Abstract
Purpose
We have shown the feasibility of administering inhaled doxorubicin to patients with cancer. This study evaluated inhaled doxorubicin combined with cisplatin and docetaxel in patients with non–small cell lung cancer. The principal objective was to determine safety and, secondarily, efficacy.
Experimental Design
Patients who had chemo-naïve advanced non–small cell lung cancer were enrolled in the study. Adequate organ and pulmonary function was required: diffusing capacity for carbon monoxide/forced expiratory volume in 1 second/forced vital capacity ≥50%, resting/exercise O2 saturation ≥90%/85%. In phase I, doxorubicin was escalated: dose level 1 (6 mg/m2) and level 2 (7.5 mg/m2). Escalation was permitted if ≤2 of 6 patients experienced pulmonary dose-limiting toxicity (grade 2 Radiation Therapy Oncology Group lung morbidity; resting O2 saturation of <85%; decrease in diffusing capacity for carbon monoxide, forced vital capacity, or forced expiratory volume in 1 second of ≥20% from baseline or ≤30% of predicted; or grade 3 Common Terminology Criteria for Adverse Events version 3.0 pulmonary toxicity). Doses of cisplatin and docetaxel were 75 mg/m2. Treatments and pulmonary function tests were repeated every 21 days, with up to eight cycles for responding patients.
Results
Twenty-eight patients were treated at level 1 and eight patients at level 2. Doxorubicin was escalated to 7.5 mg/m2, however, after two patients developed pulmonary dose-limiting toxicity; the remainder were treated at 6.0 mg/m2. Twenty-four evaluable patients received at least two courses or had progressive disease following the first course at the phase II dose. Toxicity was associated with i.v. chemotherapy although one patient had delayed pulmonary toxicity responding to corticosteroids and oxygen. Seven (29%) evaluable patients responded (six partial responses and one complete response) and 13 (54%) patients had stable disease for up to eight cycles.
Conclusion
Although this combination was safe, the primary objective was not met and will not be pursued further.
Lung cancer is a leading cause of cancer-related morbidity and mortality worldwide. It is estimated that in the United States, more than 200,000 new cases of lung cancer occur each year (1). Although newer “third-generation” combination chemotherapy regimens have resulted in higher response rates, and the recent addition of targeted antibodies to the vascular endothelial growth factor and epidermal growth factor has improved response and survival, this improvement has been relatively modest, and further study is needed (2–4). Recent data have suggested that histology is an important factor in the selection of therapeutic agents (5). The reasons for treatment failure are diverse, but one possibility is the inability to achieve adequate therapeutic concentrations of drugs at the tumor site.
An approach to improving therapeutic efficacy is localized dose intensification, which has been efficacious in a variety of tumors including ovarian, colorectal, and bladder cancers. The rationale for regional delivery of chemotherapy has been to enhance the delivery of effective therapy while minimizing systemic toxicity (6). The major limitation for this approach is the limited absorption of antineoplastic agents beyond a few cell layers to perhaps several millimeters deep (6). For these reasons, regional delivery of chemotherapy has been best evaluated in a multimodality setting (e.g., i.v. plus i.p. chemotherapy, or surgery plus i.p. chemotherapy). In the setting of ovarian cancer, several phase III trials have shown the benefit of adding i.p. chemotherapy to standard cytoreductive surgery in combination with i.v. chemotherapy (7, 8). Unfortunately, due to significant localized toxicity and logistic concerns (among others), this has not been widely accepted (6). Peritoneal carcinomatosis from colorectal or other gastrointestinal sites of tumor has been an additional place where regional/i.p. chemotherapy has been investigated (9). Although there are reasonable pharmaco-kinetic data, there are minimal controlled clinical trial data to support this use, and much of the experience is restricted to case reports and anecdotal evidence.
Inhalational chemotherapy was first described by Shevchenko and Resnik in 1968 (10). The concept was tested in dogs and subsequently in 58 patients. Antitumor efficacy was observed in 24 patients but the data were difficult to interpret because of the concurrent use of radiotherapy. Nevertheless, this early trial established the feasibility of administering chemotherapy by inhalation. Since then, a variety of chemotherapeutic agents including cisplatin, mitomycin, and 5-fluorouracil administered by inhalation have been evaluated in preclinical models. Tatsumura et al. (11) tested the administration of 5-fluorouracil by inhalation in dogs and found high levels of the drug in the trachea, hilar bronchi, and regional nodes. Subsequently, the same authors treated 10 patients with inoperable lung tumors with 5-fluorouracil administered via supersonic nebulizer at a dose of 250 mg twice daily for 2 to 3 days per week. There was antitumor efficacy with two complete and four partial responses observed.
In addition to administration of inhaled chemotherapeutic agents, Huland et al. (12) administered interleukin-2 (IL-2) by inhalation with concomitant IFN-α administered s.c. to 15 patients with metastatic renal carcinoma. There seemed to be improved response in lung compared with non-lung metastases, suggesting that inhalational IL-2 displayed enhanced antitumor efficacy in pulmonary lesions. Nebulized IL-2 in combination with systemic IL-2 has also been evaluated in patients with renal cancer (13). More recent studies have been done using formulations of cisplatin, camptothecins, and alternative biological response agents with intriguing early results (14–17).
We have previously shown the safety of delivering doxorubicin using a novel inhalational delivery device (18). Doxorubicin hydrochloride was chosen as the first agent to be evaluated in this program because of its broad level of activity and preclinical efficacy. A clinical delivery device suitable for this approach was developed, a series of preclinical studies were completed, and the device and the vehicle were evaluated in two studies conducted in healthy volunteers and patients with respiratory impairment. Subsequently, a phase I study of single-agent inhaled doxorubicin in 53 patients with cancer metastatic to the lungs showed that this could be safely done even in patients with significant metastatic burden in the lungs (18). Because non–small cell lung cancer (NSCLC) is a systemic disease and because the toxicity seen with inhaled doxorubicin was not overlapping with platinum-based doublet chemotherapy for NSCLC, we hypothesized that the addition of inhaled doxorubicin to an i.v. administered chemotherapy regimen would have beneficial effects. Docetaxel and cisplatin were chosen as the standard doublet to be combined with the inhalational compound given the efficacy of this combination and its favorable toxicity profile (2, 19). The current study determined the maximal tolerated dose and the efficacy of inhaled doxo-rubicin HCl when given in combination with i.v. docetaxel and cisplatin in patients with NSCLC.
Patients and Methods
Study design and objectives
This was a multicenter dose-escalation study in chemotherapy-naïve patients with metastatic NSCLC. The starting dose of inhaled doxorubicin HCl was 6.0 mg/m2. The dose of inhaled doxorubicin was then escalated to 7.5 mg/m2 in the next cohort of three patients. No further dose escalation was planned because 7.5 mg/m2 was the recommended phase II dose from the initial phase I study (18). The definition of dose-limiting toxicity (DLT) in the phase I portion of this study was based on pulmonary toxicity, defined as grade 2 Radiation Therapy Oncology Group lung late radiation morbidity; resting O2 saturation of <85%; decrease in diffusing capacity for carbon monoxide (DLCO), forced vital capacity (FVC), or forced expiratory volume in 1 second (FEV1) of ≥20% from baseline or ≤30% of predicted; or grade 3 Common Terminology Criteria for Adverse Events version 3.0 pulmonary toxicity. Standard 3 + 3 phase I dose escalation rules were applied. Patients were given inhaled doxorubicin between 1 and 3 h before starting i.v. chemotherapy. Docetaxel and cisplatin were given in a standard fashion (75 mg/m2 with specified dose reductions for nonpulmonary toxicity). Treatment courses were repeated every 3 wk, provided recovery from toxicities occurred.
The primary objective of the phase I portion of the study was to determine the recommended phase II dose of inhaled doxorubicin when given in combination with i.v. docetaxel and cisplatin. The primary objective of the phase II portion of the study was to obtain preliminary evidence of therapeutic activity and define the nature of toxic effects. Response to therapy was evaluated using Response Evaluation Criteria in Solid Tumors. A total of 25 patients evaluable for efficacy were planned to be treated on this regimen at the phase II dose. An overall response rate >35% (9 or more of 25 evaluable patients), which corresponded to a lower boundary greater than the 17% response rate reported by Schiller et al. (2), and if it is associated with an improvement in pulmonary symptoms would warrant evaluation in a phase III trial.
Study population
Patients had histologically or cytologically confirmed NSCLC with advanced disease and at least one lesion within the lung, of age ≥18 y, and with Eastern Cooperative Oncology Group (ECOG) performance status 0 to 1. In addition to adequate organ function, patients were required to have adequate pulmonary function: DLCO, FVC, and FEV1 ≥50% predicted; resting oxygen saturation ≥90%; exercise oxygen saturation ≥85%.
Prior chemotherapy was not allowed, and no prior thoracic radiation was permitted although extrathoracic palliative radiation was allowed. Similarly, patients with asthma, complete atelectasis, and pneumonectomy were excluded from participation. All patients provided informed consent.
OncoMyst Model CDD-2a and doxorubicin solution
The OncoMyst Model CDD-2a is a high-efficiency inhalation device that uses a Pari LC Plus nebulizer housed within a sealed containment system with apparatus for mouth-only inhalation. The details of the device are as previously described (18). Doxorubicin inhalation solution is an investigational, sterile, pyrogen-free solution supplied at two concentrations, 16 and 24 mg/mL in 20% ethanol:80% water (pH 3).
Dose delays/adjustments
Pulmonary DLT was defined as described above. Only the first course was considered for the purposes of dose escalation; however, pulmonary DLT occurring in any course was reason for removal from the study.
Dose adjustments of docetaxel and cisplatin were permitted for nonpulmonary toxicity. If the toxicity leading to i.v. therapy adjustment was prolonged neutropenia or febrile neutropenia, granulocyte colony-stimulating factor or pegylated granulocyte colony-stimulating factor was allowed for subsequent cycles and counted as a dose decrease. Dose adjustments of inhaled doxorubicin HCl were not permitted. If the docetaxel and cisplatin treatment was stopped for any reason, the patient was withdrawn from further treatment.
Response assessment and statistical analysis
Response assessment was done using Response Evaluation Criteria in Solid Tumors after every two cycles. Up to eight cycles were allowed for patients with responding disease and there was no pulmonary toxicity if the treating physician felt the patient was sustaining significant benefit. Demographic characteristics for all registered patients were summarized using appropriate descriptive statistics. The safety data were summarized for all dosed patients. The primary end point for this single-arm phase II trial was the best response based on the evaluable patients. The proportion of patients who showed at least partial response among the patients treated at the phase II dose level was reported along with a 95% confidence interval. An overall response rate >35% (>9 of 25 evaluable patients), which corresponded to a lower boundary greater than the 17% response rate reported by Schiller et al. (2), would warrant a phase III trial. The overall, intrathoracic, and extrathoracic tumor size changes relative to baseline were summarized. Estimates of survival functions for patients who received at least one course treatment at level 1 or 2 were generated by the Kaplan-Meier method and compared using the log-rank test. Confidence intervals for the median survival lengths were also computed.
Results
Forty-three patients were registered and enrolled in this study, 34 at dose level 1 (6 mg/m2) and 9 at dose level 2 (7.5 mg/m2; Table 1). Seven patients were registered but never received treatment (six at level 1 and one at level 2). The most common reason no treatment was delivered was uneven dose distribution on Tc99m scanning (three patients). After the first three patients were treated at 6 mg/m2, three patients were treated at 7.5 mg/m2, with one patient developing a drop in DLCO. Three additional patients were dosed without DLT, and accrual at that dose level continued; however, a second patient (after the first six patients at the 7.5 mg/m2 dose level) developed a drop in DLCO after the first cycle. The data safety monitoring board recommended that the dose for the phase II portion be 6 mg/m2 of inhaled doxorubicin. Further accrual at this dose level then continued. The trial was terminated before the planned 25 evaluable patients were recruited after 17 nonresponders. A total of 34 patients were registered at the phase II dose level; 28 received at least one course of treatment.
Table 1.
Baseline demographics
| Dose level 1 (6 mg/m2), n (%) |
Dose level 2 (7.5 mg/m2), n (%) |
|
|---|---|---|
| Total | 34 | 9 |
| Gender | ||
| Male | 17 (50) | 2 (22.2) |
| Female | 17 (50) | 7 (77.8) |
| Age (y), mean (range) | 58.8 (36–80) | 55.1 (50–64) |
| ECOG at screen | ||
| 0 | 15 (44.1) | 3 (33.3) |
| 1 | 17 (50.0) | 6 (66.7) |
| 2 | 1 (2.9) | 0 |
| Unknown | 1 (2.9) | 0 |
| Cancer type | ||
| Adenocarcinoma | 16 (47.1) | 3 (33.3) |
| Squamous | 5 (14.7) | 1 (11.1) |
| Large cell | 1 (2.9) | 1 (11.1) |
| NOS | 11 (32.4) | 4 (44.4) |
| Mixed | 1* (2.9) | 0 |
Abbreviation: NOS, not otherwise specified.
Squamous and adenocarcinoma.
At the phase II dose, there was equal representation of men and women, with ECOG performance status of 2 in one patient and not recorded in another. The patient with unknown performance status was never treated. As a protocol deviation, one patient with ECOG 2 received three cycles of treatment and was taken off for progressive disease. As expected, most patients had adenocarcinomas, with the next most common histology being not otherwise specified (Table 1).
Patients who were treated received one to eight cycles of treatment, with seven patients receiving only one cycle and dropped because of evidence of progressive disease (three), adverse event (two), or withdrawal of consent or physician decision (one each; Table 2). There were a total of 24 evaluable patients: 21 patients received at least two courses of treatment and 3 patients had progressive disease following the first course of treatment.
Table 2.
Number of treatment courses
| Dose level 1 (6 mg/m2), n (%) |
Dose level 2 (7.5 mg/m2), n (%) |
|
|---|---|---|
| No. of treatment courses | ||
| 0 | 6 (17.6) | 1 (11.1) |
| 1 | 7 (20.6) | 4 (44.4) |
| 2 | 0 (0.0) | 0 (0.0) |
| 3 | 2 (5.9) | 1 (11.1) |
| 4 | 6 (17.6) | 1 (11.1) |
| 5 | 5 (14.7) | 0 (0.0) |
| 6–8 | 8 (23.5) | 2 (22.2) |
The most common toxicities were those related to the i.v. chemotherapy and were generally mild (grade 1–2) including alopecia, fatigue, nausea, diarrhea, anorexia, and cough. The number of patients reporting grade 3 to 4 adverse events for both dose levels is listed in Table 3. In the phase I portion (dose level 2; 7.5 mg/m2), grade 3/4 nonhematologic toxicities included constipation (in 1 of 8 patients) and hyponatremia (in 2 of 8). Hematologic toxicities included 6 of 8 patients with grade 3/4 neutropenia. Interestingly, although there are insufficient numbers of patients treated at the higher dose level, there seemed to be a trend of greater hematologic toxicity in the patients treated at the higher dose. For example, grade 3/4 neutropenia occurred in 12 of 28 (42.9%) patients at dose level 1 whereas 6 of 8 (75%) experienced this toxicity at dose level 2 (Table 3). In addition, grade 1/2 anemia occurred in 8 of 28 (28.6%) patients at dose level 1 and in 5 of 8 (62.5%) at dose level 2 (data not shown). Our prior phase I and pharmacokinetic study did not suggest significant systemic exposure to doxorubicin (18); however, it is conceivable that limited exposure enhanced the hematologic toxicity of docetaxel and cisplatin. At dose level 1, nonhematologic toxicity was generally grade 1/2 as well, with no more than two patients reporting any specific grade 3 or 4 toxicity. Grade 3 or 4 fatigue, vomiting, and sensory neuropathy were experienced by two patients, with only one patient each reporting nausea, constipation, diarrhea, cough, dehydration, and neuropathy (Table 3). Most of these toxicities were ascribed to the i.v. chemotherapy or to the underlying disease.
Table 3.
Toxicity
| Dose level 1 (6 mg/m2), n = 28 |
Dose level 2 (7.5 mg/m2), n = 8 |
|
|---|---|---|
| Grade 3–4 |
Grade 3–4 |
|
| n (%) | n (%) | |
| Any events | 21 (75.0) | 7 (87.5) |
| Nonhematologic adverse event | ||
| Fatigue | 2 (7.1) | 0 (0.0) |
| Nausea | 1 (3.6) | 0 (0.0) |
| Constipation | 1 (3.6) | 1 (12.5) |
| Diarrhea | 1 (3.6) | 0 (0.0) |
| Cough | 1 (3.6) | 0 (0.0) |
| Vomiting | 2 (7.1) | 0 (0.0) |
| Neuropathy | 2 (7.1) | 0 (0.0) |
| Sensory | ||
| Hyponatremia | 0 (0.0) | 2 (25.0) |
| Dehydration | 1 (3.6) | 0 (0.0) |
| Neuropathy | 1 (3.6) | 0 (0.0) |
| Hematologic adverse event | ||
| Neutropenia | 12 (42.9) | 6 (75.0) |
| Leucopenia | 4 (14.3) | 1 (12.5) |
In addition, a number of patients (five) developed late (>cycle 1) decreases in pulmonary function test (PFT) parameters that were usually asymptomatic. Figure 1 shows the mean change from baseline DLCO measurements for cycles 1 to 6 and end of treatment. FEV1 and FVC measurements were similar. Although these PFT changes were generally asymptomatic, one patient experienced a drop in DLCO after completing six cycles of treatment, which was associated with symptomatic cough that responded to a short course of steroids. Whereas in many patients a drop in DLCO, FEV1, or FVC may have been associated with progression of pulmonary disease, in the patient mentioned above, the tumor had significantly decreased in response to treatment.
Fig. 1.
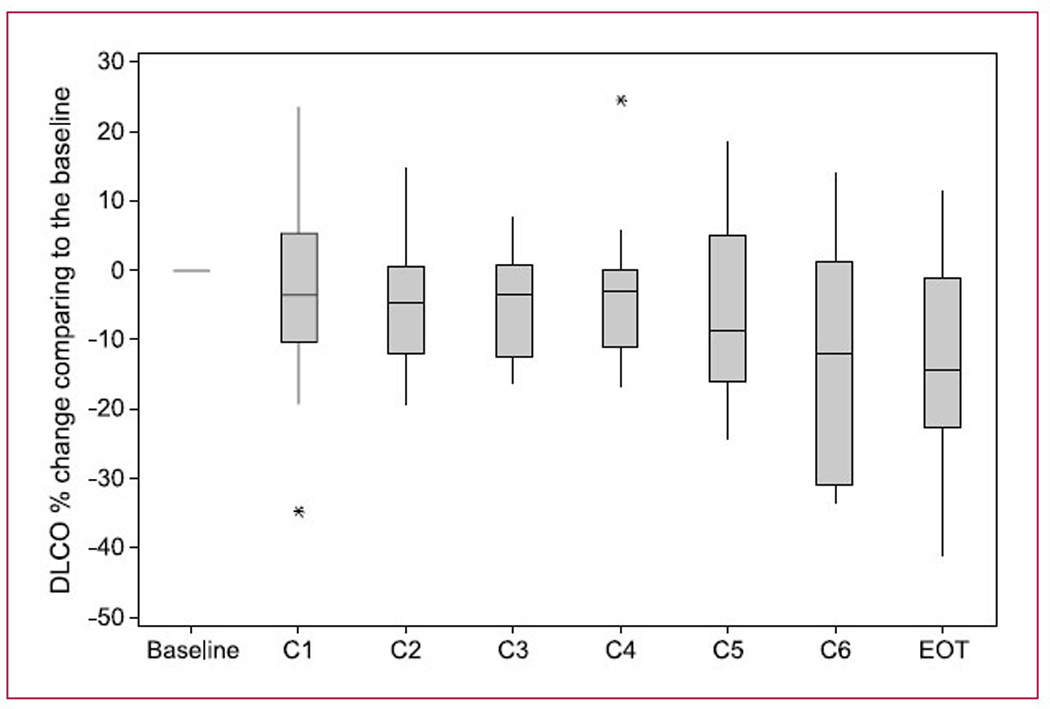
DLCO changes with cycle of treatment. This figure shows the measured DLCO changes (mean) from each patient during each cycle of treatment and at end of treatment (EOT). Asterisks, single outlier measurements.
Among the 24 evaluable patients treated at the 6.0 mg/m2 dose level, 7 patients (6 partial responses and 1 complete response), or 29% (95% confidence interval, 12.6–51.1%), responded to the therapy as assigned by the investigators. For the 17 nonresponders, 13 (54%) patients had stable disease for up to eight cycles of treatment and 4 patients had progressive disease. Eight patients had reported <30% shrinkage of their tumor as shown by the waterfall plot in Fig. 2. It was short of the expectation of having an overall response >35% (9 of 25) that would warrant further evaluation.
Fig. 2.
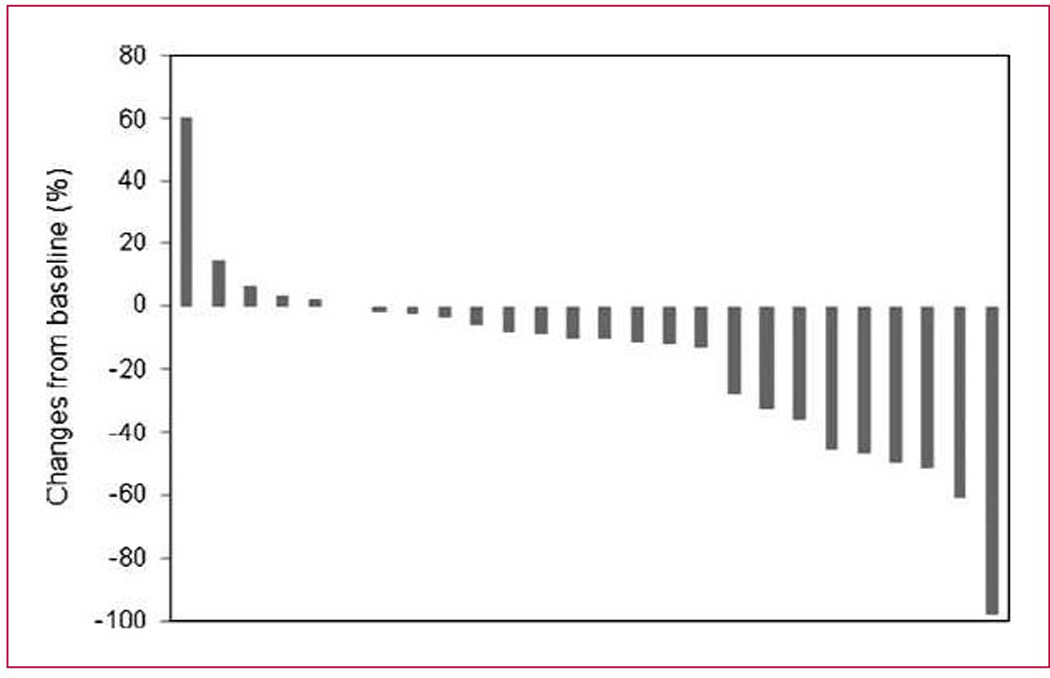
Waterfall plot of tumor size. This figure plots the percentage change from baseline (using Response Evaluation Criteria in Solid Tumors measurements) of the best response seen in treated patients.
We hypothesized that local therapy (i.e., inhaled doxo-rubicin) would have a preferentially favorable effect on intrathoracic metastatic disease, and we therefore performed a waterfall analysis of both intrathoracic and extrathoracic metastases as shown in Fig. 3A and B, respectively. As the presence of at least one measurable lesion within the lung (intrathoracic) was part of the entry criteria, there were more patients with intrathoracic disease than extrathoracic disease, but clearly there is no meaningful difference between the responses seen within the lungs versus those outside of the lungs. Overall survival was assessed at both dose levels (Fig. 4; Table 4) and was quite good in both arms: median overall survival of 433 days at dose level 1 and 584 days at dose level 2.
Fig. 3.
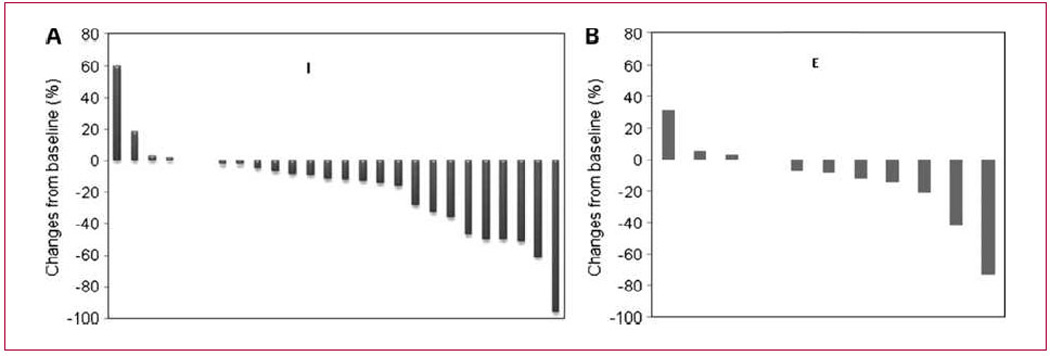
A, waterfall plot of intrathoracic tumor measurements. B, waterfall plot of measurable extrathoracic tumors. All patients were required to have measurable disease within the lungs and/or mediastinum, explaining why there were a greater number of patients with intrathoracic measurable disease.
Fig. 4.
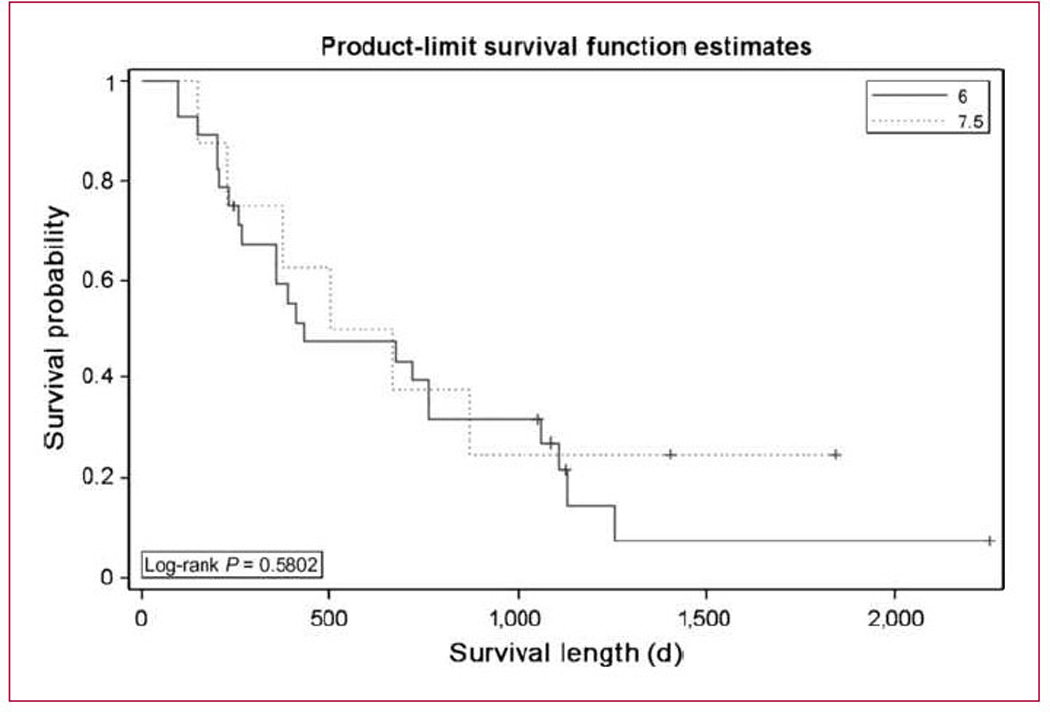
Overall survival. Kaplan-Meier estimates of overall survival for patients at the 6 mg/m2 dose level of inhaled doxorubicin (n = 28) and the 7.5 mg/m2 dose level (n = 8).
Table 4.
Survival data
| No. of subjects | Event | Censored | Median survival (95% CI), d | |
|---|---|---|---|---|
| 6 | 28 | 79% (22) | 21% (6) | 433 (264–762) |
| 7.5 | 8 | 75% (6) | 25% (2) | 584 (227-NA) |
NA, not applicable.
Discussion
Improving the results for the treatment of advanced/ metastatic lung cancer has proved difficult over the last 30 years. It was only in the mid-1990s that a meta-analysis of 52 trials showed a small but significant improvement of cisplatin-based chemotherapy against best supportive care for patients with metastatic disease (20). Subsequently, small improvements were seen with the addition of a second drug to cisplatin (21, 22) and, most recently, with the addition of monoclonal antibodies to vascular endothelial growth factor and epidermal growth factor receptor (3, 4). These advances have been able to improve median overall survival from approximately 6months (best supportive care) to 8 months (cisplatin) to 10 months (cisplatin-based doublet) to approximately 12 months (doublet plus antibody). With that said, however, there have been many more negative trials in the last 15 years, including the addition of multiple novel agents including molecularly targeted agents.
Recent data have supported a more nuanced view of the treatment of NSCLC such that histologic diagnosis over and above the simple diagnosis of a NSCLC is important for the choice of therapeutic agents. Scagliotti and colleagues (5) recently showed that cisplatin and pemetrexed was superior to cisplatin and gemcitabine in adenocarcinoma and large cell carcinoma histologic subtypes. Conversely, gemcitabine and cisplatin was superior to pemetrexed and cisplatin in squamous histology. This has also been shown in pemetrexed treatment in second-line treatment (23). Our study was designed and carried out before these data became available and, unfortunately, is not powered to show any difference in response rates or toxicity depending on histologic subtype. We, furthermore, saw no “trends” in either response or toxicity according to histology.
Localized therapy (other than surgery or radiotherapy) has been shown to benefit patients in a number of different settings including noninvasive bladder cancer (24) and ovarian cancer (25). Other localized therapies have shown benefit in small single institutional settings, but there has been relatively little activity in the use of inhalational compounds. Inhalational therapy has the potential benefit of delivering agents to the lungs with diminished systemic side effects (26, 27). This study was prompted by our earlier phase I study of inhaled doxorubicin in the treatment of patients with tumors metastatic to the lungs.
Doxorubicin as a single agent is active against many tumor types. It is active in vitro against NSCLC cell lines (28) but has not seen widespread utilization in patients given the cardiac toxicity associated with doxorubicin in patients who have other risk factors for cardiovascular disease. Initial preclinical efficacy studies with inhaled doxorubicin have shown that inhaled doxorubicin is superior to i.v. doxorubicin for the treatment of early-stage urethane-induced lung tumors in mice (29). Inhaled doxorubicin was also found to prolong survival when used as an adjuvant in dogs following resection of primary lung tumors (30). Our phase I study of inhaled doxorubicin in patients with advanced pulmonary tumors, primary or metastatic, revealed no drug-related systemic toxicity at doses ≤7.5 mg/m2 (18). With these and the above-mentioned animal data, we designed this study to examine the ability of inhaled doxorubicin to enhance response (and control) in patients with metastatic NSCLC. The rationale for combining the specific inhalational drug used and the i.v. agents used included the distinct mechanisms of action of the three cytotoxic agents and the nonoverlapping toxicity of the inhalational drug when combined with docetaxel and cisplatin. Whereas other cytotoxic and noncytotoxic agents have been tested in inhalational approaches, these (e.g., cisplatin and camptothecin; refs. 16, 17) were not available to us at the time this study was initiated.
Although we were able to show that it was safe to administer inhaled doxorubicin with systemic doses of i.v. docetaxel and cisplatin, we were not able to show a significant improvement in response rate even when we only looked at intrathoracic tumors. This, in addition to several logistic and safety challenges, makes inhalation of doxorubicin problematic. As noted above, 7 of the 43 patients who were registered onto the study never received treatment, and three of these were due to “failure” of the Tc99m testing. Generally, this meant that there was unequal distribution of the test particle to the lungs (with the concern that if >70% of the drug was delivered to one lung, this would cause a relative “overdosing” of that lung). Furthermore, although we did not maintain a log of screen failures, anecdotally, a significant number of patients with advanced lung cancer (many smokers or ex-smokers) were unable to meet the PFT requirements for entry into this study. Although there are no definitive data to prove this, it is conceivable that patients with excellent PFT parameters will have a better prognosis. Finally, although this study was “safe,” there were a significant number of patients (at least seven—two at dose level 2 during the first cycle and an additional five “late” cycle patients at dose level 1) that had >20% decrement in their PFT parameters, with most not having any symptoms associated with this decline and at least one requiring a short course of steroids for symptomatic dyspnea. For these reasons, we chose a stringent target for response to recommend this treatment for further study.
Despite the above-mentioned problems, the survival of the 34 patients treated at both dose levels in this trial was quite robust. Our median survival was 433 days for patients treated at dose level 1 (this compares quite favorably with the 7.4 months seen in the ECOG 1594 study or even with the 11.3 months seen in the TAX326 study; refs. 2, 19). At least three potential explanations can be given for this: (a) Given the small number of patients treated (36 at both dose levels, and 28 at the recommended phase II dose), there could have been unintentional selection bias. (b) As discussed above, the PFT criteria could have introduced a better prognostic group of patients that would do better regardless of the treatment administered. (c) The addition of inhalational doxorubicin to a standard cisplatin-based doublet has significant therapeutic benefit. Clearly, the only way to distinguish between these three possibilities would be through a large phase III trial randomizing patients with similar PFT characteristics to the investigational arm including the inhaled agent against standard doublet chemotherapy. Whereas inhalational doxorubicin is not moving forward, it may be that alternative agents (including novel and targeted agents) may prove to have a role in oncologic therapeutics.
Translational Relevance.
This article has translational relevance in that it attempts to address a common problem for patients with lung cancer, that of uncontrolled pulmonary metastatic disease, with a novel anatomically directed therapy, inhalational chemotherapy. The development of this strategy is described showing initial promise in animal studies, in companion dog studies, and in an earlier Clinical Cancer Research publication of inhaled doxorubicin as a single agent.
Acknowledgments
We thank the patients, staff, pharmacists, and nurses who participated in this study. John Murren, who was involved with the development of this study and our prior phase I study, died during accrual.
Grant Support
Ohio Department of Development grant ODOD AGMT TECH 04-049.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 5.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 6.Markman M. An update on the use of intraperitoneal chemotherapy in the management of ovarian cancer. Cancer J. 2009;15:105–109. doi: 10.1097/PPO.0b013e31819e31f2. [DOI] [PubMed] [Google Scholar]
- 7.Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–1955. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 9.Royal RE, Pingpank JF., Jr. Diagnosis and management of peritoneal carcinomatosis arising from adenocarcinoma of the colon and rectum. Semin Oncol. 2008;35:183–191. doi: 10.1053/j.seminoncol.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Shevchenko IT, Resnik GE. Inhalation of chemical substances and oxygen in radiotherapy of bronchial cancer. Neoplasma. 1968;15:419–426. [PubMed] [Google Scholar]
- 11.Tatsumura T, Koyama S, Tsujimoto M, Kitagawa M, Kagamimori S. Further study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: fundamental and clinical. Br J Cancer. 1993;68:1146–1149. doi: 10.1038/bjc.1993.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huland E, Heinzer H, Huland H. Inhaled interleukin-2 in combination with low-dose systemic interleukin-2 and IFN-α in patients with pulmonary metastatic renal-cell carcinoma: effectiveness and toxicity of mainly local treatment. J Cancer Res Clin Oncol. 1994;120:221–228. doi: 10.1007/BF01372560. [DOI] [PubMed] [Google Scholar]
- 13.Huland E, Burger A, Fleischer J, et al. Efficacy and safety of inhaled recombinant interleukin-2 in high-risk renal cell cancer patients compared with systemic interleukin-2: an outcome study. Folia Biol (Praha) 2003;49:183–190. [PubMed] [Google Scholar]
- 14.Enk AH, Nashan D, Rubben A, Knop J. High dose inhalation interleukin-2 therapy for lung metastases in patients with malignant melanoma. Cancer. 2000;88:2042–2046. doi: 10.1002/(sici)1097-0142(20000501)88:9<2042::aid-cncr9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Markovic SN, Suman VJ, Nevala WK, et al. A dose-escalation study of aerosolized sargramostim in the treatment of metastatic melanoma: an NCCTG Study. Am J Clin Oncol. 2008;31:573–579. doi: 10.1097/COC.0b013e318173a536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wittgen BP, Kunst PW, van der Born K, et al. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin Cancer Res. 2007;13:2414–2421. doi: 10.1158/1078-0432.CCR-06-1480. [DOI] [PubMed] [Google Scholar]
- 17.Verschraegen CF, Gilbert BE, Loyer E, et al. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(S)-camptothecin in patients with advanced pulmonary malignancies. Clin Cancer Res. 2004;10:2319–2326. doi: 10.1158/1078-0432.ccr-0929-3. [DOI] [PubMed] [Google Scholar]
- 18.Otterson GA, Villalona-Calero MA, Sharma S, et al. Phase I study of inhaled doxorubicin for patients with metastatic tumors to the lungs. Clin Cancer Res. 2007;13:1246–1252. doi: 10.1158/1078-0432.CCR-06-1096. [DOI] [PubMed] [Google Scholar]
- 19.Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003;21:3016–3024. doi: 10.1200/JCO.2003.12.046. Epub 2003 Jul 1. [DOI] [PubMed] [Google Scholar]
- 20.Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials [see comments] BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 21.Sandler AB, Nemunaitis J, Denham C, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2000;18:122–130. doi: 10.1200/JCO.2000.18.1.122. [DOI] [PubMed] [Google Scholar]
- 22.Wozniak AJ, Crowley JJ, Balcerzak SP, et al. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group study [see comments] J Clin Oncol. 1998;16:2459–2465. doi: 10.1200/JCO.1998.16.7.2459. [DOI] [PubMed] [Google Scholar]
- 23.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist. 2009;14:253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 24.Malmstrom PU. Advances in intravesical therapy of urinary bladder cancer. Expert Rev Anticancer Ther. 2004;4:1057–1067. doi: 10.1586/14737140.4.6.1057. [DOI] [PubMed] [Google Scholar]
- 25.Jaaback K, Johnson N. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2006:CD005340. doi: 10.1002/14651858.CD005340.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Shim C, Williams MH., Jr. Bronchial response to oral versus aerosol metaproterenol in asthma. Ann Intern Med. 1980;93:428–431. doi: 10.7326/0003-4819-93-3-428. [DOI] [PubMed] [Google Scholar]
- 27.Donahue JG, Weiss ST, Livingston JM, Goetsch MA, Greineder DK, Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA. 1997;277:887–891. [PubMed] [Google Scholar]
- 28.Ruckdeschel JC, Modi SP, el-Hamouly W, Portuese E, Archer S. N-Methylcarbamate derivatives of ellipticine and olivacine with cytotoxic activity against four human lung cancer cell lines. J Med Chem. 1992;35:4854–4857. doi: 10.1021/jm00104a011. [DOI] [PubMed] [Google Scholar]
- 29.Imondi AR, Scovell L, Placke ME, Kresty L, Stoner G. Antitumor activity of inhaled doxorubicin (DOX-IH) in mice with urathane (U)-induced lung tumors. Philadelphia (PA): Proceedings of the American Association for Cancer Research; 1999. [Google Scholar]
- 30.Hershey AE, Kurzman ID, Forrest LJ, et al. Inhalation chemotherapy for macroscopic primary or metastatic lung tumors: proof of principle using dogs with spontaneously occurring tumors as a model. Clin Cancer Res. 1999;5:2653–2659. [PubMed] [Google Scholar]


