Abstract
STUDY QUESTION
What is the role of the inhibitor of apoptosis proteins (IAPs) in human endometriotic tissues and a mouse model of endometriosis?
SUMMARY ANSWER
Four IAP proteins were expressed in endometriotic tissue indicating IAPs may be a key factor in the pathogenesis and progression of endometriosis.
WHAT IS KNOWN ALREADY
Overexpression of IAPs protects against a number of proapoptotic stimuli. IAPs (c-IAP1, c-IAP2, XIAP and Survivin) are expressed in human ectopic endometrial stromal cells (ESCs) from ovarian endometriomas.
STUDY DESIGN, SIZE, DURATION
Forty-eight women with or without ovarian endometrioma are included in this study. BALB/c mice (n = 24) were used for the mouse endometriosis model. Mice with surgically induced endometriosis were treated with an IAP antagonist (BV6) for 4 weeks.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Human ectopic endometrial tissues from chocolate cysts and eutopic endometrial tissue were collected. ESCs were enzymatically isolated from these tissues. ESC proliferation was examined by 5-bromo-2′-deoxyuridine—enzyme-linked immunosorbent assay. IAPs expression in tissue derived from eutopic endometria and chocolate cysts was evaluated using real-time RT–PCR and immunohistochemistry. A homologous mouse endometriosis model was established by transplanting donor mouse uterine tissue into the abdominal cavities of recipient mice. After treating the mice with BV6 (i.p. 10 mg/ml), the extent of endometriosis-like lesions in mice was measured and proliferative activity assessed by Ki67 staining. All experiments were repeated a minimum of three times.
MAIN RESULTS AND THE ROLE OF CHANCE
IAP (c-IAP1, c-IAP2, XIAP and Survivin) mRNA and protein in human ectopic endometrial tissues were expressed at higher levels than in eutopic endometrial tissues (P < 0.05). All four IAPs proteins were expressed in mouse endometriosis-like implants. BV6 inhibited BrdU incorporation of human ESCs (P < 0.05 versus control). BV6 also decreased the total number, weight, surface area and Ki67 positive cells in the endometriosis-like lesions in the mice (P < 0.05 versus control).
LIMITATIONS, REASONS FOR CAUTION
Endometriotic lesions were surgically induced in mice by transplanting mouse uterine tissue only, not human pathological endometriotic tissue. Furthermore, the effects of BV6 on human ESCs and mouse endometriosis-like lesions may differ between the species.
WIDER IMPLICATIONS OF THE FINDINGS
Our data support the hypothesis that IAPs are involved in the development of endometriosis, and therefore an inhibitor of IAPs has potential as a novel treatment for endometriosis.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by KAKENHI (Japan Society for the Promotion of Science, Grant-in-Aid: to F.T.; 21592098 and to T.H.; 24659731) and Yamaguchi Endocrine Research Foundation. The authors have no conflicts of interest to disclose.
Keywords: inhibitor of apoptosis proteins family, BV6, mouse endometriosis model, endometrial stromal cells
Introduction
Endometriosis is defined as ectopic growth of endometrial tissue outside the uterine cavity. This benign gynecologic disorder, which affects 10–15% of women of reproductive age (Lebovic et al., 2001: Winkel, 2003), causes dysmenorrhea, infertility and chronic pelvic pain. Most experts believe that prevalence and morbidity in the asymptomatic population have increased. Despite the fact that 80 years have passed since the initial description of this prevalent disease by Sampson (1927), our understanding of the etiology and pathophysiology of endometriosis remains obscure. In the current study, we utilized a mouse endometriosis model (Takai et al., 2013) as well as human cultured endometriotic cells.
Apoptosis is a normal, essential function to eliminate excess or dysfunctional cells. Impaired sensitivity of endometrial cells to spontaneous apoptosis may cause abnormal implantation and endometrial growth at ectopic sites (Dmowski et al., 2001), suggesting that a decreased susceptibility of endometrial tissue to apoptosis may contribute to the pathogenesis of endometriosis. Eutopic endometrial and endometriotic cells seem to be fundamentally different in women with endometriosis compared with those from women without endometriosis (Izawa et al., 2006; Taniguchi et al., 2013). These differences may contribute to the survival of endometrial tissue in the peritoneal cavity and to the development of endometriosis. A common characteristic of endometriotic cells is their ability to evade the apoptotic machinery. Other studies have concluded that some characteristics that differ between the eutopic endometrium of women with or without endometriosis may be involved in the pathogenesis of endometriosis (Kim et al., 2013; Laudanski et al., 2014).
The inability of endometriotic cells to transmit a ‘death’ signal thus avoiding cell death is associated with increased expression of anti-apoptotic factors. Ectopic endometrial stromal cells (ESCs) have the distinct biological characteristic of resistance to drug-induced apoptosis compared with eutopic ESCs in women without endometriosis (Izawa et al., 2006). An inhibitor of apoptosis proteins (IAPs) interacts with multiple cellular partners and inhibits apoptosis induced by a variety of stimuli (Fulda and Vucic, 2012). IAPs promote pro-survival signaling pathways and prevent activation of the effector phase of apoptosis by interfering with the activation of caspases.
Human IAP family members include neuronal apoptosis inhibitory protein (birc1), cellular IAP1 (c-IAP1, birc2), c-IAP2 (birc3), X chromosome-linked IAP (XIAP, birc4), Survivin (birc5), Apollon (birc6), melanoma IAP (ML-IAP, birc7) and IAP-like protein 2 (birc8). Overexpression of IAPs protects against a number of proapoptotic stimuli in malignant diseases (Fulda and Vucic, 2012). We demonstrated that IAPs (c-IAP1, c-IAP2, XIAP and Survivin) are expressed in the ectopic ESCs isolated from ovarian endometriomas (Taniguchi et al., 2009) and that survivin plays a critical role in the susceptibility of ectopic ESCs to drug-induced apoptosis (Watanabe et al., 2009). Survivin, exhibits a prominent cancer bias because it is undetectable in most adult tissue but is expressed at high levels in a majority of human tumors (Altieri, 2003).
Current medical treatments for endometriosis, such as GnRH agonists and progestins, are effective in suppressing the growth of endometriosis and relieving endometriosis-associated pain, yet this pain relief appears to be relatively short lived. To investigate a novel approach, we established the current experimental model using mice to evaluate the effect of an IAP inhibitor as a therapy for endometriosis. In view of the above considerations, we proposed a hypothesis that IAPs are involved in the development of endometriosis. To gain further insight into the pathogenesis of endometriosis, we investigated IAPs expression in eutopic and ectopic endometrial tissues and the efficacy of an IAP inhibitor on human ectopic ESCs and mouse endometriosis-like lesions.
Materials and Methods
Collection of human ovarian endometrioma and endometrium
This study was approved by the institutional review boards of Tottori University Faculty of Medicine, Yonago, Japan; all patients provided written informed consent. We obtained the ectopic endometriotic tissue from ovarian chocolate cysts (C: n = 16) at the time of laparoscopic surgery. We collected three types of eutopic endometrial tissues from 48 patients and classified them as follows: (i) 16 disease-free patients (F-Em) consisting of two infertility patients with no other gynecological disease and 14 fertile patients with benign ovarian tumor; (ii) 16 patients with uterine myoma (M-Em); (iii) 16 patients with endometriosis with ovarian chocolate cysts (C-Em). The menstrual phases of all specimens (proliferative/follicular: n = 8 and secretory/luteal phase: n = 8, in each group) were ascertained by pathological examination and/or the serum estradiol and progesterone values. Eutopic endometrial specimens of the disease-free patients without endometriosis were used as the control. None of the patients had received hormonal treatment for at least 2 years prior to the surgery.
Real-time RT–PCR analysis of human tissues
Tissues were preserved in RNA later™ solution (Life Technologies, Tokyo, Japan) at 4°C overnight. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Tokyo, Japan). Reverse transcription (RT) of RNA (1 µg) from the tissues into complementary DNA was performed. The mRNA levels were quantified using the ABI 7900 HT real-time PCR system (Applied Biosystems, Tokyo, Japan). The specific ABI TaqMan probes for c-IAP1, c-IAP2, XIAP, Survivin and TaqMan human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control reagents (Applied Biosystems) were used. The absolute values for IAPs were normalized to that for GAPDH, and the relative values compared with the control are presented. All samples were tested in triplicate, and each run included no-template and no-RT controls.
Immunohistochemical staining of human tissue
Formalin-fixed specimens were paraffin-embedded, cut into 5 µm sections, and immunohistochemical staining performed using a standard protocol. Sections were deparaffinized, blocked in methanol/0.3% H2O2 and then in 10% normal goat serum. A primary antibody for human c-IAP1, c-IAP2, XIAP and survivin (1:200, R&D Systems Inc., USA) was used. Sections were incubated with biotinylated goat anti-rabbit IgG (1:200) for 1 h, with streptavidin-peroxidase complex for 30 min, with diaminobenzidine for 10 min, then counterstained with hematoxylin.
For semi-quantitative analysis, each specimen was microscopically evaluated by counting IAP-positive cells in epithelial and stromal cells. Positive and negative control slides, including human malignant lymphoma (for cIAP-1 and XIAP), endometrial cancer (cIAP-2) or ovarian cancer (Survivin) tissues, were incorporated into each slide run. Negative control slides were incubated similarly, but the primary antibody was replaced with phosphate-buffered saline. Two gynecological pathologists examined immunohistochemical slides independently without prior information regarding the clinical history of patients. In cases of discrepancies in interpretation, a final consensus was achieved between the two pathologists using a multi-head microscope.
Isolation and culture of human ectopic and eutopic ESCs
Human ectopic ESCs (endometriotic cells) and eutopic ESCs were isolated from the ovarian chocolate cyst linings, the eutopic endometrial tissues of disease-free patients or uterine myomas by enzymatic digestion with collagenase (Iwabe et al., 1998; Watanabe et al., 2009). The treated tissues were passed through a nylon mesh filter to remove debris and epithelial glands. We used the stromal cells in a monolayer culture after the second passage. Cells were cultured in Dulbecco's modified Eagle's medium /F-12 medium with 10% fetal bovine serum. To confirm the purification of ESCs, immunohistochemical staining of the isolated ESCs was performed using cytokeratin (DAKO, Kyoto, Japan) as a marker of epithelial cells, vimentin (DAKO) as a marker of stromal cells, CD14 (Nichirei, Tokyo, Japan) as a marker of activated macrophages, and factor VIII (DAKO) as a marker of endothelial cells. The purity of stromal cells was more than 98% (Sakamoto et al., 2003; Watanabe et al., 2009).
Cell proliferation assay
To examine DNA synthesis in proliferating cells, 5-bromo-2′-deoxyuridine (BrdU) incorporation was assessed using the cell proliferation enzyme-linked immunosorbent assay (ELISA; Amersham Biosciences, Tokyo, Japan). Human ectopic and eutopic ESCs from the disease-free and uterine myoma specimens were cultured in the medium at a seeding density of 5 × 103 cells/well in 96-well culture plates. The antagonist for IAPs (BV6; 0.1–5 µM), which inhibits mainly cIAP-1, cIAP-2 and XIAP, was applied for 24 h. Absorbance was measured using a microplate reader. The ratio to mean value of control (% of control) was used for comparison. As the control, 0.05% dimethyl sulfoxide (DMSO) was used. Dr Domagoj Vucic supplied the IAP antagonist, BV6 (Genentech Inc., South San Francisco, CA, USA).
Animal care and treatment
Female mice (6 weeks of age, BALB/c) were purchased from Japan SLC (Shizuoka, Japan). All animal handling protocols and surgical procedures were approved by Tottori University. Before initiating the experiments, animals were allowed to acclimatize for 7 days. All 24 mice were ovariectomized through a 1 cm longitudinal skin incision then injected s.c. with estradiol valerate (0.5 µg/mouse/week; Fuji Pharma, Tokyo, Japan) once per week for 6 weeks until the experimental endometriosis induction. Two weeks after ovariectomy, the uteri of an additional eight donor mice (n = 8) were removed en bloc after euthanasia and cleaned of excess tissue in sterile saline. Each uterus was cut to include the uterine horns in each half with a linear incision longitudinally and minced (∼0.5 mm in diameter) with dissecting scissors. The ovariectomized recipient mice (n = 16) were anesthetized using pentobarbital sodium. A 0.5 cm subabdominal midline incision was made. Each recipient received half of the donor uterus (1:2 donor uterus to host ratio) minced and added to 500 µl saline, and injected into the peritoneal cavity, and the peritoneum was sutured. Injected uterine tissue weighed ∼50 mg per mouse. For the next 4 weeks, recipient mice were treated with a single i.p. injection of BV6 (n = 8; 10 mg/kg; Varfolomeev et al., 2009) or vehicle (n = 8; 1% DMSO) twice weekly.
Evaluation of endometriosis-like lesions in the mouse model
Paired mice receiving the uterine horns from the same donor mouse were administered BV6 or the vehicle, DMSO, alone. After BV6 treatment (10 mg/kg) for 4 weeks (Varfolomeev et al., 2009), the recipient mice were sacrificed and the peritoneal cavities thoroughly inspected. Endometriosis-like lesions were carefully removed and photographed to document in situ images of the lesions by microscope. The images were transferred to Image-J software (NIH, Bethesda, MD, USA) for measurement.
Formalin-fixed specimens were paraffin-embedded, cut into 5 µm sections, and stained with hematoxylin and eosin. To assess the lesions immunohistochemically, the primary antibodies for mouse c-IAP1 (1:100, Abcam, Tokyo, Japan), c-IAP-1 and -2 (1:100, IMGENEX, San Diego, CA, USA), XIAP (1:100, abcam), survivin (1:1000, Abcam), cytokeratin (for epithelial cells: Nichirei, Tokyo, Japan), vimentin (for stromal cells: Abcam, Tokyo, Japan) and calretinin (for mesothelium cells: AnaSpec, San Jose, CA, USA) were used. These antibodies for IAPs can react with both mouse and human tissues. Positive and negative control slides, including the specimens of human malignant lymphoma (for cIAP-1 and XIAP), endometrial cancer (for both cIAP-1 and 2) or ovarian cancer (for Survivin) tissues were used. An antibody for Ki67 (Novus, Littleton, CO, USA) was used to assess the proliferative activity. Proliferative activity was evaluated by counting Ki67-positive and -negative nuclei of all epithelial cells. Ki67-positive cell ratios were averaged over three fields in a single section of each lesion.
Statistical analysis
All experiments were repeated a minimum of three times. Results were analyzed using one-way analysis of variance (ANOVA) followed by Fisher's protected least significant differences post hoc test. All data sets are presented as mean with SEM. All statistical analyses were carried out using Statview 5.0 Software (SAS Institute, Cary, NC, USA). P < 0.05 was accepted as indicating statistical significance.
Results
IAPs mRNA expression in human tissues of ovarian endometrioma and eutopic endometrium
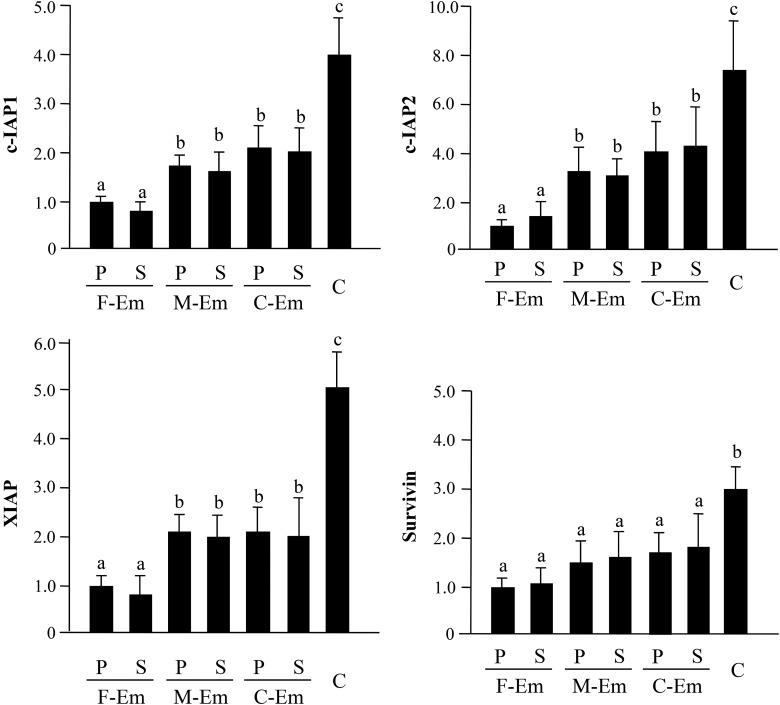
The level of mRNA expression of c-IAP1, c-IAP2, XIAP and Survivin in the ovarian endometrioma tissues (C) was enhanced compared with that in all three types of the eutopic endometrial tissues (e.g. c-IAP1; 4.1-fold, c-IAP2; 7.5-fold, XIAP; 5.1-fold, Survivin; 3.0-fold versus disease-free endometrium in the proliferative phase, P < 0.05). With regard to c-IAP1, c-IAP2 and XIAP mRNAs expression, the eutopic endometrium in women with myomas (M-Em) and ovarian endometriomas (C-Em) expression was higher than in the disease-free endometrium (F-Em) (Fig. 1). No cyclical difference was observed in these IAP mRNAs in the eutopic endometrium.
Figure 1.
Quantitative analysis of IAPs mRNA expression in human endometrial and endometriotic tissues. The mRNA levels of c-IAP1, c-IAP2, XIAP and Survivin were evaluated by real-time RT–PCR. The abbreviations of the four types of tissues are as follows: (i) eutopic endometrial tissues, disease-free patients: F-Em (n = 16); (ii) with uterine myomas: M-Em (n = 16); (iii) with ovarian chocolate cyst: C-Em (n = 16); and (iv) the ovarian chocolate cyst: C (n = 16). The mRNA level of F-Em (proliferative phase) was set arbitrarily at 1.0. P, proliferative phase; S, secretory phase. Data are the mean ± SEM of three independent experiments. Results were analyzed using one-way ANOVA followed by Fisher's protected least significant differences post hoc test. Bars that do not share a letter are significantly different (P < 0.05).
IAPs protein expression in human tissues of ovarian endometrioma and eutopic endometrium
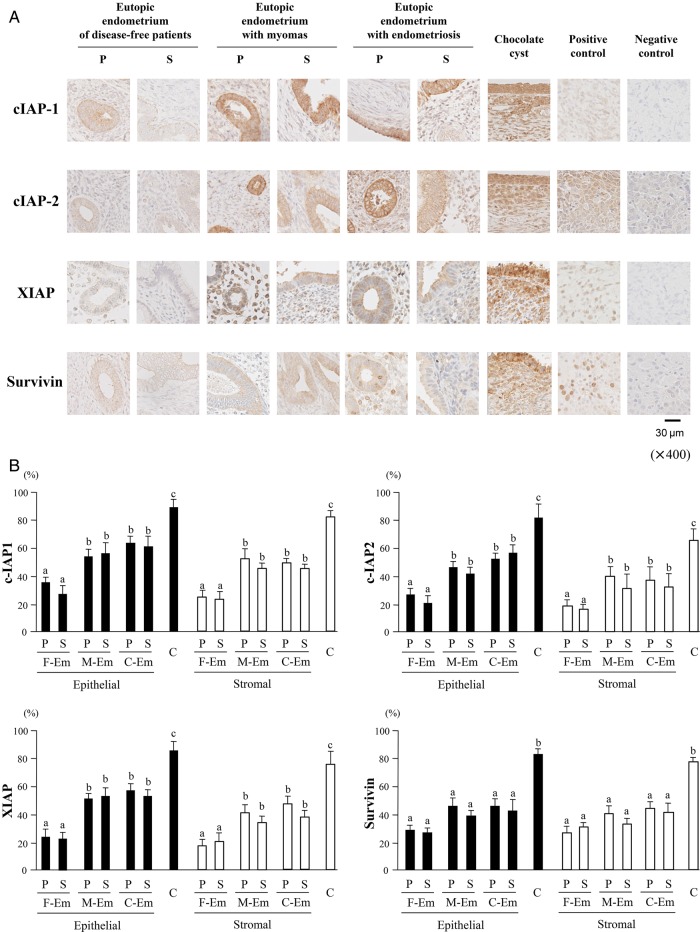
All four IAP proteins were mainly observed in the cytoplasm of both epithelial and stromal cells of endometrial or endometriotic tissues by immunohistochemical staining (Fig. 2A). According to semi-quantitative analysis of both cell types, the percentage of positive-stained cells for c-IAP1, c-IAP2, XIAP and Survivin in C was significantly higher than those in F-Em, M-Em or C-Em. In addition, c-IAP1, c-IAP2 and XIAP expression in M-Em or C-Em was more intense than in F-Em, whereas Survivin levels did not differ. No cyclical difference in IAPs expression was observed in any type of endometrium (Fig. 2B).
Figure 2.
IAP protein expression in human endometrial and endometriotic tissues. (A) Immunohistochemical staining in the eutopic endometrium, of disease-free patients, with uterine myomas, with chocolate cysts and the endometriotic tissue from chocolate cyst (each group: n = 5). Slides of human malignant lymphoma (for cIAP-1 and XIAP), endometrial cancer (for cIAP-2) or ovarian cancer (for Survivin) tissues as positive control tissues are shown. Negative control slides were incubated similarly, but the primary antibody was replaced with phosphate-buffered saline. Original magnification: ×400. (B) Semi-quantitative analysis. Each specimen was evaluated microscopically by counting IAPs-positive cells both in epithelial (glandular and surface epithelium) and stromal cells. Results are expressed as a percentage. Data are the mean ± SEM of three independent experiments. Results were analyzed using one-way ANOVA followed by Fisher's protected least significant differences post hoc test. Bars that do not share a letter are significantly different (P < 0.05). The statistical comparison was performed separately in epithelial cells (closed bars) and stromal cells (open bars).
Effect of IAP inhibitor in human ectopic and eutopic ESCs
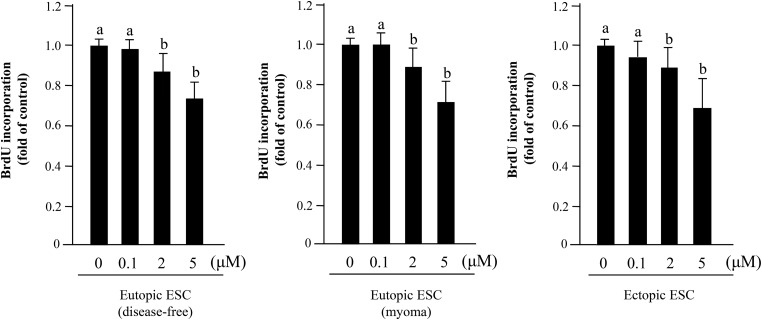
An IAPs inhibitor, BV6 (2 and 5 µM), significantly repressed BrdU incorporation in ectopic and eutopic (disease-free and myomas) ESCs. An ∼30% decrease of BrdU incorporation was observed in both groups after treatment with 5 µM BV6 (Fig. 3).
Figure 3.
Effect of IAPs inhibitor (BV6) on cell proliferation of human eutopic (disease-free or uterine myoma) and ectopic ESCs. After BV6 treatment for 24 h, BrdU-ELISA was performed. Seven different samples in each group were used. Results were analyzed using one-way ANOVA followed by Fisher's protected least significant differences post hoc test. Bars that do not share a letter are significantly different (P < 0.05).
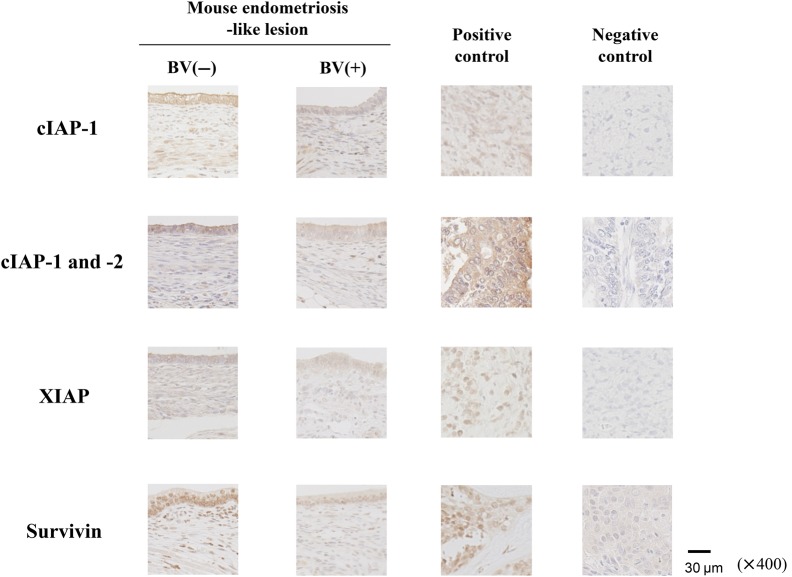
IAPs protein expression in the mouse endometriosis-like lesions and the effects of IAPs inhibitor
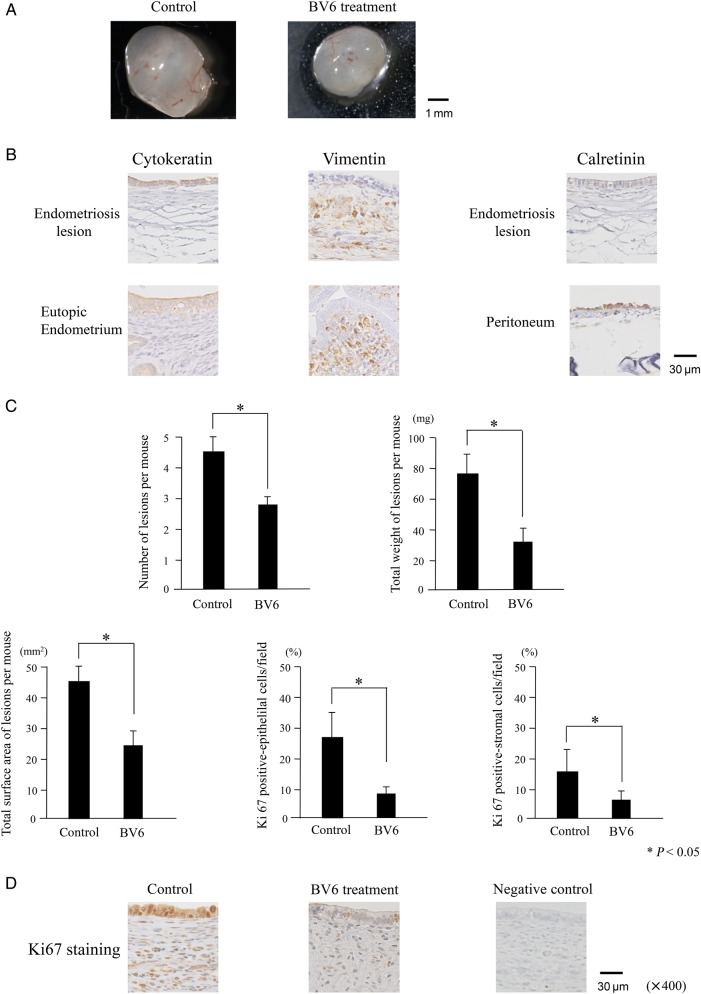
Endometriosis-like lesions grew in the abdominal cavities of all the mice. Most of the lesions were observed around the dissection site and the intestinal membrane. IAPs were expressed in mouse endometriosis-like implants, especially in the epithelial cells. Murine cIAP-1, cIAP-2 and XIAP expressions were clearly observed in the cytoplasm of both epithelial and stromal cells of implants, whereas Survivin was mainly expressed in the nuclei BV6 treatment for 4 weeks attenuated the intensity of IAPs expression (Fig. 4). The size of lesions ranged from ∼2 to 7 mm in diameter (Fig. 5A). The monolayer epithelial cell lining of the cyst was shown. After immunohistochemical staining, cytokeratin and vimentin were positively stained, whereas calretinin was negative (Fig. 5B). After BV6 treatment for 4 weeks, the total number of lesions (4.6 versus 2.8/mouse), the average weight (78.1 versus 32.0 mg/mouse) and the surface area (44.5 versus 24.6 mm2/mouse) of lesions were significantly less than in the controls (Fig. 5C). In the endometrial gland epithelia or stroma, the percentage of Ki67-positive cells decreased after BV6 treatment (epithelium: 26.8 versus 8.8%, stroma: 15.2 versus 5.0%; Fig. 5C and D).
Figure 4.
IAP protein in mouse endometriosis-like lesions. Immunohistochemical staining for IAPs in the mouse endometriosis-like implants. Positive and negative control slides, including the specimens of human malignant lymphoma (for cIAP-1 and XIAP), endometrial cancer (for both cIAP-1 and 2) or ovarian cancer (for Survivin) tissues were used. Original magnification: ×400.
Figure 5.
Characteristics of, and BV6 effects on, mouse endometriosis-like lesions. (A) A representative example of an excised implant. (B) Immunohistochemical staining of lesions for cytokeratin, vimentin and calretinin. Original magnification: ×40. As the positive control, the specimens of mouse eutopic endometrium (for cytokeratin and vimentin) and the peritoneum (for calretinin) were used. (C) Total number, weight, surface area of endometriosis-like lesions and the rate of Ki67-positive staining in epithelium and stroma of implants were assessed. Eight mice were treated with BV6 and eight with the vehicle. * P < 0.05. Results were analyzed using one-way ANOVA followed by Fisher's protected least significant differences post hoc test. (D) Ki67 staining in the implants. Original magnification: ×400.
Discussion
Our analysis focused on the effects of the IAPs inhibitor, BV6, both on human ESCs and mouse endometriosis-like lesions, as a possible therapeutic agent for endometriosis. We showed that the IAP family in the endometriotic tissues derived from ovarian endometriomas were highly expressed compared with those in the eutopic endometrial tissues (Figs 1 and 2), supporting the notion that endometriotic cells may potentially possess innate anti-apoptotic characteristics. Expression of c-IAP1, c-IAP2 and XIAP mRNA in the eutopic endometrium with chocolate cyst was higher than in the disease-free endometrium (Fig. 1). Gebel et al. (1998) also reported that apoptosis indices in the eutopic endometrium of women with endometriosis were lower than in women without endometriosis.
Endometriosis is commonly believed to arise via retrograde menstruation in which viable endometrial tissue flows retrograde into the peritoneal cavity (Sampson, 1927). According to this theory, we established a readily available animal model using the syngeneic graft of endometrial tissues (Fig. 5A; Takai et al., 2013). The lesions in the mouse model were spherical and larger than those of other models (Yoshino et al., 2006; Burns et al., 2012). As shown in Fig. 5B, cytokeratin and vimentin in the mouse endometriosis-like lesions were positive, whereas calretinin was negative, indicating that these cystic lesions originated from the injected endometrial tissues, not from the peritoneal cells.
Apoptosis helps to maintain cellular homeostasis during the menstrual cycle by eliminating senescent cells from the functional layer of endometrium during the late secretory and menstrual phases. These mechanisms are necessary but insufficient to explain why endometriosis develops only in some patients. The differences between the eutopic endometrium of patients with endometriosis and that of disease-free patients could contribute to the survival of regurgitating endometrial cells into the peritoneal cavity and the development of endometriosis. The fact that the eutopic endometrium of women with endometriosis shares the changes with ectopic tissue and that these changes are not found in the eutopic endometrium of disease-free women has advanced the view that the primary defect in endometriosis is found in the eutopic endometrium (Kruitwagen et al., 1991).
The IAP protein family contains one to three baculovirus IAP repeat (BIR) domains that are required for anti-apoptotic activity. IAPs have recently emerged as the modulators in an evolutionarily conserved step in apoptosis. IAPs were highly expressed in many types of cancer cells and may cause the resistance of apoptosis. An IAP antagonist, BV6, is the small-molecule IAP antagonist that binds and inhibits c-IAP1, c-IAP2 and XIAP (Fulda and Vucic, 2012). BV6 can neutralize IAPs action in the various cells derived from human and mouse tissues (Varfolomeev et al., 2007, 2012; Stanculescu et al., 2010). IAPs inhibition may be reasonable as a treatment for endometriosis because expression of c-IAP1, c-IAP2 and XIAP was enhanced in the endometriotic cells. The BV6 inhibited ectopic and eutopic ESC proliferation and the formation of endometriosis-like lesions (Figs 3, 5A and C), suggesting that the role of IAPs is crucial to the growth of endometriosis. The finding that IAPs expression was not excessively high suggests that eutopic ESCs might have a similar response to BV6 (Fig. 3). Differences in sensitivity and mechanisms of action of BV6 may result in reduced cell proliferation in both eutopic and ectopic ESCs. The IAPs inhibitor is therefore a potential therapeutic option for treating abnormal endometrium, such as endometrial hyperplasia, or for endometrial cancer and endometriosis.
Evidence from other studies suggests that nuclear factor (NF)-κB activity in ectopic ESCs stimulates inflammation and cell proliferation but inhibits apoptosis (Taniguchi et al., 2009; Zhang et al., 2011). We recently demonstrated that BV6 abrogated innate c-IAP1 and tumor necrosis factor (TNF)α-induced c-IAP2 protein expression in human ectopic ESCs through the NF-κB pathway, whereas it partially inhibited XIAP protein expression. Pretreatment with BV6 repressed TNFα-induced interleukin-8 protein expression and cell proliferation in ectopic ESCs (Taniguchi et al., 2014). These data suggest that NF-κB activity would be indispensable for the action of BV6 in endometriotic cells.
In this study, we did not include endometriotic epithelial cells because of the difficulty of purification and subculture. Although immortalized endometriotic epithelial cells were created by Bono et al. (2012), this cell line by combinatorial transfection of cyclin D1, cdk4 and hTERT may have the characteristics of ovarian cancer cells.
We did not determine the level of IAPs expression in ovarian endometriotic tissue and ectopic ESCs in the various menstrual phases (Figs 1 and 2). Goumenou et al. (2004) reported that the apoptotic rates as well as Bcl-2 and Bax expression in ovarian endometriotic cells are not affected by menstrual cycle phase. Although Bcl-2 expression in the eutopic endometrium of patients with endometriosis has a cyclic pattern, these cyclic changes may not be apparent in peritoneal and ovarian endometriotic tissue (Watanabe et al., 1997). The characteristics of endometriotic cells may be distinct from those of eutopic endometrial cells after the endometrioma is formed. We also previously showed that the apoptosis rates between the proliferative and secretory phases of ectopic or eutopic ESCs were similar (Izawa et al., 2006). Based on these findings and our data, we did not divide the samples into the different menstrual phases to analyze IAP expression in endometriomas. Although more study is needed to resolve this issue, the cyclic variability of apoptosis-related factors may be lost in endometriotic tissue.
Existing major pharmacological treatments for endometriosis, such as GnRH agonists and progestins, have adverse effects. In contrast, BV6 is a therapeutic agent with few side effects because it has no hormonal action (Flygare et al., 2012). In the in vivo experiments, we checked the weight and behavior of mice to assess the toxicity of BV6 and no difference between treated and untreated mice was found.
A number of studies demonstrated elevated expression levels of IAPs, particularly c-IAP1, c-IAP2 and XIAP, in many tumor types (Vucic, 2008). For instance, high expression of IAPs may contribute to colon cancer and the poor prognosis of colorectal cancer patients (Miura et al., 2011). Based on positive results from pre-clinical studies, at least five clinical trials are now testing the applicability of IAP antagonists (Flygare and Fairbrother, 2010; Fulda and Vucic, 2012). The first IAP antagonist to enter human clinical trials for patients with locally advanced or metastatic solid malignancies or non-Hodgkin's lymphoma without leukemic phase was compound GDC-0152, a potent inhibitor of c-IAP1, c-IAP2, XIAP and ML-IAP (Flygare et al., 2012). GDC-0152 showed linear pharmacokinetics over a wide range of doses in humans without significant toxicity. The XIAP antisense oligonucleotide AEG35156 proved to be well tolerated in 56 patients, two of whom experienced peripheral neuropathy (Schimmer et al., 2009). The bivalent IAP antagonists HGS1029 and TL32711 were well tolerated, with grade 2 transient lymphopaenia and neutrophilia in some patients. Clinical trials must now explore the safety and efficacy of IAP antagonists for treating human diseases other than cancer.
In conclusion, our results suggest that since IAPs exhibit increased expression in endometriotic tissues, they may be effective therapeutic targets for treating endometriosis. Continued research efforts to elucidate IAPs function and the pharmacological potential of IAP inhibitors in the treatment of endometriosis should be conducted.
Authors' roles
T.U., F.T., F.O., O.Y. and T.H. participated in the study design, analysis and manuscript drafting. T.U., F.T., K.N. and M.O. executed the experiments.
Funding
This work was supported by KAKENHI (Japan Society for the Promotion of Science, Grant-in-Aid: to F.T.; 21592098 and to T.H.; 24659731) and Yamaguchi Endocrine Research Foundation to F.T.
Conflict of interest
None declared.
Acknowledgements
We appreciate the work of Drs Katherine A. Burns and Kenneth S. Korach (National Institute of Environmental Health Sciences/National Institutes of Health, NC, USA) for establishing the mouse endometriosis model, and Dr Domagoj Vucic (Genentech, South San Francisco, CA, USA) for providing the IAP antagonist, BV6.
References
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- Bono Y, Kyo S, Takakura M, Maida Y, Mizumoto Y, Nakamura M, Nomura K, Kiyono T, Inoue M. Creation of immortalised epithelial cells from ovarian endometrioma. Br J Cancer. 2012;106:1205–1213. doi: 10.1038/bjc.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Rodriguez KF, Hewitt SC, Janardhan KS, Young SL, Korach KS. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology. 2012;153:3960–3971. doi: 10.1210/en.2012-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmowski WP, Ding J, Shen J, Rana N, Fernandez BB, Braun DP. Apoptosis in endometrial glandular and stromal cells in women with and without endometriosis. Hum Reprod. 2001;16:1802–1808. doi: 10.1093/humrep/16.9.1802. [DOI] [PubMed] [Google Scholar]
- Flygare JA, Fairbrother WJ. Small-molecule pan-IAP antagonists: a patent review. Expert Opin Ther Pat. 2010;20:251–267. doi: 10.1517/13543770903567077. [DOI] [PubMed] [Google Scholar]
- Flygare JA, Beresini M, Budha N, Chan H, Chan IT, Cheeti S, Cohen F, Deshayes K, Doerner K, Eckhardt SG, et al. Discovery of a potent small-molecule antagonist of inhibitor of apoptosis (IAP) proteins and clinical candidate for the treatment of cancer (GDC-0152) J Med Chem. 2012;55:4101–4113. doi: 10.1021/jm300060k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012;11:109–124. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- Gebel HM, Braun DP, Tambur A, Frame D, Rana N, Dmowski WP. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil Steril. 1998;69:1042–1047. doi: 10.1016/s0015-0282(98)00073-9. [DOI] [PubMed] [Google Scholar]
- Goumenou AG, Matalliotakis IM, Tzardi M, Fragouli YG, Mahutte NG, Arici A. Apoptosis and differential expression of apoptosis-related proteins in endometriotic glandular and stromal cells. J Soc Gynecol Investig. 2004;11:318–322. doi: 10.1016/j.jsgi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Iwabe T, Harada T, Tsudo T, Tanikawa M, Onohara Y, Terakawa N. Pathogenetic significance of increased levels of interleukin-8 in the peritoneal fluid of patients with endometriosis. Fertil Steril. 1998;69:924–930. doi: 10.1016/s0015-0282(98)00049-1. [DOI] [PubMed] [Google Scholar]
- Izawa M, Harada T, Deura I, Taniguchi F, Iwabe T, Terakawa N. Drug-induced apoptosis was markedly attenuated in endometriotic stromal cells. Hum Reprod. 2006;21:600–604. doi: 10.1093/humrep/dei372. [DOI] [PubMed] [Google Scholar]
- Kim SH, Ihm HJ, Oh YS, Chae HD, Kim CH, Kang BM. Increased nuclear expression of nuclear factor kappa-B p65 subunit in the eutopic endometrium and ovarian endometrioma of women with advanced stage endometriosis. Am J Reprod Immunol. 2013;70:497–508. doi: 10.1111/aji.12161. [DOI] [PubMed] [Google Scholar]
- Kruitwagen RF, Poels LG, Willemsen WN, Jap PH, Thomas CM, Rolland R. Retrograde seeding of endometrial epithelial cells by uterine-tubal flushing. Fertil Steril. 1991;56:414–420. doi: 10.1016/s0015-0282(16)54533-6. [DOI] [PubMed] [Google Scholar]
- Laudanski P, Charkiewicz R, Kuzmicki M, Szamatowicz J, Swiatecka J, Mroczko B, Niklinski J. Profiling of selected angiogenesis-related genes in proliferative eutopic endometrium of women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2014;172:85–92. doi: 10.1016/j.ejogrb.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. doi: 10.1016/s0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- Miura K, Fujibuchi W, Ishida K, Naitoh T, Ogawa H, Ando T, Yazaki N, Watanabe K, Haneda S, Shibata C, et al. Inhibitor of apoptosis protein family as diagnostic markers and therapeutic targets of colorectal cancer. Surg Today. 2011;41:175–182. doi: 10.1007/s00595-010-4390-1. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Harada T, Horie S, Iba Y, Taniguchi F, Yoshida S, Iwabe T, Terakawa N. Tumor necrosis factor-alpha-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-kappa B activation: gonadotropin-releasing hormone agonist treatment reduced IL-8 expression. J Clin Endocrinol Metab. 2003;88:730–735. doi: 10.1210/jc.2002-020666. [DOI] [PubMed] [Google Scholar]
- Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3:93–110.143. [PMC free article] [PubMed] [Google Scholar]
- Schimmer AD, Estey EH, Borthakur G, Carter BZ, Schiller GJ, Tallman MS, Altman JK, Karp JE, Kassis J, Hedley DW, et al. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. J Clin Oncol. 2009;27:4741–4746. doi: 10.1200/JCO.2009.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanculescu A, Bembinster LA, Borgen K, Bergamaschi A, Wiley E, Frasor J. Estrogen promotes breast cancer cell survival in an inhibitor of apoptosis (IAP)-dependent manner. Horm Cancer. 2010;1:127–135. doi: 10.1007/s12672-010-0018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai E, Taniguchi F, Nakamura K, Uegaki T, Iwabe T, Harada T. Parthenolide reduces cell proliferation and prostaglandin E synthesis in human endometriotic stromal cells and inhibits development of endometriosis in the murine model. Fertil Steril. 2013;100:1170–1178. doi: 10.1016/j.fertnstert.2013.06.028. [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Harada T, Miyakoda H, Iwabe T, Deura I, Tagashira Y, Miyamoto A, Watanabe A, Suou K, Uegaki T, et al. TAK1 activation for cytokine synthesis and proliferation of endometriotic cells. Mol Cell Endocrinol. 2009;307:196–204. doi: 10.1016/j.mce.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Higaki H, Azuma Y, Deura I, Iwabe T, Harada T, Terakawa N. Gonadotropin-releasing hormone analogues reduce the proliferation of endometrial stromal cells but not endometriotic cells. Gynecol Obstet Invest. 2013;75:9–15. doi: 10.1159/000343748. [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Higaki H, Izawa M, Azuma Y, Hirakawa E, Deura I, Iwabe T, Hata K, Harada T. The cellular inhibitor of apoptosis protein-2 is a possible target of novel treatment for endometriosis. Am J Reprod Immunol. 2014;71:278–285. doi: 10.1111/aji.12193. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Alicke B, Elliott JM, Zobel K, West K, Wong H, Scheer JM, Ashkenazi A, Gould SE, Fairbrother WJ, et al. X chromosome-linked inhibitor of apoptosis regulates cell death induction by proapoptotic receptor agonists. J Biol Chem. 2009;284:34553–34560. doi: 10.1074/jbc.M109.040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varfolomeev E, Goncharov T, Maecker H, Zobel K, Komuves LG, Deshayes K, Vucic D. Cellular inhibitors of apoptosis are global regulators of NF-kappaB and MAPK activation by members of the TNF family of receptors. Sci Signal. 2012;5:ra22. doi: 10.1126/scisignal.2001878. [DOI] [PubMed] [Google Scholar]
- Vucic D. Targeting IAP (inhibitor of apoptosis) proteins for therapeutic intervention in tumors. Curr Cancer Drug Targets. 2008;8:110–117. doi: 10.2174/156800908783769373. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kanzaki H, Narukawa S, Inoue T, Katsuragawa H, Kaneko Y, Mori T. Bcl-2 and Fas expression in eutopic and ectopic human endometrium during the menstrual cycle in relation to endometrial cell apoptosis. Am J Obstet Gynecol. 1997;176:360–368. doi: 10.1016/s0002-9378(97)70499-x. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Taniguchi F, Izawa M, Suou K, Uegaki T, Takai E, Terakawa N, Harada T. The role of survivin in the resistance of endometriotic stromal cells to drug-induced apoptosis. Hum Reprod. 2009;24:3172–3179. doi: 10.1093/humrep/dep305. [DOI] [PubMed] [Google Scholar]
- Winkel CA. Evaluation and management of women with endometriosis. Obstet Gynecol. 2003;102:397–408. doi: 10.1016/s0029-7844(03)00474-5. [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Koga K, Hirota Y, Hirata T, Ruimeng X, Na L, Yano T, Tsutsumi O, Taketani Y. FR 167653, a p38 mitogen-activated protein kinase inhibitor, suppresses the development of endometriosis in a murine model. J Reprod Immunol. 2006;72:85–93. doi: 10.1016/j.jri.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Xu ZM, Zhang CM, Dai HY, Ji XQ, Wang XF, Li C. Pyrrolidine dithiocarbamate inhibits nuclear factor-kappaB pathway activation, and regulates adhesion, migration, invasion and apoptosis of endometriotic stromal cells. Mol Hum Reprod. 2011;17:175–181. doi: 10.1093/molehr/gaq090. [DOI] [PubMed] [Google Scholar]