 | 26.044493 | 14747 | Cmklr1 | chemokine-like receptor 1 |
| chr7 | 135258809 | 135259228 | 25.773195 | 16409 | Itgam | integrin alpha M |
| chr13 | 37488117 | 37488288 | 25.773195 | 17084 | Ly86 | lymphocyte antigen 86 |
| chr3 | 106592873 | 106593332 | 25.472342 | 12508 | Cd53 | CD53 antigen |
| chr16 | 38789019 | 38789447 | 25.28691 | 22268 | Upk1b | uroplakin 1B |
| chr11 | 46280548 | 46280823 | 24.811836 | 171285 | Havcr2 | hepatitis A virus cellular receptor 2 |
| chr6 | 40535749 | 40536059 | 24.257126 | 23845 | Clec5a | C-type lectin domain family 5, member a |
| chr11 | 78799024 | 78799325 | 23.375689 | 16859 | Laals9 | lectin, galactose binding, soluble 9 |
| chr13 | 103496918 | 103497108 | 22.680412 | 17079 | Cd180 | CD180 antigen |
| chr3 | 105735367 | 105735926 | 22.315817 | 433638 | l830077J02Rik | RIKEN cDNA I830077J02 gene |
| chr5 | 65352028 | 65352431 | 21.753523 | 21899 | Tlr6 | toll-like receptor 6 |
| chr16 | 36642687 | 36643132 | 21.577559 | 12524 | Cd86 | CD86 antigen |
| chr1 | 140026784 | 140027054 | 21.477663 | 19264 | Ptprc | protein tyrosine phosphatase, receptor type, C |
| chr7 | 106859678 | 106859981 | 21.273113 | 101488 | Slco2b1 | solute carrier organic anion transporter family, member 2b1 |
| chr6 | 122805926 | 122806252 | 20.940722 | 12267 | C3ar1 | complement component 3a receptor 1 |
| chr7 | 16849252 | 16849697 | 20.837904 | 12273 | C5ar1 | complement component 5a receptor 1 |
| chr4 | 132139773 | 132139985 | 20.618557 | 19204 | Ptafr | platelet-activating factor receptor |
| chr19 | 40782595 | 40783023 | 20.34726 | 12495 | Entpd1 | ectonucleoside triphosphate diphosphohydrolase 1 |
| chr11 | 69479440 | 69480081 | 20.306154 | 12514 | Cd68 | CD68 antigen |
| chr1 | 172903138 | 172903598 | 20.071161 | 14130 | Fcar2b | Fc receptor, IgG, low affinity lIb |
| chr10 | 19317003 | 19317223 | 19.63672 | 15979 | Ifngrl | interferon gamma receptor 1 |
| chr3 | 87180536 | 87180918 | 19.435524 | 229499 | FcrM | Fc receptor-like 1 |
| chr6 | 125030472 | 125030733 | 19.091257 | 381810 | Lpar5 | lysophosphatidic acid receptor 5 |
| chr3 | 59064135 | 59064423 | 19.032515 | 70839 | P2ry12 | purinergic receptor P2Y, G-protein coupled 12 |
| chr16 | 33947248 | 33947454 | 18.990776 | 16419 | Itab5 | integrin beta 5 |
| chr1 | 172989424 | 172989659 | 18.93541 | 14131 | Fcar3 | Fc receptor, IgG, low affinity III |
| chr4 | 133654539 | 133654895 | 18.744143 | 23833 | Cd52 | CD52 antigen |
| chr7 | 4018702 | 4019029 | 18.126204 | 52855 | Lairl | leukocyte-associated lg-like receptor 1 |
| chrX | 103346689 | 103346910 | 17.842981 | 279572 | TIr13 | toll-like receptor 13 |
| chr9 | 119977884 | 119978196 | 17.774618 | 13051 | Cx3cr1 | chemokine (C-X3-C) receptor 1 |
| chr1 | 174408269 | 174408572 | 17.634293 | 98365 | Slamf9 | SLAM family member 9 |
| chr3 | 100835299 | 100835620 | 16.568483 | 630146 | Cd101 | CD101 antigen |
| chr2 | 93289154 | 93289478 | 16.108248 | 12521 | Cd82 | CD82 antigen |
| chr4 | 47408473 | 47408654 | 16.108248 | 21812 | Tgfbr1 | transforming growth factor, beta receptor I |
| chr3 | 105716542 | 105717356 | 15.942183 | 11542 | Adora3 | adenosine A3 receptor |
| chr15 | 103140748 | 103141572 | 14.66406 | 80910 | Gpr84 | G protein-coupled receptor 84 |
| chr7 | 31198532 | 31198988 | 14.567458 | 22177 | Tyrobp | TYRO protein tyrosine kinase binding protein |
| chr5 | 138278454 | 138278742 | 14.318442 | 231805 | Pilra | paired immunoglobin-like type 2 receptor alpha |
| chr3 | 96097068 | 96098277 | 14.087213 | 14129 | Fcqrl | Fc receptor, IgG, high affinity I |
| chr9 | 114661993 | 114662369 | 14.058106 | 67213 | Cmtm6 | CKLF-like MARVEL transmembrane domain containing 6 |
| chr17 | 48491514 | 48491850 | 12.772557 | 83433 | Trem2 | triggering receptor expressed on myeloid cells 2 |
| chr15 | 78135354 | 78135537 | 12.326311 | 12984 | Csf2rb2 | colony stimulating factor 2 receptor, beta 2, low-affinity (granulocyte-macrophage) |
| chr18 | 61280165 | 61280583 | 10.698308 | 12978 | Csf1r | colony stimulating factor 1 receptor |
| chr1 | 172948811 | 172949254 | 10.376222 | 246256 | Fcqr4 | Fc receptor, IgG, low affinity IV |
| chr4 | 144830530 | 144830848 | 9.163803 | 21938 | Tnfrsf1b | tumor necrosis factor receptor superfamily, member 1 b |
| chr10 | 59834857 | 59835131 | 8.8232565 | 74048 | 4632428N05Rik | RIKEN cDNA 4632428N05 gene |
| chr9 | 20820541 | 20820889 | 7.9916887 | 15894 | Icam1 | intercellular adhesion molecule 1 |
Notes: From the ChIP-Seq dataset, we identified 5,264 Spi1-target genes in BV2 mopuse microglia showing fold enrichment (FE) ≥5. Among them, those corresponding to microglial sensome genes (Supplementary Table 2) are listed with the chromosome, the position of the peak (start, end), FE, Entrez Gene ID, Gene Symbol, and Gene Name. The Cebpa-target genes are underlined.
Molecular networks of ChIP-Seq-based Spi1 target genes in microglia
Next, we studied molecular networks of the set of 5,264 ChIP-Seq-based Spi1 target genes by using three distinct pathway analysis tools of bioinformatics. By using DAVID, we identified functionally associated gene ontology (GO) terms. The most significant GO terms included “phosphate metabolic process” (GO:0006796; P = 2.21E-16 corrected by Bonferroni multiple comparison test) for biological process, “plasma membrane” (GO:0005886; P = 1.26E-15) for cellular component, and “GTPase regulator activity” (GO:0030695; P = 1.27E-22) for molecular function.
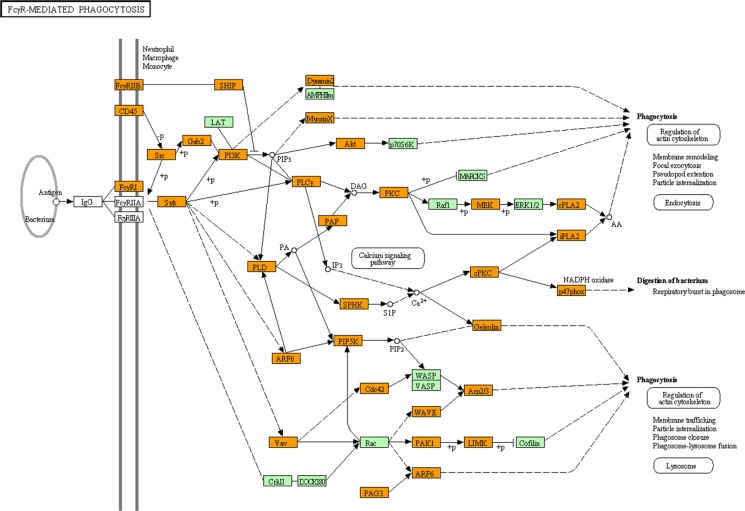
By using KEGG, we found that the set of 5,264 Spi1 targets showed a significant relationship with the pathways defined as “Lysosome” (mmu04142; P = 5.08E-08 corrected by Bonferroni multiple comparison test), “Focal adhesion” (mmu04510; P = 1.27E-07), “Endocytosis” (mmu04144; P = 4.54E-07), “Fcγ receptor-mediated phagocytosis” (mmu04666; P = 5.03E-07) (Fig. 4), and “MAPK signaling pathway” (mmu04010; P = 6.23E-06) (Table 2). Furthermore, they also exhibited significant association with the pathways defined as “Pathways in cancer” (mmu05200; P = 1.39E-04), “B cell receptor signaling pathway” (mmu04662; P = 2.18E-04), “Apoptosis” (mmu04210; P = 4.23E-04), “Leukocyte transendothelial migration” (mmu04670; P = 6.57E-04), “Chemokine signaling pathway” (mmu04062; P = 8.52E-04), and “Chronic myeloid leukemia“ (mmu05220; P = 1.16E-03) (Table 2). Importantly, the top-ranked “Lysosome” pathway included the set of 10 cathepsin genes, such as Ctsa, Ctsb, Ctsc, Ctsd, Ctse, Ctsf, Ctsk, Ctsl, Ctss, and Ctsz, essential for degradation of lysosomal proteins in microglia (Table 2).
Figure 4.
KEGG “Fcγ receptor-mediated phagocytosis” pathway relevant to Spi1 target genes. Entrez Gene IDs of 5,264 ChIP-Seq-based Spi1 target genes were imported into the Functional Annotation tool of DAVID. It extracted the KEGG “Fcγ receptor-mediated phagocytosis” pathway (mmu04666) as the fourth rank significant pathway as listed in Table 2. Spi1 target genes are colored by orange.
Table 2.
KEGG pathways relevant to ChIP-Seq-based Spi1 target genes in microglia.
| RANK | CATEGORY | FOCUSED GENES IN THE PATHWAY | P-VALUE CORRECTED BY BONFERRONI | FDR |
|---|---|---|---|---|
| 1 | mmu04142: Lysosome | Abcb9, Ap1b1, Ap1g1, Ap1s3, Ap3b1, Ap3b2, Ap3m2, Ap3s2, Ap4e1, Ap4s1, Arsa, Arsb, Arsg, Atp6v0a1, Atp6v0c, Atp6v0d1, Atp6v0d2, Cd68, Clta, Cltb, Cltc, Ctsa, Ctsb, Ctsc, Ctsd, Ctse, Ctsf, Ctsk, Ctsl, Ctss, Ctsz, Ctns, Fuca1, Galc, Gga1, Gla, Gnptab, Gns, Gusb, Hexa, Hexb, Hyal1, Igf2r, Lampl, Lamp2, Laptm4a, Laptm4b, Laptm5, Lgmn, LipA, Man2b1, NagA, Neu1, Npd, Pla2g15, Ppt1, Psap, Scarb2, Sgsh, Slc11a1, Slc11a2,Slc17a5, Sorti, Tcirg1 | 5.08E-08 | 3.30E-07 |
| 2 | mmu04510: Focal adhesion | Actb, Actn1, Akt1, Akt3, Arhgap5, Bcar1, Bcl2, Birc2, Birc3, Capn2, Cav2, Ccnd2, Ccnd3, Cdc42, Col1 a1, Col2a1, Col4a1, Col4a2, Col4a6, Col5a1, Col5a3, Diap1, Dock1, Egf, Flna, Flnb, Fine, Fyn, Grb2, Grlf1, Gsk3b, Igf1, Igf1r, Itga2, Itga4, Itga5, Itga6, Itga7, Itga8, Itga9, Itgav, Itgb3, Itgb5, Itgb7, Kdr, Lama5, Lamb1, Lamc1, Map2k1, Mapk9, Met, Myl10, Myl12a, Mylk, Pak1, Pak2, Parvb, Parvg, Pdgfc, Pgf, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Pip5k1c, Ppp1r12a, Prkca, Prkcb, Pten, Ptk2, Pxn, Rap1a, Rapgef1, Rasgrf1, Rock2, Shc1, Shc2, Shc4, Spp1, Src, Tln1, Tln2, Tnr, Vav1, Vav2, Vav3, Vcl, Vegfa, Vwf, Zyx | 1.27E-07 | 8.24E-07 |
| 3 | mmu04144: Endocytosis | Acap2, Acvr1b7, Adrb2, Adrbk1, Agap1, Ap2m1, Arap1, Arap3, Arf6, Arrb1, Asap1, Asap3, Cbl, Cblb, Cdc42, Chmp3, Chmp4b, Chmp6, Clta, Cltb, Cltc, Csf1r, Cxcr2, Cxcr4, Dab2, Dnm1, Dnm2, Dnm3, Eea1, Egf, Ehd1, Ehd2, Ehd4, Epn1, Epn2, Eps15, Fgfr2, Fgfr4, Git1, Glit2, Grk5, Grk6, H2-D1, H2-K1, H2-Q6, H2-Q9, H2-T3, Hspa1a, Igf1r, II2ra, Iqsec1, Itch, Kdr, Met, Mvb12b, Nedd4l, Ntrk1, Pard3, Pard6b, Pip4k2b, Pip5k1a, Pip5k1b, Pip5k1c, Pld1, Prkcz, Psd, Psd3, Psd4, Rabila, Rab11b, Rab11fip1, Rab11fip2, Rab11fip5, Rab22a, Rab31, Rab5b, Rab5c, Rabep1, Sh3gl1, Sh3gl3, Sh3glb1, Sh3kbp1, Smap2, Smurf1, Src, Tfrc, Tgfbrl, Tgfbr2, Tsg101, Vps37b, Vps37C, Vps45, Vps4b | 4.54E-07 | 2.94E-06 |
| 4 | mmu04666: Fe gamma R-mediated phagocytosis | Akt1, Akt3, Arf6, Arpdb, Arpc2, Arpc4, Arpc5l, Asapl, Asap3, Cdc42, Dnm1, Dnm2, Dnm3, Fcgr1, Fcgr2b, Gab2, HCck, Igh, Inpp5d, Limk1, Limk2, Lyn, Map2k1, Myo10, Ncf1, Pak1, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Pip4k2b, Pip5k1a, Pip5k1b, Pip5k1c, Pla2g4a, Pla2g6, Plcg2, Pld1, Ppap2a, Ppap2b, Prkca, Prkcb, Prkcd, Prkce, Ptprc, Scin, Sphk2, Syk, Vav1, Vav1, Vav3, Wasf2 | 5.03E-07 | 3.26E-06 |
| 5 | mmu04010: MAPK signaling pathway | Acvr1b, Akt1, Akt3, Arrbl, Atf2, B230120H23Rik, Cacna1a, Cacna1b, Cacna1d, Cacna1f, Cacna1g, Cacna2d3, Cacnb2, Cacnb4, Cacng2, Cacng4, Casp3, Cdc42, Chuk, Dusp16, Dusp2, Dusp3, Dusp4, Dusp5, Dusp6, Egf, Fas, Fgf14, Fgf18, Fgfr1, Fgfr2, Fgfr4, Fina, Flnb, Fine, Gadd45a, Gna12, Gng12, Grb2, Hspala, Ikbkb, II1a, II1r1, II1r2, Map2k1, Map2k3, Map2k6, Map3k1, Map3k11, Map3k12, Map3k13, Map3k14, Map3k2, Map3k3, Map3k5, Map3k7, Map4k2, Map4k3, Map4k4, Mapk14, Mapk9, Mapkapk2, Mapt, Max, Mef2c, Mknk1, Mras, Myc, Nf1, Nfatc2, Nfatc4, Nfkb1, Nr4a1, Ntrk1, Pak1, Pak2, Pla2g12a, Pla2g2e, Pla2g4a, Pla2g5, Pla2g6, Ppm1a, Ppm1b, Ppp3ca, Ppp5c, Prkaca, Prkca, Prkcb, Ptpn5, Ptpn7, Rapla, Rapgef2, Rasgrf1, Rasgrf2, Rasgrp1, Rasgrp3, Rps6ka2, Rps6ka4, Rps6ka5, Rras2, Srf, Stk3, Tab2, Taok3, Tgfb1, Tgfb2, Tgfbr1, Tgfbr2, Tm4sf19, Tnfrsf1a | 6.23E-06 | 4.04E-05 |
| 6 | mmu04810: Regulation of actin cytoskeleton | Abi2, Actb, Actnl, Arhgef12, Arhgef6, Arhgef7, Arpc1b, Arpc2, Arpc4, Arpc5l, Baiap2, Bcarl, Cdc42, Chrm3, Csk, Cyfip1, Cyfip2, Diap1, Diap2, Diap3, Dock1, Egf, Fgd1, Fgd3, Fgf14, Fgf18, Fgfr1, Fgfr2, Fgfr4, Git1, Gna12, Gng12, Iqgap1, Iqgap3, Itga2, Itga4, Itga5, Itga6, Itga7, Itga8, Itga9, Itgad, Itgae, Itgam, Itgav, Itgax, Itgb3, Itgb5, Itgb7, Limk1, Limk2, Map2k1, Mras, Msn, Myh10, Myh14, Myh9, Myl10, Myl12a, Mylk, Nckap1, Nckap1l, Pak1, Pak2, Pdgfc, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Pip4k2b, Pip5k1a, Pip5k1b, Pip5k1c, Ppp1r12a, Ptk2, Pxn, Rock2, Rras2, Scin, Ssh1, Ssh2, Tiam1, Tmsb4x, Vav1, Vav2, Vav3, Vcl, Wasf2 | 1.38E-04 | 8.96E-04 |
| 7 | mmu05200: Pathways in cancer | Abl1, Acvr1b, Akt1, Akt3, Arnt, Arnt2, Axin1, Bcl2, Bcl2l1, Bcr, Bid, Birc2, Birc3, Casp3, Casp8, Casp9, Cbl, Cblb, Ccdc6, Ccne1, Cdc42, Cdk6, Cdkn1a, Cdkn1b, Chuk, Col4a1, Col4a2, Col4a6, Crebbp, Csf1r, Csf3r, Ctbp2, Ctnna2, Dapk1, Dapk2, E2f1, Egf, Egln3, Epas1, Fas, Fgf14, Fgf18, Fgfrl, Fgfr2, Flt3, Fzd3, GN2, Grb2, Gsk3b, Hsp90ab1, Igf1, Igf1r, Ikbkb, Itga2, Itga6, Itgav, Jak1, Lama5, Lambí, Lamc1, Lef1, Map2k1, Mapk9, Max, Met, Mitf, Msh3, Msh6, Myc, Nfkb1, Ntrk1, Pax8, Pgf, Piasl, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Plcg2, Pml, Ppard, Pparg, Prkca, Prkcb, Pten, Ptk2, Ralb, Ralgds, Rara, Rassf1, Rassf5, Rb1, Runx1, Rxra, Rxrb, Spi1, Skp2, Slc2a1, Smad3, Smo, Stat1, Stat3, Stat5a, Stat5b, SufU, Tceb1, Tcf7l2, Tfg, Tgfb1, Tgfb2, Tgfbr1, Tgfbr2, Tpm3, Traf1, Traf3, Traf5, Vegefa, Wnt1, Wnt2b, Wnt5b, Wnt7b, Zbtb16 | 1.39E-04 | 8.99E-04 |
| 8 | mmu04662: B cell receptor signaling pathway | Akt1, Akt3, Blnk, Btk, Card11, Cd72, Cd81, Chuk, Dapp1, Fcgr2b, Grb2, Gsk3b, Igh, Ikbkb, Inpp5d, Lyn, Map2k1, Nfat5, Nfatc1, Nfatc2, Nfatc3, Nfatc4, Nfkb1, Nfkbie, Pik3ap1, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Pirb, Plcg2, Ppp3ca, Prkcb, Ptpn6, Rasgrp3, Syk, Tm4sf19, Vav1, Vav2, Vav3 | 2.18E-04 | 0.001413938 |
| 9 | mmu04210: Apoptosis | Akt1, Akt3, Apaf1, Atm, Bcl2, Bcl2l1, Bid, Birc2, Birc3, Capn2, Casp3, Casp7, Casp8, Casp9, Cflar, Chuk, Csf2rb, Csf2rb2, Endod1, Fas, Ikbkb, II1a, II1r1, II1rap, Irak2, Irak3, Irak4, Map3k14, Myd88, Nfkb1, Ntrk1, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Ppp3ca, Prkaca, Prkar1b, Prkar2a, Prkar2b, Tm4sf19, Tnfrsf1A | 4.23E-04 | 0.002744042 |
| 10 | mmu05222: Small cell lung cancer | Akt1, Akt3, Apaf1, Bcl2, Bcl2l1, Birc2, Birc3, Casp9, Ccne1, Cdk6, Cdkn1b, Chuk, Col4a1, Col4a2, Col4a6, E2f1, Fhit, Ikbkb, Itga2, Itga6, Itgav, Lama5, Lamb1, Lamc1, Max, Myc, Nfkb1, Pias1, Pik3cb, Pik3cd, Plik3cg, Pik3r1, Pik3r3, Pik3r5, Pten, Ptk2, Rb1, Rxra, Rxrb, Skp2, Traf1, Traf3, Traf5 | 5.60E-04 | 0.003631259 |
| 11 | mmu04670: Leukocyte transendothelial migration | Actb, Actn1, Arhgap5, Bcar1, Cdc42, Cldn14, Cldn23, Ctnna2, Ctnnd1, Cxcr4, Cyba, Cybb, Esam, F11r, Gnai2, Gnai3, Grlf1, Icam1, Itga4, Itgam, Jam3, Mapk14, Mllt4, Msn, Myl10, Myl12a, Ncf1, Ncf2, Ncf4, Nox1, Pecam1, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Plcg2, Prkca, Prkcb, Ptk2, Ptk2b, Ptpn11, Pxn, Rap1a, Rapgef4, Rassf5, Rock2, Sipa1, Txk, Vav1, Vav2, Vav3, Vcam1, Vcl | 6.57E-04 | 0.004260774 |
| 12 | mmu04062: Chemokine signaling pathway | Adcy3, Adcy7, Adrbk1, Akt1, Akt3, Arrb1, BcaM, Ccl1, Ccl2, Ccl3, Ccl4, Ccl5, Ccl9, Ccr1, Ccr6, Cdc42, Chuk, Csk, Cx3cl1, Cx3cr1, Cxcr2, Cxcr3, Cxcr4, Elmo1, Fgr, Foxo3, Gnai2, Gnai3, Gnb1, Gng12, Gng2, Gng4, Gngt2, Grb2, Grk5, Grk6, Gsk3a, Gsk3b, Hck, Ikbkb, Lyn, Map2k1, Ncf1, Nfkb1, Pak1, Pard3, Pf4, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Plcb2, Plcb4, Prex1, Prkaca, Prkcb, Prkcd, Prkcz, Ptk2, Ptk2b, Pxn, Rap1a, Rock2, Shc1, Shc2, Shc4, Stat1, Stat3, Stat5b, Tiam1, Vav1, Vav2, Vav3, Xcr1 | 8.52E-04 | 0.005526401 |
| 13 | mmu05220: Chronic myeloid leukemia | Abl1,Acvr1b, Akt1, Akt3, Bcl2l1, Bcr, Cbl, Cblb, Cdk6, Cdkn1a, Cdkn1b, Chhuk, Ctbp2, E2f1, Gab2, GRrb2, Ikbkb, Map2k1, Myc, Nfkb1, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Ptpn11, Rb1, Runx1, Shc1, Shc2, Shc4, Smad3, Stat5a, Stat5b, Tgfb1, Tgfb2, Tgfbr1, Tgfbr2 | 0.001156477 | 0.007503174 |
| 14 | mmu04070: Phosphatidylinositol signaling system | Calm1, Calm3, Cds2, Dgkd, Dgkg, Dgki, Dgkz, Inpp4a, Inpp5a, Inpp5d, Inpp5k, Itpk1, Itpkb, Itpr1, Itpr2, Itpr3, Ocrl, PI4kb, Pik3c2a, Pik3c2b, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Pip4k2b, Pip5k1a, Pip5k1b, Pip5k1c, Plcb2, Plcb4, Plcd3, Plce1, Plcg2, Prkca, Prkcb, Pten, Synj1 | 0.002271981 | 0.014748224 |
| 15 | mmu04660: T cell receptor signaling pathway | Akt1, Akt3, Card11, Cbl, Cblb, Cd247, Cd28, Cd4, Cdc42, Chuk, Ctla4, Fyn, Grap2, Grb2, Gsk3b, Icos, Ikbkb, II10, II4, Lcp2, Map2Kk1, Map3k14, Map3k7, Mapk14, Mapk9, Nck1, Nck2, Nfat5, Nfatc1, Nfatc2, Nfatc3, Nfatc4, Nfkb1, Nfkbie, Pak1, Pak2, Pdk1, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Ppp3ca, Prkcq, PtpnN6, Ptprc, Rasgrp1, Tec, Tm4sf19, Vav1, Vav2, Vav3 | 0.002792449 | 0.018131185 |
| 16 | mmu05212: Pancreatic cancer | Acvr1b, Akt1, Akt3, Arhgef6, Bcl2l1, Casp9, Cdc42, Cdk6, Chuk, E2f1, Egf, Ikbkb, Jak1, Map2k1, Mapk9, Nfkbl, Pgf, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Pld1, Ralb, Ralgds, Rb1, Smad3, Stat1, Stat3, Tgfb1, Tgfb2, Tgfbr1, Tgfbr2, Vegfa | 0.015811557 | 0.103295105 |
| 17 | mmu04650: Natural killer cell mediated cytotoxicity | Bid, Casp3, Cd244, Cd247, Fas, Fcgr3, Fcgr4, Fyn, Grb2, H2-D1, H2-K1, Hcst, Icam1, Icam2, Ifnab, Ifngr1, Ifngr2, Igh, Klrb1c, Lcp2, Map2k1, Nfat5, Nfatd, Nfatc2, Nfatc3, Nfatc4, Pak1, Pik3cb, Pik3cd, Pik3cg, Pik3r1, Pik3r3, Pik3r5, Plcg2, Ppp3ca, Prkca, Prkcb, Ptk2b, Ptpn11, Ptpn6, Raet1d, Raet1e, Sh3bp2, Shd, Shc2, Shc4, Syk, Tm4sf19, Tyrobp, Vav1, Vav2, Vav3 | 0.038233058 | 0.252464917 |
Notes: By importing Entrez Gene IDs of 5,264 ChIP-Seq-based Spi1 target genes into the Functional Annotation tool of DAVID, KEGG pathways showing significant relevance to the set of imported genes were identified. They are listed with pathways, focused genes, p-value corrected by Bonferroni multiple comparison test, and false discovery rate (FDR).
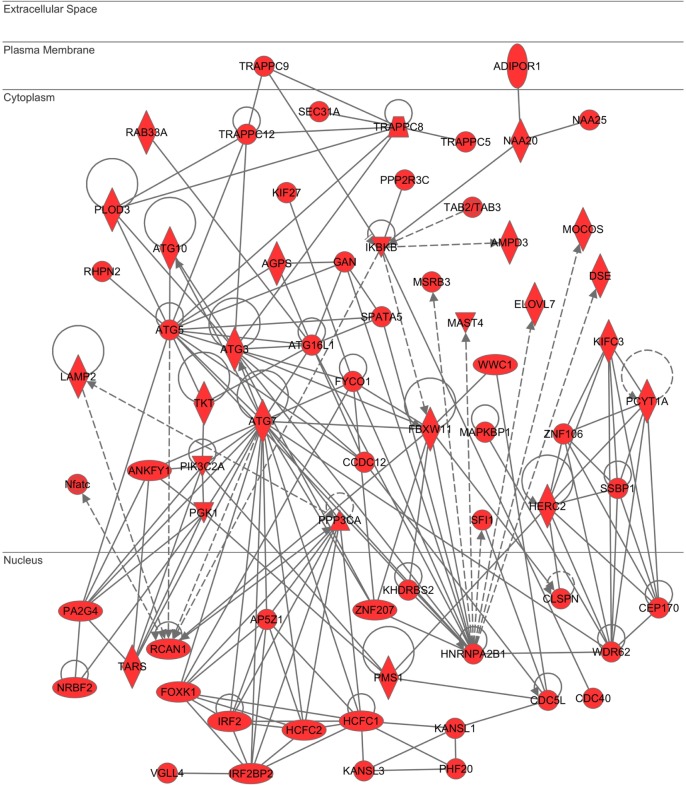
Next, we studied molecular networks of 5,264 Spi1 target genes by using the core analysis tool of IPA. They showed a significant relationship with canonical pathways defined as “Fcγ receptor-mediated phagocytosis in macrophages and monocytes” (P = 2.11E-15), “Molecular mechanisms of cancer” (P = 6.92E-15), “B cell receptor signaling” (P = 2.77E-14), “Role of NFAT in regulation of the immune response” (P = 1.17E-12), and “PI3K signaling in B lymphocytes” (P = 2.15E-12). The results of KEGG and IPA combined together indicated that Spi1 regulates expression of not only the genes crucial for normal function of monocytes/macrophages and B cells but also those involved in oncogenesis, particularly in leukemogenesis. IPA also identified functional networks relevant to Spi1 target genes (Supplementary Table 3). The most significant network was defined as “Cell Morphology, Cellular Function and Maintenance, Cell Death and Survival” (P = 1.00E-53), where key components of autophagosomes, such as ATG3, ATG5, ATG7, and ATG10, are clustered (Fig. 5). The second rank network represented “RNA Post-Transcriptional Modification, Cellular Assembly and Organization, Infectious Disease” (P = 1.00E-53).
Figure 5.
IPA “Cell Morphology, Cellular Function and Maintenance, Cell Death and Survival” network relevant to Spi1 target genes. Entrez Gene IDs of 5,264 ChIP-Seq-based Spi1 target genes were imported into the Core Analysis tool of IPA. It extracted the “Cell Morphology, Cellular Function and Maintenance, Cell Death and Survival” network as the first rank significant functional network as listed in Supplementary Table 3. Spi1 target genes are colored by red.
Finally, we studied molecular networks of 5,264 Spi1 target genes by using KeyMolnet. The neighboring network-search algorithm extracted the highly complex network composed of 5,788 molecules and 13,719 molecular relations (Supplementary Fig. 4). It showed the most significant relationship with “Transcriptional regulation by RB/E2F” (P = 7.27E-193). We identified Rb1 (FE = 17.7), Rbl1 (FE = 25.8), Rbl2 (FE = 37.1), and E2f1 (FE = 18.6) as a group of Spi1 target genes (Supplementary Table 1).
Discussion
Mice lacking PU.1/Spi1 are devoid of microglia, indicating that PU.1 acts as an indispensable transcription factor for development and differentiation of microglia.13,31,32 By analyzing a ChIP-Seq dataset, we identified 5,264 Spi1 target protein-coding genes in BV2 mouse microglial cells. BV2 cells are derived from immortalized microglia of newborn mouse origin that express morphological, phenotypical, and functional characteristics of primary microglia.33 We extracted the genes overlapping between data derived from two distinct peak-finding algorithms MACS and PICS termed as the set of most reliable Spi1 targets. Enrichment of the PU-box consensus sequences within the genomic regions surrounding ChIP-Seq peaks validated the reliability of our analysis. By using pathway analysis tools named KEGG and IPA, we found that ChIP-Seq-based Spi1 target genes show a significant relationship with diverse pathways essential for normal function of monocytes/macrophages, such as endo-cytosis, Fcγ receptor-mediated phagocytosis, and lysosomal degradation, along with the pathway closely related to leuke-mogenesis. Relevantly, mice with reduced expression of PU.1 develop acute myeloid leukemia.34 Approximately two-thirds (63%) of “microglial sensome” genes that reflect the micro-glia-specific gene signature30 corresponded to Spi1 targets. These observations suggest that Spi1 plays a pivotal role in regulation of the genes relevant to specialized functions of microglia. KeyMolnet constructed the complex network of Spi1 target genes showing the most significant relationship with transcriptional regulation by RB/E2F. PU.1, capable of interacting physically with the C pocket of phosphorylated retinoblastoma (Rb) protein, blocks erythroid differentiation by repressing GATA-1 in mouse erythroleukemia cells.35
The set of 5,264 Spi1 target genes include Spi1 itself, supporting the previous observations that PU.1 activates its own promoter elements via an autoregulatory loop.36 We found that approximately one-third of Spi1 target genes in microglia are potentially coregulated by Cebpa, a transcription factor essential for the development of monocytic and granulocytic lineage cells.37 Importantly, Cebpa directly activates PU.1 gene transcription by binding to its promoter and distal enhancer.38 A previous study showed that the Cebpa– Spi1 pathway plays a central role in regulation of microglial proliferation in a mouse model of prion diseases.39
NHD is a rare autosomal recessive disorder characterized by progressive dementia and multifocal bone cysts, caused by genetic mutations of either DAP12 or TREM2.29 Pathologically, NHD brains exhibit extensive demyelination and gliosis distributed predominantly in the frontal and temporal lobes and the basal ganglia, accompanied by marked accumulation of axonal spheroids and microglia.40 TREM2 acts as a phagocytic receptor expressed on osteoclasts, dendritic cells, macrophages, and microglia, where it constitutes a signaling complex with an adaptor molecule DAP12, leading to phos-phorylation and activation of the downstream kinase Syk. TREM2 expressed on microglia plays a key role in the clearance of damaged neural tissues to resolve damage-induced inflammation.41 We identified Trem2, Tyrobp (Dap12), and Syk as a group of Spi1 target genes, consistent partly with previous observations.42 Importantly, Dap12 serves as a hub of the “microglial sensome” network, on which major molecular connections are concentrated.30 These observations indicate that aberrant function of microglia plays a central role in the pathogenesis of NHD.
A recent study by combining genome-wide linkage analysis and exome sequencing identified several mutations in the CSF1R gene in patients with hereditary diffuse leukoencephalopathy with spheroids (HDLS), a rare autosomal dominant disease that affects predominantly the CNS white matter.43 Clinically, HDLS exhibits early-onset personality and behavioral disturbances, dementia, and parkinsonism. HDLS shows striking similarities to the pathology of NHD, in view of diffuse demyelination and gliosis with morphologically abnormal microglia and marked accumulation of axonal spheroids, although HDLS never exhibits bone cysts and basal ganglia calcification, both of which are characteristic features of NHD. We identified Csf1r, Csf1, and Il34 as another group of Spi1 target genes, indicating that HDLS represents a disease entity designated as “microgliopathy” caused by microglial dysfunction. Based on these observations, we could propose a hypothesis that microglial dysfunction caused by aberrant regulation of PU.1 target genes contributes to the pathogenesis of various neurodegenerative and neuroinflammatory diseases. Importantly, a recent study indicates that DAP12 acts as a central regulator in gene networks of the late-onset AD.44
Although ChIP-Seq serves as a highly efficient method for genome-wide profiling of transcription factor-binding sites, the method intrinsically requires several technical considerations to achieve reproducibility of the results.45 The specificity of antibodies, the sequencing depth and coverage, the source of target cell types and relevant controls, developmental stages, and culture conditions constitute critical factors capable of affecting both genetic and epigenetic features. Motif analysis of a defined set of high-quality peaks makes it possible to evaluate the antibody specificity and to predict the specificity of DNA–protein interaction to some extent.45 In general, DNA-binding by transcription factors is a highly dynamic process following recruitment of the complex of auxiliary factors, such as coactivators and corepressors. However, in most occasions, ChIP-Seq data generally reflect a snapshot of binding actions of limited DNA-binding factors onto responsive elements, not always corresponding to their biological activities. Because of these limitations, it is highly important to validate main results by examining technical and biological replicates of samples with different sources of ChIP-quality antibodies, along with transcriptome analysis.
Conclusions
By analyzing a ChIP-Seq dataset numbered SRP036026 with the Strand NGS program, we identified 5,264 Spi1 target protein-coding genes in BV2 mouse microglial cells. They included Spi1, Irf8, Runx1, Csf1r, Csf1, Il34, Aif1 (Iba1), Cx3cr1, Trem2, and Tyrobp. Motif analysis identified the PU-box consensus sequences in the genomic regions surrounding ChIP-Seq peaks. By using pathway analysis tools of bioinformatics, we found that ChIP-Seq-based Spi1 target genes show a significant relationship with diverse pathways essential for normal function of monocytes/macrophages, such as endocytosis, Fcγ receptor-mediated phagocytosis, and lysosomal degradation. These results suggest that PU.1/Spi1 plays a pivotal role in regulation of the genes relevant to specialized functions of microglia. Therefore, aberrant regulation of PU.1 target genes might contribute to the development of neurodegenerative diseases with accumulation of activated microglia.
Supplementary Materials
Supplementary Figure 1. FastQC analysis of ChIP-Seq data. FASTQ format files composed of cleaned NGS data derived from Spi1 (panel a) or Cebpa (panel b) ChIP-Seq were imported into the FastQC program. The per base sequence quality score is shown with the median (red line), the mean (blue line), and the interquartile range (yellow box).
Supplementary Figure 2. Genomic locations of Spi1 ChIP-Seq peaks on the Trem2 gene. The genomic locations of Spi1 ChIP-Seq peaks were determined by importing the processed data into GenomeJack. An example of triggering receptor expressed on myeloid cells 2 (Trem2; Entrez Gene ID 83433) is shown, where a MACS peak numbered 51970 in the Spi1.bam Coverage lane is located in the intronic region of the Trem2 gene (panel a) with a Spi1-binding consensus sequence motif highlighted by orange square (panel b).
Supplementary Figure 3. Genomic locations of Spi1 ChIP-Seq peaks on the Tyrobp gene. The genomic locations of Spi1 ChIP-Seq peaks were determined by importing the processed data into GenomeJack. An example of TYRO protein kinase binding protein (Tyrobp, Dap12; Entrez Gene ID 22177) is shown, where a MACS peak numbered 100752 in the Spi1.bam Coverage lane is located in the promoter region of the Tyrobp gene (panel a) with a Spi1-binding consensus sequence motif (reverse complement) highlighted by orange square (panel b).
Supplementary Figure 4. KeyMolnet molecular network relevant to Spi1 target genes. Entrez Gene IDs of 5,264 Spi1 target genes were imported into KeyMolnet. The neighboring network-search algorithm extracted the extremely complex network composed of 5,788 molecules and 13,719 molecular relations, showing the most significant relationship with “Transcriptional regulation by RB/E2F”. Red nodes indicate those closely related to imported genes. White nodes exhibit additional nodes extracted automatically from the core contents of KeyMolnet to establish molecular connections. The molecular relation is indicated by solid line with arrow (direct binding or activation), solid line with arrow and stop (direct inactivation), solid line without arrow (complex formation), dash line with arrow (transcriptional activation), and dash line with arrow and stop (transcriptional repression).
Supplementary Table 1. The set of 5,264 ChIP-Seq-based Spi1 target genes in microglia. From the ChIP-Seq dataset, we identified 5,264 Spi1-target genes in BV2 mouse microglia showing fold enrichment (FE) ≥5. They are listed with the chromosome, the position of the peak (start, end), FE, Entrez Gene ID, Gene Symbol, and Gene Name. The set of 1,844 Cebpa-target genes are underlined.
Supplementary Table 2. The list of 100 microglial sen-some genes. The set of 100 microglial sensome genes (Ref. 30) are listed in order of their expression levels with Entrez Gene ID, Gene Symbol, Gene Name, and an existence of ChIP-Seq-based peaks for Spi1 or Cebpa.
Supplementary Table 3. IPA functional networks relevant to ChIP-Seq-based Spi1 target genes in microglia. By importing Entrez Gene IDs of 5,264 ChIP-Seq-based Spi1 target genes into the core analysis tool of IPA, functional networks showing significant relevance to the imported genes were identified. They are listed with rank, category of functional networks, focused molecules, and P-value by the Fisher’s exact test.
Acknowledgments
The authors thank Ms. Mutsumi Motouri for her invaluable help.
Footnotes
Author Contributions
JS designed the methods, analyzed the data, and drafted the manuscript. NA, SK, and YK helped with the data analysis. All authors have read and approved the final manuscript.
ACADEMIC EDITOR: James Willey, Editor in Chief
FUNDING: This work was supported by the JSPS KAKENHI (C25430054), the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and the grant from the National Center for Geriatrics and Gerontology (NCGC26–20). The authors confirmed that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: The authors declare no competing interests.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11(11):775–87. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 2.Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz C, Perdiguero EG, Wieghofer P, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16(3):273–80. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 4.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 5.Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. 2014;14(7):463–77. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 6.Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Ab uptake and clearance. Brain. 2006;129(pt 11):3006–19. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halle A, Hornung V, Petzold GC, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol. 2008;9(8):857–65. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Béraud D, Twomey M, Bloom B, et al. α-Synuclein alters toll-like receptor expression. Front Neurosci. 2011;5:80. doi: 10.3389/fnins.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crotti A, Benner C, Kerman BE, et al. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat Neurosci. 2014;17(4):513–21. doi: 10.1038/nn.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turkistany SA, DeKoter RP. The transcription factor PU.1 is a critical regulator of cellular communication in the immune system. Arch Immunol Ther Exp (Warsz) 2011;59(6):431–40. doi: 10.1007/s00005-011-0147-9. [DOI] [PubMed] [Google Scholar]
- 11.Pham TH, Minderjahn J, Schmidl C, et al. Mechanisms of in vivo binding site selection of the hematopoietic master transcription factor PU.1. Nucleic Acids Res. 2013;41(13):6391–402. doi: 10.1093/nar/gkt355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKercher SR, Torbett BE, Anderson KL, et al. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15(20):5647–58. [PMC free article] [PubMed] [Google Scholar]
- 13.Beers DR, Henkel JS, Xiao Q, et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103(43):16021–6. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith AM, Gibbons HM, Oldfield RL, et al. M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia. J Neuroinflammation. 2013;10:85. doi: 10.1186/1742-2094-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith AM, Gibbons HM, Oldfield RL, et al. The transcription factor PU.1 is critical for viability and function of human brain microglia. Glia. 2013;61(6):929–42. doi: 10.1002/glia.22486. [DOI] [PubMed] [Google Scholar]
- 16.Horiuchi M, Wakayama K, Itoh A, et al. Interferon regulatory factor 8/interferon consensus sequence binding protein is a critical transcription factor for the physiological phenotype of microglia. J Neuroinflammation. 2012;9:227. doi: 10.1186/1742-2094-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zusso M, Methot L, Lo R, Greenhalgh AD, David S, Stifani S. Regulation of postnatal forebrain amoeboid microglial cell proliferation and development by the transcription factor Runx1. J Neurosci. 2012;32(33):11285–98. doi: 10.1523/JNEUROSCI.6182-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park PJ. ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet. 2009;10(10):669–80. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satoh J, Kawana N, Yamamoto Y. Pathway analysis of ChIP-Seq-based NRF1 target genes suggests a logical hypothesis of their involvement in the pathogenesis of neurodegenerative diseases. Gene Regul Syst Bio. 2013;7:139–52. doi: 10.4137/GRSB.S13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27(2–3):229–37. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Liu T, Meyer CA, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9(9):R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Robertson G, Krzywinski M, et al. PICS: probabilistic inference for ChIP-seq. Biometrics. 2011;67(1):151–63. doi: 10.1111/j.1541-0420.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 23.Li L. GADEM: a genetic algorithm guided formation of spaced dyads coupled with an EM algorithm for motif discovery. J Comput Biol. 2009;16(2):317–29. doi: 10.1089/cmb.2008.16TT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 25.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40(Database Issue):D109–14. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh J, Tabunoki H. Comprehensive analysis of human microRNA target networks. BioData Min. 2011;4:17. doi: 10.1186/1756-0381-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin H, Li L, Xu J, et al. Runx1 regulates embryonic myeloid fate choice in zebrafish through a negative feedback loop inhibiting Pu.1 expression. Blood. 2012;119(22):5239–49. doi: 10.1182/blood-2011-12-398362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Szretter KJ, Vermi W, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13(8):753–60. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klünemann HH, Ridha BH, Magy L, et al. The genetic causes of basal ganglia calcification, dementia, and bone cysts: DAP12 and TREM2. Neurology. 2005;64(9):1502–7. doi: 10.1212/01.WNL.0000160304.00003.CA. [DOI] [PubMed] [Google Scholar]
- 30.Hickman SE, Kingery ND, Ohsumi TK, et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16(12):1896–905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kierdorf K, Prinz M. Factors regulating microglia activation. Front Cell Neurosci. 2013;7:44. doi: 10.3389/fncel.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7:45. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henn A, Lund S, Hedtjärn M, Schrattenholz A, Pörzgen P, Leist M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX. 2009;26(2):83–94. doi: 10.14573/altex.2009.2.83. [DOI] [PubMed] [Google Scholar]
- 34.Rosenbauer F, Wagner K, Kutok JL, et al. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat Genet. 2004;36(6):624–30. doi: 10.1038/ng1361. [DOI] [PubMed] [Google Scholar]
- 35.Rekhtman N, Choe KS, Matushansky I, Murray S, Stopka T, Skoultchi AI. PU.1 and pRB interact and cooperate to repress GATA-1 and block erythroid differentiation. Mol Cell Biol. 2003;23(21):7460–74. doi: 10.1128/MCB.23.21.7460-7474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H, Ray-Gallet D, Zhang P, et al. PU.1 (Spi-1) autoregulates its expression in myeloid cells. Oncogene. 1995;11(8):1549–60. [PubMed] [Google Scholar]
- 37.Wang D, D’Costa J, Civin CI, Friedman AD. C/EBPα directs monocytic commitment of primary myeloid progenitors. Blood. 2006;108(4):1223–9. doi: 10.1182/blood-2005-12-008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman AD. C/EBPa induces PU.1 and interacts with AP-1 and NF-kB to regulate myeloid development. Blood Cells Mol Dis. 2007;39(3):340–3. doi: 10.1016/j.bcmd.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gómez-Nicola D, Fransen NL, Suzzi S, Perry VH. Regulation of microglial proliferation during chronic neurodegeneration. J Neurosci. 2013;33(6):2481–93. doi: 10.1523/JNEUROSCI.4440-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satoh J, Tabunoki H, Ishida T, et al. Immunohistochemical characterization of microglia in Nasu-Hakola disease brains. Neuropathology. 2011;31(4):363–75. doi: 10.1111/j.1440-1789.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells−2. J Exp Med. 2005;201(4):647–57. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weigelt K, Ernst W, Walczak Y, et al. Dap12 expression in activated microglia from retinoschisin-deficient retina and its PU.1-dependent promoter regulation. J Leukoc Biol. 2007;82(6):1564–74. doi: 10.1189/jlb.0707447. [DOI] [PubMed] [Google Scholar]
- 43.Rademakers R, Baker M, Nicholson AM, et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet. 2011;44(2):200–5. doi: 10.1038/ng.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang B, Gaiteri C, Bodea LG, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153(3):707–20. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landt SG, Marinov GK, Kundaje A, et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 2012;22(9):1813–31. doi: 10.1101/gr.136184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. FastQC analysis of ChIP-Seq data. FASTQ format files composed of cleaned NGS data derived from Spi1 (panel a) or Cebpa (panel b) ChIP-Seq were imported into the FastQC program. The per base sequence quality score is shown with the median (red line), the mean (blue line), and the interquartile range (yellow box).
Supplementary Figure 2. Genomic locations of Spi1 ChIP-Seq peaks on the Trem2 gene. The genomic locations of Spi1 ChIP-Seq peaks were determined by importing the processed data into GenomeJack. An example of triggering receptor expressed on myeloid cells 2 (Trem2; Entrez Gene ID 83433) is shown, where a MACS peak numbered 51970 in the Spi1.bam Coverage lane is located in the intronic region of the Trem2 gene (panel a) with a Spi1-binding consensus sequence motif highlighted by orange square (panel b).
Supplementary Figure 3. Genomic locations of Spi1 ChIP-Seq peaks on the Tyrobp gene. The genomic locations of Spi1 ChIP-Seq peaks were determined by importing the processed data into GenomeJack. An example of TYRO protein kinase binding protein (Tyrobp, Dap12; Entrez Gene ID 22177) is shown, where a MACS peak numbered 100752 in the Spi1.bam Coverage lane is located in the promoter region of the Tyrobp gene (panel a) with a Spi1-binding consensus sequence motif (reverse complement) highlighted by orange square (panel b).
Supplementary Figure 4. KeyMolnet molecular network relevant to Spi1 target genes. Entrez Gene IDs of 5,264 Spi1 target genes were imported into KeyMolnet. The neighboring network-search algorithm extracted the extremely complex network composed of 5,788 molecules and 13,719 molecular relations, showing the most significant relationship with “Transcriptional regulation by RB/E2F”. Red nodes indicate those closely related to imported genes. White nodes exhibit additional nodes extracted automatically from the core contents of KeyMolnet to establish molecular connections. The molecular relation is indicated by solid line with arrow (direct binding or activation), solid line with arrow and stop (direct inactivation), solid line without arrow (complex formation), dash line with arrow (transcriptional activation), and dash line with arrow and stop (transcriptional repression).
Supplementary Table 1. The set of 5,264 ChIP-Seq-based Spi1 target genes in microglia. From the ChIP-Seq dataset, we identified 5,264 Spi1-target genes in BV2 mouse microglia showing fold enrichment (FE) ≥5. They are listed with the chromosome, the position of the peak (start, end), FE, Entrez Gene ID, Gene Symbol, and Gene Name. The set of 1,844 Cebpa-target genes are underlined.
Supplementary Table 2. The list of 100 microglial sen-some genes. The set of 100 microglial sensome genes (Ref. 30) are listed in order of their expression levels with Entrez Gene ID, Gene Symbol, Gene Name, and an existence of ChIP-Seq-based peaks for Spi1 or Cebpa.
Supplementary Table 3. IPA functional networks relevant to ChIP-Seq-based Spi1 target genes in microglia. By importing Entrez Gene IDs of 5,264 ChIP-Seq-based Spi1 target genes into the core analysis tool of IPA, functional networks showing significant relevance to the imported genes were identified. They are listed with rank, category of functional networks, focused molecules, and P-value by the Fisher’s exact test.