Abstract
Background
Acisarcuatus variradius gen. et sp. nov., an extinct new species representing a new genus, is described from the Middle Jurassic Jiulongshan Formation in Daohugou Village, Inner Mongolia, China.
Methodology/Principal Findings
In this paper, we revised the diagnosis of Necrotauliidae Handlirsch, 1906. One new genus and species of Necrotauliidae is described. An analysis based on the fossil morphological characters clarified the taxonomic status of the new taxa.
Conclusions/Significance
New fossil evidence supports the viewpoint that the family Necrotauliidae belongs to the Integripalpia.
Introduction
The Amphiesmenoptera, comprising two distinctive insect orders: the Trichoptera and the Lepidoptera [1]. Trichoptera, or caddisflies, are holometabolous insects. Their bodies and wings are covered by bushy hairs, and the adults resemble moths in appearance. They are among the largest group of aquatic insects [1], [2] and one of the most diverse groups of insects overall with more than 14,000 extant species and more than 680 fossil species [3]. Trichoptera include three living suborders: Annulipalpia, Integripalpia, and Spicipalpia [4], but the monophyly of Spicipalpia is disputable [2], [5]. Species of the Permian suborder Protomeropina Sukatcheva (1980) [6] are sometimes placed in Trichoptera [7], [8] and sometimes are considered representatives of the Amphiesmenoptera stem group or more distant lineages [9], [10].
The Necrotauliidae Handlirsch, 1906, an extinct caddisflies family, has been considered as representatives of the Amphiesmenoptera stem-group [1], [11]–[16]. Since the original description definition imprecise, at one time the family was deemed to “primitive” Trichoptera-like Mesozoic insects [12], [17]–[21]. However, the stem-group of Trichoptera is exactly similar to that of Lepidoptera. This ambiguity has augmented the heterogeneity of the Necrotauliidae [22]. Necrotauliidae have been described in the Late Triassic of Western Europe and the Late Mesozoic of Asia. Most fossil specimens of Necrotauliidae collected from Germany, Russia, China, and United Kingdom [13], [14], [16], [23], [24]. In China four Mesozoic Necrotauliidae, including Necropsis paludis Hong, 1983 Necrotaulius fascialatus Hong, 1983, N. kritus Lin, 1986, and N. qingshilaense Hong, 1984 have been described [13], [25].
Here, we describe a new and unique male adult fossil specimen collected from the Daohugou beds. The beds consist of 100–150 m thick succession of grey-white or locally reddish, thinly bedded claystones, shales, siltstones and sandy mudstones with intercalated ash-fall tuffs and ignimbrites. It was radiometrically dated by 40K/40Ar at 164–165 Ma [26], which accorded with the Callovian–Oxfordian boundary interval of the latest Middle Jurassic, using the latest standard international time scale [27]. Although there has been controversial to the precise age and stratigraphic position [28], [29]. The well-preserved fossils of insects and other animals also prove that the Daohugou fauna assemblages may correlate with the Middle Jurassic Yan-Liao biota [30]. This new fossil specimen is significant because of its well-preserved head, maxillary palps, fore- and hind wings, abdomen, and male genitalia. Most previously described representatives of the family were based only on fragmentary remnants and/or isolated wings [23], [31]–[33]. Thus, this new fossil is an important supplement to Necrotauliidae records and provides new evidence for studying their origin and evolution. The complete preservation of the new specimen enables us to determine the phylogenetic status of Necrotauliidae.
Materials and Methods
Material
The part and counterpart of the fossil specimen (CNU-TRI-NN2013001pc) are deposited in the Key Lab of Insect Evolution & Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China. No specific permits were required for the described field studies.
Nomenclatural Acts
The electronic version of this document does not represent a published work according to the International Code of Zoological Nomenclature (ICZN), and hence the nomenclatural acts contained in the electronic version are not available under that Code from the electronic edition. Therefore, a separate edition of this document was produced to ensure that numerous identical and durable copies were simultaneously obtainable (from the publication date noted on the first page of this article) to provide a public and permanent scientific record in accordance with Article 8.1 of the Code.
This published work and the nomenclatural acts it contains have been registered in Zoobank, the online registration system for the ICZN. The Zoobank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix “http://zoobank.org”. The ISID for this publication is: urn:lsid:zoobank.org:pub:64E83F92-0277-4F41-BC5B-9732B6691611. The electronic edition of this work was published in a journal with an ISSN, and has been archived and is available from the following digital repositories: PubMed Central and LOCKSS.
Methods
The fossil specimen was examined using a Leica MZ12.5 dissecting microscope (Wetzlar, Germany) and illustrated with the aid of a drawing tube attachment. When observing the details, the specimen was put under pure alcohol. Line drawings were made by Photoshop 9.0 graphic software (Adobe Systems, San Jose, CA, USA). Photographs were taken with a Nikon Digital Camera DXM 1200C (Tokyo, Japan).
Body length was measured from the apex of the head to the apex of the abdomen. The wing length was measured from the base to the apex of the wing. The length of antennae was measured from the base to the apex.
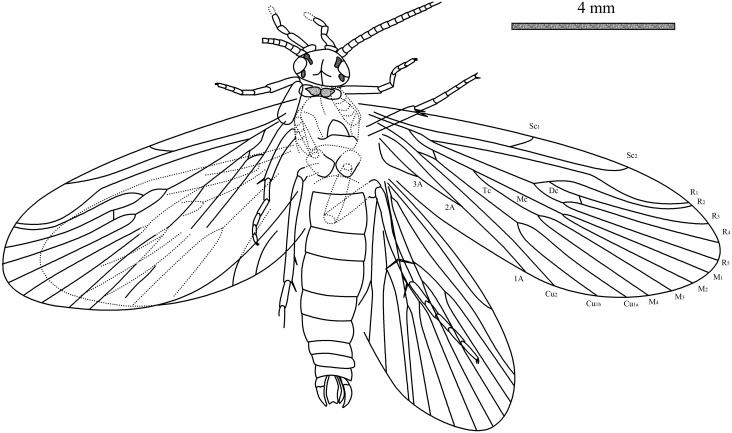
Interpretation and terminology used herein follow Holzenthal et al. [5]: C, costa; Sc, subcosta; R, radius; R1, branches of anterior radius; Rs, posterior branch of radius (composed of R2, R3, R4, and R5); M, media; M1+2, anterior branch of media, composed of M1 and M2; M3+4, posterior branch of media, composed of M3 and M4; Cu, cubitus; Cu1, anterior branch of cubitus (composed of Cu1a and Cu1b); Cu2, posterior branch of cubitus; 1A, 2A, and 3A, first, second, and third branches of anal vein; the forks giving rise to R2 and R3, R4 and R5, M1 and M2, M3 and M4, CuA1a and CuA1b, are referred to as F1, F2, F3, F4, and F5, respectively; the discoidal cell (Dc) is the cell formed by the branching of Rs into R2+3 and R4+5 and is closed apically by the sectorial crossvein (s); the medial cell (Mc) is formed by the branching of M into M1+2 and M3+4; the anal cells delimited by 1A, 2A, and 3A.
Results
Systematic Paleontology
Family Necrotauliidae Handlirsch, 1906.
Type genus
Necrotaulius Handlirsch, 1906.
Revised diagnosis
Head with setal warts. Antennae filiform. Maxillary palps five-segmented, segment V terminal, invisibly annulated, not covered densely hair or scales. Pronotum with one pair of setal warts. Mesothorax with triquetrous scutellum. Tibial spur formula: 0: 2: 3 or 4?. Forewing with vein Sc long, extending into pterostigma region; pterostigma variously developed; Rs with 4 branches; M usually with 4, rarely 3 branches, crossveins weakly developed or absent. Hind wing, Sc long; Rs with 4 branches; M with 3 or 4 branches; anal veins not joined.
Remark
According to the new fossil, we added the characters on head, antennae, maxillary palps and tibial spur formula. On hind wing, M with only 3 branches on the previously reported specimens, but our specimen has M four-branched, thus we revised this character.
Genera included. Acisarcuatus gen. nov.; Cretotaulius Sukacheva, 1982 [19]; Karatauliodes Sukacheva, 1968 [34]; Karataulius Sukacheva, 1968 [34]; Mesotrichopteridium Handlirsch, 1906 [12]; Necropsis Hong, 1983 [35]; Necrotaulius Handlirsch, 1906 [12]; Pteromixanum Sukatcheva and Jarzembowski, 2001 [23]; Scyphindusia Sukacheva, 1985 [36].
Acisarcuatus gen. nov.
urn:lsid:zoobank.org:act:85FB6752-52E4-4BFE-8549-9D43976642BB.
Type Species
Acisarcuatus variradius gen. et sp. nov.
Diagnosis
Warts present on head. Forewing with 5 forks (I–V); Sc forked, Sc2 straight and long, extending into pterostigma region; anal veins form a typical anal loop; discoidal cell short and closed, median cell and thyridial cell very long and open. Male genitalia harpagones regularly curving mesad, narrowing at apex; median phallic apparatus seems to be spicular and arcuate.
Etymology
The generic name is a combination of the Latin word acis (tip) and arcuatus (arc, curve), describing the peculiar curving of R1; gender masculine.
Distribution
China.
Remark
We assigned Acisarcuatus variradius gen. et sp. nov. to the Necrotauliidae on the basis of the following characters: head with anterior and posterior setal warts; maxillary palps five-segmented, first segment stout; tibial spur formula: 0: 2: 3 or 4?; forewing Sc long, extending into pterostigma region; Rs with 4 branches; M with 4 branches; crossvein rare; hind wing Sc extending about 2/3 length of hind wing, Rs and M with 4 branches; anal veins not joined; male genitalia inferior appendages two-segmented gonopods, with dense hairs around margin.
This new specimen shows affinity on vein characters with some other genera of Necrotauliidae. Acisarcuatus share several characters with Necrotaulius Handlirsch, 1906 [12] such as warts on the head are present, forks I–V long and slender, and crossvein m-cu1 present. However, Acisarcuatus differs from Necrotaulius in: 1) Sc forked (vs. Sc unforked); 2) R1 unforked, straight in basal part but curved in pterostigma area (vs. R1 forked and straight in pterostigma area); 3) Dc short and closed by r3–r4 (vs. Dc open); 4) Rs1+2 furcation before Rs1+2 furcation (vs. Rs1+2 furcation beyond Rs1+2 furcation).
Acisarcuatus differs from Mesotrichopteridium Handlirsch, 1906 [12] in the following characters: 1) forewing length 0.9 mm (vs. forewing length 3.5–4.5 mm); 2) forewing without crossvein sc-r (vs. crossvein sc-r present); 3) M four-branched on the hind wing, (vs. M4 reduced).
Acisarcuatus differs from Pteromixanum Sukatcheva and Jarzembowski, 2001 [23] in the following characters: 1) body size relatively large, length 0.9 mm (vs. length 0.5 mm); 2) Sc forked (vs. Sc unforked); 3) forewing with forks I–V (vs. forewing with forks I, II, III, V); 4) M forking before Rs forking (vs. M forking beyond Rs forking).
Acisarcuatus variradius gen. et sp. nov. ( Figs. 1 – 3 ).
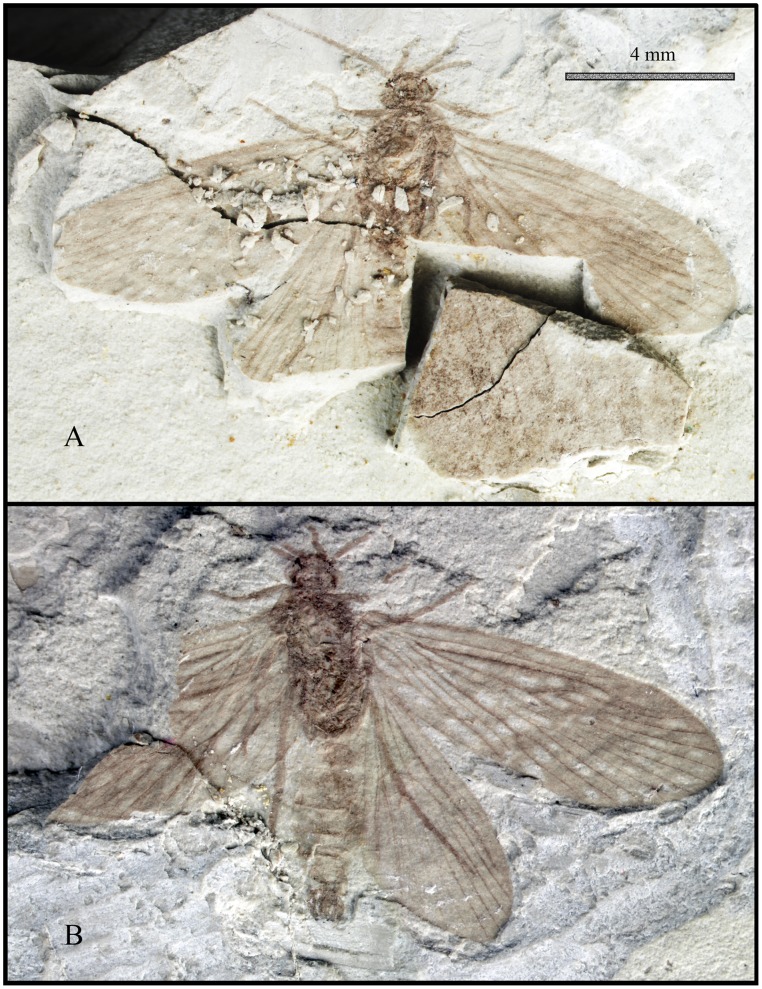
Figure 1. Photographs of the holotype of Acisarcuatus variradius gen. et sp. nov. CNU-TRI-NN2013001pc.
A, ventral view, B, dorsal view.
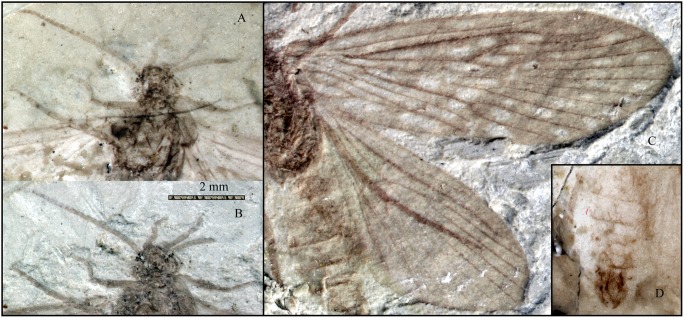
Figure 3. Photographs of Acisarcuatus variradius gen. et sp. nov. CNU-TRI-NN2013001pc.
A, head, antennae, maxillary palps, fore leg, and mid leg in alcohol. B, head, antennae, maxillary palps, fore leg, and mid leg. C, forewing and hind wing. D, male genitalia in alcohol.
Figure 2. Line drawings of Acisarcuatus variradius gen. et sp. nov. CNU-TRI-NN2013001pc.
urn:lsid:zoobank.org:act:D1107F5B-EAB9-4C76-B0F2-89CF7264A77F.
Type Material
Holotype, male: CNU-TRI-NN2013001pc (part and counterpart, dorsoventrally compressed). Antennae, maxillary palps, setal warts on head and thorax, tibial spurs, forewing, hind wing and male genitalia are well-preserved.
Locality and horizon
Daohugou Village, Shantou Township, Ningcheng County, Inner Mongolia, China (N41°18.979′, E119°14.318′), Jiulongshan Formation, Middle Jurassic.
Etymology. Specific name is a combination of the Latin word vari (different) and radius, indicating peculiar R1; gender masculine.
Diagnosis
Body small; Sc 2-branched; R1 unforked, straight basally and curved in pterostigma area, R1 closed to Rs1 terminally.
Description
Head with saponaceous triangle, compound eye at head sides, oval. Anterior setal warts and posterior setal warts present surrounding compound eye, irregularly oval. Antennae filiform but not well-preserved, scape slightly thicker than pedicel and flagellum, pedicel cylindrical, flagellum slender, length of segments equal to their diameter. Maxillary palps five-segmented; segment I swollen, segment II longest, segment III subequal to IV, segment V indistinct.
Thorax: Pronotum with one pair of setal warts, symmetrically drop-shaped. Mesothorax with triquetrous scutellum. Legs well-preserved. Foretibial spur invisible, mesotibia with two apical spurs; metatibia with two preapical spurs and one or two apical spurs; tibial spur formula: 0: 2: 3 or 4. Fore tarsus five-segmented, slender, segment I longest, II-IV subequal in length; mesotarsus five-segmented, all tarsal segments with terminal spinules. Tarsal claw visible. Forewings elongated elliptic; Rs4 terminating slightly below apex of forewing. Forewing with forks I–V; Sc forked, Sc2 slightly bend terminally and ending into C at about 2/3 the length of forewing, Sc1 terminating into C at about 2/3 length of Sc; R1 unforked distally, straight in basal part and curved in pterostigma area; Rs forked at mid-length of the forewing; Dc short and closed by r3–r4; F1 forks before than F2; Rs1 slight bent towards R1 at terminus; M originating from base of R; M forking before Rs forks; F3 and F4 longer than their stems; F3 forks later than F4; Mc very long and apparently open; Cu1 bifurcated into Cu1a and Cu1b, and then F5 forks as same level as Rs fork; crossvein m-cu1 present; Tc open; Cu2 straight and simple; anal veins visible, 1A straight, 2A reaches the median of 1A, 3A strongly curved and reaches median of 2A. Hind wing narrower and shorter than forewing. Hind wing with forks I–V; Dc, Mc, and Tc open; Sc simple; R1 straight and simple; F1 forks later than F2, F3 forks slightly before F4, F5 forks earliest.
Abdomen: In dorsal view, eight sternites visible and male genitalia prominent, bearing pair of two-segmented gonopods; coxopodite broad at base and shorter than harpago. Harpagones regularly curving mesad, narrowing at apex. Coxopodites and harpagos with dense hairs around margin. Middle preanal appendage and periphallus visible, median phallic apparatus seems to be spicular and arcuate. External structural details of male genitalia indistinct in fossil.
Remark
In our specimen, only one apical spur is visible, but the presence of another apical spur cannot be excluded (i.e. absent due to incomplete preservation).
Measurements (in mm)
Body length 9.92, width 1.74. Head length 0.91, width 1.43. Interocular space 0.75. Maxillary palp segments I–IV: 0.15, 0.52, 0.30, 0.30. Scutellum length 0.42, width 0.57. Forewing length 9.36, width 3.40. Hind wing length 6.79, width 3.02. Fore leg length: femur 1.09, tibia 0.79, tarsus I–V: 0.38, 0.30, 0.23, 0.23, 0.23; middle leg length: tarsus I–V: 0.52, 0.34, 0.30, 0.26, 0.26; hind leg length: tibia 2.38, tarsus I–V: 0.64, 0.42, 0.42, 0.42, 0.49.
Discussion
Kristensen provided a summary of 21 apomorphies supporting the monophyletic group of Amphiesmenoptera, with both Trichoptera and Lepidoptera certain features (e.g. forewing the terminal of the anal vein fusion) [37]. Furthermore, the monophylies of Trichoptera and Lepidoptera are also generally accepted [9]. Insect fossil caddisflies are generally preserved incompletely or indistinctly, and often only forewing is visible on the fossil [32], [33]. Many paleontologists considered Necrotauliidae to be a representative of the amphiesmenopteran stem-group, and proximal to the common ancestor of trichopterans and lepidopterans that survived after the Triassic [1], [11]–[14], [17]–[21], [38]. This viewpoint is mainly based on the characteristics of forewing.
Our specimen possesses very clear male genitalia with harpagones (coxopodite broad at base and shorter than harpago), middle preanal appendage, spicular and arcuate median phallic apparatus. The harpagones is a synapomorphy of Trichoptera [5], [24]. Beside that, maxillary palps of the new fossil specimen correspond to the apomorphy of Integripalpia [39], [40]: maxillary palps upturned, with segment I swollen, densely hairs or scales invisible, segment II longest, segment III subequal to IV. The character that crossvein m absent is also similar to suborder Integripalpia [40]. On the basis of these characters, we believe Necrotauliidae is belongs to Integripalpia (Trichoptera).
Meanwhile A. variradius gen. et sp. nov. has some plesiomorphies of Integripalpia: Sc forked; forewing with five forks; crossveins very rare on both forewing and hind wing; only two crossveins, r and m-cu1 present. These characters also can be found in the extinct suborder Protomeropina [6], [41]–[43]. It is interesting to speculate that necrotauliids are representatives of the Integripalpia stem-group rather than the amphiesmenopteran.
Acknowledgments
We are grateful to Yan Gao of Capital Normal University, Beijing, for their detailed comments and valuable suggestions.
Funding Statement
This research was funded by grants from the National Basic Research Program of China (973 Program) (No. 2012CB821900), The National Natural Science Foundation of China (Nos. 31372242, 41272006, 31230065, 41402009), the Project of Outstanding Young Talents of Beijing Municipal Commission of Education (No. CIT&TCD201304163), the Fok Ying-Tong Education Foundation for Young Teachers in the Higher Education Institutions of China (No. 131021), the General Program of Science and Technology Development Project of Beijing Municipal Education Commission of China (No. KM201210028016), the Project of Great Wall Scholars and KEY Project of Beijing Municipal Commission of Education Project (grant KZ201310028033), and the PhD Research Startup Foundation of Shijiazhuang University of Economics (No. BQ201319). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Grimaldi DA, Engel MS (2005) Amphiesmenoptera: the caddisflies and Lepidoptera. In: Grimaldi DA, Engel MS editors. Evolution of the insects. New York: Cambridge University Press. 548–606. [Google Scholar]
- 2.Wiggins GB, Morse JC, Yang LF, Tian LX, Li YW (1994) Trichoptera. In: Morse JC, Yang LF, Tian LX, editor, Aquatic Insects of China Useful for Monitoring Water Quality. Nanjing: Hohai University Press. 260–329. [Google Scholar]
- 3.Morse JC. Trichoptera world checklist. Available:http://entweb.clemson.edu/database/trichopt/. [accessed22.13.8].
- 4.Weaver JS (1984) The evolution and classification of Trichoptera. Part I: the groundplan of Trichoptera. In: Morse JCeditors. Proceedings of the 4th International Symposium on Trichoptera. Dr. W. Junk, The Hague. 413–419.
- 5. Holzenthal RW, Blahnik RJ, Prather AL, Kjer KM (2007) Order Trichoptera Kirby, 1813 (Insecta), Caddisflies. Zootaxa 1668:639–698. [Google Scholar]
- 6. Sukatcheva ID (1980) Evolution of the caddisfly (Trichoptera) larval case construction. Zhurnal Obshchey Biol 41:457–469. [Google Scholar]
- 7. Novokshonov VG (1993) The early evolution of the caddisflies (Trichoptera). Entomol Rev 72:138–48 (1992. Zool Zhurn 71: 58–68).. [Google Scholar]
- 8.Sukatsheva ID (1976) Caddisflies of the suborder Permotrichoptera. Paleontol Zh 1976 ((2):): 94–105. (In Russian; transl. in Paleontol J 10: 198–209).
- 9. Morse JC (1997) Phylogeny of Trichoptera. Annu Rev Entomol 42:427–450. [DOI] [PubMed] [Google Scholar]
- 10. Kukalová-Peck J, Willmann R (1990) Lower Permian “mecopteroid-like” insects from central Europe (Insecta, Endopterygota). Can J Earth Sci 27:459–468. [Google Scholar]
- 11.Handlirsch A (1906–1908) Die fossilen Insekten und die Phylogenie der rezenten Formen: Ein Handbuch für Palaöntologen und Zoologen. Leipzig: Engelmann. 1430 p. [Google Scholar]
- 12. Ansorge J (2002) Revision of the “Trichoptera” described by Geinitz and Handlirsch from the Lower Toarcian of Dobbertin (Germany) based on new material. Nova Suppl. Entomol Keltern 15:55–74. [Google Scholar]
- 13.Gao Y, Liu SY, Yang G, Yao YZ, Ren D (2012) Progress in the study of Trichoptera (caddisfly) fossils. Chin J App Entomol 49 ((2):): 543–555.
- 14.Ansorge J (2003) Upper Liassic Amphiesmenopterans (Trichoptera + Lepidoptera) from Germany– a review. Acta Zool Cracov 46 (Suppl. Fossil Insects): 285–290.
- 15. Willmann R (1989) Evolution und phylogenetisches System der Mecoptera (Insecta, Holometabola). Abh Senckb Naturforsch Ges 544:1–153. [Google Scholar]
- 16.Ivanov VD, Sukatsheva ID (2002) Order Trichoptera Kirby, 1813. The caddisflies. In: Rasnitsyn AP, Quicke DLJeditors. History of Insects, Dordrecht, Kluwer Academic Publishers. 199–219. [Google Scholar]
- 17.Tillyard RJ (1933) The panorpoid complex in the British Rhaetic and Lias. London: British Museum (Natural History). 79 p. [Google Scholar]
- 18. Martynova OM (1962) Otryad Trichoptera. Rucheiniki. In: Osnovy Paleontologii Rodendorf BB, editor. Chlenistonogie, trakheinye I khelitserovye. 9:294–302. [Google Scholar]
- 19. Sukacheva ID (1982) Historical development of the order of caddisflies. Trudy Paleontol Inst 197:1–112 (In Russian).. [Google Scholar]
- 20.Hennig W (1981) Insect phylogeny. New York: Wiley and Sons. 514 p. [Google Scholar]
- 21.Jarzembowski EA (1991) New insects from the weald clay of the Weald. Proc Gecl Ass 102 (2): 93–108.
- 22. Sohn JC, Labandeira C, Davis D, Mitter C (2012) An annotated catalog of fossil and subfossil Lepidoptera (Insecta: Holometabola) of the world. Zootaxa 3286:1–132. [Google Scholar]
- 23. Sukatchvea ID, Jarzembowski EA (2001) Fossil caddisflies (Insecta: Trichoptera) from the Early Cretaceous of southern England. Cret Res 22:685–694. [Google Scholar]
- 24. Ross HH (1967) The evolution and past dispersal of the Trichoptera. A Rev Ent 12:169–206. [Google Scholar]
- 25.Lin QB (1986) Early Mesozoic fossil insect from the South China. Beijing: Palaeontologica Sinica Science Press. 85p. [Google Scholar]
- 26. He HY, Wang XL, Zhou ZH, Zhu RX, Jin F, et al. (2004) 40Ar/39Ar dating of ignimbrite in Inner Mongolia, northeastern China, indicates a post-Middle Jurassic age for the overlying Daohugou bed. Geophys. Res Lett 31:206–209. [Google Scholar]
- 27.Gradstein FM, Ogg JG, Schmitz MD, Ogg GM (2012) The Geologic Time Scale 2012, Amsterdam, Elsevier, 2 vols.1144 p. [Google Scholar]
- 28. Gao K, Ren D (2006) Radiometric dating of ignimbrite from Inner Mongolia provides no indication of a post-Middle Jurassic age for the Daohugou Beds. Acta Geol Sin 80:42–45. [Google Scholar]
- 29. Wang X, Zhou Z, He H, Jin F, Wang Y, et al. (2005) Stratigraphy and age of the Daohugou Bed in Ningcheng, Inner Mongolia. Chin Sci Bull 50:2369–2376. [Google Scholar]
- 30. Huang D, Nel A, Shen Y, Selden PA, Lin Q (2006) Discussions on the age of the Daohugou fauna – evidence from invertebrates. Prog Nat Sci 16:309–312. [Google Scholar]
- 31. Jarzembowski EA (1995) Fossil caddisflies (Insecta: Trichoptera) from the Early Cretaceous of southern England. Cret Res 16:695–703. [Google Scholar]
- 32. Wootton RJ (1988) The historical ecology of aquatic insects: an overview. Palaeogeogr Palaeoclimatol Palaeoecol 62:477–492. [Google Scholar]
- 33. Williams NE (1988) The use of caddisflies (Trichoptera) in palaeoecology. Palaeogeogr Palaeoclimatol Palaeoecol 62:493–500. [Google Scholar]
- 34.Sukacheva ID (1968) Jurassic caddis flies from Karatau (Trichoptera). Yurskie nasekomye Karatau, 175–179. (In Russian).
- 35.Hong YC (1983) Middle Jurassic Fossil Insects in North China. Beijing: Geological Publishing House, 104p. [Google Scholar]
- 36.Sukacheva ID (1985) Jurassic Insects of Siberia and Mongolia: Trichoptera. Trudy Paleontologicheskogo Instituta Akadamii Nauk 96–114. (In Russian). [Google Scholar]
- 37.Kristensen NP (1984) Studies on the morphology and systematic of primitive Lepidoptera (Insecta). Steenstrupia 10 (5): 141–191.
- 38. Shields O (1988) Mesozoic history and neontology of Lepidoptera in relation to Trichoptera, Mecoptera and angiosperms. J Paleontol 62:251–258. [Google Scholar]
- 39. Wiggins GB, Wichard W (1989) Phylogeny of pupation in Trichoptera, with proposals on the origin and higher classification of the order. J N Am Benthol Soc 8:260–276. [Google Scholar]
- 40. Kristensen NP, Scoble MJ, Karsholt O (2007) Lepidoptera phylogeny and systematics: the state of inventorying moth and butterfuly diversity. Zootaxa 1668:699–747. [Google Scholar]
- 41.Tillyard RJ (1926) Upper Permian Insects of New South Wales. 2. The orders Mecoptera, Paramecoptera and Neuroptera. Proc Linn Soc N S W. 265–282.
- 42. Riek EF (1955) Fossil insects from the Triassic beds at Mt. Crosby, Queensland. Aust J Zool 654–691. [Google Scholar]
- 43. Martynova OM (1958) New insects from Permian and Mesozoic deposits of the USSR. Materialy k Osnovam Palaeontologiy 69–94. [Google Scholar]