Abstract
Neuroblastoma is a common solid malignant tumor of the sympathetic nervous system, which contributes to 15% of cancer-related mortality in children. The differentiation status of neuroblastoma is correlated with clinical outcome, and the induction of differentiation thus constitutes a therapeutic approach in this disease. However, the molecular mechanisms that control the differentiation of neuroblastoma remain poorly understood. The present study aimed to define whether GATA3 is involved in the differentiation of neuroblastoma cells. The results demonstrated that GATA3 is a prognostic marker for survival in patients with neuroblastoma, and that high-level GATA3 expression is associated with increased self-renewal and proliferation of neuroblastoma cells. Retinoic acid treatment led to GATA3 downregulation together with neuronal differentiation, suppression of cell proliferation and inhibition of tumorigenecity in neuroblastoma cells. These findings suggest that GATA3 is a key regulator of neuroblastoma differentiation, and provide a novel potential therapeutic strategy for the induction of neuroblastoma differentiation.
Keywords: cell proliferation, differentiation, GATA3, neuroblastoma
Introduction
Neuroblastoma is a common childhood malignant tumor of the sympathetic nervous system, accounting for up to 10% of pediatric cancers and 15% of cancer-related mortality in children (1–3). Neuroblastoma comprises a heterogeneous group of tumors, in which the level of differentiation is known to be of prognostic significance (4,5). Histologically, neuroblastomas range from tumors containing poorly-differentiated neuroblasts to those composed of fully-differentiated sympathetic neurons (6,7). Patients with poorly differentiated neuroblastomas have a significantly poorer survival than those with neuroblastomas that are shown to be well-differentiated on histological examination (4,5,8).
Retinoic acid (RA) is an effective inducer of the differentiation of neuroblastoma cells (9,10), which has been used in clinical practice as a therapeutic agent in high-risk neuroblastomas in order to improve the differentiation state of the cells (11,12). In addition, GATA transcription factors are involved in the regulation of hematopoiesis, and the development of the cardiovascular, nervous, and urogenital systems (13–17). The GATA family contains six members, which are reported to be expressed in distinct spatiotemporal patterns (18–20). GATA2 and GATA3 are the only members of this family that are present in the nervous system (21,22), and the pattern of the expression of these two proteins is known to overlap. GATA3 has been reported to be involved in the development of serotonergic neurons during formation of the ear, and in the development of the caudal raphe nuclei and the peripheral nervous system (22–27). The present study investigated the role of GATA3 in neuroblastoma proliferation and differentiation.
Materials and methods
Cell culture
SHEP1, SK-N-DZ, SK-N-AS, and SK-N-SH human neuroblastoma cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). SK-N-BE (2), IMR32, BE (2)-C and SY5Y human neuroblastoma cell lines were grown in a 1:1 mixture of DMEM and Ham’s nutrient mixture F12 (F12/DMEM), supplemented with 10% FBS and nonessential amino acids. LAN-6 and SMS-KCNR human neuroblastoma cell lines were grown in RPMI-1640 supplemented with 10% FBS. The growth media and FBS were obtained from Invitrogen Life Technologies (Carlsbad, CA, USA). All cells were obtained from American Type Culture Collection (Manassus, VA, USA) and cultured at 37°C in a 5% CO2 humidified incubator. The 293GPG retroviral packaging cell line was cultured as described previously (28).
Retroviral production and infection
The retroviral constructs, pBabe-green fluorescent protein (GFP) and pBabe-GATA3, were used in the overexpression experiments. Retroviruses were produced using the 293GPG packaging cell line as described previously (28). At 24 h following the final round of retroviral infection, cells were cultured in the growth medium containing 1.0 μg/ml puromycin for three days, and drug-resistant cells were pooled. The percentage of retrovirus-infected cells ranged between 80 and 90%, as estimated in parallel infections using the retrovirus-expressing GFP. Over-expression of relevant proteins was verified by an immunoblotting assay.
Immunoblot analysis
Following RA (Sigma-Aldrich, St. Louis, MO, USA) treatment or retroviral infection, cells in the exponential growth phase at 70–80% confluence were harvested at various time points and washed once with ice-cold phosphate-buffered saline. Cell pellets were suspended in SDS sample buffer and boiled for 10 min prior to centrifugation at 211 × g for 10 min. Samples were subjected to 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). The membrane was probed with antibodies and binding was visualized using enhanced chemiluminescence (ECL; Beyotime Institute of Biotechnology, Haimen, China). The following primary antibodies were used: Rabbit polyclonal anti-GATA3 (1:100; H-48, sc-9009; Santa Cruz Biotechnology Inc., Dallas, TX, USA), mouse monoclonal anti-Mash1 (1:100; clone 24B72D11.1; BD Pharmingen, San Diego, CA, USA), rabbit polyclonal anti-peripherin (1:2,000; AB1530; Chemicon International, Inc., Billerica, MA, USA) and mouse monoclonal anti-α-tubulin (1:10,000; B-5-1-2; Sigma-Aldrich). Horseradish peroxidase-conjugated goat anti-mouse and goat anti-rabbit IgG (1:5,000, ICN, Bryan, OH, USA) were used as secondary antibodies.
Cell growth and differentiation assays
For differentiation assays, RA was dissolved in dimethyl sulfoxide (DMSO) and 10 mM stock solutions were prepared. SK-N-SH cells were treated with 1 μM RA. DMSO (0.1%; Sigma-Aldrich) was used as negative control. Cell growth was observed under a microscope (Olympus IX71; Olympus, Tokyo, Japan) and determined by MTT analysis (Sigma-Aldrich)
Patient data analysis
Patient data and gene expression datasets were obtained from the Oncogenomics Section Data Center (http://pob.abcc.ncifcrf.gov/cgi-bin/JK). Kaplan-Meier analysis and resulting survival curves were created using GraphPad Prism (version 6.0; GraphPad Software, Inc, La Jolla, CA, USA). All data and P-values (log-rank test) for these experiments were downloaded online (http://pob.abcc.ncifcrf.gov/cgi-bin/JK) and all cutoff values for separating the groups with high and low expression were determined using the online database algorithm (29). A good prognosis of neuroblastoma patients was considered to be associated with better survival.
Soft agar clonogenic assay and sphere formation assay
Cells were mixed in 0.3% Noble agar (Sigma-Alrdich) in DMEM supplemented with 10% FBS and plated at 4,000 cells/well into 6-well plates, which contained a solidified bottom layer composed of 0.6% Noble agar in the same growth medium. At 14 days, colonies were stained with 5 mg/ml MTT and photographed (Olympus, IX71; Olympus). For sphere formation assays, cells were plated at 4,000 cells/well in serum-free DMEM, and supplemented with 20 ng/ml epidermal growth factor and basic fibroblast growth factor (Invitrogen Life Technologies) in Matrigel ultra-low attachment plates (Thermo Fisher Scientific, Pitsburgh, PA, USA). Spheres that arose within 1–2 weeks were counted.
In vivo tumorigenic assay
For the tumorigenic assays, six female NOD/SCID mice (4 weeks old) were used and were maintained under SPF conditions. For the tumorigenic assays, the mice were randomly divided into two groups, control group and GATA3-overexpressing group. Mice were injected subcutaneously in both flanks with 1×107 SK-N-SH cells or SK-N-SH-GATA3 cells in 200 μl DMEM. At one week following the injection of tumor cells, tumor growth was estimated using calipers, and tumor volume was calculated using the formula 4/3πr3, where r is the radius of the tumor. Tumors were removed and weighed following three weeks of tumor growth. The present study was approved by the Institutional Animal Care and Use Committee of Southwest University (Chonqing, China).
Statistical analysis
Data are presented as the mean ± standard deviation. Two-tailed Student’s t-test was conducted for paired samples and was performed using GraphPad Prism version 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). P≤0.05 was considered to indicate a statistically significant difference.
Results
High GATA3 expression predicts poor survival in neuroblastoma patients
The correlation between GATA3 expression levels and prognosis in primary neuroblastoma was investigated using the Seeger microarray dataset, which is available from the online Oncogenomics database. This dataset includes a cohort of 102 neuroblastoma patients with metastatic tumors lacking MYCN amplification (30). Kaplan-Meier analysis of progression-free survival for the Seeger dataset showed that low GATA3 expression was associated with a good prognosis, whereas high GATA3 expression was associated with a poor outcome (Fig. 1A). Furthermore, a box plot of GATA3 expression levels in tumors from patients with either a good or a poor prognosis demonstrated the same result (Fig. 1B). This analysis indicated that GATA3 is a prognostic marker in neuroblastoma, which is independent of the status of MYCN amplification.
Figure 1.
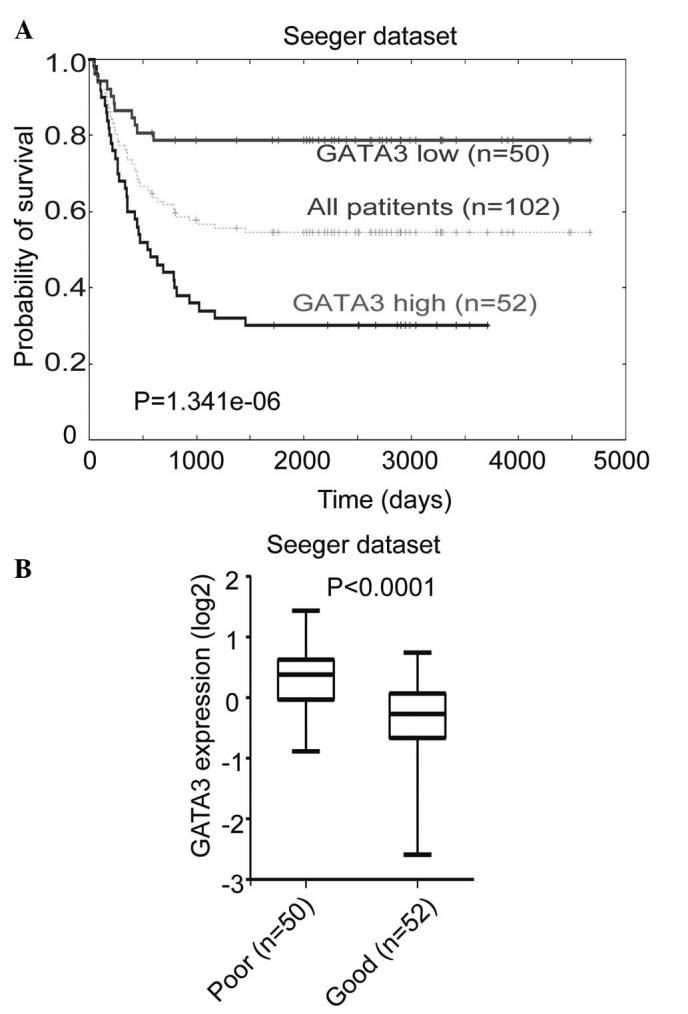
Association between GATA3 expression and survival in patients with neuroblastoma. (A) Kaplan-Meier analysis of progression-free survival for the Seeger database, with the log rank test P-value indicated. A cutoff value of 0.0365 was used to separate the patients into high and low GATA3 expression groups. (B) Box plot of GATA3 expression levels in tumors from groups of patients with good and poor prognoses.
GATA3 is commonly expressed in neuroblastoma cells
The expression of GATA3 in various neuroblastoma cell lines was examined. GATA3 was found to be widely expressed in the majority of neuroblastoma cell lines (Fig. 2A), including BE (2)-C, IMR32, SK-N-DZ, SK-N-AS, and SK-N-BE, which are malignant cell lines. The expression of GATA3 was also investigated in the SHEP1 cell line, which is a benign neuroblastoma cell line with a highly differentiated status (31,32). The results indicated that GATA3 may be associated with the degree of neuroblastoma differentiation and the consequent prognosis. The SK-N-SH cells were a group of mixed cells which were isolated into SY5Y and SHEP1 in different conditions (33), and SY5Y is a type of malignant cell comparing with SHEP1 (32). As hypothesized, GATA3 expression was relatively high in the SY5Y and SK-N-SH cell lines, but there was no detectable expression in the SHEP1 cell line (Fig. 2B). This suggests that GATA3 may be used as a prognostic marker in neuroblastoma.
Figure 2.
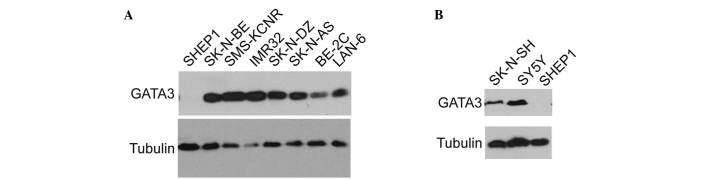
Expression of GATA3 in various neuroblastoma cell lines. (A) Western blot analysis of GATA3 expression in eight neuroblastoma cell lines. (B) Western blot analysis of GATA3 expression in SK-N-SH, SK-N-SY5Y and SHEP1 cell lines. α-tubulin levels were used as a loading control.
GATA3 is associated with neuronal differentiation in neuroblastoma cells
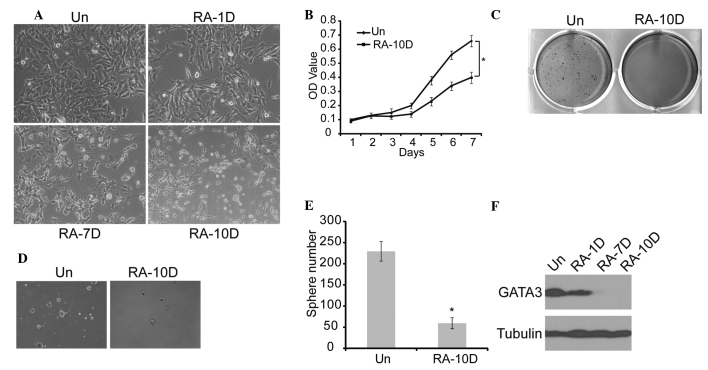
Since RA is commonly used to induce neuronal differentiation in neuroblastoma (34), SK-N-SH cells were treated with RA for 10 days. On examination the cells displayed morphological features of neuronal differentiation, such as small and rounded cell bodies, scant cytoplasm, and extensive neurite-like processes (Fig. 3A). Furthermore, MTT, sphere formation and soft agar analyses showed that RA treatment resulted in the suppression of cell proliferation, tumorigenicity and self-renewal of neuroblastoma cells (Fig. 3B–E). The GATA3 expression in this process was measured, and the results showed that RA induction led to marked downregulation of GATA3 expression with time (Fig. 3F). These findings suggest that RA-induced neuronal differentiation is accompanied by GATA3 downregulation, which leads to weakened self-renewal of neuroblastoma cells.
Figure 3.
Association between GATA3 expression and neuronal differentiation in neuroblastoma cells. (A) Morphological examination of SK-N-SH cells treated with RA or DMSO (magnification, ×20). (B) SK-N-SH cells were treated with RA or DMSO, and cell proliferation was analyzed with an MTT assay. (C) SK-N-SH cells were plated at 4×103 cells per well in six-well culture plates. At days 14 to 21 soft agar colonies grew from the cells treated with DMSO. Cells treated with RA were observed to give rise to small and scanty colonies in soft agar. (D) and (E) SK-N-SH cells were plated at 4×103 cells per well in Matrigel ultra-low attachment plates. At days 14 to 21, spheres grew from cells treated with DMSO, and were recorded. (F) Western blot analysis of GATA3 expression in SK-N-SH cells treated with RA or DMSO (magnification, ×10). α-tubulin levels are shown as a loading control. Data in (B) and (E) are presented as the average obtained from three independent experiments. Error bars, represent standard deviation. *P≤0.05. RA, retinoic acid; Un, untreated; DMSO, dimethyl sulfoxide; RA-(1D, 7D and 10D), one day, seven days and ten days following RA treatment, respectively; OD, optical density.
GATA3 promotes proliferation and tumorigenicity of neuroblastoma cells
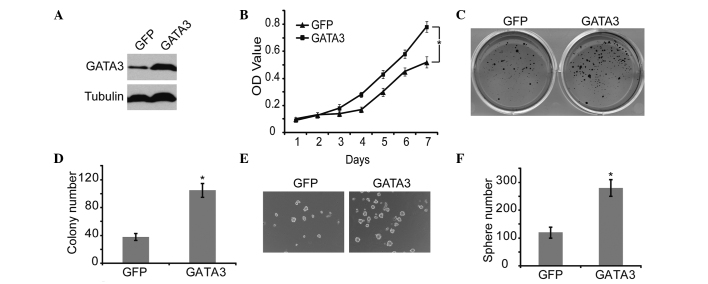
To confirm the correlation between GATA3 and self-renewal of neuroblastoma cells, GATA3 was overexpressed in neuroblastoma cells, using GFP as a control (Fig. 4A). GATA3 significantly increased cell proliferation, which was verified by MTT analysis (Fig. 4B). In addition, GATA3 markedly upregulated the self-renewal ability, including the colony forming and sphere forming capability, of neuroblastoma cells (Fig. 4C and 4F). These results demonstrated that high expression of GATA3 is associated with increased self-renewal and cell proliferation in neuroblastoma cells.
Figure 4.
Effect of GATA3 overexpression on the proliferation and self-renewal of neuroblastoma cells. (A) Western blot analysis of GATA3 expression in SK-N-SH cells with GFP or GATA3 overexpression. α-Tubulin levels were used as a loading control. (B) SK-N-SH cells with GFP or GATA3 overexpression were analyzed for cell growth curve with an MTT assay. (C) SK-N-SH cells with GFP or GATA3 overexpression were plated at 4×103 cells per well in six-well culture plates. At days 14 to 21, soft agar colonies grew from cells with GFP or GATA3 overexpression. Cells with GATA3 overexpression were observed to give rise to larger and more colonies in soft agar. (D) Colonies >0.5 mm or that contained >50 cells were recorded. (E) and (F) SK-N-SH cells with GFP or GATA3 overexpression were plated at 4×103 cells per well in Matrigel ultra-low attachment plates. At days 14 to 21, spheres grew and were recorded (magnification, ×10). Data in (B), (D) and (F) are presented as the average obtained from three independent experiments. Error bars represent the standard deviation. *P≤0.05. GFP, green fluorescent protein; OD, optical density.
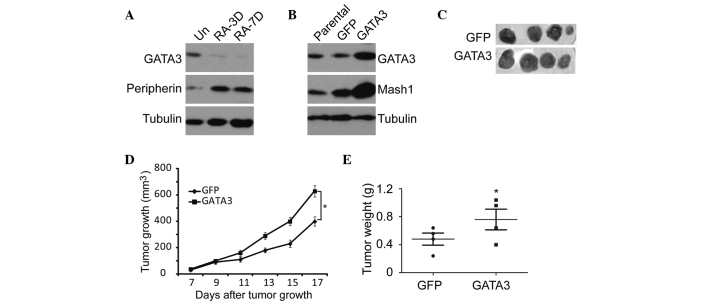
Furthermore, the RA-induced neuroblastoma differentiation was accompanied by GATA3 downregulation and the upregulation of peripherin, a neuronal differentiation marker (35) (Fig. 5A). GATA3 upregulation led to a significant increase in the expression of Mash1 (Fig. 5B), which is a potential stem cell or progenitor cell marker (36,37). Similar results were obtained from the in vivo tumorigenicity analysis using the SK-N-SH neuroblastoma cells. Overexpression of GATA3 in SK-N-SH cells significantly enhanced tumor growth and development in NOD/SCID mice (Fig. 5C–E). These data indicate that GATA3 is not only a prognostic marker, but also an important mediator of cell proliferation and differentiation.
Figure 5.
Effect of GATA3 overexpression on the tumorigenicity of neuroblastoma cells. (A) Western blot analysis of GATA3 and peripherin expression in SK-N-SH cells treated with RA or DMSO. (B) Western blot analysis of GATA3 and Mash1 expression in SK-N-SH cells with GFP or GATA3 overexpression. α-tubulin levels were used as a loading control. (C) Tumor growth in NOD/SCID mice injected with indicated SK-N-SH cells. (D) Scatter plot of xenograft tumor weight with horizontal lines indicating the mean per group. (E) Xenograft tumor volume was measured using calipers. *P≤0.05. Scale bar=5 cm. GFP, green fluorescent protein; Un, untreated; RA-3D/RA-7D, three and seven days, respectively, following retinoic acid administration.
Discussion
The current study provided a number of lines of evidence to support the hypothesis that GATA3 acts as an important mediator of neuroblastoma differentiation. GATA3 was shown to be expressed at significantly lower levels following RA-induced differentiation. Overexpression of GATA3 expression significantly increased cell growth and self-renewal in neuroblastoma cells. Furthermore, RA-induced neuronal differentiation resulted in the upregulation of peripherin, a neuronal differentiation marker, and the downregulation of GATA3. In turn, GATA3 overexpression increased the expression of a marker of a self-renewal marker, Mash1. These results suggest a possible molecular mechanism linking neuronal differentiation and self-renewal. Using a gene expression dataset of 102 metastatic neuroblastoma tumors, it was shown that high GATA3 expression is a prognostic marker of poor outcome, which supported the results of the other experiments.
MYCN is an important oncogene in the pathogenesis of neuroblastoma (38), and is known to regulate various cellular processes, including cell growth, cell proliferation, cell differentiation and apoptosis (39,40). The oncogene MYCN was originally identified in neuroblastoma cells (41,42), and it has been reported as a prognostic marker in patients with neuroblastoma (43). Amplification of MYCN occurs in 22% of cases of neuroblastoma and is associated with advanced stages of this disease and a poor prognosis (7). N-myc is currently the only marker commonly used in the diagnosis of neuroblastoma. It is therefore necessary to identify further genetic markers for neuroblastoma. The current study showed that high GATA3 expression was correlated with poor survival in patients with neuroblastoma. Low expression of GATA3 was associated with a high degree of differentiation, indicating that GATA3 may be of use as a prognostic marker in neuroblastoma.
Neuroblastoma originates from precursor neuroblasts of the sympathetic nervous system, and is characterized by a unique capacity for complete spontaneous regression, at least partly through the process of neuronal differentiation (8). In clinical practice, patients with advanced neuroblastoma may be successfully treated by the administration of RA, which induces tumor cells to differentiate and leads to growth inhibition (9,11,44). In the current study, neuronal differentiation, induced by RA, was accompanied by GATA3 downregulation in neuroblastoma cells, whereas upregulation of GATA3 was associated with increased self-renewal and proliferation of neuroblastoma cells. In conclusion, the present results confirmed that GATA3 has an important function in neuroblastoma differentiation and proliferation. Therefore, GATA3 may be useful as a prognostic marker in patients with neuroblastoma, and may also serve as a potential therapeutic target for neuroblastoma.
Acknowledgements
This study was supported by the Research Fund for the Doctoral Program of Higher Education of China (grant no. 20130182110003), the Natural Science Foundation of Chongqing (grant no. cstc2013jcyjys0007) and the Fundamental Research Funds for the Central Universities (grant no. SWU111014).
References
- 1.Zhu S, Yan X, Xiang Z, Ding HF, Cui H. Leflunomide reduces proliferation and induces apoptosis in neuroblastoma cells in vitro and in vivo. PLoS One. 2013;8:e71555. doi: 10.1371/journal.pone.0071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li T, Wang L, Ke XX, et al. DNA-damaging drug-induced apoptosis sensitized by N-myc in neuroblastoma cells. Cell Biol Int. 2012;36:331–337. doi: 10.1042/CBI20110231. [DOI] [PubMed] [Google Scholar]
- 3.Li T, Cui ZB, Ke XX, et al. Essential role for p53 and caspase-9 in DNA damaging drug-induced apoptosis in neuroblastoma IMR32 cells. DNA Cell Biol. 2011;30:1045–1050. doi: 10.1089/dna.2011.1255. [DOI] [PubMed] [Google Scholar]
- 4.Mao L, Ding J, Zha Y, et al. HOXC9 links cell-cycle exit and neuronal differentiation and is a prognostic marker in neuroblastoma. Cancer Res. 2011;71:4314–4324. doi: 10.1158/0008-5472.CAN-11-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambros IM, Hata J, Joshi VV, et al. Morphologic features of neuroblastoma (Schwannian stroma-poor tumors) in clinically favorable and unfavorable groups. Cancer. 2002;94:1574–1583. doi: 10.1002/cncr.10359. [DOI] [PubMed] [Google Scholar]
- 6.Shimada H, Ambros IM, Dehner LP, et al. The international neuroblastoma pathology classification (the Shimada system) Cancer. 1999;86:364–372. doi: 10.1002/(SICI)1097-0142(19990715)86:2<364::AID-CNCR21>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 8.Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013;13:397–411. doi: 10.1038/nrc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sidell N. Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J Natl Cancer Inst. 1982;68:589–596. [PubMed] [Google Scholar]
- 10.Hämmerle B, Yañez Y, Palanca S, et al. Targeting neuroblastoma stem cells with retinoic acid and proteasome inhibitor. PLoS One. 2013;8:e76761. doi: 10.1371/journal.pone.0076761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds CP, Matthay KK, Villablanca JG, Maurer BJ. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003;197:185–192. doi: 10.1016/S0304-3835(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 12.Volchenboum SL, Cohn SL. Progress in defining and treating high-risk neuroblastoma: lessons from the bench and bedside. J Clin Oncol. 2009;27:1003–1004. doi: 10.1200/JCO.2008.20.2739. [DOI] [PubMed] [Google Scholar]
- 13.Pevny L, Simon MC, Robertson E, et al. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 14.Asnagli H, Afkarian M, Murphy KM. Cutting edge: Identification of an alternative GATA-3 promoter directing tissue-specific gene expression in mouse and human. J Immunol. 2002;168:4268–4271. doi: 10.4049/jimmunol.168.9.4268. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Lim KC, Onodera K, et al. Rescue of the embryonic lethal hematopoietic defect reveals a critical role for GATA-2 in urogenital development. EMBO J. 1998;17:6689–6700. doi: 10.1093/emboj/17.22.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craven SE, Lim KC, Ye W, Engel JD, de Sauvage F, Rosenthal A. Gata2 specifies serotonergic neurons downstream of sonic hedgehog. Development. 2004;131:1165–1173. doi: 10.1242/dev.01024. [DOI] [PubMed] [Google Scholar]
- 17.Dasen JS, O’Connell SM, Flynn SE, et al. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell. 1999;97:587–598. doi: 10.1016/S0092-8674(00)80770-9. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Ko LJ, Leonard MW, Beug H, Orkin SH, Engel JD. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990;4:1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- 19.Whyatt DJ, deBoer E, Grosveld F. The two zinc finger-like domains of GATA-1 have different DNA binding specificities. EMBO J. 1993;12:4993–5005. doi: 10.1002/j.1460-2075.1993.tb06193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsarovina K, Pattyn A, Stubbusch J, et al. Essential role of Gata transcription factors in sympathetic neuron development. Development. 2004;131:4775–4786. doi: 10.1242/dev.01370. [DOI] [PubMed] [Google Scholar]
- 21.Kornhauser JM, Leonard MW, Yamamoto M, LaVail JH, Mayo KE, Engel JD. Temporal and spatial changes in GATA transcription factor expression are coincident with development of the chicken optic tectum. Brain Res Mol Brain Res. 1994;23:100–110. doi: 10.1016/0169-328X(94)90216-X. [DOI] [PubMed] [Google Scholar]
- 22.Pata I, Studer M, van Doorninck JH, et al. The transcription factor GATA3 is a downstream effector of Hoxb1 specification in rhombomere 4. Development. 1999;126:5523–5531. doi: 10.1242/dev.126.23.5523. [DOI] [PubMed] [Google Scholar]
- 23.Tsarovina K, Reiff T, Stubbusch J, et al. The Gata3 transcription factor is required for the survival of embryonic and adult sympathetic neurons. J Neurosci. 2010;30:10833–10843. doi: 10.1523/JNEUROSCI.0175-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Esch H, Devriendt K. Transcription factor GATA3 and the human HDR syndrome. Cell Mol Life Sci. 2001;58:1296–1300. doi: 10.1007/PL00000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattyn A, Simplicio N, van Doorninck JH, Goridis C, Guillemot F, Brunet JF. Ascl1/Mash1 is required for the development of central serotonergic neurons. Nat Neurosci. 2004;7:589–595. doi: 10.1038/nn1247. [DOI] [PubMed] [Google Scholar]
- 26.Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet. 2000;25:209–212. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- 27.Milo M, Cacciabue-Rivolta D, Kneebone A, et al. Genomic analysis of the function of the transcription factor gata3 during development of the mammalian inner ear. PLoS One. 2009;4:e7144. doi: 10.1371/journal.pone.0007144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen QR, Song YK, Wei JS, et al. An integrated cross-platform prognosis study on neuroblastoma patients. Genomics. 2008;92:195–203. doi: 10.1016/j.ygeno.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asgharzadeh S, Pique-Regi R, Sposto R, et al. Prognostic significance of gene expression profiles of metastatic neuroblastomas lacking MYCN gene amplification. J Natl Cancer Inst. 2006;98:1193–1203. doi: 10.1093/jnci/djj330. [DOI] [PubMed] [Google Scholar]
- 31.Cui H, Hu B, Li T, et al. Bmi-1 is essential for the tumorigenicity of neuroblastoma cells. Am J Pathol. 2007;170:1370–1378. doi: 10.2353/ajpath.2007.060754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui H, Ma J, Ding J, Li T, Alam G, Ding HF. Bmi-1 regulates the differentiation and clonogenic self-renewal of I-type neuroblastoma cells in a concentration-dependent manner. J Biol Chem. 2006;281:34696–34704. doi: 10.1074/jbc.M604009200. [DOI] [PubMed] [Google Scholar]
- 33.Lambert DG, Ghataorre AS, Nahorski SR. Muscarinic receptor binding characteristics of a human neuroblastoma SK-N-SH and its clones SH-SY5Y and SH-EP1. Eur J Pharmacol. 1989;165:71–77. doi: 10.1016/0014-2999(89)90771-1. [DOI] [PubMed] [Google Scholar]
- 34.Sidell N, Altman A, Haussler MR, Seeger RC. Effects of retinoic acid (RA) on the growth and phenotypic expression of several human neuroblastoma cell lines. Exp Cell Res. 1983;148:21–30. doi: 10.1016/0014-4827(83)90184-2. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen WA, Becker LE, Yeger H. Expression and distribution of peripherin protein in human neuroblastoma cell lines. Int J Cancer. 1993;53:463–470. doi: 10.1002/ijc.2910530319. [DOI] [PubMed] [Google Scholar]
- 36.Sommer L, Shah N, Rao M, Anderson DJ. The cellular function of MASH1 in autonomic neurogenesis. Neuron. 1995;15:1245–1258. doi: 10.1016/0896-6273(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 37.Horton S, Meredith A, Richardson JA, Johnson JE. Correct coordination of neuronal differentiation events in ventral forebrain requires the bHLH factor MASH1. Mol Cell Neurosci. 1999;14:355–369. doi: 10.1006/mcne.1999.0791. [DOI] [PubMed] [Google Scholar]
- 38.Alam G, Cui H, Shi H, et al. MYCN promotes the expansion of Phox2B-positive neuronal progenitors to drive neuroblastoma development. Am J Pathol. 2009;175:856–866. doi: 10.2353/ajpath.2009.090019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–699. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- 40.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6:635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 41.Schwab M, Alitalo K, Klempnauer KH, et al. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983;305:245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- 42.Kohl NE, Kanda N, Schreck RR, et al. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell. 1983;35:359–367. doi: 10.1016/0092-8674(83)90169-1. [DOI] [PubMed] [Google Scholar]
- 43.Akter J, Takatori A, Hossain MS, et al. Expression of NLRR3 orphan receptor gene is negatively regulated by MYCN and Miz-1, and its downregulation is associated with unfavorable outcome in neuroblastoma. Clin Cancer Res. 2011;17:6681–6692. doi: 10.1158/1078-0432.CCR-11-0313. [DOI] [PubMed] [Google Scholar]
- 44.Seeger RC, Siegel SE, Sidell N. Neuroblastoma: clinical perspectives, monoclonal antibodies, and retinoic acid. Ann Intern Med. 1982;97:873–884. doi: 10.7326/0003-4819-97-6-873. [DOI] [PubMed] [Google Scholar]