Abstract
Domino liver transplantation is a method used to increase the number of liver grafts available for orthotopic liver transplantation (OLT). Reports indicate that livers from patients with metabolic liver disease can be safely transplanted into select recipients if the donor’s defect and the recipient’s metabolic needs are carefully considered. The liver of patients with many types of metabolic liver disease are morphologically and biochemically normal, except for the mutation that characterizes that disease. Other biochemical functions normally performed by liver are present and presumably “normal” in these hepatocytes. Hepatocytes were isolated from the liver of 35 organ donors and 35 liver tissues taken at OLT from patients with liver disease were analyzed for 9 different measures of viability and function. The data indicate that cells isolated from some diseased livers performed as well or better than those isolated from organ donors with respect to viability, cell yield, plating efficiency and in assays of liver function, including drug metabolism, conjugation reactions and ammonia metabolism. Cells from metabolic diseased livers rapidly and efficiently repopulated a mouse liver upon transplantation. Conclusions: As with domino liver transplantation, domino cell transplantation deserves consideration as method to extend the pool of available organs and cells for transplantation.
Keywords: Hepatocyte Transplantation, Human Hepatocytes, Metabolic Liver Disease, Domino Transplant
INTRODUCTION
Transplantation of hepatocytes (HTx) has been shown to be useful for patients with chronic or acute liver failure or genetic defects in liver function(1–12). The most common indications of HTx are liver-based inborn metabolic disorders. Because of large redundancies of function, the entire hepatic capacity is not normally required to maintain homeostasis. The main source of cells for HTx is livers rejected for transplantation (4, 13, 14). Steatosis is the most common cause for rejection of a tissue for transplantation. Low cell viability, yield and drug-metabolizing enzyme activity has been reported in hepatocytes obtained from fatty livers (15).
Domino liver transplantation (DLT) of organs from patients with metabolic diseases has been used to increase the number of organs available for transplant (16–18). The world registry lists 790 such transplants through 2009 (19). The metabolic disease in the donor liver either should not induce the disease in the recipient, or would be expected to produce symptoms of the disease only after many years. Unlike DLT, where the entirety of the liver is rapidly replaced with one with a metabolic disease, HTx generally results in replacement of <10% of the recipient liver with donor cells. Since most metabolic disease hepatocytes would not induce the symptoms of the disease in the recipient, hepatocytes from donors with metabolic diseases might be useful for transplantation if they could be isolated in useful numbers and retained sufficient viability and function. Here we examined the viability, cell yield and metabolic activity of hepatocytes isolated from organ donors and tissues obtained from patients receiving OLT for metabolic and other types of liver diseases. The results indicate that hepatocytes with high viability and function can be isolated from organs removed at the time of OLT from patients with diseased livers. If the diseases of the donor and recipient are carefully considered, these organs could provide a useful source of cells for clinical transplantation.
MATERIALS AND METHODS
Human liver tissues were collected under IRB protocol 0411142 and hepatocytes were isolated from 35 organ donors (OD) and from 35 liver tissues obtained from patients receiving OLT for metabolic and other liver diseases (MD). Liver tissue from organ donors were flushed with either Belzer’s, UW solution or HTK depending on the solution preferred by the procurement agency. Metabolic disease cases were recovered in the OR and flushed with either Belzer’s UW solution if stored for more than 90 minutes or ice-cold Eagles MEM if the tissues were taken to the lab immediately for isolation. Hepatocyte isolation and culture were performed as previously described (20, 21). Methods to assess cell viability, plating efficiency, or ammonia, testosterone and 7-ethoxyresorufin metabolism, resorufin conjugation, media and culture conditions were as previously described (20). Cell-based assays specific for specific CYP (1A2; 2C9; 3A7; 3A4, Promega Corporation, Madison, WI, U.S.A.) were used according to manufacturer’s instructions. Results were expressed as Luminescent Counting Unit (LCU)/min and normalized to a million of viable cells (suspension cultures) or to the double strand DNA content (adherent cultures) measured, after the Glo -assays are complete, by Quant-iT™ PicoGreen® dsDNA kit according manufacturer’s instructions (Molecular Probes, Invitrogen, Camarillo, CA).
Animal transplant
Animal studies were reviewed and approved by the Institutional Animal Care and Use Committee. Human hepatocytes (106/ml in Eagle’s Minimal Essential Medium; Lonza) were transplanted in FRG mice as previously described (22).
Statistical Analysis
Statistical differences were determined by comparing means using analysis of variance with repeated measurements and Dunnett post hoc test, p < 0.05 was chosen as the minimum level of significance. Data analyzed by GraphPad Prism (version 5.03, GraphPad Software Inc.).
RESULTS
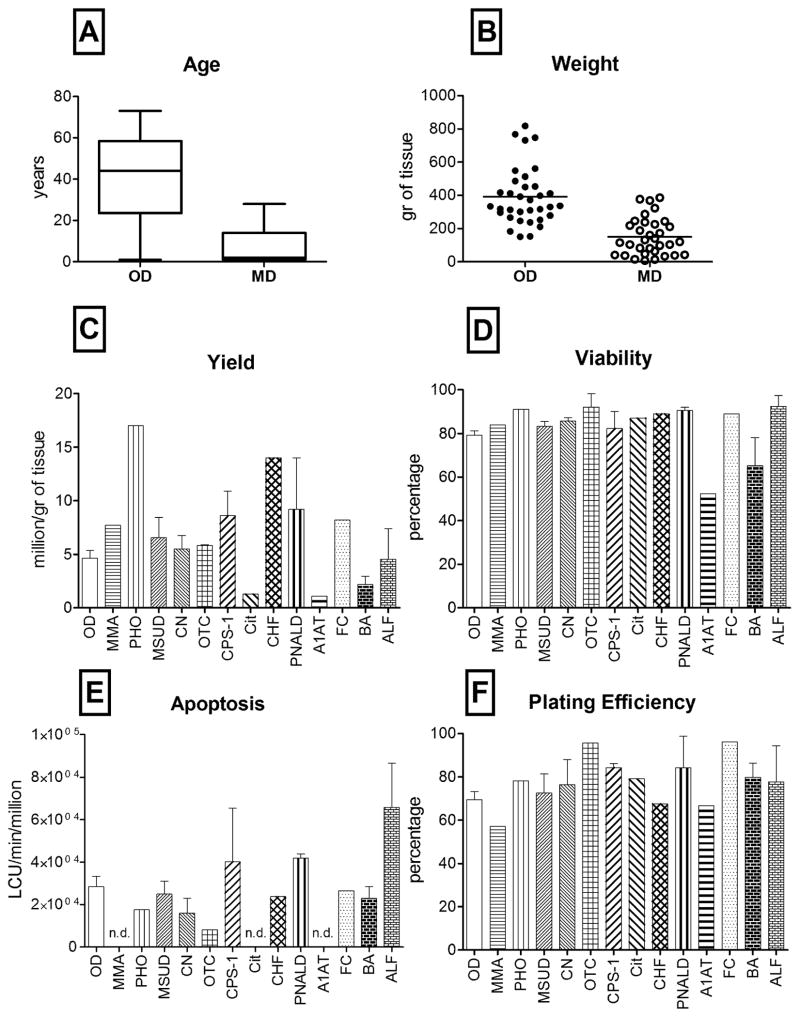
Hepatocytes were successfully isolated from 70 hepatic tissues; 35 were obtained from organ donors (OD) rejected for OLT, and 35 were tissues were obtained from the explanted liver of patients who received OLT for a metabolic and other types of liver disease (MD) including, biliary atresia, PNALD as described in Table 1. The average age of the OD group was 41±4 (mean±SEM) years (Figure 1). Metabolic and disease livers were obtained from younger individuals (8±2 years), with a gender distribution of 2:1 (M/F). The cell yield, normalized to millions of viable cells per gram of tissue, was nearly 1.5 times higher in the MD group as compared to the OD group. Gender does not significantly affect the isolation outcome, while younger donors are normally providing more viable hepatocytes per gram of processed tissue. Nevertheless, no significant differences were observed between ODs and MDs in term of cell recovery.
Table 1.
Characteristics of the liver tissues investigated. Livers from organ donors were those rejected for transplantation, mainly for prolonged cold ischemia time (up to 22hrs), anoxia, drug abuse or steatosis. Also listed are the metabolic and diseased livers, the number of cases collected, the age and gender of the donor. A small portion of the right lobe was dissected in the OR immediately after explant, flushed and transported to the lab for cell isolation. Cold ischemic times ranged from 1 to 12 hours.
| Metabolic disease | abbreviation | n | gender | age |
|---|---|---|---|---|
| Methyl Malonic Acidemia | MMA | 1 | M | 24yrs |
| Primary Hyperoxaluria | PHO | 1 | M | 13mos |
| Maple Syrup Urine Disease | MSUD | 9 | M (6)/F(3) | 16mos–28yrs |
| Crigler-Najjar syndrome | CN | 4 | M (2)/F (2) | 13mos–23yrs |
| Ornithine Transcarbamylase deficiency | OTC | 2 | M (1)/F (1) | 2–25yrs |
| Carbamoyl-Phosphate Synthetase 1 deficiency | CPS-1 | 2 | F | 6–15mos |
| Citrullinemia | Cit | 1 | F | 2yrs |
| Congenital Hepatic Fibrosis | CHF | 1 | F | 14yrs |
| Parenteral Nutrition Associated Liver Disease | PNALD | 2 | M (1)/F (1) | 18mos |
| Alpha-1-antitrypsin deficiency | A1AT | 1 | M | 10yrs |
| Familial Cholestasis | FC | 1 | M | 14mos |
| Biliary Atresia | BA | 7 | M (4)/F (3) | 6–12mos |
| Acute Liver Failure | ALF | 3 | M (2)/F (1) | 18mos–12yrs |
Figure 1. Age, tissue weight, viable cell yield, viability, apoptosis and plating efficiency of hepatocytes.
(A) Box-and-Whisker plot showing median, 25- and 75-percentiles and min and max values of OD and MD tissue samples (n=70); (B) scatter plots of wet tissue weights of tissues used for cell isolation (n=70). Bar graphs representing (C) viable cell recovery (n=70); (D) percentage of viable hepatocytes (n=70); (E) caspase 3/7 activity measured immediately after isolation (n= 34); (F) percent of cells attached to a collagen substrate after 24 hours in culture (n=56). Bars show mean and standard error in groups with more than one sample; nd = evaluations not done.
A viability of 60% would still be considered useful for a clinical transplant. Only one sample from the OD group and 3 from the MD groups were below this level of viability (Figure 1D). As expected, the MD cases with low viability were from cirrhotic livers, from an A1AT patient and two cases with biliary atresia. Taken together, there were no significant differences in viability between the OD and MD groups (79±2% and 82±3%, respectively).
The level of apoptosis, as measured by caspase activity in cells, were not different between the two groups: specimens with the highest caspase activity were those obtained from the liver failure (ALF and PNALD) and cirrhotic (biliary atresia) cases (Figure 1E).
Plating efficiency measures the ability of cells to attach to culture dishes and remain attached for the first 24 hrs. Since attachment requires continued energy expenditure by the cells, it is a more robust test of viability than Trypan blue exclusion. Plating efficiency was measured on 56 samples, equally distributed between the OD and MD groups. There were no significant difference in the plating efficiency between MD and the OD groups (Figure 1F). Only two of the MD cases and 4 of the OD cases displayed less than a 50% plating efficacy. In both groups, cells showed typical hepatocyte morphology: polygonal shape, granular cytoplasm with small vesicular inclusions, one or more nuclei and sharp and bright cell membranes with canalicular structures readily visible between adjacent hepatocytes (Figure 2). Isolated cells from the MD cases showed remarkably normal morphology, particularly when the gross morphology of the native liver tissue was considered (Figure 2F) parenchymal hepatocytes made up >95% of the attached cells by visual inspection.
Figure 2. Phase-contrast photographs of Human Hepatocytes isolated from OD and MD cases.
Representative photographs taken on day 5 of culture, Bar = 40 microns (A) OD (B) Primary Hyperoxaluria; (C) Maple Syrup Urine Disease; (D) Crigler-Najjar syndrome; (E) Biliary Atresia; (F) Explant liver from a biliary atresia patient prior to cell isolation.
Metabolic Activity of Isolated Cells
Isolated cells must display robust biotransformation activity to support liver function. Hepatic cytochrome P450 enzymes metabolize drugs and other xenobiotics as well as endogenous substances such as hormones. Data in figures 3 and 4 summarize activities measured in isolated cells with substrates for CYP1A, CYP2C9, CYP3A4 and CYP3A7. For CYP1A we employed two different measures of activity, a luminescent (Luciferin-ME) and a fluorescent assay (EROD). Testosterone is metabolized in a regioselective manner by CYP3A4. Hydroxylation of testosterone on the 6β position is a common measure of CYP3A4 activity. High-pressure liquid chromatography was used to separate and quantify testosterone metabolism to 6-betahydroxytestosterone to assess CYP3A4 mediated activity. Cells obtained from the two different tissue sources, ODs and MDs, were analyzed as a rapid readout protocol, within 2 hours of isolation for CYPs1A1, 1A2, 2C9, 3A7 and 3A4, n=61 (Figure 3) and, when possible (n=50), with cells maintained in culture with and without exposure to prototypical inducing agents on the final 3 days of a 5 day culture period, β-naphthoflavone (BNF), rifampicin (Rif) or phenobarbital (PB)(Figure 4).
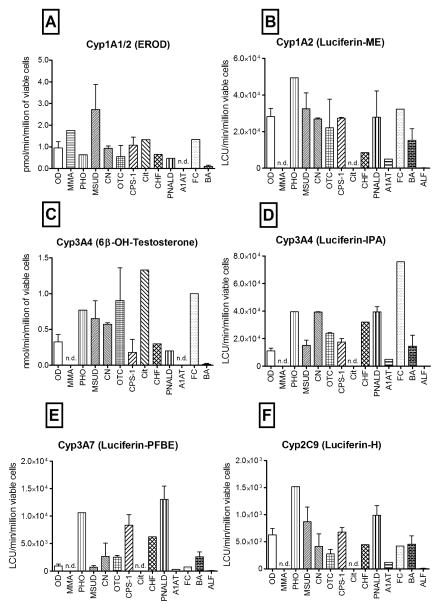
Figure 3. Metabolic functions in freshly isolated hepatocytes from OD and MD cases.
CYP1A1/2 activity measured as (A) EROD activity or (B) Luciferin-ME; CYP3A4 measured as (C) 6-betahydroxytestosterone formation or (D) Luciferin-IPA; (E) CYP3A7 activity measured with Luciferin-PFBE; (F) CYP2C9 activity quantified with Luciferin-H; (n=61); nd = evaluation not done.
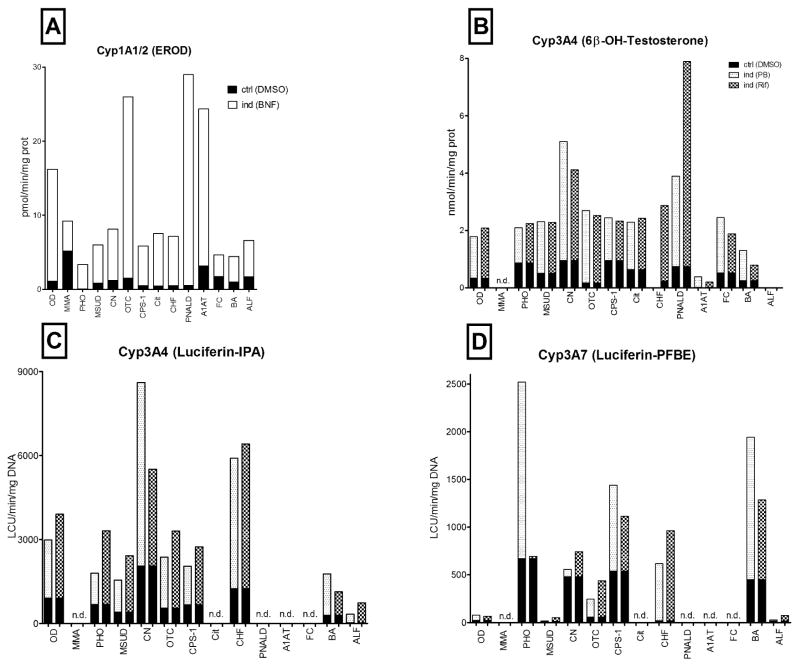
Figure 4. Long-term metabolic activity and CYP450 inductions.
Metabolic activities measured in hepatocytes isolated from OD or MD. Cells were cultured for 2 days in regular maintenance media. In the indicated cultures, the inducing agents were added to culture media for 3 additional days. Control cultures were replenished daily with fresh media without inducers but with the vehicle (0.1% DMSO). All activities were measured on day 5 of culture (n=50). The small black area at the base of each bar represents the activity measured in control cultures treated with vehicle (DMSO) and the open or shaded area above, represents the activity measured following a 3-day exposure to the indicated compound. (A) CYP1A1/2 activity, white bars are data from cells induced with BNF; (B,C) CYP3A4 activity, shaded bars represent cells pretreated with PB or Rif, respectively; (D) CYP3A7 activity in control (black) vs PB or Rif-treated hepatocytes (shaded bars); nd = evaluation not done.
The conversion of 7-ethoxyresorufin to resorufin (EROD) is a measure of CYP1A activities. Similar EROD activities were measured in both the OD and MD groups immediately after isolation (1.0±0.3 and 1.1±0.3 pmol/min/million in the OD and MD hepatocytes, respectively, Figure 3A). The metabolism of a luminescent probe (Luciferin-ME), reported to be specific for CYP1A2 activity was also examined. In general, the results with Luciferin-ME agree well with the EROD assay. Although specific cases in each group showed low activity, taken together, no significant differences were observed between the hepatocytes isolated from the OD and MD groups (figure 3B). Metabolism dependent on CYP3A4 activity in hepatocytes was measured by testosterone and with Luciferin-IPA metabolism. A 1.6 fold higher capacity to metabolize testosterone was noted in MD, as compared to OD hepatocytes immediately after isolation (Figure 3C). Using the luminescent CYP3A4 assay, Luciferin-IPA showed a 2-fold increase in metabolism. Both CYP3A4 assays showed a similar profile of activity with specific samples (Figure 3D). Another CYP3A family member, CYP3A7, is expressed at highest levels in fetal liver and its expression gradually declines over the late fetal, neonatal and pediatric life (23). Since one OD sample and 50% of the MD samples were obtained from patients under 3-years of age, a time when significant levels of CYP3A7 could still be expressed, we examined CYP3A7 activity with Luciferin-PFBE as the substrate. The OD group contained older donors and, as expected, showed significantly lower CYP3A7 activity compared to the younger MD group (p=0.0125)(Figure 3E). Measurements of CYP2C9 activity showed a similar profile as that observed with CYP3A4 and 7 (Figure 3F).
Cytochromes in the 1A, 2C and 3A families have a characteristic that prior exposure to specific compounds will result in the induction of the expression and activity of these enzymes in hepatocytes. Thus, the ability to induce the expression of these CYPs with prototypical inducers is a robust test of the capacity of the cells to survive in culture for 5 days, and respond as they would, in vivo, with regulated RNA expression, increased de novo synthesis of proteins that are fully functional and can be quantified as increased metabolism of specific CYP substrates.
Data presented in figure 4 show the response of hepatocytes from the OD and MD groups to specific CYP induction protocols. As shown in panel 4A, prior exposure to BNF induced CYP1A activity in both OD and MD cases. Even cases with low basal activity such as PHO and BA could be induced more than 2-fold by BA exposure. Cells from the MMA patient showed the highest basal levels of activity, more than 3-fold higher than the OD controls. Metabolism mediated by CYP3A4 measured as testosterone metabolism (4B) or the luminescent IPA assay (4C) was measured in the OD and MD groups. While MD donors tended towards higher basal activities than the OD, the results were not significantly different. Both groups were readily induced by prior exposure to PB or Rif with induction greater than 7-fold over basal levels. The lowest CYP3A4 levels were measured in the cirrhotic cases, A1AT and BA.
As described earlier, CYP3A7, is the CYP3A family member expressed at highest levels in fetal and early postnatal life. As shown in figure 4D, CYP3A7 is expressed at low levels in the OD group and was not significantly induced by prior exposure to PB or Rif. However, the MD group contains many pediatric patients, and CYP3A7 activity was readily measured and was induced by Rif or PB in most of the MD cases. On average, the basal CYP3A7 activity is 10-fold higher in the MD than in the OD group. There were noticeable differences among the inborn errors in the MD groups: the urea cycle defects (OTC and CPS-1) showed robust induction, both in term of 3A7 (5–12 fold increase) and 3A4 isoforms (10–27 fold). CHF showed a modest induction of CYP3A4, but the greatest induction in terms of CYP3A7 activity, 35–54-fold, but this high ratio is due in part, to an extremely low basal level.
Conjugation
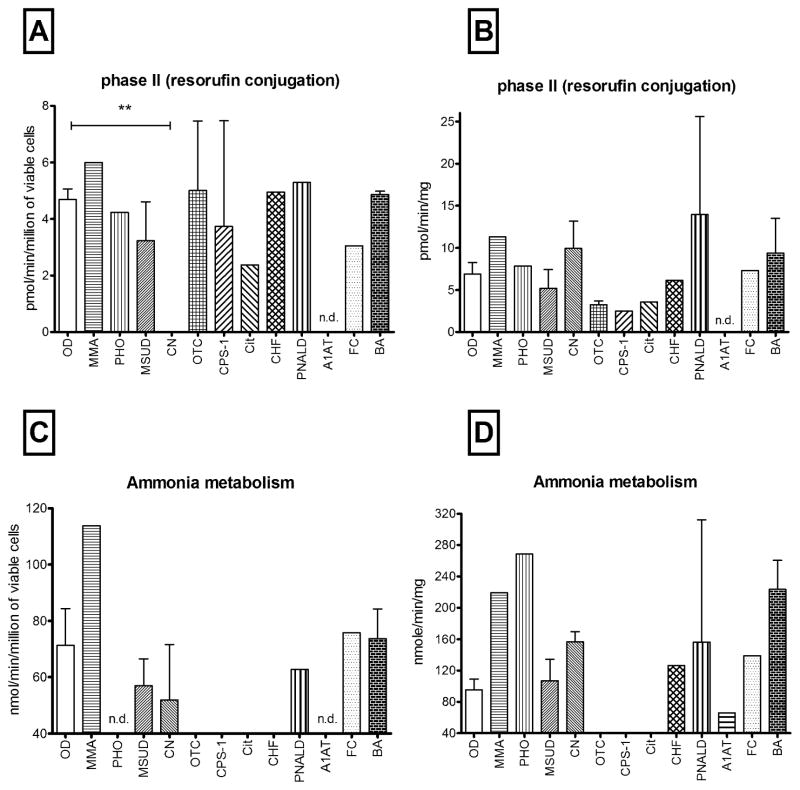
Phase II activities, such as conjugation reactions with sulphate, glucuronide or glutathione, are generally considered to be detoxification processes that aid in the elimination of endogenous or xenobiotics from the body. In freshly isolated cells conjugation of resorufin was comparable in OD and MD-derived hepatocytes (Figure 5A) and was well maintained in cells in longer-term cultures (5B). Interestingly, cells from the Crigler-Najjar cases, showed no capacity to conjugate resorufin immediately upon isolation (p=0.0004), but normal metabolic activity was restored to normal levels by day 5 when cells were maintained in culture.
Figure 5. Conjugation and ammonia metabolism.
Bars represent conjugation or ammonia metabolism from OD and MD hepatocytes (A,C) immediately after isolation (n=54) and (B,D) in cells cultured in maintenance media for an additional 5 days. (n=50). No CN cases (n=3) displayed any conjugation capacity towards resorufin, ** p=0.0004. As expected, cells from urea cycle defect patients (OTC, CPS-1 and Cit) showed no capacity to metabolize ammonia, nd = evaluation not done.
Ammonia metabolism
Ammonia metabolism was measurable in 8 of 11 MD cases examined and the range of activities were similar to that observed in the OD group. Ammonia metabolic capacity was completely absent in PHO and CHF cells immediately after isolation (Figure 5C) but was restored to normal levels (or above) when the cells were cultured for 5 days (Figure 5D). As expected, cells from patients with urea cycle defects displayed no capacity to metabolize ammonia at any time point. If urea cycle defect cases are removed from the analysis, the MD group displayed a greater ability to metabolize ammonia, compared to OD cases (147±18 vs 95±13 nmol/min/mg, respectively).
Cell Transplantation
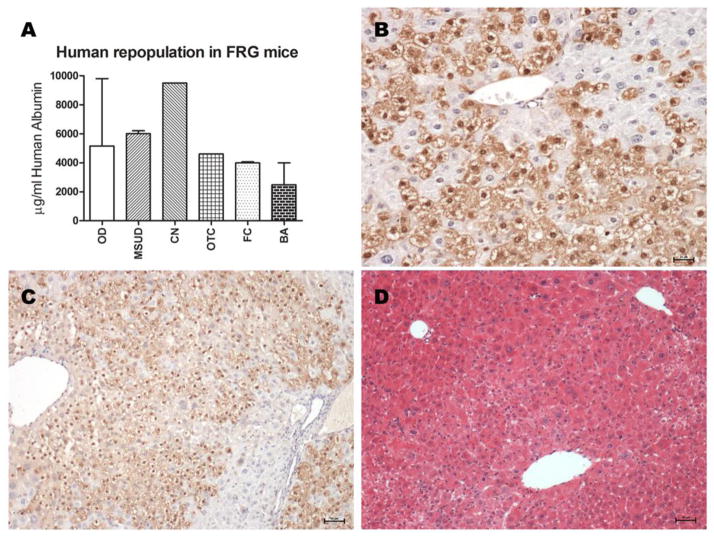
Hepatocytes isolated from the metabolic disease cases showed excellent viability and function so their transplant potential was examined as the ability to repopulate the liver of an immunodeficient host. Hepatocytes from 5 different MDs were transplanted into Fah−/−/Rag2−/−/Il2rg−/− (FRG) mice as described by Azuma, et al. (22), and human albumin levels were recorded (Figure 6A). Robust growth of donor hepatocytes was evident from elevations in circulating albumin, to values that correspond to 50–99% repopulation with human cells. Staining by FAH antibody allowed identification of human hepatocytes (positive cells)(Figure 6B) distributed in the liver lobules, around portal and central veins, and inside the parenchima, in a range between 40% and 80% of repopulation (Figure 6C,D).
Figure 6. Human hepatocyte transplantation in fah−/ −/rag−/ −/il-2rg−/ − mice.
(A) Repopulation of the liver of FRG mice with human hepatocytes isolated from organ donors (n=3) or 5 different metabolic disease patients including MSUD, CN-1, FC, BA or OTC-deficiency. Each MD case repopulated the liver as quickly and effectively as cells from normal organ donors. (B) Human hepatocytes in the mouse liver from an OTC deficient patient identified by reactivity with antibodies to FAH (brown color). Animals were maintained for approximately 3 months prior to sacrifice, scale bar: 25μm. (C) Serial sections of transplanted human hepatocyte stained with anti-FAH antibodies, scale bar = 50μm and (D) H&E, showing the histology of the liver tissue repopulated with human hepatocytes; scale bar: 50μm.
DISCUSSION
Experiments presented here examined the viability, cell yield and metabolic activity of hepatocytes isolated from liver tissue obtained from organ donors or explanted liver tissue obtained at the time of OLT from patients with metabolic and other types of liver disease including biliary atresia, acute liver failure and parenteral nutrition associated liver disease. To simplify of the discussion of a wide range of liver diseases, we refer to all tissues derived from explanted livers obtained at the time of OLT as MD.
Domino liver transplantation is used to increase the number of liver grafts available for OLT (16, 24). In a recent review Popescue and Dima stress that livers from patients with metabolic liver disease can be safely transplanted if the donor’s defect and the recipient’s metabolic needs are carefully considered (18). Liver from patients with many metabolic diseases are morphologically and biochemically normal, except for the mutation that characterizes that disease. Other biochemical functions performed by the liver are “normal” in these hepatocytes. Data presented here supports this hypothesis, as cells from most MD cases performed as well or better than OD cases in assays of liver function, including drug and ammonia metabolism and conjugation. Thus, hepatocytes from a Crigler-Najjar explant, could be expected to metabolize ammonia normally if transplanted into an OTC patient (Figure 6).
The aim of DLT is to correct the immediate liver problem and not to produce symptoms of the donor’s disease in the recipient for many years, if at all. With whole organ DLT, the entirety of the metabolic defect in the liver is immediately transferred to the recipient. Since hepatocyte transplantation only replaces a small portion of the liver, domino hepatocyte transplantation may have greater appeal than DLT. Because of the redundancy in function for most biochemical pathways in the liver, as little as 5–20% normal function of specific pathways may be sufficient to keep the individual symptom free. Hepatocyte transplants aim to provide the 5–20% of missing enzyme activity. Far less than 20% repopulation has been accomplished in clinical HTx. Transplant procedures and techniques are currently being revised to include pretreatments such as portal embolization or low dose radiation to a defined portion of the liver to improve engraftment and repopulation of recipient liver with donor cells, however, there are no reports of clinical experience with these techniques. With current techniques, if OTC-deficient cells were transplanted into Crigler-Najjar recipients, there would be no risk of transferring OTC deficiency to the recipient. The 80–95% of native hepatocytes easily perform the ammonia metabolism required, while the 5–20% OTC-deficient donor cells could conjugate bilirubin to correct the defect in the CN recipient.
Hepatocyte isolation from organs removed from metabolic disease patients have some advantages for HTx procedures. These organs are readily available from OLT cases at the major transplant centers; the same places where HTx would be conducted. The MD organs are immediately available for cell isolation with little to no cold ischemic time. Another important advantage is that these donors are living donors and they would not have the negative effects typical of a braindead donors. A major advantage of MD donors is their age. In our study the average age of the MD donors was approximately 8 years, and 22 of the cases procured for this study were from donors 5 years or younger. Hepatocytes from younger donors would be expected to have higher proliferative capacity than cells from aged donors. As reported here, viability and cell yields are generally excellent (Figures 1). Since these organs can be brought to the GMP facility for cell isolation, no additional facilities would be needed. Like all organ donors, these donors would require regular serological screening prior to their use for transplantation. Although all tissues received from MD donors over a 30-month period were analyzed and reported here, one would not expect that every MD organ could be used for hepatocyte transplant procedures. Cells from patients with metabolic diseases such as MSUD, where extrahepatic organs and tissues can contribute to the overall function of that pathway, would be considered extremely useful for HTx. Domino transplants of livers from MSUD patients have clearly shown that recipients have normal branched chain amino acids levels even while on a normal protein diet (17). One would expect virtually no risk of imparting MSUD to any HTx recipient. Organs that show minimal histopathological abnormalities are the most useful, while those from cirrhotic livers the least useful. Organs from patients with urea cycle defects, Crigler-Najjar and MSUD display normal to near normal gross morphology, histology and vascularity. Cell isolation from these tissues is efficient and billions of viable hepatocytes could be isolated from these organs.
Careful matching of the metabolic capabilities of the donor and need of the recipient are critical. If the donor defect would not be expected to have an immediate adverse effect on the recipient, their use could be considered. While cells from a MSUD or a Crigler-Najjar donor might be useful for an acute liver failure patient, cells from donors with urea cycle defects would not likely be useful because of the immediate need for ammonia metabolism in the ALF recipient.
We do not advocate the use of cells from every diseased liver. Although we performed isolation on biliary atresia and acute liver failure cases, these cells would not be recommended for clinical transplants because of concerns for cell yield, viability and function.
There is evidence from our study, of extreme resilience in hepatocytes isolated from patients with metabolic liver disease and for recovery of hepatic function when the hepatocytes are removed from the donor. For example, ammonia metabolism improved in the PHO, CN, CHF cases in cells cultured for 5 days as compared to measurements made immediately after isolation. Conjugation of resorufin was completely absent in the cells from the CN cases, however, after 5 days in culture the cells showed normal activity. Resorufin conjugation could be expected to recover, even in CN cases, because resorufin is conjugated by up to 9 different UGT enzymes, with UGT1A6 and 1A9 the most active (25). While UGT1A1 (the form mutated in CN cases) remains inactive in the CN cells, data presented here indicate that the remaining UGTs recover activity in hepatocytes removed from the CN environment.
The observations made from hepatocytes explanted from metabolic disease cases have provided clinical guidance for hepatocyte transplants. High CYP3A4 activities were noted in the OTC patients. When HTx was performed on these patients, because of the high CYP3A4 levels, normal doses of Tacrolimus were rapidly cleared resulting in insufficient immunosuppression until dosage could be adjusted. Significantly higher doses of Tacrolimus were required to maintain therapeutic levels. Two recent CN patients received HTx and whose immunosuppression post hepatocyte transplantation included Tacrolimus, prednisolone and mycophenolate mofetil (MMF). As we predicted form CN hepatocytes, conjugation was impaired, and a complete absence of mycophenolate glucuronide was noted in blood samples taken during the first post-transplant week. The patient registered extremely high mycophenolyc acid levels when a normal dose of MMF was administered (533 mg*h/L; therapeutic range 30–60 mg*h/L). Their inability to metabolize the drug necessitated that MMF be discontinued. A subsequent patient was treated with a reduced dose of MMF. Samples taken during the first two post-transplant weeks showed a small, but gradually growing peak in the chromatograms at a retention time similar to mycophenolate glucuronide, suggesting that conjugation of mycophenolyc acid is returning to normal over time, again, as predicted from experiments with the cultured cells. We are currently investigating, drugs, diet and other factors that might influence these metabolic activities in such a disease-specific manner.
Bhogal, et al., recently reported the isolation of hepatocytes from diseased livers (26). Their disease selections were quite different from those in the current study, in that they focused on cirrhotic and fibrotic tissues from patients with alcoholic liver disease, primary biliary cirrhosis and primary sclerosing cholangitis. They reported an experience similar to ours in that the viability, total cell yield and the success rate with cirrhotic tissues were low. Like theirs, our experience suggests that cirrhotic tissues would not provide enough cells from a single donor for clinical transplantation. For the studies reported here with tissue from metabolic disease patients, we generally procured less than 1/10 of the total liver weight for isolation. Given the viability and cell yield reported here, even one lobe from many metabolic disease organs could provide billions of cells, enough for one or more clinical transplants. Transplants of MD cells to FRG immunodeficient mice support the hypothesis that these cells would perform well post transplantation as was evident from the robust growth and albumin secretion from cells from 5 metabolic donors in parallel with 3 “normal” ODs (Figure 6A). Since each mg of human albumin correlates with 15–20% repopulation, these animals show 50–99% repopulation with human hepatocytes by 3 months post transplant. Analysis of the liver of FRG mice transplanted with human hepatocytes isolated from metabolic diseased patients resulted in a rapid and efficient repopulation of the liver. There were no differences in the levels of repopulation of the liver with diseased cells or those isolated from normal organ donors (Figure 6B,C).
While it is clear that not all cases will be useful, as with DLT, domino hepatocyte transplantation should be considered a transplant option. The use of cells from a different metabolic disease would require careful consideration of donor function and recipient’s needs. However, in an age where a number of options to extend the pool of available organs and cells for transplantation are being considered, including xenotransplantation of organs or cells from animals, recycling human hepatocytes from patients with metabolic liver disease for domino hepatocytes transplantation deserves consideration. During the writing of this manuscript, a paper from Stephenne et al., appeared reporting that a domino hepatocyte transplant was conducted on a child with phenylketonuria from a donor liver with a glycogen storage disease, affirming the hypothesis that these organs should be investigated for safety, efficacy and possible transplantation (27).
Highlights.
Hepatocyte transplantation is limited by the availability of useful cells.
Some metabolic liver diseases show little liver pathology and organs from these patients have been used for whole organ transplantation.
Hepatocytes isolated from patients with certain types of metabolic liver disease display high viability and normal function.
Metabolic disease livers could be a useful source of hepatocytes if donor function and recipients needs are carefully considered.
Acknowledgments
Support:
Supported by NIH. N01-DK-7-0004/HHSN26700700004C and RC1DK086135 (SCS), National PKU Alliance (RG and KJS), and R01DK051592 to M.G.
Support of the COPEV Associazione per la Prevenzione e Cura dell’Epatite Virale “Beatrice Vitiello” ONLUS to RG.
Abbreviations
- HTx
Hepatocyte Transplant
- OLT
Orthotopic Liver Transplantation
- CYP
Cytochrome P450
- DLT
Domino Liver Transplantation
- OD
Organ Donor
- MD
Metabolic and Diseased Livers
- MMA
MethylMalonic Acidemia
- PHO
Primary HyperOxaluria
- MSUD
Maple Syrup Urine Disease
- CN
Crigler-Najjar syndrome
- OTC
Ornithine TransCarbamylase deficiency
- CPS-1
Carbamoyl-Phosphate Synthetase 1 deficiency
- Cit
Citrullinemia
- CHF
Congenital Hepatic Fibrosis
- PNALD
Parenteral Nutrition Associated Liver Disease
- A1AT
Alpha-1-antiTrypsin deficiency
- FC
Familial Cholestasis
- BA
Biliary Atresia
- ALF
Acute Liver Failure
- HMM
Hepatocyte Maintenance Medium
- PE
Plating Efficiency
- EROD
Ethoxyresorufin-O-Deethylase
- LCU
Luminescent Counting Unit
- BNF
beta-Naphthoflavone
- PB
Phenobarbital
- Rif
Rifampicin
- UGT
Uridine 5′-diphospho-glucuronosyltransferase
- FRG
Fah−/−/Rag2−/−/Il2rg−/−
- MMF
Mycophenolate Mofetil
Footnotes
Potential Conflict of Interest:
R.G., V.T., K.D., K.J.S, M.C.H., W.Z., R.V. E.C.S.E, C.J., B.E., K.A.S., G.V.M, and I.J.F declare no competing financial interests, while M.G., E.M.W, and S.C.S have financial interests in Yecuris Corporation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, Posner MP. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63:559–569. doi: 10.1097/00007890-199702270-00014. [DOI] [PubMed] [Google Scholar]
- 2.Fisher RA, Bu D, Thompson M, Tisnado J, Prasad U, Sterling R, Posner M, et al. Defining hepatocellular chimerism in a liver failure patient bridged with hepatocyte infusion. Transplantation. 2000;69:303–307. doi: 10.1097/00007890-200001270-00018. [DOI] [PubMed] [Google Scholar]
- 3.Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–449. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- 4.Strom S, Bruzzone P, Cai H, Ellis E, Lehmann T, Mitamura K, Miki T. Hepatocyte Transplantation: Clinical Experience and Potential for Future Use. Cell Transplantation. 2006;15:S105–S110. doi: 10.3727/000000006783982395. [DOI] [PubMed] [Google Scholar]
- 5.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 6.Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC, Fox IJ. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003;111:1262–1267. doi: 10.1542/peds.111.6.1262. [DOI] [PubMed] [Google Scholar]
- 7.Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, et al. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317–318. doi: 10.1016/S0140-6736(02)07529-3. [DOI] [PubMed] [Google Scholar]
- 8.Dhawan A, Mitry RR, Hughes RD. Hepatocyte transplantation for liver-based metabolic disorders. J Inherit Metab Dis. 2006;29:431–435. doi: 10.1007/s10545-006-0245-8. [DOI] [PubMed] [Google Scholar]
- 9.Stephenne X, Najimi M, Sibille C, Nassogne MC, Smets F, Sokal EM. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130:1317–1323. doi: 10.1053/j.gastro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Lee KW, Lee JH, Shin SW, Kim SJ, Joh JW, Lee DH, Kim JW, et al. Hepatocyte transplantation for glycogen storage disease type Ib. Cell Transplant. 2007;16:629–637. doi: 10.3727/000000007783465019. [DOI] [PubMed] [Google Scholar]
- 11.Meyburg J, Alexandrova K, Barthold M, Kafert-Kasting S, Schneider AS, Attaran M, Hoerster F, et al. Liver cell transplantation: basic investigations for safe application in infants and small children. Cell Transplant. 2009;18:777–786. doi: 10.3727/096368909X470775. [DOI] [PubMed] [Google Scholar]
- 12.Mitry RR, Dhawan A, Hughes RD, Bansal S, Lehec S, Terry C, Heaton ND, et al. One liver, three recipients: segment IV from split-liver procedures as a source of hepatocytes for cell transplantation. Transplantation. 2004;77:1614–1616. doi: 10.1097/01.tp.0000122224.98318.19. [DOI] [PubMed] [Google Scholar]
- 13.Meyburg J, Das AM, Hoerster F, Lindner M, Kriegbaum H, Engelmann G, Schmidt J, et al. One liver for four children: first clinical series of liver cell transplantation for severe neonatal urea cycle defects. Transplantation. 2009;87:636–641. doi: 10.1097/TP.0b013e318199936a. [DOI] [PubMed] [Google Scholar]
- 14.Hughes RD, Mitry RR, Dhawan A, Lehec SC, Girlanda R, Rela M, Heaton ND, et al. Isolation of hepatocytes from livers from non-heart-beating donors for cell transplantation. Liver Transpl. 2006;12:713–717. doi: 10.1002/lt.20732. [DOI] [PubMed] [Google Scholar]
- 15.Donato MT, Lahoz A, Jimenez N, Perez G, Serralta A, Mir J, Castell JV, et al. Potential impact of steatosis on cytochrome P450 enzymes of human hepatocytes isolated from fatty liver grafts. Drug metabolism and disposition: the biological fate of chemicals. 2006;34:1556–1562. doi: 10.1124/dmd.106.009670. [DOI] [PubMed] [Google Scholar]
- 16.Ericzon BG, Larsson M, Wilczek HE. Domino liver transplantation: risks and benefits. Transplantation proceedings. 2008;40:1130–1131. doi: 10.1016/j.transproceed.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Mazariegos GV, Morton DH, Sindhi R, Soltys K, Nayyar N, Bond G, Shellmer D, et al. Liver transplantation for classical maple syrup urine disease: long-term follow-up in 37 patients and comparative United network for organ sharing experience. The Journal of pediatrics. 2012;160:116–121. e111. doi: 10.1016/j.jpeds.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popescu I, Dima SO. Domino liver transplantation: How far can we push the paradigm? Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012;18:22–28. doi: 10.1002/lt.22443. [DOI] [PubMed] [Google Scholar]
- 19.Roels L, Rahmel A. The European experience. Transplant international : official journal of the European Society for Organ Transplantation. 2011;24:350–367. doi: 10.1111/j.1432-2277.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 20.Gramignoli R, Green M, Tahan V, Dorko K, Skvorak KJ, Marongiu F, Zao W, et al. Development and appliation of purified dissociation enzyme mistures for human hepatocyte isolation. Cell Transplantation. 2012;21:1245–1260. doi: 10.3727/096368911X600939. [DOI] [PubMed] [Google Scholar]
- 21.Kostrubsky VE, Ramachandran V, Venkataramanan R, Dorko K, Esplen JE, Zhang S, Sinclair JF, et al. The use of human hepatocyte cultures to study the induction of cytochrome P-450. Drug Metab Dispos. 1999;27:887–894. [PubMed] [Google Scholar]
- 22.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, et al. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hines RN, McCarver DG. The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J Pharmacol Exp Ther. 2002;300:355–360. doi: 10.1124/jpet.300.2.355. [DOI] [PubMed] [Google Scholar]
- 24.Wilczek HE, Larsson M, Yamamoto S, Ericzon BG. Domino liver transplantation. Journal of hepato-biliary-pancreatic surgery. 2008;15:139–148. doi: 10.1007/s00534-007-1299-1. [DOI] [PubMed] [Google Scholar]
- 25.Tolando R, Rose T, Moeller TA. Selective conjugation of 7-hydroxycoumarin by recombinant human uridine-5′-diphospho-glucuronosyltransferase (UGT) 2012 http://www.celsisivt.com/the-cryo-advantage/32-our-company/ivt-resources/88-ivt-publications-and-posters.
- 26.Bhogal RH, Hodson J, Bartlett DC, Weston CJ, Curbishley SM, Haughton E, Williams KT, et al. Isolation of primary human hepatocytes from normal and diseased liver tissue: a one hundred liver experience. PloS one. 2011;6:e18222. doi: 10.1371/journal.pone.0018222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephenne X, Debray FG, Smets F, Jazouli N, Sana G, Trondreau T, Menten R, Goffette P, Boemer F, Schoos R, Gersting SW, Najimi M, Muntau AC, Goyens P, Sokal EM. Hepatocyte transplantation using the domino concept on a child with tetrabiopterin nonresponsive phenylketonuria. Cell Transplantation. 2012;21:2765–2770. doi: 10.3727/096368912X653255. [DOI] [PubMed] [Google Scholar]