Abstract
Background
While the ergogenic effect of sodium bicarbonate (BICA) on short-term, sprint-type performance has been repeatedly demonstrated, little is known about its effectiveness during prolonged high-intensity exercise in well-trained athletes. Therefore, this study aims to examine the influence of BICA on performance during exhaustive, high-intensity endurance cycling.
Methods
This was a single-center, double-blind, randomized, placebo-controlled cross-over study. Twenty-one well-trained cyclists (mean ± SD: age 24±8 y, BMI 21.3±1.7, VO2peak 67.3±9.8 ml·kg−1·min−1) were randomly allocated to sequences of following interventions: oral ingestion of 0.3 g·kg−1 BICA or 4 g of sodium chloride (placebo), respectively. One h after ingestion subjects exercised for 30 min at 95% of the individual anaerobic threshold (IAT) followed by 110% IAT until exhaustion. Prior to these constant load tests stepwise incremental exercise tests were conducted under both conditions to determine IAT and VO2peak. Analysis of blood gas parameters, blood lactate (BLa) and gas exchange measurements were conducted before, during and after the tests. The main outcome measure was the time to exhaustion in the constant load test.
Results
Cycling time to exhaustion was improved (p<0.05) under BICA (49.5±11.5 min) compared with placebo (45.0±9.5 min). No differences in maximal or sub-maximal measures of performance were observed during stepwise incremental tests. BICA ingestion resulted in an increased pH, bicarbonate concentration and BLa before, throughout and after both exercise testing modes.
Conclusion
The results suggest that ingestion of BICA may improve prolonged, high-intensity cycling performance.
Trial Registration
German Clinical Trials Register (DRKS) DRKS00006198.
Introduction
During prolonged cycling the ability to sustain a high power output is limited by the resources of the athlete to resist fatigue. To date, the cause of fatigue is controversial and performance may be limited by numerous factors, including physiological and psychological components [1]. However, for high-intensity exercise perturbations in both blood and muscle acid-base balance are associated with a decline in work rate and performance [2]–[4]. Thus, it has been assumed that muscle fatigue occurring in high-intensity exercise is mainly caused by the accumulation of hydrogen ions (H+) in the myoplasm [2], [5]. The cellular mechanisms include detrimental effects on myofilament interaction [6], glycolytic flux [4] and sarcoplasmatic reticulum function [7]. The ability of the body to prevent or delay these force limiting processes is determined by the capacity of its intrinsic buffering systems [5], which counteract the accumulation of H+ both inside and outside the cell [8].
Supplementation of alkalizing agents such as sodium bicarbonate (BICA) indirectly affects intramuscular pH by increasing the H+ concentration gradient across the sarcolemma [9]. As a result, the co-transport of H+ and lactate from the myoplasm is enhanced [10]. These findings suggest that increased extracellular buffering capacity may influence muscle function and potentially improve exercise performance during continuous cycling.
Consequently, BICA has been studied as a potential ergogenic substance in several trials, mainly with a focus on short-term, sprint-type exercise. In a recently published meta-analysis it was concluded that BICA can be used as an ergogenic aid in short-term, high-intensity exercise [11]. By contrast, only few studies investigated the ergogenic potential of BICA in prolonged, high-intensity exercise with controversial findings [12]–[16]. These studies are based on small sample sizes and, in part [14], [15], on subjects who are not habituated to the applied exercise mode. This is despite the relevance for typical competition modes (especially in cycling) and the lower variability of performance in trained subjects. Therefore the aim of this study was to investigate the effects of BICA supplementation on performance during prolonged, high-intensity cycling to exhaustion in well-trained athletes.
Materials and Methods
Subjects
Participants were recruited between May 2011 and October 2011 through email. Twenty-one healthy, well-trained cyclists (16 male, 5 female; mean ± SD: age 24±8 y, BMI 21.3±1.7, VO2peak 67.3±9.8 ml·kg−1·min−1) met the following criterion for inclusion in the study: a maximal performance during a stepwise incremental cycle test of at least 4.5 W·kg−1 for females and 5.0 W·kg−1 for males, respectively. The sample consisted of nine racing bikers, nine mountain bikers and three triathletes. On their first visit to the laboratory each subject received a medical check-up consisting of 12-lead ECG, blood pressure measurement, whole-body plethysmography and blood analysis (routine blood count and clinical chemistry). During the test period subjects were instructed to continue their normal training routine but to refrain from exercise, alcohol and caffeine the day before each trial.
All subjects reached the age of majority (at least 18 years) and were fully informed of the experimental procedures and gave written informed consent prior to participation. The study was carried out in accordance with the declaration of Helsinki and approved by the local ethics committee (Aerztekammer des Saarlandes, Saarbruecken, Germany; approval number: 35/11).
Reasons for not registering this study before enrolment of participants started were low subject number and ready recruitement of subjects from a local pool of athletes. The authors confirm that all ongoing and related trials for this intervention are registered. The protocol for this trial and supporting CONSORT checklist are available as supporting information; see S1 and S2 Protocols and S1 Checklist. In addition, all relevant data are presented in tabular form; see S1 Data.
General Design
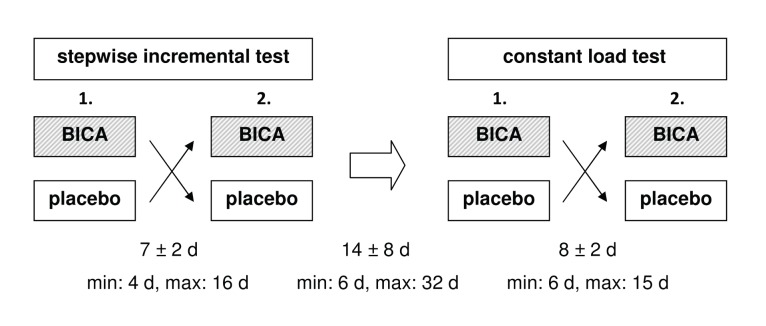
In a double-blind, randomized cross-over design, subjects underwent two stepwise incremental exercise tests and two constant load tests (with two phases) on an electrically braked cycle ergometer (Excalibur Sport, Lode, Groningen, The Netherlands). An overview of the design is given in Fig. 1. Each test type was completed under the following conditions: either after oral ingestion of 0.3 g·kg−1 BICA, or 4 g sodium chloride (placebo). Both supplements were solved in 0.7 l water. Randomization was performed as simple random allocation; each subject identifier was forwarded to a person who had no further relationship to the conduct of the study, and who performed the randomization blindly using a random draw of numbers. This person also prepared the BICA or placebo drinks, respectively and deposited them in a blank sports bottle in the ergometry lab before entry of subject or testing staff.
Figure 1. Schematic representation of the general design.
Both stepwise incremental tests and constant load tests were conducted under two conditions: either after oral ingestion of 0.3 g·kg−1 sodium bicarbonate (BICA) or 4 g of sodium chloride (placebo). Time interval between tests is specified in days (d). Data are presented as means ± standard deviation respectively, with minimum (min) and maximum (max) values.
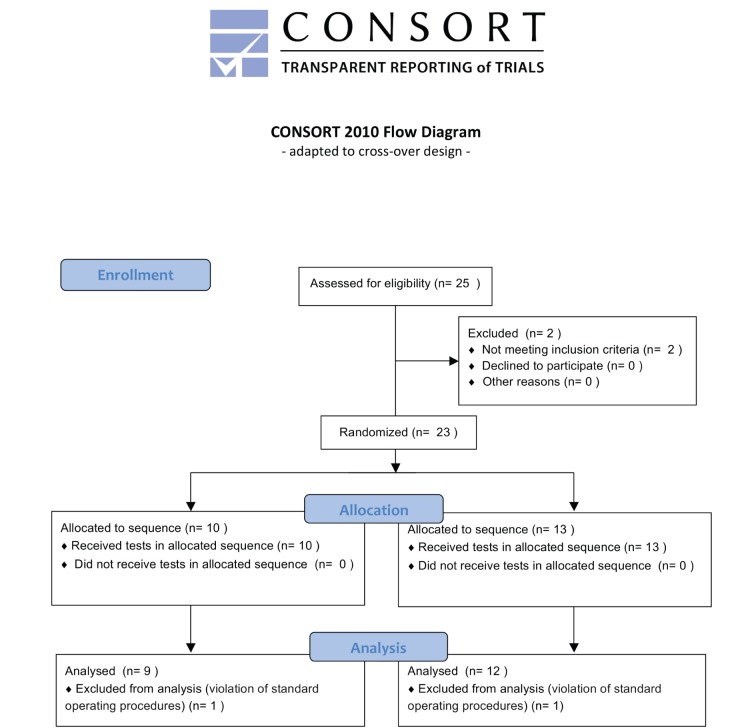
Of twenty-five participants included in the study, twenty-one could be included in the analysis (cp. CONSORT flowchart in Fig. 2).
Figure 2. Flow of participants through the study.
The incremental test with placebo was used to evaluate peak oxygen uptake (VO2peak) and the individual anaerobic threshold (IAT). IAT from the placebo test was used to determine the workload applied in the constant load tests.
Outcome measures
Time to exhaustion in a constant load test, a measure of performance during prolonged, high-intensity exercise to exhaustion, was the main outcome measure in this study. Parameters related to mechanisms for potential improvements in performance (blood lactate [BLa], pH, bicarbonate concentration), as well as parameters of maximum performance in the stepwise incremental exercise test (maximal workload, VO2peak), were selected as secondary outcome measures.
Exercise testing
Test substances were drunk in sips from 2 h to 1 h prior to each test. All trials were performed with clipless pedals and seat position on the cycle ergometer was individualized for each subject. All trials were carried out by the same investigator who was blinded for the administered test solution. Subjects received verbal encouragement throughout the tests. Tests were terminated when subjects signalized maximum exhaustion or were unable to maintain a cadence of 50 rpm (revolutions per minute) for more than 10 s.
Incremental exercise test
The stepwise incremental exercise test protocol was as follows: subjects started at an individually chosen workload ranging from 50 to 150 W. Load was increased every 3 min by 50 W until volitional fatigue. During the first trial heart rate was recorded employing 12-lead ECG whereas in the second trial, and the ensuing constant load tests, a telemetric system (Polar S-series, Kempele, Finnland) was used continuously. BLa was determined at rest, at the end of each 3 min step, at exhaustion and 1, 3, 5, 7 and 10 min after cessation of exercise. IAT was determined according to previous methods used by Stegmann et al. [17].
Constant load test
The protocol for the constant load tests consisted of 30 min cycling at a resistance equal to 95% IAT followed by exercising at 110% IAT until exhaustion. Prior to each trial subjects completed a standardized warm-up of 2 min at 50 or 100 W. BLa was determined every 5 min and at cessation of exercise.
Gas exchange measurements
Gas exchange parameters were measured continuously during all exercise tests. Subjects breathed through a Hans-Rudolph-facemask, which was attached to a “Triple V” digital flow meter measuring ventilation volume. Expired gases were continuously collected and analyzed with the MetaMax II metabolic test system (Cortex Biophysik, Leipzig, Germany; mixing chamber; sampling frequency 10 s). Before each test the flow meter was calibrated using a 3-L syringe and gas sensors were gauged by commercial calibration gas (2-point calibration) according to the manufactureŕs recommendations. Test-specific maximal respiratory parameters were calculated as the mean of the 3 highest values during exercise.
Blood sampling and analysis
Arterialized capillary blood was obtained from the hyperemized earlobe and analyzed for BLa (enzymatic-amperometric method, Greiner, Flacht, Germany) and blood gas parameters (ABL5 blood gas analyzer, Radiometer, Willich, Germany). Measured blood gas parameters were pH, pO2 and pCO2 from which bicarbonate concentration, oxygen saturation and base excess (BE) were calculated [18]. The blood gas analyzer was calibrated prior to each test. Blood gas parameters were measured 2 h and immediately before testing (pre and post drink), every 5 min during the constant load tests, at exhaustion and 2 min post assuming that this represents the time point of largest extracellular acidosis [19].
Statistical analysis
Sample size was estimated with the program G*Power (version 3.1.5) using the following parameters: effect size to be detected 0.6, alpha error probability 0.05, 1-beta error probability 0.8; 1-tailed testing. The calculated sample size of 19 was rounded up to 20. To allow for potential drop-outs 25 subjects were included in the study.
Because normal distribution was present for all dependent variables (Shapiro-Wilk-W-test), parametric tests were applied and data are presented as means ± standard deviation. Differences between the BICA and placebo conditions were tested for significance using one-factorial analysis of variance (ANOVA; factor: condition). Courses of BLa and blood gas parameters during constant load tests were compared using two-factorial ANOVA for repeated measures (factor one: condition, factor two: time of measurement). When the difference between means was significant, post-hoc comparisons were performed with the Scheffé test. The significance level for the alpha error was set at p<0.05.
Results
Performance
Time to exhaustion during the constant load tests was significantly increased (p<0.05) under BICA (49.5±11.5 min) compared to placebo (45.0±9.5 min). Maximal workload in the stepwise incremental tests (BICA: 341±66 W; placebo: 339±67 W) and workload at IAT (BICA: 234±5.5 W; placebo 233±5.7 W) did not differ between conditions. In this context, exercise lactate curves showed a parallel shift along the y-axis (factor condition) but not along the x-axis (interaction).
Acid-base-status
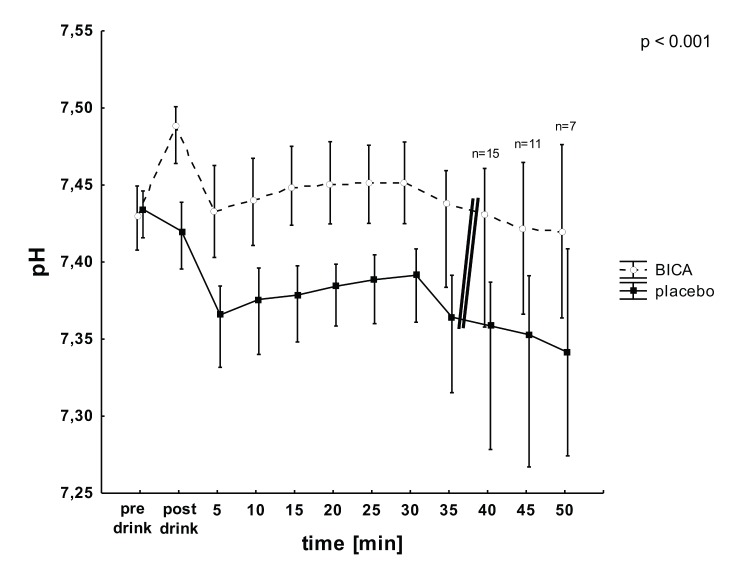
All baseline blood gas parameters were similar and within normal ranges. In both test types BICA was associated with extracellular metabolic alkalosis 1 h after ingestion at rest indicated by increased pH, bicarbonate concentration and BE. Changes in acid-base-status during the constant load tests and incremental tests are shown in table 1 and 2, respectively. Throughout the constant load tests pH remained elevated in the BICA-treatment as compared to placebo condition (Fig. 3). Under both conditions a pH nadir was reached 2 min after exercise. While the lowest pH with BICA was similar to resting-value just before placebo-trial, the lowest pH with placebo was slightly within acidic range.
Table 1. Blood gas parameters during constant load tests.
| BICA (n = 21) | Placebo (n = 21) | p-value | |
| pH | |||
| Post drink | 7.48±0.02 | 7.42±0.02 | <0.001 |
| 30 min | 7.45±0.03 | 7.38±0.03 | <0.001 |
| 2 min post-exercise | 7.39±0.05 | 7.32±0.07 | <0.001 |
| Bicarbonate [mmol*l−1] | |||
| Post drink | 31.3±1.4 | 24.9±1.5 | <0.001 |
| 30 min | 27.6±1.7 | 21.4±2.0 | <0.001 |
| 2 min post-exercise | 20.2±4.0 | 16.4±2.9 | <0.001 |
| Base Excess [mmol*l−1] | |||
| Post drink | 7.4±1.2 | 0.7±1.5 | <0.001 |
| 30 min | 3.1±1.6 | −2.6±1.7 | <0.001 |
| 2 min post-exercise | −3.2±3.7 | −8.6±3.8 | <0.001 |
| pCO2 [mmHg] | |||
| Post drink | 42.2±2.3 | 39.2±2.4 | <0.001 |
| 30 min | 38.8±3.2 | 36.5±3.9 | <0.001 |
| 2 min post-exercise | 33.8±4.9 | 32.5±3.4 | 0.33 |
| pO2 [mmHg] | |||
| Post drink | 81.8±8.1 | 89.0±7.6 | <0.01 |
| 30 min | 71.2±6.1 | 75.7±6.9 | <0.01 |
| 2 min post-exercise | 83.4±6.5 | 88.0±6.2 | <0.01 |
| Oxygen saturation [%] | |||
| Post drink | 96.4±1.3 | 96.7±0.8 | 0.30 |
| 30 min | 94.5±1.1 | 94.6±1.5 | 0.72 |
| 2 min post-exercise | 96.0±1.4 | 95.7±1.4 | 0.35 |
pH, bicarbonate concentration, base excess, partial pressure of carbon dioxide (pCO2), partial pressure of oxygen (pO2) and oxygen saturation were determined at rest (post drink), after 30 minutes cycling and 2 minutes post-exercise. Values are means ± SD.
Significance testing: one-factorial repeated measures analysis of variance (factor: condition; post-hoc: Scheffé test).
Table 2. Blood gas parameters before and after incremental tests.
| BICA (n = 21) | Placebo (n = 21) | p-value | |
| pH | |||
| Pre drink | 7.43±0.02 | 7.44±0.02 | 0.14 |
| Post drink | 7.48±0.02 | 7.42±0.01 | <0.001 |
| 2 min post-exercise | 7.24±0.07 | 7.18±0.07 | <0.001 |
| Bicarbonate [mmol*l−1] | |||
| Pre drink | 25.0±1.5 | 24.9±1.4 | 0.41 |
| Post drink | 31.1±1.5 | 24.1±1.9 | <0.001 |
| 2 min post-exercise | 13.6±2.4 | 11.4±2.2 | <0.001 |
| Base Excess [mmol*l−1] | |||
| Pre drink | 1.2±1.2 | 1.4±1.4 | 0.44 |
| Post drink | 7.3±1.3 | 0.3±1.5 | <0.001 |
| 2 min post-exercise | −12.5±3.2 | −16.2±3.6 | <0.001 |
| pCO2 [mmHg] | |||
| Pre drink | 38.6±2.7 | 37.6±2.5 | 0.13 |
| Post drink | 42.0±2.7 | 38.3±2.9 | 0.03 |
| 2 min post-exercise | 32.8±4.6 | 31.2±3.1 | 0.46 |
| pO2 [mmHg] | |||
| Pre drink | 83.3±6.9 | 83.8±5.9 | 0.14 |
| Post drink | 80.2±7.8 | 85.6±5.2 | <0.001 |
| 2 min post-exercise | 89.7±4.9 | 95.1±5.1 | <0.001 |
| Oxygen saturation [%] | |||
| Pre drink | 96.2±0.8 | 96.4±0.6 | 0.29 |
| Post drink | 96.2±1.2 | 96.6±0.8 | 0.34 |
| 2 min post-exercise | 95.7±1.2 | 95.5±1.1 | 0.56 |
pH, bicarbonate concentration, base excess, partial pressure of carbon dioxide (pCO2), partial pressure of oxygen (pO2) and oxygen saturation were determined at rest (pre and post drink) and 2 minutes post-exercise. Values are means ± SD.
Significance testing: one-factorial repeated measures analysis of variance (factor: condition; post-hoc: Scheffé test).
Figure 3. pH-levels before (pre drink), after ingestion (post drink) and during constant load tests (mean ± SD) for the BICA (open circles) and placebo (closed squares) trials.
Double bar = decrease in number of exercising subjects (n<21). p = significant interaction between condition and time.
In the BICA-trial bicarbonate concentration remained higher compared to pre-ingestion value after 30 min cycling but was lower again at exhaustion. Compared to placebo, post-ingestion bicarbonate-levels remained higher with BICA throughout the constant load tests (p<0.001). Similarly BE remained increased with BICA after 30 min and dropped considerably below its initial value when the constant load test was finished. The pCO2-values were continuously elevated after ingestion of BICA. The time course of pO2 did not show an interaction effect between the condition and time factors. No difference was observed for oxygen saturation throughout the constant load tests.
During the incremental tests the increase of pH, BE and bicarbonate concentration after ingestion of BICA at rest was maintained until 2 minutes post-exercise, when largest acidosis occured. In addition, pO2 was decreased with BICA just after cessation of exercise, while oxygen saturation remained unchanged throughout the test.
Blood lactate concentration
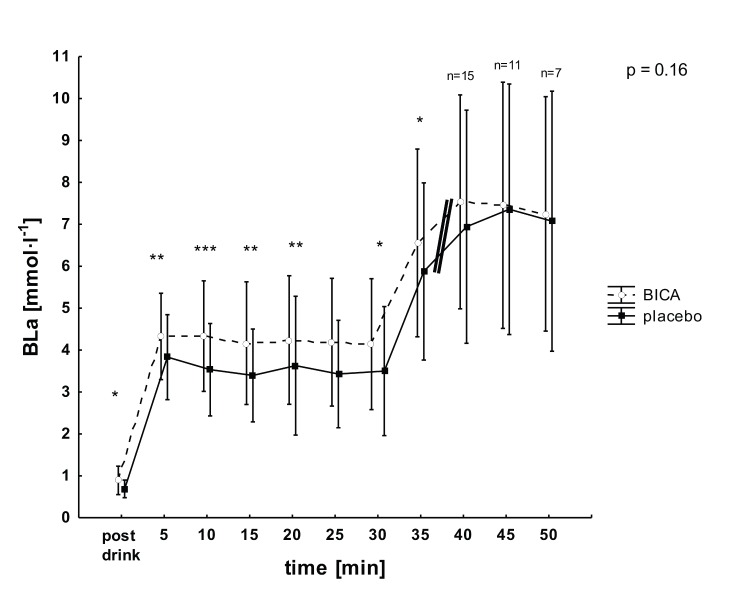
Baseline BLa with BICA was elevated compared to placebo for both test types (stepwise incremental tests 0.9±0.3 vs. 0.7±0.2 mmol·l−1, p = 0.04; constant load tests 0.8±0.3 vs. 0.7±0.2 mmol·l−1, p = 0.02). Time courses of BLa during the constant load tests did not differ between conditions as indicated by the absence of significant interaction between the condition and time factors. However, a significant global effect was present for the condition factor and post-hoc tests confirmed higher BICA concentrations for all time points except for the 25-minute value (Fig. 4). Moreover, BLa at exhaustion was elevated with BICA in the constant load tests (p<0.01) as well as in the incremental tests (p<0.01).
Figure 4. Blood lactate (BLa) concentrations after ingestion (post drink) and during constant load tests (mean ± SD) for the BICA (open circles) and placebo (closed squares) trials.
Double bar = decrease in number of exercising subjects (n<21). p = interaction between condition and time. * significant effect of condition, p<0.05; ** significant effect of condition, p<0.01; *** significant effect of condition, p<0.001.
Gas exchange measurements
The test specific highest oxygen uptake during the constant load tests did not differ significantly between conditions (BICA: 58.6±8.6 ml·kg−1·min−1, placebo: 57.6±8.8 ml·kg−1·min−1). However, during stepwise incremental tests VO2peak was slightly decreased (p<0.05) with BICA (64.0±8.6 ml·kg−1·min−1) compared to placebo (67.3±9.8 ml·kg−1·min−1). Respiratory exchange ratio at exhaustion did not differ significantly for the constant load tests but was increased with BICA for the stepwise incremental test (p<0.001).
Heart rate
The course of exercise heart rate as well as maximum heart rate did not differ significantly between conditions for both test types.
Discussion
The present study demonstrates an ergogenic effect of oral BICA ingestion for prolonged cycling slightly above the anaerobic threshold. Specifically the intake of 0.3 g·kg−1 BICA was associated with an increase in time to exhaustion at 110% IAT. No subject reported gastrointestinal discomfort or felt impaired in performance. While in a recent meta-analysis evidence was shown in support of an ergogenic effect of BICA for sprint-type exercise [11], only few studies with small samples examined the effect of BICA on prolonged cycling performance [12]–[14].
In the study of McNaughton et al. [12] cyclists performed on average 14% more work with BICA compared to placebo in a 60-min time trial [12]. In contrast, Stephens et al. [13] reported a significant muscle alkalosis but no difference in performance between BICA and placebo in a trial consisting of 30 min cycling at 77% VO2peak followed by completing as much work as possible in another 30 min (mean intensity equivalent to 80% VO2peak) [13]. Our subjects’ characteristics were similar to Stephens et al. [13], whereas exercise intensity was higher after 30 min in our study (on average 85% VO2peak equivalent to 110% IAT). It is conceivable that subjects in the study of Stephens et al. [13] worked just below, or at IAT, and thus did not benefit from the increased buffering capacity. Nevertheless, McNaughton et al. [12] assumed that their participants did not exercise for a long period of time above the IAT though BICA ingestion showed an ergogenic effect. Mitchell et al. [14] observed no difference in cycling time to exhaustion at exercise intensity similar to that of Stephens et al. [13] (∼80% VO2max) when comparing BICA to sodium chloride. In these studies [13], [14] exercise intensity above the IAT remains unclear, since exercising at given percentages of VO2max is associated with high variability of BLa responses [20]. During more anaerobic prolonged intermittent cycling, BICA improves sprint performance, although this protocol may be more representative of team sports [21].
Apart from cycling, two studies have previously examined the effect of BICA on running endurance performance [15], [16]. George et al. [15] reported a numerical increase of 17% in time to exhaustion, which failed to reach statistically significance based on the small sample size (n = 7). Likewise, Potteiger et al. [16] observed no statistically significant difference between BICA and placebo in seven runners using a test protocol similar to our study. Statistical power is not specified in either of these cases.
The significant elevation of blood pH (0.05±0.02) and bicarbonate concentration (6.1±1.6 mmol·l−1) after ingestion of BICA was of similar magnitude compared to previous investigations [2], [21]. Both values remained unchanged after placebo and differences between conditions were maintained throughout the constant load tests. Moreover, the exercise-induced decline in pH from preingestion values to exhaustion was smaller with BICA (0.03±0.05) compared to placebo (0.11±0.06). 2 min after cessation of constant load tests only a slight acidosis occurred with placebo, whereas pH remained within standard range with BICA. We therefore agree with others that pH does not seem to be the limiting factor during such endurance performance [13], [14]. Likewise, the ergogenic effect of BICA does not seem to be limited to – or ensured by - exercise protocols leading to severe lactic acidosis. This is further substantiated by the results of stepwise incremental test, where no difference in performance became apparent between BICA and placebo despite a severe acidosis.
In agreement with earlier reports BLa-levels remained higher during exercise after BICA-induced metabolic alkalosis in both test types [2], [4], [13]. This may be due to a higher efflux of lactate from the working muscle resulting from an increased H+-concentration gradient across the sarcolemma due to extracellular alkalosis [3], [9]. Lactate removal from muscle is mediated by membrane associated lactate/H+-cotransporters (monocarboxylate-transporters), which are sensitive to the concentration gradients of both ions [22]. However, H+ seems to be the main effector with induced alkalosis [9]. In addition, it has been reported that imposed metabolic alkalosis results in acceleration of glycogenolysis and glycolysis leads to a mismatch in pyruvate production and oxidation and subsequent increase in BLa production [9]. Therefore an elevated glycolytic flux is likely to be responsible for the elevated BLa after BICA ingestion.
Increased glycolysis and glycogenolysis [9] may lead to accelarated consumption of glycogen stores and premature exhaustion in the case of long term endurance exercise. However, the relevance of this potential side effect remains to be investigated.
In contrast to constant load tests, maximal and submaximal performance were unchanged during the stepwise incremental cycle test in our subjects. Probably, exercise time at maximal intensity was not long enough to profit from increased buffering capacity. Moreover, the absence of an ergogenic effect with BICA during stepwise incremental tests could also be explained by a limitation of aerobic metabolism. Despite similar maximum heart rate, VO2peak was significantly lower with BICA compared to placebo. This may be due to the well-known left shift of the oxyhemoglobin dissociation curve during alkalosis [23], evidenced by lowered pO2 with BICA and unchanged oxygen saturation between conditions. It is possible that this impairment of oxygen respiration was compensated for by the aforementioned increase in glycolytic flux and concomitant adenosine triphosphate production. In contrast, oxygen uptake was unaltered during the (submaximal) constant load test. Therefore, the accelerated glycolytic flux and consecutive performance enhancement came into effect.
A comprehensive assessment of the physiological consequences of BICA-induced metabolic alkalosis is beyond the scope of the present study. However, it seems clear that increased buffering capacity and its influence on glycolytic flux are not the only factors with a potential influence on physical performance. While the classical theory of lactate production causing muscular fatigue is strongly challenged [24], [25], it has been shown that lactate triggers positive effects on muscle performance resulting in preservation of muscle excitability [24]. In addition, lactate as a weak base is able to buffer H+-ions. Thus, the higher lactate level by itself may have contributed to performance enhancement with BICA. Moreover, BICA reduces the extracellular accumulation of potassium (K+) after high-intensity exercise [26], [27], thereby potentially counteracting K+-induced depression of muscle force [8].
Limitations
BICA has the potential to improve performance during prolonged high-intensity cycling. However, several aspects have to be considered when practical application of BICA is envisaged. First, although prolonged high-intensity exercise is representative of road-cycling competitions, the variation in load will be higher than during a constant-load exercise test. This is particularly true for pack-based competitions, but holds true even for time-trials due to individual pacing strategies. Therefore, the effectiveness of BICA under race conditions warrants further study. Second, time-trial performance is generally regarded as having a lower coefficient of variation indicating better reproducibility as compared to TTE [28]. However, as a significant effect of BICA could be demonstrated, this potential decrease in standardized effect sizes seems unlikely to have affected our results.
With respect to maximum performance in the incremental exercise test, which was mainly conducted to allow for the determination of IAT, it has to be kept in mind that stepwise tests are less optimized for determination of maximal parameters of performance than ramp-shaped tests [29].
Conclusion
The results of the present study have shown that the ergogenic potential of BICA, which has been conclusively demonstrated in previous work for sprint-type exercise, may also be seen with prolonged exercise. However, the interindividual variability in the difference between tests under BICA or placebo conditions was high. Moreover, the transferability of these results to real-world race conditions remains to be demonstrated.
Supporting Information
CONSORT Checklist.
(PDF)
Tabular presentation of all relevant data.
(PDF)
Study protocol. The original study protocol has been carefully translated into English language.
(PDF)
Study protocol in original language (German).
(PDF)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Abbiss CR, Laursen PB (2005) Models to explain fatigue during prolonged endurance cycling. Sports Med 35:865–898. [DOI] [PubMed] [Google Scholar]
- 2. Costill DL, Verstappen F, Kuipers H, Janssen E, Fink W (1984) Acid-base balance during repeated bouts of exercise: influence of HCO3. Int J Sports Med 5:228–231. [DOI] [PubMed] [Google Scholar]
- 3. Siegler JC, Keatley S, Midgley AW, Nevill AM, McNaughton LR (2008) Pre-exercise alkalosis and acid-base recovery. Int J Sports Med 29:545–551. [DOI] [PubMed] [Google Scholar]
- 4. Sutton JR, Jones NL, Toews CJ (1981) Effect of PH on muscle glycolysis during exercise. Clin Sci (Lond) 61:331–338. [DOI] [PubMed] [Google Scholar]
- 5. Linderman JK, Gosselink KL (1994) The effects of sodium bicarbonate ingestion on exercise performance. Sports Med 18:75–80. [DOI] [PubMed] [Google Scholar]
- 6. Allen DG, Westerblad H, Lannergren J (1995) The role of intracellular acidosis in muscle fatigue. Adv Exp Med Biol 384:57–68. [DOI] [PubMed] [Google Scholar]
- 7. Baker AJ, Brandes R, Weiner MW (1995) Effects of intracellular acidosis on Ca2+ activation, contraction, and relaxation of frog skeletal muscle. Am J Physiol 268:C55–63. [DOI] [PubMed] [Google Scholar]
- 8. Douroudos, II, Fatouros IG, Gourgoulis V, Jamurtas AZ, Tsitsios T, et al. (2006) Dose-related effects of prolonged NaHCO3 ingestion during high-intensity exercise. Med Sci Sports Exerc 38:1746–1753. [DOI] [PubMed] [Google Scholar]
- 9. Hollidge-Horvat MG, Parolin ML, Wong D, Jones NL, Heigenhauser GJ (2000) Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am J Physiol Endocrinol Metab 278:E316–329. [DOI] [PubMed] [Google Scholar]
- 10. Granier PL, Dubouchaud H, Mercier BM, Mercier JG, Ahmaidi S, et al. (1996) Effect of NaHCO3 on lactate kinetics in forearm muscles during leg exercise in man. Med Sci Sports Exerc 28:692–697. [DOI] [PubMed] [Google Scholar]
- 11. Carr AJ, Hopkins WG, Gore CJ (2011) Effects of acute alkalosis and acidosis on performance: a meta-analysis. Sports Med 41:801–814. [DOI] [PubMed] [Google Scholar]
- 12. McNaughton L, Dalton B, Palmer G (1999) Sodium bicarbonate can be used as an ergogenic aid in high-intensity, competitive cycle ergometry of 1 h duration. Eur J Appl Physiol Occup Physiol 80:64–69. [DOI] [PubMed] [Google Scholar]
- 13. Stephens TJ, McKenna MJ, Canny BJ, Snow RJ, McConell GK (2002) Effect of sodium bicarbonate on muscle metabolism during intense endurance cycling. Med Sci Sports Exerc 34:614–621. [DOI] [PubMed] [Google Scholar]
- 14. Mitchell TH AG, Wing S, Magder SA, Cosio MG, Deschamps A, Marliss EB (1990) Intravenous bicarbonate and sodium chloride both prolong endurance during intense cycle ergometer exercise. Am J Med Sci 300:88–97. [DOI] [PubMed] [Google Scholar]
- 15. George KP, MacLaren DP (1988) The effect of induced alkalosis and acidosis on endurance running at an intensity corresponding to 4 mM blood lactate. Ergonomics 31:1639–1645. [DOI] [PubMed] [Google Scholar]
- 16. Potteiger JA, Webster MJ, Nickel GL, Haub MD, Palmer RJ (1996) The effects of buffer ingestion on metabolic factors related to distance running performance. Eur J Appl Physiol Occup Physiol 72:365–371. [DOI] [PubMed] [Google Scholar]
- 17. Stegmann H, Kindermann W, Schnabel A (1981) Lactate kinetics and individual anaerobic threshold. Int J Sports Med 2:160–165. [DOI] [PubMed] [Google Scholar]
- 18. Siggaard-Andersen O WP, Fogh-Andersen N, Gøthgen IH (1988) Measured and derived quantities with modern pH and blood gas equipment: Calculation algorithms with 54 equations. Scand J Clin Lab Invest 48:7–15.2851165 [Google Scholar]
- 19. Cairns SP (2006) Lactic acid and exercise performance: culprit or friend? Sports Med 36:279–291. [DOI] [PubMed] [Google Scholar]
- 20. Scharhag-Rosenberger F, Meyer T, Gassler N, Faude O, Kindermann W (2010) Exercise at given percentages of VO2max: heterogeneous metabolic responses between individuals. J Sci Med Sport 13:74–79. [DOI] [PubMed] [Google Scholar]
- 21. Price M, Moss P, Rance S (2003) Effects of sodium bicarbonate ingestion on prolonged intermittent exercise. Med Sci Sports Exerc 35:1303–1308. [DOI] [PubMed] [Google Scholar]
- 22. Juel C (1997) Lactate-proton cotransport in skeletal muscle. Physiol Rev 77:321–358. [DOI] [PubMed] [Google Scholar]
- 23. Bellingham AJ, Detter JC, Lenfant C (1971) Regulatory mechanisms of hemoglobin oxygen affinity in acidosis and alkalosis. J Clin Invest 50:700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG (2004) Intracellular acidosis enhances the excitability of working muscle. Science 305:1144–1147. [DOI] [PubMed] [Google Scholar]
- 25. Robergs RA, Ghiasvand F, Parker D (2004) Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 287:R502–516. [DOI] [PubMed] [Google Scholar]
- 26. Sjogaard G, Adams RP, Saltin B (1985) Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol 248:R190–196. [DOI] [PubMed] [Google Scholar]
- 27. Fraley DS, Adler S (1977) Correction of hyperkalemia by bicarbonate despite constant blood pH. Kidney Int 12:354–360. [DOI] [PubMed] [Google Scholar]
- 28. Palmer GS, Dennis SC, Noakes TD, Hawley JA (1996) Assessment of the reproducibility of performance testing on an air-braked cycle ergometer. Int J Sports Med 17:293–298. [DOI] [PubMed] [Google Scholar]
- 29. Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, et al. (1983) Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol Respir Environ Exerc Physiol 55:1558–1564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT Checklist.
(PDF)
Tabular presentation of all relevant data.
(PDF)
Study protocol. The original study protocol has been carefully translated into English language.
(PDF)
Study protocol in original language (German).
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.