Abstract
Prior studies have indicated that post-encoding stress can protect memories from the effects of forgetting, and this has been taken as evidence that stress facilitates memory consolidation. However, it is not known whether stress acts by directly influencing the strength of the underlying memories or whether it influences the generation process that plays a critical role in tests such as free recall. To address this issue, we examined the effects of stress produced by skydiving on recognition memory for negative and neutral pictures. Relative to a non-stress control condition, post-encoding stress in males was found to increase recognition memory for neutral pictures. However, stress was not found to improve recognition for emotional pictures, nor was it found to influence recognition memory in female participants. Additional analysis of recognition performance suggested that stress increased familiarity-based recognition rather than recollection. The current study indicates that stress can improve familiarity-based recognition, thus showing that that stress directly increases the strength of the underlying memories.
Introduction
It is well established that chronic stress can lead to profound memory deficits, and that acute stress experienced during the time of retrieval impairs accurate recall (for reviews see McEwen & Sapolsky, 1995; Schwabe, Wolf & Melly, 2010). However, several recent studies have suggested that brief periods of stress immediately after encoding may protect recent memories from the effects of forgetting. For example, Cahill, Gorski and Le (2003) presented subjects with pictures, then either stressed the subjects using a ‘cold-pressor’ manipulation in which the subject held their arm in ice water for up to 3 minutes, or had subjects hold their arm in warm water for the same period. In a subsequent free recall test, the cold-pressor stress group recalled more of the studied items than did the non-stress control group (for similar results see Andreano & Cahill, 2006; Beckner, Tucker, Delville, & Mohr, 2006; Smeets, Otgaar, Candel, & Wolf, 2008). These results are consistent with the claim that stress facilitates a post-encoding consolidation process whereby recently encoded memories are strengthened or ‘consolidated’ and are thus made more resistant to the effects of forgetting (Cahill & Alkire, 2003; McGaugh, 2000). This account is consistent with an extensive animal literature showing the importance of stress related glucocorticoids in facilitating consolidation (McGaugh, 2000; Roozendaal, 2000).
However, a critical limitation of the prior human studies of post-encoding stress is that they have used only free recall measures of memory, and have yet to examine recognition measures. Free recall tasks require subjects to first generate items and then conduct a recognition check to determine if the memory strength of the generated item is strong enough to warrant a recall response (Kintsch, 1970). Thus, it is not known whether stress influences the generation process involved in recall or the strength of the underlying memories. In studies of post-encoding stress, the stressful event itself is highly memorable, and thus it should serve as an effective retrieval cue that can be used to facilitate the generation process (i.e., “What were the items presented just prior to that very stressful event?”). In fact, prior studies have shown that free recall benefits when the encoding context is reinstated at time of test, whereas recognition can show little or no such benefit (e.g., Smith, Glenberg & Bjork, 1978). Thus, stress manipulations, may act as an effective retrieval cue that benefits item generation without directly influencing memory strength.
A more direct test of the consolidation hypothesis is to examine if stress influences recognition memory. If post-encoding stress increases memory strength then recognition memory should be better after stress than after a no-stress control condition. The effect of post-encoding stress on recognition memory is not known, but a number of related studies suggest that post-encoding stress may increase recognition. For example, administration of cortisol shortly after encoding can increase overall recognition (e.g., Van Stegeren et al., 2010). Moreover, presenting positive or negative arousing visual images shortly after encoding can increase subsequent recognition (e.g., Nielson & Meltzer, 2009; Nielson & Powless, 2007). Although note that the latter psychological arousal manipulations do not appear to directly influence cortisol responses (e.g., van Stegeren et al., 2008).
Another important question is whether stress influences recollection or familiarity-based recognition responses. Recognition judgments can be based either on the recollection of qualitative information about a study event such as where or when the event took place, or on assessments of stimulus familiarity (for review see Yonelinas, 2002). Prior studies have indicated that negative compared to neutral materials lead to an increase in recollection, but do not influence familiarity-based responses (Sharot, Verfaellie, & Yonelinas, 2007). Whether stress influences recollection or familiarity-based recognition is unknown, but prior studies have indicated that recollection and familiarity reflect hippocampal and cortical processes, respectively (Eichenbaum, Yonelinas, & Ranganath, 2007). Thus, if stress facilitates a consolidation process in which memories become more dependent on the cortex rather than the hippocampus over time, then one expects stress to benefit familiarity-based recognition, rather than recollection.
The effects of stress on memory may be modulated by a number of important factors. First, the existing studies of post-encoding stress have been limited primarily to relatively mild stress manipulations (e.g., cold-pressor), and it is not known if these effects generalize to other stronger manipulations of stress. It is possible that more robust consolidation effects may be produced by more extreme stress manipulations. Alternatively, stress may only be beneficial at moderate levels (Andreano & Cahill, 2006), and thus high levels of stress may disrupt consolidation. Second, in some prior studies, the beneficial effect of stress has been observed for negative materials, but not for neutral materials (Cahill et al., 2003; Smeets et al., 2008), whereas other studies have found stress related improvements in the recall of neutral materials (Andreano & Cahill, 2006; Beckner et al., 2006). Thus, the extent to which stress influences memory for negative versus neutral information is not yet clear. Finally, there is some evidence that gender may play a critical role in moderating the effects of stress on memory. For example, one recent study reported that the beneficial effects of stress were only observed in male participants (Andreano & Cahill, 2006; but also see Beckner et al., 2006).
In the current study we asked whether relatively high levels of post-encoding stress would lead to an increase in memory strength as measured using recognition memory. Participants first encoded a series of negative and neutral color photos. Then, subjects in the stress condition boarded a plane and completed a tandem skydive with a trained instructor, whereas subjects in the control condition remained on the ground. Following a two hour delay period, subjects were then given a recall test and a recognition test for the materials they had studied earlier. Salivary cortisol was measured prior to encoding (Time 1), 20 minutes after the stress group completed their jump (Time 2), and then 2 hours later just prior to the memory tests (Time 3). Salivary cortisol measures served as a manipulation check to ensure that participants in the stress condition mounted an acute stress response characterized by a rise in cortisol (i.e., an increase in salivary cortisol from Time 1 to Time 2), and, if so, whether or not the system had returned to pre-stress levels prior to the final memory test phase. Recognition was assessed using a combined confidence judgment procedure and remember/know procedure (Yonelinas & Jacoby, 1995), which was used to assess recognition sensitivity and to separate recollection and familiarity-based recognition responses.
The primary aim of the study was to determine the effects of post-encoding stress on recognition memory. If stress leads to a strengthening of memory then recognition accuracy should be higher after stress than after the no-stress control condition. In addition, if stress increases underlying memory strength, then stress should increase familiarity-based recognition responses.
Methods
Participants
A total of 50 subjects participated in the experiment. Thirty-five subjects (15 women) were individuals who had enrolled to complete a tandem skydive at the Skydance Skydiving School in Davis, CA; for all but four of the individuals, this was to be their first skydiving experience (for the remaining four, it was their second). Twenty subjects from this group (9 women) were assigned to the stress condition (Mean age = 27, Mean years education = 17) and 15 (6 women) were assigned to the control group (Mean age = 26, Mean years education =17). Skydance subjects were not aware of the experiment when they enrolled for the skydiving class; they were all approached by an experimenter on the day of their class at the Skydance school and were asked if they would be willing to participate in a study on cognition and stress. Individuals who were interested were given a brief description of the study, its length, and general procedures. Those who agreed to participate were then given consent forms to sign before beginning the study. An additional 15 undergraduate students (10 women) enrolled in a psychology course at the University of California, Davis were recruited as additional control subjects and participated in a laboratory setting (Mean age = 22, Mean years education = 13). Student controls signed up for the study online and were compensated with credit to apply toward their course; Skydance subjects were given $15 an hour for their time. The undergraduate controls were slightly younger that the Skydance controls (t(28) = 1.64, p=0.11), and had few years of completed education (t(28) = 7.20, p<0.01). However, preliminary analyses indicated that the control subjects from the skydiving cohort did not differ from the laboratory control subjects on any of the memory measures indicating that these factors did not impact the observed memory effects, so the subgroups were combined. Subjects were asked about smoking, oral contraceptives and medications. Only one participant smoked, one was taking Adderall for ADHD and one was taking Lezapro for anxiety. Excluding these participants did not influence the pattern of results. The study was approved by the Internal Review Board at the University of California, Davis.
Stimuli
Stimuli consisted of 120 negatively arousing photos, and 120 neutral photos, selected from the International Affective Photo Series (IAPS), based on their standard scores for emotional arousal and emotional valence (Lang, Bradley, & Cuthbert, 1998), and from our own set of negative and neutral pictures to equate the two sets for the presence of humans and visual complexity (Sharot et al., 2007) and ability for experimenters to identify the images from participants’free recall descriptions. An additional 6 images were used for practice and instruction. Images were approximately 315 pixels square, although there was minor variation in size and shape; this variation was kept minimal so as not to provide memory cues that were independent of the images. A newly randomized mix of 60 negative and 60 neutral images were presented to each subject in the encoding phase and all 240 images were presented on the recognition test in a new random order for each subject.
Procedure
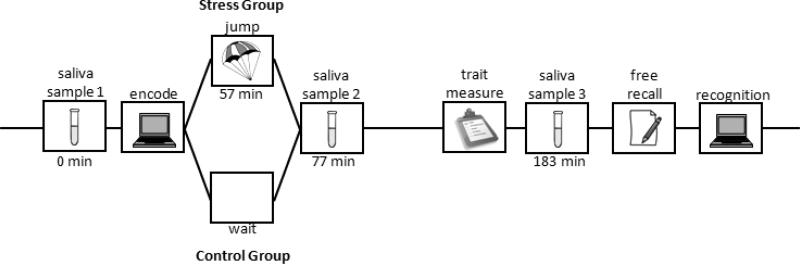
Figure 1 illustrates the procedure and timeline of the experiment. The experimental sessions began at approximately 9:30 a.m. Prior to the experiment proper, subjects first completed consent forms, filled out a demographic questionnaire and completed a sensation seeking scale (Zuckerman, 1994). Next, the first saliva sample was taken for subsequent analysis of plasma cortisol concentrations. After the first saliva sample, participants completed the memory encoding phase of the study in which they were presented with a mixture of 60 neutral and 60 negative pictures presented on a black background on a laptop computer. Each picture was presented for 2 s, and followed by a 6-point scale that subjects used to rate the visual complexity of the picture. Subjects were informed that some images might be disturbing but that we were interested in people's interpretation of visual complexity.
Figure 1.
The procedures and timeline of the experiment. The experimental session began at approximately 9:30 a.m.
Subjects in the stress group then boarded a plane and completed a tandem skydive with a certified instructor. Twenty minutes after the skydiving subjects landed, a second saliva sample was collected. Control subjects at the skydiving site waited for a later flight whereas the laboratory control subjects waited near the lab for the second phase of the experiment. Subjects were free to fill their time as they chose, but were asked to refrain from meals, drinking alcohol or caffeine. The control subjects received cortisol and behavioral tests at times that were matched to that of the stress group.
After a 1.5 hour delay period, subjects completed a personality questionnaire (NEO-FFI, Costa & McCrae, 1992), then they provided third saliva sample. Note that none of the personality measures were a related to any of the memory measures, and are not discussed further. Immediately after the questionnaire, participants completed the memory test phase of the experiment. First, participants were given a 10 minute free recall test in which they were asked to recall as many images as they could from the earlier visual complexity task and to write a description of each image. They were asked to describe the images succinctly, but to provide sufficient detail to uniquely identify each image. Then, a recognition test was administered on a laptop computer. Subjects were presented with 240 pictures at test, consisting of a mixture of 120 studied images, 60 new neutral and 60 new negative pictures. Each picture was displayed for 2 s and subjects were required to make recognition responses using a modified remember/know confidence procedure (Yonelinas, 2001). They made a “Recollect” response if they were sure it was old and could remember specific details about the picture's prior presentation, such as how they reacted to it, what it made them think about, or how they rated it for complexity. Otherwise they made a recognition confidence rating on a 5-point scale from 1 (sure new) to 5 (sure old).
Cortisol Analysis
Prior to assay, samples were centrifuged at 3000 rpm for 20 min to separate the aqueous component from mucins and other suspended particles. Salivary concentrations of cortisol were estimated in duplicate using commercial radioimmunoassay kits (Siemens Medical Solutions Diagnostics, Los Angeles, CA). Assay procedures were modified to accommodate overall lower levels of cortisol in human saliva relative to plasma as follows: 1) standards were diluted to concentrations ranging from 2.76 to 345 nmol/L; 2) sample volume was increased to 200 μl, and 3) incubation times were extended to 3 h. Serial dilution of samples indicates that the modified assay displays a linearity of .98 and a least detectable dose of 1.3854 nmol/L. Intra- and inter-assay coefficients of variation are 6.53 and 5.319, respectively.
Statistical analysis
The effect of stress on cortisol was assessed using an ANOVA with time of test (test 1 vs test 2 vs test 3) as a within-subject variable, and stress condition (stress vs control) and gender (males vs females) as between-subject variables. In addition, each memory measure was assessed using an ANOVA with stimulus valence (negative vs neutral) as a within-subject variable, and with stress condition (stress vs control) and gender (male vs female) as between-subject variables. Interactions were followed up using appropriate independent or paired-samples t-tests. An alpha level of less that or equal to 0.05 was considered significant for all statistical tests. All statistical analyses were completed using the SPSS software package 16.0.
Recognition memory was examined using confidence scores to plot receiver operating characteristics (ROCs) (see MacMillan & Creelman, 2005). ROC analysis is critical in assessing memory sensitivity and in evaluating recollection and familiarity. That is, percent correct measures of recognition, even when corrected for false alarm rates, do not provide unbiased measures of memory sensitivity (MacMillan & Creelman, 2005), and they cannot be used to assess recollection and familiarity (Yonelinas & Parks, 2007).
Results
Salivary Cortisol
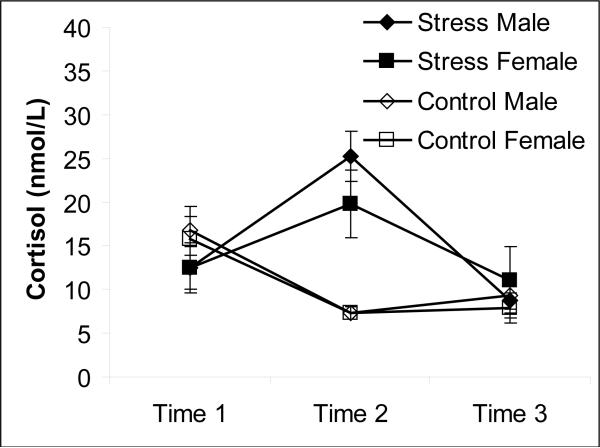
An examination of the salivary cortisol measures indicated that the stress manipulation was effective at increasing cortisol (see Figure 2). A group (stress vs control) by time (time 1 vs time 2 vs time 3) by gender (male vs female) ANOVA indicated that there was a main effect of time, F(2,92) = 8.149, MSe = 56.84, p < 0.01, that was qualified by a significant time by group interaction, F(2,92) = 20.14, MSe = 56.84, p < 0.001). At the onset of the experiment (Time 1), cortisol concentrations did not differ for the stress and control groups, t(48) = 1.36, p = 0.18. However, 20 minutes after the jump (Time 2), cortisol was significantly higher in the stress than the control group, t(48) = 7.58, p < 0.001. Finally, just prior to the retrieval phase 140 minutes after the jump (Time 3), cortisol in the stress group returned to the same level as the control group, t(48) = 0.57, p = 0.57. In addition, within the stress group, cortisol at Time 2 was greater than both Time 1, t(19) = 3.62, p < 0.01, and Time 3, t(19) = 5.41, p < 0.001. Note that cortisol at Time 2 was numerically higher for males than females in the stress condition, but the difference was not significant, t(18) = 1.15, p = 0.27.
Figure 2.
Salivary cortisol concentrations for the male and female participants in the stress and control groups taken at the onset of the experiment (Time 1), 20 minutes after the skydive (Time 2) and 140 minutes after the skydive just prior to memory retrieval (Time 3). Standard errors of the means are presented in parentheses. A gender (male vs. female), stress (stress vs. control), and time (Time 1 vs. Time 2 vs. Time 3) mixed ANOVA revealed a significant interaction between time and stress on cortisol concentration (p < 0.01). The interaction demonstrated that cortisol concentrations were increased by stress (i.e., skydiving) then returned to baseline. Post hoc t-tests confirmed that cortisol concentration were greater in the stress group than the control group shortly after skydiving (Time 2; p < 0.001), but the groups did not differ prior to the skydive (Time 1; p = .18) or prior to the memory test (Time 3; p = .57). Males showed slightly higher cortisol concentrations at Time 2, but no significant gender differences were observed (p = .27).
Recognition Memory Performance
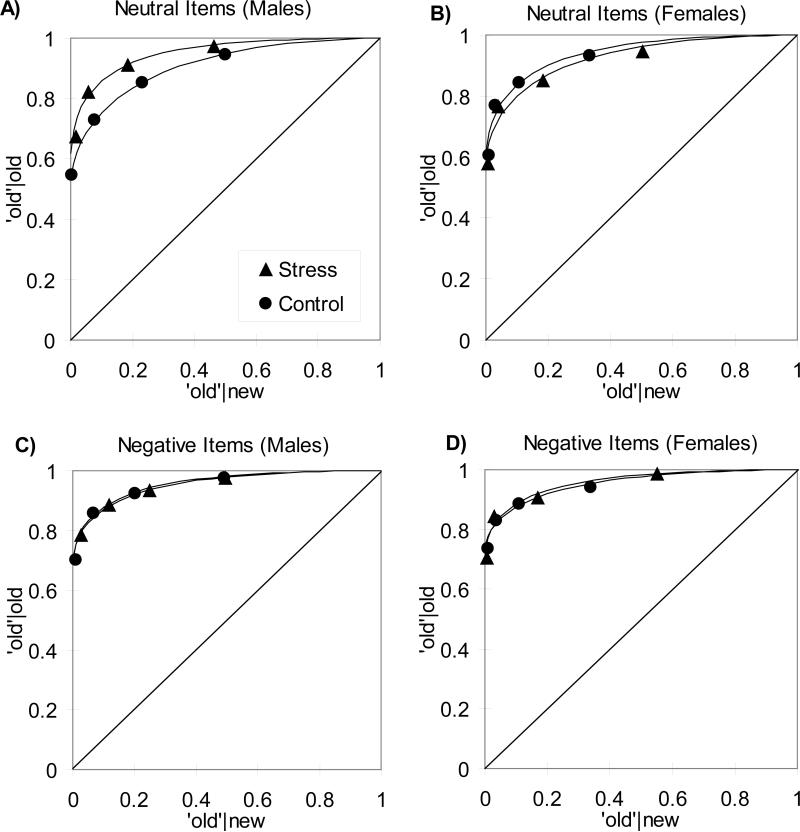
All memory performance measures are presented in Table 1. Recognition memory performance was assessed by plotting receiver operating characteristics (ROCs), whereby the proportion of old items correctly accepted as old was plotted against the proportion of new items incorrectly accepted as old (Figure 3; see MacMillan & Creelman, 2005 for review). The left most point on each function reflects the proportion of items receiving a high confidence recognition response (i.e., either an ‘R’ or a ‘5’ response), whereas each successive point includes the next most confident responses (e.g., either an R, 5, or 4 response). An examination of Figure 3 reveals that stress led to an increase in recognition accuracy (i.e., a higher ROC) for neutral items in the male subjects (Figure 3A), but not in the female subjects (Figure 3B). In fact, recognition was slightly lower in the stress than the control condition in females. In addition, there was no evidence that stress influenced recognition for negative items in either male or female participants (Figure 3C & D).
Table 1.
Average recognition memory performance, and estimates of recollection and familiarity derived from the receiver operating characteristic (ROC) and remember/know (RK) analyses as a function of stimulus valence, stress condition, and gender. Standard errors of the means are presented in parentheses.
| Recognition | ROC Estimates | RK Estimates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d’ | Recollection | Familiarity (d’) | Recollection | Familiarity | ||||||
| Neutral | Negative | Neutral | Negative | Neutral | Negative | Neutral | Negative | Neutral | Negative | |
| Skydiving | ||||||||||
| Male (N=11) | 2.70 (.19) | 2.87 (.22) | .47 (.10) | .77 (.05) | 2.00 (.22) | 1.61 (.31) | .31 (.09) | .39 (.10) | .69 (.05) | .66 (.06) |
| Female (N=9) | 2.44 (.19) | 2.84 (.13) | .56 (.05) | .68 (.03) | 1.69 (.18) | 1.95 (.08) | .11 (.03) | .30 (.04) | .70 (.04) | .74 (.02) |
| Control | ||||||||||
| Male (N=14) | 2.12 (.19) | 2.71 (.19) | .50 (.06) | .64 (.06) | 1.42 (.12) | 1.81 (.16) | .23 (.06) | .38 (.06) | .58 (.05) | .68 (.06) |
| Female (N=16) | 2.66 (.18) | 2.91 (.18) | .58 (.05) | .67 (.05) | 1.87 (.19) | 1.83 (.22) | .22 (.05) | .36 (.05) | .66 (.05) | .67 (.05) |
Figure 3.
Recognition memory receiver operating characteristics for the stress and non-stress control groups, for neutral (top panels) and negative items (bottom panels), for males (left panels, N=11 and 14 in the stress and control conditions, respectively) and females (right panels, N=9 and 16 in the stress and control conditions, respectively). A gender (male vs. female) by stress (stress vs. contol) ANOVA on recognition memory performance (i.e., d’) for neutral items revealed a significant gender by stress interaction. Post hoc t-tests demonstrated that stress improved recognition memory of neutral items (i.e., d’ measures were greater for the stress group than the control group) for males (p < 0.05), but not females (p = 0.44). There were no significant effects of gender or stress on recognition memory for negative items.
To quantify these effects, each subject's ROCs were fit to an equal-variance signal detection model to obtain measures of discriminability (d’). Negative items (M=2.84) were better recognized than neutral items (M = 2.48), F(1,46) = 17.79, MSe = 0.17, p < 0.001. Moreover, the three-way interaction between stress group, time and gender approached significance, F(1,46) = 3.00, MSe = 0.17, p = 0.09. This effect was further examined by conducting a stress by gender ANOVA for negative and neutral items separately. For neutral items, there was a significant stress by gender interaction, F(1,46) = 4.21, MSe = 0.46, p < 0.05. Follow-up t-tests revealed that for males, stress led to a significant increase in recognition memory for neutral items, t(23) = 2.16, p <0 .05. In contrast, for females, stress did not influence recognition memory performance for neutral items, t(23) < 1, p = 0.44. Recognition memory for negative items was not influenced by stress or gender, and there was no evidence of a stress by gender interaction, all p's > 0.58.
Recollection and Familiarity
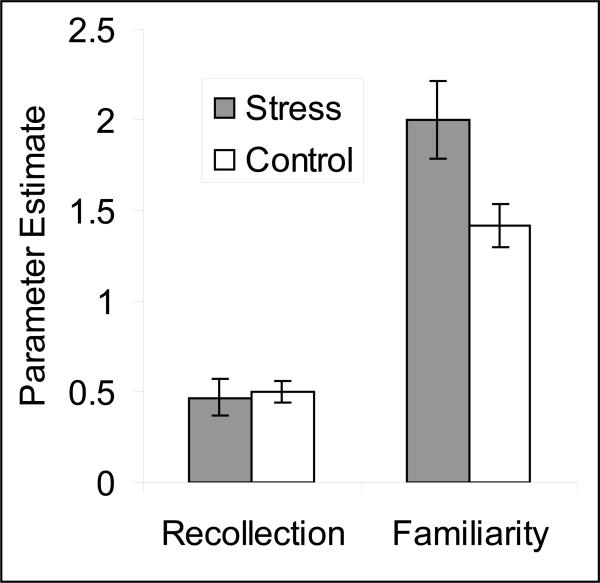
To determine whether the effect of stress on recognition memory arose because of effects on recollection or familiarity-based recognition, we estimated recollection and familiarity by fitting the recognition ROC data with the dual process signal detection model (Yonelinas, 1994) and by examining the remember reports (Table 1). First, for the ROC estimates of recollection there was a main effect of valence demonstrating that recollection was greater for negative (M = 0.69) than neutral (M = 0.53) pictures, F(1,46) = 23.22, MSe = 0.03, p < 0.001. However, there was no effect of stress, gender, nor any interactions, all p's > .10. In contrast, for the ROC estimates of familiarity, there was a significant three-way interaction, F(1,46) = 10.81, MSe = 0.16, p <0 .01, which reflected the fact that stress increased familiarity for neutral items in the males, t(23) = 2.44, p < 0.05 (see Figure 4), but did not influence familiarity in any other condition, all p's >0.53.
Figure 4.
Estimates of recollection (probability) and familiarity (d’) for male participants based on the receiver operating characteristics (ROCs) for neutral items. Standard errors of the means are presented in parentheses. A t-test demonstrated that stress induced by skydiving increased estimates of familiarity (p<0.05), but did not influence recollection (N=11 and 14 for the stress and control conditions respectively).
A second analysis examined recollection and familiarity using the proportion of remember responses and the proportion of items judged to be recognized in the absence of recollection (5 or 4 responses; Yonelinas & Jacoby, 1995). Mirroring the ROC estimates of recollection, there was a significant main effect of stimulus valence showing that recollection was greater for negative pictures (M=0.36) compared to neutral pictures (M=0.22), F(1,46) = 100.24, MSe = 0.01, p < 0.001. However, in contrast to the ROC analysis, there were no significant effects on familiarity estimates. Note that numerically, male's familiarity estimates for the neutral items were larger in the stress than in control conditions, but this difference failed to reach significance, p = 0.15. Thus, the ROC analyses indicated that stress significantly increased familiarity for neutral items in males, whereas the RK analysis revealed only a non-significant stress-related increase in familiarity. Both methods indicated that recollection was greater for negative than neutral items, and this effect was not significantly modulated by stress.
Free Recall Performance
The mean number of negative and neutral items recalled is presented in Table 2. There was a significant effect of stimulus valence such that negative items were recalled at a higher rate (M = 10.43) than neutral items (M = 4.55), F(1,46) = 107.62, p < 0.001. However, there was no effect of stress, gender, or any higher-order interactions, all p's > 0.12. Thus, there was no evidence that stress modulated recall performance in either the male or female subjects.
Table 2.
Average number of items recalled as a function of stimulus valence, stress condition, and gender. Standard errors of the means are presented in parentheses.
| Stimulus Valence | ||
|---|---|---|
| Neutral | Negative | |
| Skydiving | ||
| Male (N=11) | 3.27 (0.59) | 8.82 (0.88) |
| Female (N=9) | 4.33 (0.78) | 11.44 (1.07) |
| Control | ||
| Male (N=14) | 4.79 (0.70) | 10.57 (1.04) |
| Female (N=16) | 5.81 (0.91) | 10.87 (1.11) |
The relationship between the individual cortisol responses observed in the stress group and each of the memory measures (recall and recognition) was also examined using regression methods. However, no significant relationships were observed, which is likely due to the fact that these secondary analyses relied on only half of the sample (i.e., those in the stress condition).
Discussion
The current study examined the effects of post-encoding stress on recognition memory for negative and neutral pictures. Stress induced by jumping from a plane led to a significant increase in salivary cortisol levels and produced better recognition memory performance than a non-stress control condition. This memory effect, however, was emotion and gender specific in the sense that stress increased recognition memory for neutral pictures only in male participants. Stress had no effect on memory for negative pictures, and it did not moderate recognition memory performance in the female participants. Moreover, the recognition effects appeared to influence familiarity rather than recollection-based recognition.
Several prior studies have found that post-encoding stress can enhance free recall performance (Andreano & Cahill, 2006; Beckner et al., 2006; Smeets et al., 2008), but whether this occurred because stress increased memory strength or whether it influenced retrieval processes underlying the generation of items in free recall is unknown. By showing that stress increased recognition performance, the current study indicates that stress can have direct effects on memory strength rather than simply influencing the generation process specific to free recall tasks. The finding that stress increased familiarity-based recognition and did not influence either recollection or free recall, provides additional evidence that stress had a direct impact on memory strength.
The skydiving manipulation used in the current study was likely more stressful than the cold-pressor manipulations used in most previous studies of post-encoding stress on memory. Although it is difficult to compare across studies, skydiving led salivary cortisol to increase from approximately 10 to over 20 nmol/L. The cortisol increases caused by cold press have been much more modest (Andreano & Cahill, 2006; Cahill et al., 2003; Smeets et al., 2008). Note that one previous study examined the effects of a social stressor experienced after encoding - which would presumably also be less stressful that skydiving - showed that socially-induced stress could also increase free recall (Beckner et al. 2006). In addition, a number of prior studies have indicated that presenting positive or negative arousing films shortly after encoding can increase recognition accuracy (e.g., Nielson & Meltzer, 2009). Arousal manipulations of this sort can have little or no effect on salivary cortisol concentrations (e.g., van Stegeren et al., 2008). Thus, stress related improvements in memory can be observed across a variety of different manipulations that vary considerably in levels of induced stress. However, to what extent these various different stressors influence the recollection and familiarity processes supporting recognition awaits further investigation.
The finding that only male participants benefited from stress is consistent with prior studies showing that males are more likely to show beneficial effects of stress on memory (Andreano & Cahill, 2006). In the current study, males showed a numerically larger increase in cortisol than did the female subjects. Thus, one possibility is that skydiving was not sufficiently stressful to produce a stress advantage in the women. An alternative possibility is that the high levels of stress produced in the current study may have exceeded the optimal level of stress for the females, potentially masking any positive effects of stress. Future studies that parametrically manipulate stress levels will be useful in testing these alternatives. In addition, it will be useful to examine the effects of hormonal variations across the estrus cycle in females, as these factors may also critically impact whether stress benefits memory (Andreano, Arjomandi & Cahill, 2008; Burgess & Handa, 1992; Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999).
Why stress did not influence free recall in the current study is not clear. Several previous studies have reported stress-related increases in free recall (e.g., Andreano & Cahill, 2006; Beckner et al., 2006; Cahill, Gorski & Le, 2003; Smeets et al., 2008). One potential reason we did not observe an effect of stress on free recall is that recall performance was quite low and thus may have been subject to floor effects. Arguing against this possibility, however, is the fact that recall performance was sensitive to the effects of arousal level. Alternatively, rehearsal or reminiscence of the study materials in the control subjects during the delay period may have masked the stress effect. That is, controls may have thought of the study items during the delay period and this may have reduced the potential recall advantage in the stress group. The subjects in the current study were not informed that their memory was to be tested until the memory test began, so it is unlikely that they intentionally rehearsed the studied items, but they may have been incidentally reminded of the study items during the delay period. Importantly, this rehearsal account could explain the null effects of stress on recall, but it could not account for the positive effect of stress on recognition. Another possibility is that the positive effects of stress on recall may have been masked by opposing effects of stress during the time of retrieval. That is, although cortisol concentrations had returned to baseline by the time of the memory tests, there may have been residual effects of stress still acting at the time of retrieval. Prior studies have indicated that stress during retrieval reduces free recall. Thus, lingering effects of stress acting at time of retrieval may have masked the beneficial effects of post-encoding stress on recall. However, why this was not the case for recognition is not clear. In any case, determining the conditions under which recall is influenced by stress should be examined in future studies. Importantly, however, the current study indicates that stress conditions that lead to improvements in recognition do not necessarily benefit free recall, suggesting that the mechanisms underlying the stress effects in these two memory tasks might be quite different.
In the current study stress was found to improve recognition memory for neutral, but not negative items. In contrast, two previous studies demonstrated that stress benefited memory for negative more than positive materials (Cahill et al., 2003; Smeets et al., 2008). One possible account for this discrepancy is that recognition of negative items was quite high in the current study, and this may have made it more difficult to observe a beneficial effect of stress. Another possibility is that different types of stress may influence memory for different types of materials. For example, pain induced by cold-pressor stress is presumably a negative experience and thus may preferentially influence memory for negative events, whereas skydiving is presumably a pleasurable experience and as such may preferentially influence memory for positive or neutral events.
Post-encoding stress protects recently encoded memories from the effects of forgetting, but what is the underlying mechanism? The present results are consistent with the consolidation hypothesis whereby stress acts to strengthen recently encoded information (Cahill & Alkire, 2003; McGaugh, 2000; Roozendaal, 2000). Moreover, the beneficial effects of stress appeared to be familiarity-based rather than recollection-based, indicating that stress influences the strength of the underlying memories, rather than facilitating the generation process that is involved in free recall. Although the current study does not provide insight into the neural mechanisms underlying the stress effects on recognition memory, the effects of stress on familiarity that were observed here are consistent with the notion that stress facilitates the storage of recent information within cortical networks rather than within the hippocampus per se. That is, prior work has indicated that recollection relies on the hippocampus whereas familiarity relies on cortical regions such as the perirhinal cortex (Eichenbaum et al., 2007). Thus, stress may act by preserving or strengthening otherwise fragile cortical memory representations.
Whether the current stress effects reflect ‘systems’ or ‘synaptic’ forms of consolidation is unclear (Dudai & Morris, 2000). The effects of stress that we observed were apparent quite soon after the stress manipulation (i.e., memory was tested 2 hours after the skydive). Synaptic changes can occur minutes to hours after initial encoding, thus they may have played a role in producing the current effects (for evidence that consolidation effects may be observed over even shorter time periods see Anderson, Wais, & Gabrieli, 2006). In contrast, systems consolidation – whereby hippocampal representations are either transferred to or overshadowed by cortical representations (Squire, Cohen & Nadel, 1984; Nadel & Moscovitch, 1997) - is often thought to operate on a time frame from months to decades. However, whether such effects might also occur over the shorter delay period in the current study is unknown.
In sum, high levels of stress induced by skydiving can rescue recently encoded memories from the effects of forgetting by enhancing familiarity-based recognition. These findings suggest that stress may facilitate consolidation by preserving the memory strength of recently encoded events.
References
- Anderson AK, Wais PE, Gabrieli JDE. Emotion enhances remembrance of neutral events past. Proc Natl Acad Sci USA. 2006;103(5):1599–1604. doi: 10.1073/pnas.0506308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychol Sci. 2006;17(6):466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 33(6):874–882. doi: 10.1016/j.psyneuen.2008.03.009. 2998. [DOI] [PubMed] [Google Scholar]
- Beckner VE, Tucker DM, Delville Y, Mohr DC. Stress facilitates consolidation of verbal memory for a film but does not affect retrieval. Behav Neurosci. 2006;120(3):518–527. doi: 10.1037/0735-7044.120.3.518. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131(3):1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol Learn Mem. 2003;79(2):194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learn Mem. 2003;10(4):270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI); Professional Manual. Psychological Assessment Resources; Odessa, FL: 1992. [Google Scholar]
- Dudai Y, Morris RGM. To consolidate or not to consolidate: What are the questions. In: Bulhuis JJ, editor. Brain, Perception, Memory. Advances in Cognitive Sciences. Oxford University Press; Oxford, UK: 2000. pp. 149–162. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintsch W. Learning, memory, and conceptual processes. Wiley; New York, NY: 1970. [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion and motivation: Measuring affective perception. J Clin Neurophysiol. 1998;15(5):397–408. doi: 10.1097/00004691-199809000-00004. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5(2):205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- MacMillan NA, Creelman CD. Detection theory: A user's guide. 2nd ed. Lawrence Erlbaum Associates; Mahwah, NJ: 2005. [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin NeuroBiol. 1997;7(2):217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Meltzer MA. Modulation of long-term memory by arousal in alexithymia: The role of interpretation. Conscious Cogn. 2009;18(3):786–793. doi: 10.1016/j.concog.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Powless M. Positive and negative sources of emotional arousal enhance long-term word-list retention when induced as long as 30 minutes after learning. Neurobiol Learn Mem. 2007;88(1):40–47. doi: 10.1016/j.nlm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25(3):213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT, Melly SO. Memory formation under stress: Quality and quantity. Neurosci Biobehav Rev. 2010;34:584–591. doi: 10.1016/j.neubiorev.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Sharot T, Verfaellie M, Yonelinas AP. How emotion strengthens the recollective experience: A time-dependent hippocampal process. PLoS One. 2007;2(10):e1068. doi: 10.1371/journal.pone.0001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T, Otgaar H, Candel I, Wolf OT. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 2008;33(10):1378–1386. doi: 10.1016/j.psyneuen.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Smith SM, Glenberg A, Bjork RA. Environmental context and human memory. Mem Cogn. 1978;6(4):342–353. [Google Scholar]
- Squire LR, Cohen NJ, Nadel L. The medial temporal region and memory consolidation: A new hypothesis. In: Weingartner H, Parker E, editors. Memory Consolidation. Erlbaum; Hillsdale, NJ: 1984. pp. 185–210. [Google Scholar]
- van Stegeren AH, Wolf OT, Kindt M. Salivary alpha amylase and cortisol responses to different stress tasks: Impact of sex. Int J Psychophysiol. 2008;69(1):33–40. doi: 10.1016/j.ijpsycho.2008.02.008. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joels M. Interacting noradrenergic and corticosteroid systems shift human brain activity patterns during encoding. Neurobio Learn Mem. 2010;93:56–65. doi: 10.1016/j.nlm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J Exp Psychol Learn Mem Cogn. 1994;20(6):1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Consciousness, control, and confidence: The 3 Cs of recognition memory. J Exp Psychol Gen. 2001;130(3):361–379. doi: 10.1037//0096-3445.130.3.361. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46(3):441–517. [Google Scholar]
- Yonelinas AP, Jacoby LL. The relation between remembering and knowing as bases for recognition: Effects of size congruency. J Mem Lang. 1995;34(5):622–643. [Google Scholar]
- Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: A review. Psychol Bull. 2007;133(5):800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral Expressions and Biosocial Bases of Sensation Seeking. Cambridge University Press; New York, NY: 1994. [Google Scholar]