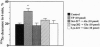Abstract
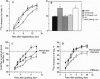
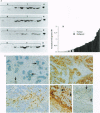
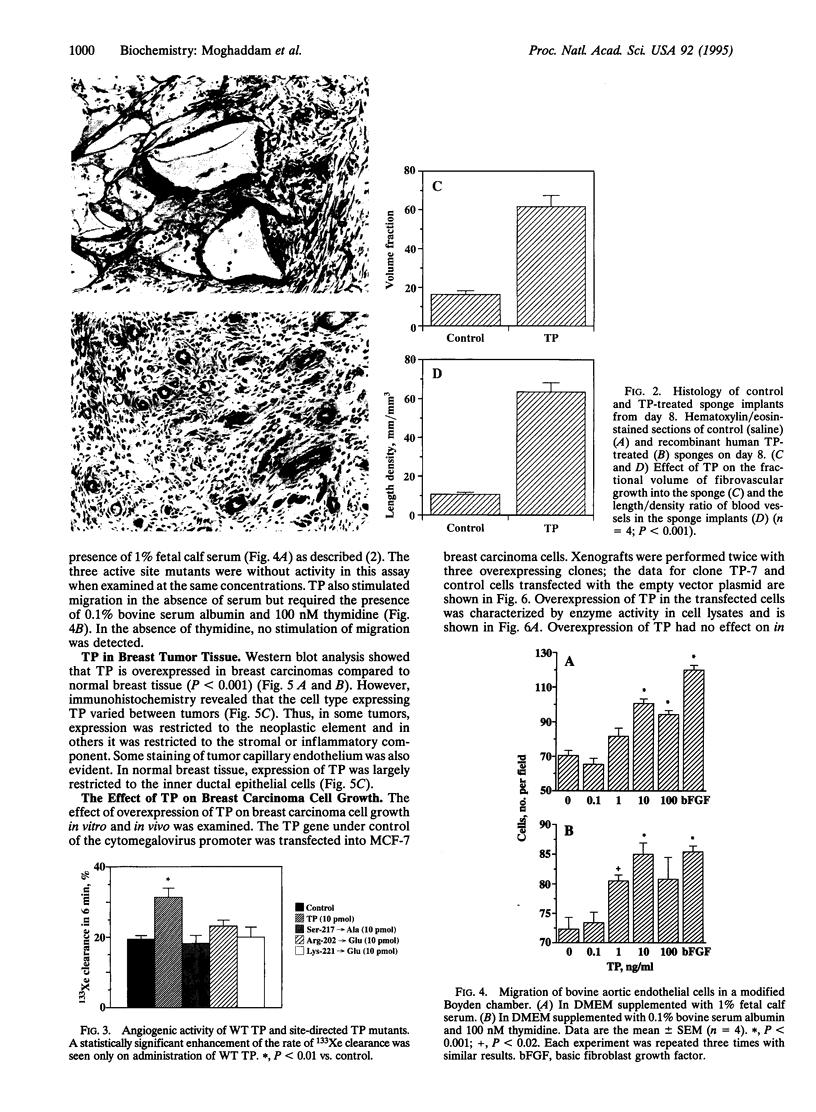
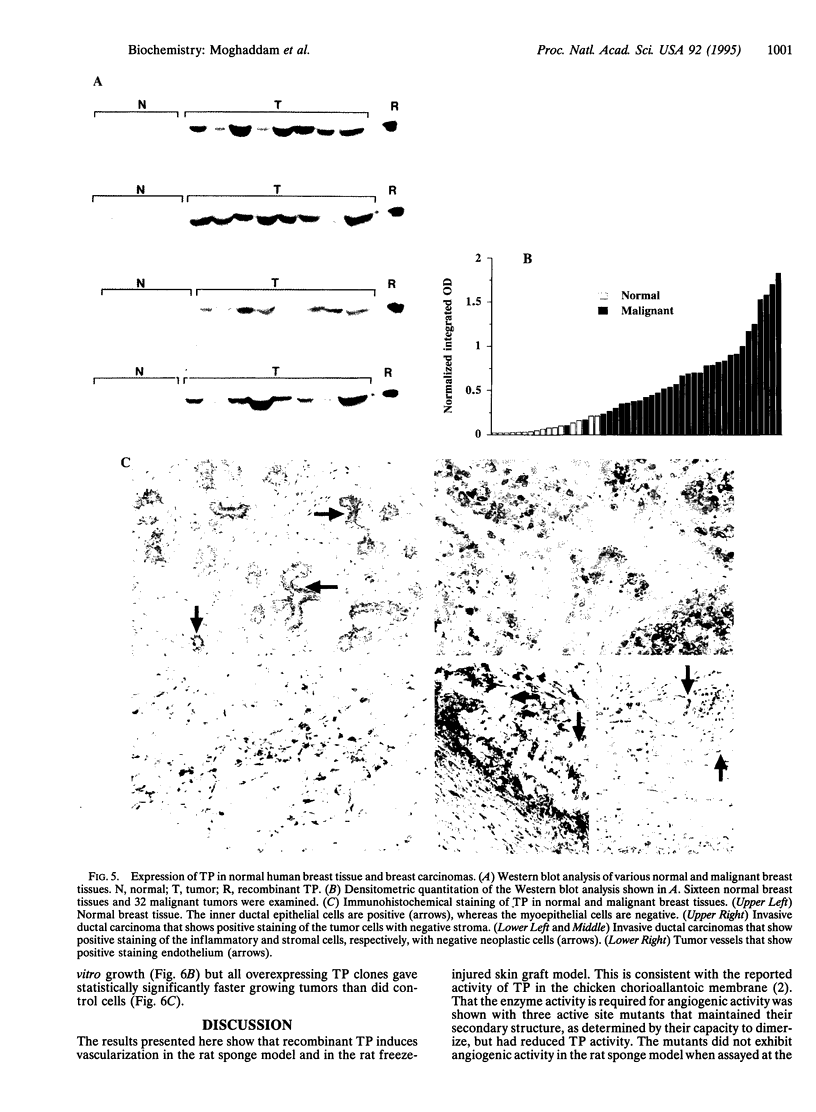
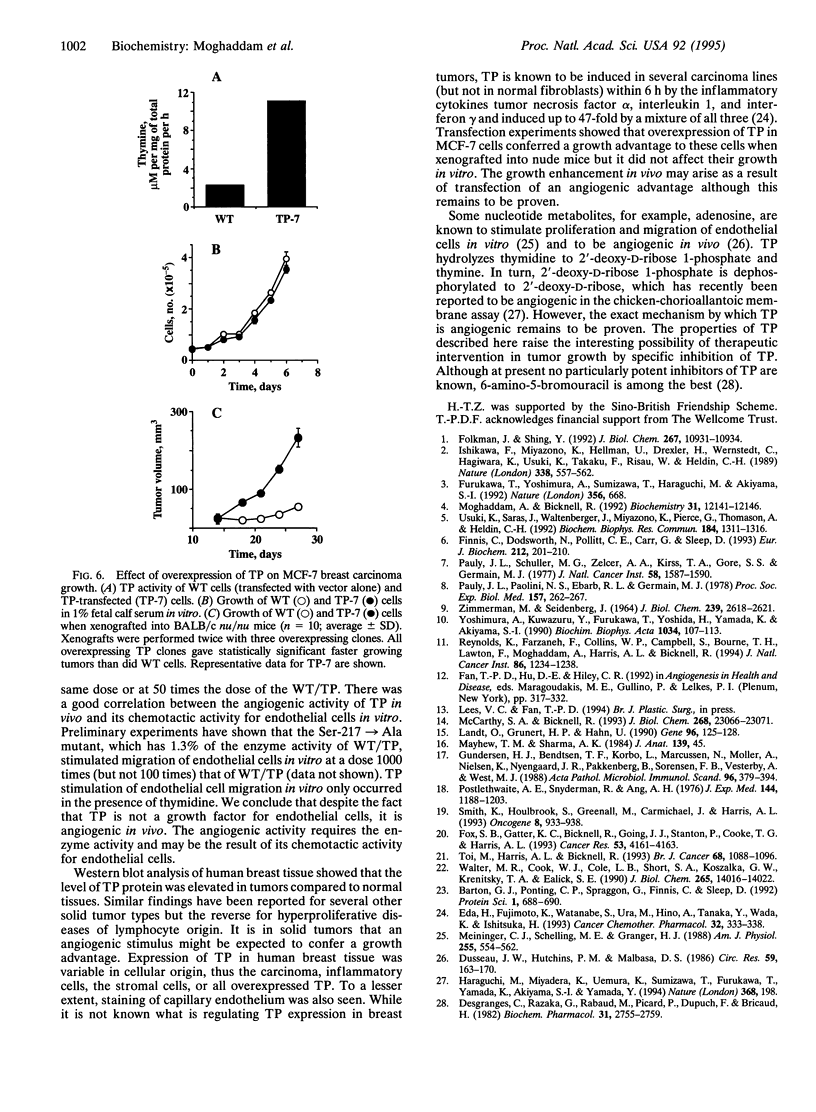
Platelet-derived endothelial cell growth factor was previously identified as the sole angiogenic activity present in platelets; it is now known to be thymidine phosphorylase (TP). The effect of TP on [methyl-3H]thymidine uptake does not arise from de novo DNA synthesis and the molecule is not a growth factor. Despite this, TP is strongly angiogenic in a rat sponge and freeze-injured skin graft model. Neutralizing antibodies and site-directed mutagenesis confirmed that the enzyme activity of TP is a condition for its angiogenic activity. The level of TP was found to be elevated in human breast tumors compared to normal breast tissue (P < 0.001). Overexpression of TP in MCF-7 breast carcinoma cells had no effect on growth in vitro but markedly enhanced tumor growth in vivo. These data and the correlation of expression in tumors with malignancy identify TP as a target for antitumor strategies.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton G. J., Ponting C. P., Spraggon G., Finnis C., Sleep D. Human platelet-derived endothelial cell growth factor is homologous to Escherichia coli thymidine phosphorylase. Protein Sci. 1992 May;1(5):688–690. doi: 10.1002/pro.5560010514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgranges C., Razaka G., Rabaud M., Picard P., Dupuch F., Bricaud H. The human blood platelet: a cellular model to study the degradation of thymidine and its inhibition. Biochem Pharmacol. 1982 Sep 1;31(17):2755–2759. doi: 10.1016/0006-2952(82)90129-0. [DOI] [PubMed] [Google Scholar]
- Dusseau J. W., Hutchins P. M., Malbasa D. S. Stimulation of angiogenesis by adenosine on the chick chorioallantoic membrane. Circ Res. 1986 Aug;59(2):163–170. doi: 10.1161/01.res.59.2.163. [DOI] [PubMed] [Google Scholar]
- Eda H., Fujimoto K., Watanabe S., Ura M., Hino A., Tanaka Y., Wada K., Ishitsuka H. Cytokines induce thymidine phosphorylase expression in tumor cells and make them more susceptible to 5'-deoxy-5-fluorouridine. Cancer Chemother Pharmacol. 1993;32(5):333–338. doi: 10.1007/BF00735915. [DOI] [PubMed] [Google Scholar]
- Finnis C., Dodsworth N., Pollitt C. E., Carr G., Sleep D. Thymidine phosphorylase activity of platelet-derived endothelial cell growth factor is responsible for endothelial cell mitogenicity. Eur J Biochem. 1993 Feb 15;212(1):201–210. doi: 10.1111/j.1432-1033.1993.tb17651.x. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Fox S. B., Gatter K. C., Bicknell R., Going J. J., Stanton P., Cooke T. G., Harris A. L. Relationship of endothelial cell proliferation to tumor vascularity in human breast cancer. Cancer Res. 1993 Sep 15;53(18):4161–4163. [PubMed] [Google Scholar]
- Furukawa T., Yoshimura A., Sumizawa T., Haraguchi M., Akiyama S., Fukui K., Ishizawa M., Yamada Y. Angiogenic factor. Nature. 1992 Apr 23;356(6371):668–668. doi: 10.1038/356668a0. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Bendtsen T. F., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B., Sørensen F. B., Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988 May;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Haraguchi M., Miyadera K., Uemura K., Sumizawa T., Furukawa T., Yamada K., Akiyama S., Yamada Y. Angiogenic activity of enzymes. Nature. 1994 Mar 17;368(6468):198–198. doi: 10.1038/368198a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa F., Miyazono K., Hellman U., Drexler H., Wernstedt C., Hagiwara K., Usuki K., Takaku F., Risau W., Heldin C. H. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989 Apr 13;338(6216):557–562. doi: 10.1038/338557a0. [DOI] [PubMed] [Google Scholar]
- Landt O., Grunert H. P., Hahn U. A general method for rapid site-directed mutagenesis using the polymerase chain reaction. Gene. 1990 Nov 30;96(1):125–128. doi: 10.1016/0378-1119(90)90351-q. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M., Sharma A. K. Sampling schemes for estimating nerve fibre size. I. Methods for nerve trunks of mixed fascicularity. J Anat. 1984 Aug;139(Pt 1):45–58. [PMC free article] [PubMed] [Google Scholar]
- McCarthy S. A., Bicknell R. Inhibition of vascular endothelial cell growth by activin-A. J Biol Chem. 1993 Nov 5;268(31):23066–23071. [PubMed] [Google Scholar]
- Moghaddam A., Bicknell R. Expression of platelet-derived endothelial cell growth factor in Escherichia coli and confirmation of its thymidine phosphorylase activity. Biochemistry. 1992 Dec 8;31(48):12141–12146. doi: 10.1021/bi00163a024. [DOI] [PubMed] [Google Scholar]
- Pauly J. L., Paolini N. S., Ebarb R. L., Germain M. J. Elevated thymidine phosphorylase activity in the plasma and ascitis fluids of tumor-bearing animals. Proc Soc Exp Biol Med. 1978 Feb;157(2):262–267. doi: 10.3181/00379727-157-40034. [DOI] [PubMed] [Google Scholar]
- Pauly J. L., Schuller M. G., Zelcer A. A., Kirss T. A., Gore S. S., Germain M. J. Identification and comparative analysis of thymidine phosphorylase in the plasma of healthy subjects and cancer patients. J Natl Cancer Inst. 1977 Jun;58(6):1587–1590. doi: 10.1093/jnci/58.6.1587. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K., Farzaneh F., Collins W. P., Campbell S., Bourne T. H., Lawton F., Moghaddam A., Harris A. L., Bicknell R. Association of ovarian malignancy with expression of platelet-derived endothelial cell growth factor. J Natl Cancer Inst. 1994 Aug 17;86(16):1234–1238. doi: 10.1093/jnci/86.16.1234. [DOI] [PubMed] [Google Scholar]
- Smith K., Houlbrook S., Greenall M., Carmichael J., Harris A. L. Topoisomerase II alpha co-amplification with erbB2 in human primary breast cancer and breast cancer cell lines: relationship to m-AMSA and mitoxantrone sensitivity. Oncogene. 1993 Apr;8(4):933–938. [PubMed] [Google Scholar]
- Toi M., Harris A. L., Bicknell R. cDNA transfection followed by the isolation of a MCF-7 breast cell line resistant to tamoxifen in vitro and in vivo. Br J Cancer. 1993 Dec;68(6):1088–1096. doi: 10.1038/bjc.1993.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuki K., Saras J., Waltenberger J., Miyazono K., Pierce G., Thomason A., Heldin C. H. Platelet-derived endothelial cell growth factor has thymidine phosphorylase activity. Biochem Biophys Res Commun. 1992 May 15;184(3):1311–1316. doi: 10.1016/s0006-291x(05)80025-7. [DOI] [PubMed] [Google Scholar]
- Walter M. R., Cook W. J., Cole L. B., Short S. A., Koszalka G. W., Krenitsky T. A., Ealick S. E. Three-dimensional structure of thymidine phosphorylase from Escherichia coli at 2.8 A resolution. J Biol Chem. 1990 Aug 15;265(23):14016–14022. doi: 10.2210/pdb1tpt/pdb. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Kuwazuru Y., Furukawa T., Yoshida H., Yamada K., Akiyama S. Purification and tissue distribution of human thymidine phosphorylase; high expression in lymphocytes, reticulocytes and tumors. Biochim Biophys Acta. 1990 Apr 23;1034(1):107–113. doi: 10.1016/0304-4165(90)90160-x. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN M., SEIDENBERG J. DEOXYRIBOSYL TRANSFER. I. THYMIDINE PHOSPHORYLASE AND NUCLEOSIDE DEOXYRIBOSYLTRANSFERASE IN NORMAL AND MALIGNANT TISSUES. J Biol Chem. 1964 Aug;239:2618–2621. [PubMed] [Google Scholar]