Abstract
Inhibitor of DNA binding 2 (ID2) is a helix-loop-helix transcriptional repressor rhythmically expressed in many adult tissues. Our previous studies have demonstrated that Id2 null mice have altered expression of circadian genes involved in lipid metabolism, altered circadian feeding behavior, and sex-specific enhancement of insulin sensitivity and elevated glucose uptake in skeletal muscle and brown adipose tissue. Here we further characterized the Id2−/− mouse metabolic phenotype in a sex-specific context and under low and high fat diets, and examined metabolic and endocrine parameters associated with lipid and glucose metabolism. Under the low-fat diet Id2−/− mice showed decreased weight gain, reduced gonadal fat mass, and a lower survival rate. Under the high-fat diet, body weight and gonadal fat gain of Id2−/− male mice was comparable to control mice and survival rate improved markedly. Furthermore, the high-fat diet treated Id2−/− male mice lost the enhanced glucose tolerance feature observed in the other Id2−/− groups, and there was a sex-specific difference in white adipose tissue storage of Id2−/− mice. Additionally, a distinct pattern of hepatic lipid accumulation was observed in Id2−/− males: low lipids on the low-fat diet and steatosis on the high-fat diet. In summary, these data provides valuable insights into the impact of Id2 deficiency on metabolic homeostasis of mice in a sex-specific manner.
Keywords: lipid accumulation, survivorship, glucose tolerance, adipogenesis, hepatic steatosis, sexual dimorphism
Introduction
Inhibitor of DNA binding, (Id) genes, encode helix-loop-helix (HLH) transcription factors lacking a DNA binding domain, that act as dominant negative regulators of basic HLH transcription factors [1,2]. The Id gene family includes four genes (Id1-4), which are involved in the regulation of many biological processes, including the cell cycle, circadian rhythms and adipocyte differentiation [1,2,3,4]. Recent studies have revealed a role for Id1 in the regulation of insulin secretion and β-cell differentiation [5], and Id4 in adipocyte differentiation and adipose tissue formation [6].
ID2 is rhythmically expressed in many mammalian tissues and is involved in the input pathway, core clock function and output pathways of the circadian clock [3,7,8]. ID2 contributes to the output pathways of the circadian clock as demonstrated in Id2−/− mice by the expression profiles of clock controlled genes in the liver, including those involved in lipid metabolism [3]. Moreover, studies have shown that absence of Id2 results in impaired adipogenesis in vitro and that Id2−/− mice have reduced gonadal white adipose tissue (WAT) and lipid content in the liver [3,4]. Our previous findings demonstrated that Id2−/− mice exhibit altered feeding and locomotor rhythms, sex- and age-dependent enhanced glucose tolerance and insulin sensitivity, and sex-dependent elevated glucose uptake in skeletal muscle and brown adipose tissue [9]. It is well known that risk, development and manifestation of obesity, metabolic syndrome and insulin-resistance are sexually dimorphic [10,11]. Here we extend our studies on the characterization of the Id2−/− mouse metabolic phenotype under energy-rich diet challenge in a sex-specific context.
Materials and Methods
Animals
The generation and husbandry of Id2−/− mice, and determination of genotypes, was performed as described previously [7,9]. Id2+/+ wild-type (WT) and Id2−/− mice were on a mixed background for breeding purposes: 129sv/C57BL6J/FBVN [7,9]. Mice were maintained on a regular chow diet (Kal – 22:23:55 by % calculation as fat: protein: carbohydrate; Teklad Global diet 2919) provided ad libitum, and with sterile water containing antibiotic [3,7,9]. All mice were housed in laboratory cages at temperature of 20°–21°C and humidity of 50–65% under a 12:12 light: dark (LD) cycle with lights on at Zeitgeber time (ZT) 0 and lights off at ZT12. Starting at age wk 8–10, mice were fed ad libitum with either a low fat diet (LFD; Kal – 12:22:66; Harlan Laboratories: 2916) or a high fat diet (HFD; Kal – 45:20:35, Research Diet Inc: D12451). Littermate mice were used as control animals, assignment to diet groups was randomized, and apart from the change in diet all other conditions were as detailed above. All animal experiments were approved by the University of Notre Dame Animal Care and Use Committee and performed in accordance with NIH Guidelines for the Care and Use of Laboratory Animals.
For the growth curves, 48 WT and 39 Id2−/− mice were weighed weekly for 17 wk. For the survival rate, the percentage of surviving animals over a period of 20 wk was determined. The in vivo body fat composition of WT and Id2−/− mice was measured ranging from age 12 to 19 wk. Mice were aged 32–34 wk at end of experiment when tissues were harvested for phenotyping.
Glucose tolerance tests
After 18 wk on LFD or HFD, mice were subjected to glucose tolerance testing. Mice were fasted overnight (16 h) and given an intra-peritoneal injection of D-glucose (1.5g/kg of body mass) at ZT4. Subsequent blood glucose were measured at 0, 10, 20, 30, 60, 90, and 120 min from a distal tail vein as previously described [9].
Fat mass estimation
In vivo X-ray Micro-Computed Tomography (MicroCT) was used to quantify percent body fat (see Supplementary information for full methods).
Analysis of serum and liver lipid and endocrine panels
At the age of 32–34 wk, and 22 wk of feeding experiment, serum and liver were harvested at ZT4 for lipid and endocrine analysis, and conducted at the UC Davis Mouse Metabolic Phenotyping Center (MMPC; Davis, CA) (see Supplementary information for full methods).
Tissue mass and histology
At the end of the feeding experiment, gonadal WAT deposits and interscapular brown adipose tissue (iBAT) were excised and weighed. Cryostat cut WAT sections were prepared and stained with hematoxylin-eosin as described [9]. For lipid analysis, cryostat cut liver sections were stained for Oil-red-O and hematoxylin-eosin, as described [12]. Multiple images were captured at 20x and 10x magnification for WAT and liver, respectively, using a Nikon 90i wide field microscope with a Nikon DS-Fi1 digital camera. To measure WAT cell area and liver lipid accumulation, three to five sections from each animal were analyzed manually using NIH ImageJ software and using methods previously described [9,12].
Statistics
Data were analyzed using Sigma Plot 12.0 software (Chicago, IL) to run two-factor ANOVA, two-factor repeated measures (RM) ANOVA and three-factor ANOVA with genotype, sex and diet as the independent variables. Tukey’s post hoc tests were performed when significant ANOVA results between factors were revealed. Where necessary, data were natural log, square root or ranks transformed to correct for non-normal distributions. The survival rate data were analyzed by using χ2 analyses (and Fisher’s exact tests) for trend (Prism 5.0, Graphpad, La Jolla, CA).
Results
High fat diet ameliorates Id2−/− male mice phenotype and survival rate
The lean and lower body mass phenotype of Id2−/− mice reported in our previous studies raised the question as to whether this animal phenotype is affected by a HFD [3,7,9]. 8–10-wk-old Id2−/− mice and their WT littermates were put on LFD and HFD for 22 wk. WT mice gained weight indistinguishably on both diets, presumably due to their mixed genetic background, which may cause resistance to diet-induced obesity on a HFD [13] (Fig. 1A). Conversely, Id2−/− males gained more weight and became heavier on HFD than on a LFD by the end of experiment (Time (T), p<0.001; Diet (D), n.s.; Interaction (I), p<0.05) (Fig. 1A). However, this pattern of weight gain was not observed in Id2−/− females (T, p<0.001; D, n.s.; I, n.s.) (Fig. 1A). When the body weights of WT and Id2−/− mice were normalized to their initial weight, the growth rate of Id2−/− males was found to be lower than their WT counterparts when fed the LFD (T, p<0.001; Genotype (G), n.s.; I, p<0.01) (Fig. 1B). However, under HFD, Id2−/− males showed nearly the same growth rate as their WT littermates (T, p<0.001; G, n.s.; I, n.s.). Id2−/− females grew significantly less than WTs under LFD (T, p<0.001; G, p<0.01; I, p<0.001) and exhibited a lower growth rate than WTs under HFD (T, p<0.001; G, n.s.; I, p<0.05) (Fig. 1B). We also monitored the survival rate of the WT and Id2−/− mice during the 20 wk experiment. Surprisingly, none of the Id2−/− males died on the HFD, whereas the survival rate of Id2−/− males on LFD dropped to under 65%; and only 56% and 67% of Id2−/− females on LFD and HFD, respectively, reached the age of 20 wk, (χ2 test for trend: HFD male, p<0.001, different from other 3 groups) (Fig. 1C). The remaining three Id2−/− groups were not different from one another (χ2 test for trend, n.s.). There was no significant decline in body weight prior to death, as compared with susceptible Id2−/− mice groups, measured over 4 weeks prior to death (two factor ANOVA, n.s.). Note that all WT mice survived irrespective of sex and diet.
Fig. 1. Sex- and diet specific regulation of growth and survival rate in Id2−/− mice.
(A) Weekly body weight measurements of WT and Id2−/− male (left) and female (right) mice fed with low fat diet (LFD) or high fat diet (HFD) over 17 weeks. (B) Upper: Growth rate (fold change) of WT and Id2−/− male (Left: LFD; Right: HFD). Lower: Growth rate (fold change) of WT and Id2−/− female (Left: LFD; Right: HFD). Values are mean ± S.E.M. RM-ANOVA were performed followed by Tukey’s post-hoc tests, *p<0.05, **p<0.01, ***p<0.001. (C) Percentage survival rate of the WT and Id2−/− mice during the 20 wk experiment.
High fat diet modulates Id2−/− male mice glucose homeostasis
Our previous study revealed that male, but not female, Id2−/− mice exhibit an enhanced glucose tolerance when fed with a regular chow diet (22% kcal% from fat) [9]. To assess the consequences of diet on Id2−/− mouse glucose homeostasis, glucose tolerance tests were performed on these mice after 17 wk on LFD or HFD. Id2−/− males had an enhanced glucose tolerance under LFD compared to WTs (Time (T), p<0.001; Genotype (G), p<0.001; Interaction (I), n.s.) (Fig. 2A). In contrast, no difference in glucose tolerance between male Id2−/− and WT mice on HFD was observed (T, p<0.001; G, n.s.; I, n.s.) In the female mice, an enhanced glucose tolerance was observed in Id2−/− mice fed with either LFD (T, p<0.001; G, p<0.001; I, n.s.) or fed with HFD (T, p<0.001; G, p<0.001; I, n.s.) (Fig. 2B).
Fig. 2. Sex-specific modulation of Id2−/− mice glucose homeostasis under high fat diet.
(A) Glucose tolerance tests of WT and Id2−/− male mice (Left: LFD; Right: HFD). (B) Glucose tolerance tests of WT and Id2−/− female mice. (Left: LFD; Right: HFD). Values are mean ± S.E.M. Two- factor ANOVA were performed followed by Tukey’s post-hoc tests, *p<0.05, **p<0.01.
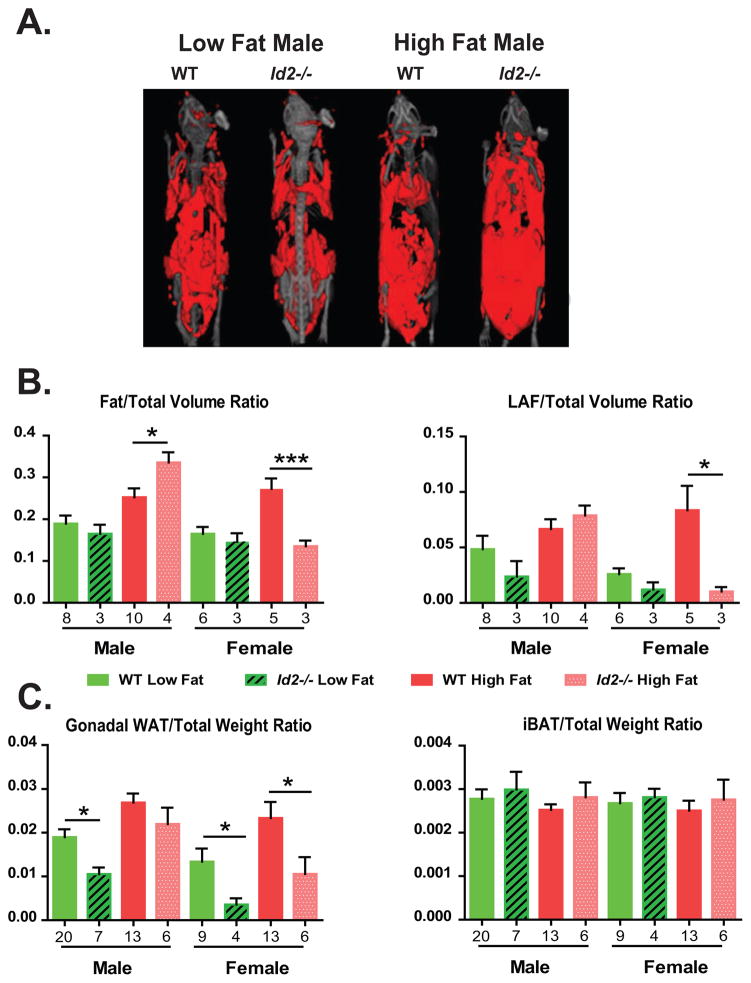
Modulation of fat storage of Id2−/− male mice under high fat diet
The apparent weight gain of Id2−/− males under HFD raised a question as to whether the increased body mass in these animals was associated with an increase in fat mass. Therefore, we examined and quantified the amount of fat tissue present in WT and Id2−/− animals fed the LFD and HFD. A representative 3D visual display of fat deposits in WT and Id2−/− males is shown in Fig. 3A. Analysis by MicroCT revealed no difference in fat/total volume ratio between Id2−/− and WT males fed with LFD, whereas Id2−/− males on HFD had a significant increase in this ratio compared to WTs (Genotype (G), n.s.; Diet (D), p<0.001, Interaction (I), p=0.05) (Fig. 3B). There was no difference in fat/total volume ratio between WT and Id2−/− females when fed with LFD. However, Id2−/− females had a significantly lower fat/total volume ratio compared to WTs when fed with HFD (G, p<0.01.; D, p<0.05, I, p<0.05) (Fig. 3B). The trends observed from global body fat of WT and Id2−/− mice fed with the two diets were consistent with those of the lower abdominal fat (LAF) trends (male: G, n.s.; D, p<0.01; I, n.s.; female: G, p<0.01; D, n.s.; I, n.s.) (Fig. 3B). Moreover, at the end of the experiment, at wk 22, perigonadal adipose tissue in WT and Id2−/− mice was harvested and weighed (Fig. 3C). The weight ratio of fat from gonadal WAT was lower in Id2−/− males than that from WT males fed with the LFD. However, these differences were not observed in Id2−/− male fed with HFD (G, p<0.05; D, p<0.001; I, n.s.). Id2−/− females had a lower gonadal WAT weight ratio in both diets when compared to WT females (G, p<0.001; D, p<0.05; I, n.s.). No difference in iBAT weight ratios was observed between WT and Id2−/− mice when fed with LFD and HFD (two factor ANOVA: n.s.) (Fig. 3C). The actual weight trends of gonadal WAT and iBAT were comparable to those of weight ratio trends (Supplemental Fig. 1A,C). Histological evaluation of gonadal WAT revealed no significant difference between genotype, although the trends of adipocyte size in the female LFD group were comparable to our previous study, but less pronounced (Supplemental Fig. 1B) [9].
Fig. 3. Sex-specific modulation of fat storage of Id2−/− mice under high fat diet.
(A) MicroCT representative 3D visual display of global fat deposits of WT and Id2−/− male mice. (B) MicroCT examination of the ratio of total fat to body volume (left) and of lower abdominal fat (LAF) volume to total volume (right) from WT and Id2−/− mice fed with LFD or HFD. (C) Ratio of weight of gonadal white adipose tissue to total body mass (left) and of weight of iBAT to total body mass (Right) from WT and Id2−/− fed with LFD and HFD. Values are mean ± S.E.M. Two factor-ANOVAs were performed followed by Tukey’s post-hoc tests, *p<0.05, ***p<0.001.
High fat diet modulates lipid metabolic homeostasis of Id2−/− mice
At the end of the 22 wk of controlled diet, and at the age of 32–34 wk, serum and liver were collected from mice following overnight fasting. Table 1 shows the lipid and endocrine parameters measured for these surviving mice. No difference of serum triglyceride (TG) levels was observed between genotypes in either males and females when fed with LFD or HFD (Table 1, Supplemental Fig. 2A). However, the serum total cholesterol levels were significantly lower in Id2−/− males fed with HFD (Table 1, Fig. 4A). Also, serum LDL cholesterol levels were lower in Id2−/− males fed with HFD in comparison with diet and sex-matched WTs (Table 1, Fig. 4A). A similar pattern was observed in serum HDL cholesterol levels, although this pattern was less pronounced and did not reach statistical significance (Table 1, Supplemental Fig. 2A). There was no significant difference in insulin levels between genotype, sex and diet (Table 1, Supplemental Fig. 2B). However, Id2−/− females were found to have elevated glucagon levels when fed with HFD (Table 1, Supplemental Fig. 2B). The serum leptin levels were found to be consistently lower in Id2−/− mice, irrespective of diet and sexes. However, the reduction between male Id2−/− and WT mice fed with HFD was not observed to be significant in post hoc analysis (Table 1, Fig. 4A). The Id2−/− mice serum adiponectin levels were not significantly different from WTs when fed with LFD and HFD (Table 1, Supplemental Fig. 2C). The liver total cholesterol levels in males showed a similar pattern across experimental groups as serum total cholesterol, but not reaching statistical significance (Table 1, Fig. 4B). Surprisingly, although the liver TG levels in Id2−/− males fed with LFD were significantly lower than WTs, there was striking accumulation of TG in Id2−/− males when fed with HFD compared to WTs (Table 1, Fig. 4B). Furthermore, these differences were observed in males only. Oil-red-O staining of liver sections confirmed this distinct pattern of lipid accumulation in Id2−/− male mice: a depletion of lipids on LFD but significantly higher levels of lipid on HFD, as compared to WTs (Fig. 4C).
Table 1.
Serum and Liver lipid/endocrine parameters.
| Male | Female | Three Factor ANOVA Statistic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LFD | HFD | LFD | HFD | Sex | Diet | Genotype | |||||
| WT | Id2−/− | WT | Id2−/− | WT | Id2−/− | WT | Id2−/− | Male vs Female | LFD vs HFD | Id2−/− vs WT | |
| Serum | |||||||||||
| Triglyceride (TG; mg/dl) | 105.6 ± 11.4 | 86.3 ± 33.7 | 94.4 ± 12.3 | 62.5 ± 8.5 | 93.8 ± 13.9 | 89.5 ± 13.6 | 88.1 ± 6.7 | 125.6 ± 33.5 | n.s. | n.s. | n.s. |
| Total Cholesterol (TC; mg/dl) | 165.5 ± 11.2 | 127.2 ± 7.8 | 198.5 ± 14.6 | 141.5 ± 11.2 a | 119.0 ± 8.2 | 103.8 ± 13.1 | 127.9 ± 6.7 | 120.7 ± 10.4 | p<0.001 | n.s | p<0.01 |
| HDL Cholesterol (mg/dl) | 59.2 ± 6.5 | 48.4 ± 5.6 | 80.0 ± 7.8 | 69.2 ± 4.9 | 42.5 ± 6.3 | 53.8 ± 10.7 | 42.0 ± 5.5 | 37.0 ± 7.4 | p<0.01 | n.s. | n.s. |
| p<0.001 (within HFD) | p<0.05 (within male) | ||||||||||
| LDL Cholesterol (mg/dl) | 48.2 ± 3.6 | 39.7 ± 2.8 | 58.0 ± 4.2 | 43.2 ± 2.6 a | 35.0 ± 2.9 | 30.7 ± 4.3 | 37.9 ± 2.1 | 35.5 ± 2.4 | p<0.001 | n.s. | p<0.05 |
| Insulin (ng/ml) | 0.8 ± 0.1 | 0.9 ± 0.4 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.8 ±0.5 | 1.1 ± 0.3 | 0.6 ± 0.4 | n.s. | n.s. | n.s. |
| Glucagon (pg/ml) | 92.1 ± 18.1 | 69.3 ± 6.8 | 59.5 ± 14.4 | 48.0 ± 5.6 | 90.2 ± 15.8 | 206.6 ± 88.3 | 64.9 ± 9.6 | 102.1 ± 20.4 a | p<0.01 | p<0.01 | n.s. |
| Leptin (ng/ml) | 2.8 ± 0.3 | 0.8 ± 0.4 a | 4.1 ± 0.9 | 2.6 ± 0.6 | 3.7 ± 0.7 | 0.6 ± 0.2 b | 5.9 ± 0.9 | 2.1 ± 0.8 b | n.s. | p<0.01 | p<0.001 |
| Adiponectin (μg/ml) | 7.2 ± 0.3 | 8.2 ± 1.8 | 6.9 ± 0.4 | 8.0 ± 0.8 | 13.2 ± 0.7 | 12.1 ± 0.7 | 14.2 ± 0.8 | 12.7 ± 2.0 | p<0.001 | n.s. | n.s. |
| Liver | |||||||||||
| TG (mg/g tissue) | 14.4 ± 1.8 | 4.2 ± 0.6 c | 15.0 ± 2.1 | 44.5 ± 9.4 c | 22.1 ± 5.0 | 28.4 ± 1.9 | 23.5 ± 3.7 | 15.8 ± 7.4 | n.s. | n.s. | n.s. |
| p<0.001 (within LFD-Id2−/−) | p<0.001 (within male-Id2−/−) | p<0.001 (Diet X Genotype X Sex) | |||||||||
| p<0.001(within HFD-Id2−/−) | p<0.05 (within female-Id2−/−) | ||||||||||
| TC (mg/g tissue) | 2.0 ± 0.1 | 1.9 ± 0.0 | 2.8 ± 0.2 | 2.4 ± 0.1 | 2.0 ± 0.1 | 2.4 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.3 | p<0.001 | p<0.001 | n.s. |
| p<0.001 (within Id2−/−) | p<0.001 (within WT) | p<0.05 (within male) | |||||||||
| p<0.01 (within Id2−/−) | |||||||||||
Serum and liver lipid and endocrine parameters were analyzed as described in Materials and Methods. Values are mean ± S.E.M. Three factor ANOVAs were performed with sex, diet and genotype as independent variables. n.s., non-significant. Two factor-ANOVA were performed followed by Tukey’s post-hoc tests,
p<0.05,
p<0.01,
p<0.001, and significantly different values highlighted in bold underline in table.
Figure 4. High fat diet differentially modulates lipid metabolic homeostasis of Id2−/− male mice.
(A) Serum total cholesterol (left), LDL cholesterol (middle) and leptin (right) of WT and Id2−/− under LFD or HFD. (B) Liver total cholesterol (left) and triglyceride (right) of WT and Id2−/− under LFD or HFD. (C) Representative Oil-red-O and Hematoxylin-Eosin stained liver section from WT and Id2−/− male mice liver fed with LFD or HFD. Scale bar, 100 μm. Oil-red-O quantification values, mean ± S.E.M., on the right. Two factor-ANOVAs were performed followed by Tukey’s post-hoc tests, *p<0.05, **p<0.01, ***p<0.001.
Discussion
In this study, we have explored the effects of ID2 deficiency on energy metabolism in mice. Loss of ID2 inhibited weight gain in animals exposed to a LFD. This could be attributed, in part, to reduced adiposity, as shown in the current study and prior work, as well as impaired adipogenesis, and increased energy expenditure from iBAT and skeletal muscle [3,4,9]. High fat diet moderately ameliorates the gaining weight capacity of Id2−/− male mice only. This sexual dimorphism may be attributed to multiple factors, such as the sex difference in the fat deposition and storage, hormones, and metabolism [14,15]. Interestingly, we also found that the survival rate of Id2−/− male mice was significantly improved by a HFD. One possibility might be amelioration of body weight loss and reduced fat mass, as mice with no WAT fat (A-ZIP/F-1 mice) show a high mortality rate [16]. Moreover, no significant decline in body weight prior to death was found. It is also plausible that the cause or contribution to the sudden occurrence of death was due to heart failure; it has been reported previously that Id2−/− mice have an abnormal cardiac morphology and intraventricular conduction [17]. Since HDL cholesterol levels are particularly higher in Id2−/− male mice on HFD compared to other groups of Id2−/− mice, this may help improve their cardiac function.
In this study, both female and male Id2−/− mice showed increased glucose tolerance, except for HFD males. MicroCT analysis revealed sex differences in total fat storage of Id2−/− mice under HFD. Moreover, assessment of gonadal WAT mass suggests that there are increased visceral fat depots in Id2−/− male mice under HFD, whereas there is less stored in visceral fat in females on both diets. Furthermore, serum leptin levels were consistent with the appropriate WAT/body mass ratio. Since previous studies show a clear association between visceral fat accumulation and insulin sensitivity, increased visceral fat depots in Id2−/− males might contribute to a loss of enhanced glucose tolerance [18,19]. In addition, hepatic steatosis may also compromise glucose metabolism in the HFD-fed Id2−/− mice. Our previous findings also revealed that increased energy expenditure is compensated in Id2−/− male mice through increasing food intake and reducing locomotor activity [9]. However, an energy-rich diet may undermine this compensation effect in a sex-specific manner, which may contribute to fat accumulation, loss of the lean phenotype and loss of enhanced glucose tolerance.
Interestingly, despite the lack of a marked change in circulating adiponectin levels, ectopic lipid accumulation occurred in the liver of Id2−/− mice in a sex-specific manner. Id2−/− male liver showed an opposite effect on TG levels under low and high fat diets. This observation could be explained in part by earlier findings that lipoprotein lipase (Lpl) was found to be up-regulated in the liver of Id2−/− mice under regular chow diet in a time-specific manner [3]. Hepatic-specific overexpression of LPL leads results in hepatic steatosis [20,21]. Another possibility for the steatosis is the spillover of fat from adipose tissue, as the total fat mass is increased in the Id2−/− male mice.
According to our data, we propose the following possible mechanisms to explain the sexual dimorphism observed in Id2−/− mice. First, female Id2−/− mice have higher adiponectin levels than their male counterparts. Since adiponectin is an important adipokine that regulates systemic insulin sensitivity and lipid metabolism [22], female Id2−/− mice maintain glucose tolerance and moderate levels of hepatic lipid contents even on high-fat diet. Second, an increase in circulated glucagon in Id2−/− females may also improve lipid homeostasis in adipose tissue and liver lipid degradation [23]. In addition, sex-specific adipocyte programing [9,14] and differences in hepatic lipid uptake and export might play important roles as well. Given that Id2 is implicated in regulating circadian clock output [3,9], it is also possible that the Id2−/− mouse phenotypes could be a result of poor intra- and inter-organ temporal coordination of metabolic processes, including central nervous system control and intestinal lipid absorption [24,25]. These data and other pieces of evidence suggest that Id2−/− mice have defects in adipogenesis and lipid metabolism, which might include disturbances to rhythmic and non-rhythmic regulation of genes and processes necessary for adipocyte differentiation and function, lipid synthesis/uptake, metabolism and/or storage [26,27]. In conclusion, our current data and earlier reports suggest that ID2 is a potential therapeutic target for treating metabolic disorders. Further, these data reinforce the relevance of sex-specific analyses in studying models of metabolic function as they pertain to human health and disease.
Supplementary Material
High fat diet ameliorates Id2−/− mice phenotype and survival rate sex specifically
High fat diet modulates Id2−/− mice glucose homeostasis in a sex dependent manner
High fat diet modulates lipid metabolic homeostasis of Id2−/− mice sex specifically
Acknowledgments
The work was supported from grants to GED from NIGMS (R01-GM087508) and AHA (10SDG4030011). The authors are grateful to the Freimann Life Science Center for assistance with animal care and training, the Notre Dame Integrated Imaging Facility (W.M. Leevy and S. Chapman) for assistance with CT and histology, and the UC Davis MMPC (NIDDK grant U24-DK092993) for phenotyping services.
Abbreviations
- HFD
high fat diet
- HLH
helix-loop-helix
- iBAT
interscapular brown adipose tissue
- Id2
Inhibitor of DNA binding 2
- LAF
lower abdominal fat
- LFD
low fat diet
- TG
triglyceride
- WAT
white adipose tissue
- ZT
Zeitgeber time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patil M, Sharma BK, Satyanarayana A. Id transcriptional regulators in adipogenesis and adipose tissue metabolism. Front Biosci (Landmark Ed) 2014;19:1386–1397. doi: 10.2741/4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113(Pt 22):3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- 3.Hou TY, Ward SM, Murad JM, Watson NP, Israel MA, Duffield GE. ID2 (inhibitor of DNA binding 2) is a rhythmically expressed transcriptional repressor required for circadian clock output in mouse liver. J Biol Chem. 2009;284:31735–31745. doi: 10.1074/jbc.M109.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park KW, Waki H, Villanueva CJ, Monticelli LA, Hong C, Kang S, MacDougald OA, Goldrath AW, Tontonoz P. Inhibitor of DNA binding 2 is a small molecule-inducible modulator of peroxisome proliferator-activated receptor-gamma expression and adipocyte differentiation. Mol Endocrinol. 2008;22:2038–2048. doi: 10.1210/me.2007-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akerfeldt MC, Laybutt DR. Inhibition of Id1 augments insulin secretion and protects against high-fat diet-induced glucose intolerance. Diabetes. 2011;60:2506–2514. doi: 10.2337/db11-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murad JM, Place CS, Ran C, Hekmatyar SK, Watson NP, Kauppinen RA, Israel MA. Inhibitor of DNA binding 4 (ID4) regulation of adipocyte differentiation and adipose tissue formation in mice. J Biol Chem. 2010;285:24164–24173. doi: 10.1074/jbc.M110.128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffield GE, Watson NP, Mantani A, Peirson SN, Robles-Murguia M, Loros JJ, Israel MA, Dunlap JC. A role for Id2 in regulating photic entrainment of the mammalian circadian system. Curr Biol. 2009;19:297–304. doi: 10.1016/j.cub.2008.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward SM, Fernando SJ, Hou TY, Duffield GE. The transcriptional repressor ID2 can interact with the canonical clock components CLOCK and BMAL1 and mediate inhibitory effects on mPer1 expression. J Biol Chem. 2010;285:38987–39000. doi: 10.1074/jbc.M110.175182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew D, Zhou P, Pywell CM, van der Veen DR, Shao J, Xi Y, Bonar NA, Hummel AD, Chapman S, Leevy WM, Duffield GE. Ablation of the ID2 gene results in altered circadian feeding behavior, and sex-specific enhancement of insulin sensitivity and elevated glucose uptake in skeletal muscle and brown adipose tissue. PLoS One. 2013;8:e73064. doi: 10.1371/journal.pone.0073064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Link JC, Chen X, Arnold AP, Reue K. Metabolic impact of sex chromosomes. Adipocyte. 2013;2:74–79. doi: 10.4161/adip.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, Roemisch-Margl W, Polonikov A, Peters A, Theis FJ, Meitinger T, Kronenberg F, Weidinger S, Wichmann HE, Suhre K, Wang-Sattler R, Adamski J, Illig T. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011;7:e1002215. doi: 10.1371/journal.pgen.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou P, Ross RA, Pywell CM, Liangpunsakul S, Duffield GE. Disturbances in the murine hepatic circadian clock in alcohol-induced hepatic steatosis. Sci Rep. 2014;4:3725. doi: 10.1038/srep03725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champy MF, Selloum M, Zeitler V, Caradec C, Jung B, Rousseau S, Pouilly L, Sorg T, Auwerx J. Genetic background determines metabolic phenotypes in the mouse. Mamm Genome. 2008;19:318–331. doi: 10.1007/s00335-008-9107-z. [DOI] [PubMed] [Google Scholar]
- 14.Bloor ID, Symonds ME. Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Horm Behav. 2014;66:95–103. doi: 10.1016/j.yhbeh.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E, Jensen MD. Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr. 2008;87:56–63. doi: 10.1093/ajcn/87.1.56. [DOI] [PubMed] [Google Scholar]
- 16.Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moskowitz IP, Kim JB, Moore ML, Wolf CM, Peterson MA, Shendure J, Nobrega MA, Yokota Y, Berul C, Izumo S, Seidman JG, Seidman CE. A molecular pathway including Id2, Tbx5, and Nkx2-5 required for cardiac conduction system development. Cell. 2007;129:1365–1376. doi: 10.1016/j.cell.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17:644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, Hucking K, Ader M. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14(Suppl 1):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 20.Merkel M, Weinstock PH, Chajek-Shaul T, Radner H, Yin B, Breslow JL, Goldberg IJ. Lipoprotein lipase expression exclusively in liver. A mouse model for metabolism in the neonatal period and during cachexia. J Clin Invest. 1998;102:893–901. doi: 10.1172/JCI2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ, Breslow JL, Shulman GI. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci U S A. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee B, Shao J. Adiponectin and energy homeostasis. Rev Endocr Metab Disord. 2014;15:149–156. doi: 10.1007/s11154-013-9283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marroqui L, Alonso-Magdalena P, Merino B, Fuentes E, Nadal A, Quesada I. Nutrient regulation of glucagon secretion: involvement in metabolism and diabetes. Nutr Res Rev. 2014;27:48–62. doi: 10.1017/S0954422414000031. [DOI] [PubMed] [Google Scholar]
- 24.Douris N, Kojima S, Pan X, Lerch-Gaggl AF, Duong SQ, Hussain MM, Green CB. Nocturnin regulates circadian trafficking of dietary lipid in intestinal enterocytes. Curr Biol. 2011;21:1347–1355. doi: 10.1016/j.cub.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcheva B, Ramsey KM, Peek CB, Affinati A, Maury E, Bass J. Circadian clocks and metabolism. Handb Exp Pharmacol. 2013:127–155. doi: 10.1007/978-3-642-25950-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reitman ML. Metabolic lessons from genetically lean mice. Annu Rev Nutr. 2002;22:459–482. doi: 10.1146/annurev.nutr.22.010402.102849. [DOI] [PubMed] [Google Scholar]
- 27.Reue K, Phan J. Metabolic consequences of lipodystrophy in mouse models. Curr Opin Clin Nutr Metab Care. 2006;9:436–441. doi: 10.1097/01.mco.0000232904.82038.db. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.