Abstract
Preclinical HIV prevention models use either a single high-dose viral challenge in depot medroxyprogesterone acetate (DMPA)-treated macaques or repeated viral challenges in cycling macaques. We tested the efficacy of an intravaginal tenofovir disoproxil fumarate (TDF) ring in a model combining repeated 30 mg injections of DMPA every 6 weeks with vaginal viral challenges weekly for 12 weeks. Twelve macaques were randomized to TDF or placebo rings. All placebo macaques became infected after a median of 2 exposures, whereas only one TDF macaque became infected at the eighth exposure (p=0.0012). The TDF ring provides durable protection in a stringent challenge model.
Keywords: Macaque, intravaginal ring, TDF, Depo-Provera, PrEP
Introduction
Pre-exposure prophylaxis (PrEP) has become one of the frontline strategies to reduce HIV acquisition and transmission. Coitally-dependent topical PrEP with a 1% tenofovir (TFV) gel provided modest protection in one human study, but difficulties with adherence limit the effectiveness of gel formulations1. Intravaginal rings (IVR) have the potential to overcome some adherence hurdles and, by providing sustained drug delivery, may prove more effective compared to vaginal gels2. The concentrations of drug that must be delivered to protect against HIV are not known and may be affected by hormonal contraception, which could modulate drug pharmacokinetics (PK) and pharmacodynamics (PD) in ways that have yet to be fully delineated.
Epidemiological studies suggest that the use of injectable depot medroxyprogesterone (DMPA) may increase the risk of HIV acquisition3, 4. The underlying mechanisms are not yet established, although in vitro and animal studies indicate that DMPA may have pleiotropic effects including thinning of the epithelial barrier, alterations in the vaginal microbiome, and immunomodulatory effects5. Concerns about DMPA have fostered the design of multipurpose IVRs to deliver antiretroviral drugs in combination with levonorgestrol or other hormonal contraceptives6, 7.
The nonhuman primate model provides the opportunity to evaluate the potential safety and efficacy of oral and topical PrEP as illustrated by results with oral Truvada, TFV gel, and a polyurethane reservoir IVR delivering the prodrug, tenofovir disoproxil fumarate (TDF)8, 9. The vaginal exposure models include a single high dose (500-10,000 TCID50) SIV or SHIV challenge in DMPA-treated (single 30 mg injection) rhesus macaques, and a lower dose (50 TCID50) multiple SHIV challenge model in cycling pigtail macaques10. We hypothesized that combining DMPA treatment with repeat viral challenges may provide insights into how DMPA might impact TDF PK and PrEP efficacy. We elected to treat pigtail macaques with 30 mg DMPA every 6 weeks, a dose that is approximately 10 fold higher in mg/kg than the human dose, which results in marked thinning of the epithelium5 and compared efficacy of the TDF IVR to a placebo IVR in DMPA-treated pigtail macaques challenged with SHIV weekly for 12 weeks. We also compared the findings to results obtained in a previous study in which the TDF ring completely protected 6 cycling macaques from 16 weekly challenges with SHIV9.
Methods
Pharmacokinetics and efficacy
Twelve sexually mature female pigtailed macaques received intramuscular injections of 30mg DMPA every 6 weeks starting 3 weeks prior to insertion of a TDF (T1-T6, N=6) or a placebo IVR (P1-P6, N=6); details on ring design were previously described9. DMPA doses ranged from 3.8 to 5.8 mg/kg. The first of 12 weekly vaginal exposures to SHIV162p3 (50 TCID50) was administered one week after insertion of the IVR. The final IVR was removed at week 14. IVRs were replaced every 4 weeks, three days after virus exposure. Macaques were monitored weekly for infection by RT-PCR with a lower limit of detection of 50 copies/mL of plasma9. Resistance mutations of SHIV162p3 in plasma were assessed by PCR with a mean limit of detection for the K65R mutation of 0.4%11.
Vaginal secretions were collected at each IVR exchange and during the follow up period with Ultracell surgical sponges (3.5×4mm). TFV content was quantified with a lower limit of detection of 5 ng/mL9. Residual TDF in used rings and plasma progesterone levels were assayed as previously described12, 13. All macaques were housed at the Centers for Disease Control and Prevention under the Guide for the Care and Use of Laboratory Animals (National Research Council of the National Academies, 2010).
Statistical analysis
SHIV infection-free survival curves for the TDF + DMPA, DMPA placebo, historical TDF IVR cycling and historical cycling no ring groups were performed using Kaplan-Meier plots. Each treatment group was compared to its own control group using a log-rank test. Wilcoxon rank-sum tests with Bonferroni adjustment for multiple comparisons were employed to compare TFV levels in vaginal secretions between DMPA and cycling macaques. Student t-tests with Bonferroni adjustment for multiple comparisons were used to compare residual drug levels in used rings between DMPA and cycling macaques. Statistical analyses were performed with GraphPad Prism Version 6 (GraphPad Software, La Jolla, CA) and SAS Version 9.3 (SAS Institute Inc, Cary, NC).
Results
Pharmacokinetics of progesterone, MPA and TFV
To determine if DMPA fully suppressed progesterone production, plasma progesterone was measured weekly beginning 6 weeks prior to first DMPA dose and ending at the week of the last virus exposure (Figure 1A). The plasma progesterone levels fell below 400pg/mL within 2 weeks of the first DMPA dose and remained low throughout the experimental period (Figure 1B). One animal (T3) had a single spike in plasma progesterone at week 10 and a second animal (T6) continued to show plasma progesterone peaks similar to cycling animals, despite having detectable MPA levels after each DMPA injection (Supplemental Table 1).
Figure 1. Study design and progesterone monitoring.
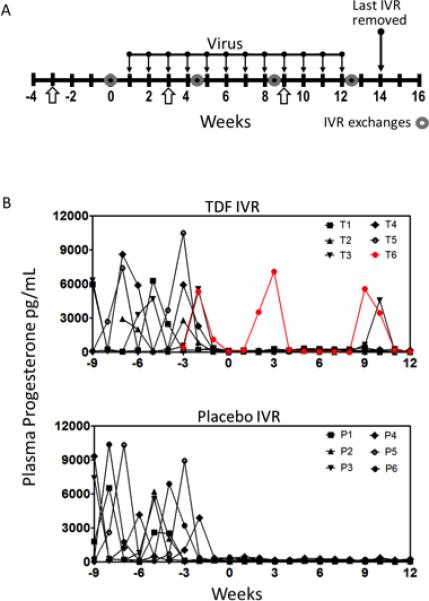
A) Twelve sexually mature pigtailed macaques were enrolled in the study (n=6 TDF IVR, n=6 Placebo IVR) and received 30mg DMPA injections at weeks −3, 3 and 9. IVRs were inserted at week 0 and exchanged at weeks 4.5, 8.5 and 12.5. The last IVR was removed at week 14. B) Progesterone levels for individual macaques confirmed that all animals were cycling prior to the DMPA injections, and progesterone levels were suppressed after DMPA administration. After each DMPA injection, MPA was detected in plasma for all animals. Two animals (T3 at week 10, and T6 at weeks 2, 3, 9 and 10) had high levels of progesterone after DMPA injections. T, TDF animals; P, Placebo animals.
Vaginal secretions were collected for TFV analysis in the TDF ring treated animals at each IVR exchange and at weeks 13, 14 and 15, and were compared to those obtained in the previous study in cycling macaques9. Median TFV levels in both proximal (circles) and distal (triangle) vaginal secretions were greater than 1×105 ng/mL throughout the 12 week exposure period and persisted at week 15, one week after removal of the last IVR (Figure 2A). Proximal TFV drug levels in macaques that received DMPA were significantly higher compared to cycling macaques at 4½ weeks (Wilcoxon rank-sum, Bonferroni adjustment p=0.012), at 8½ weeks (p = 0.007), and at 12½ weeks (p= 0.007); distal TFV levels were also significantly higher at 4½ weeks in DMPA-treated compared to cycling animals (p=0.012). Similarly, the average daily release rate of TDF from the rings removed at 4½ weeks was significantly higher in the DMPA-treated compared to the cycling macaques (t-test, Bonferroni adjustment, p < 0.001) (Figure 2B)9. Notably, plasma TFV levels, which were measured weekly, were below the limit of detection (5ng/mL) in all animals.
Figure 2. Tenofovir levels remain high throughout the study and protect macaques from SHIV acquisition.
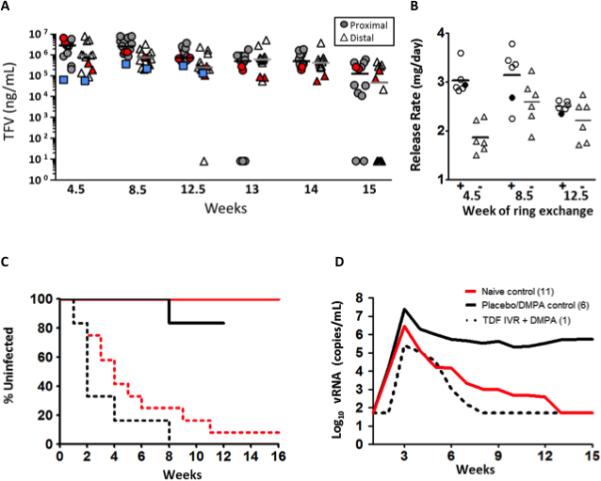
A) Tenofovir levels were measured from vaginal secretions collected proximal (circles) and distal (triangle) to the ring at the time of ring exchanges (weeks 4.5, 8.5 and 12.5) and during the follow up phase. The last sample collected was one week after removal of the last ring. Each symbol represents a single animal and the median values are indicated by bars. Blue squares are median values from the previous study in cycling macaques. Red symbols are levels obtained for T6. B) Average release rate of TDF at each ring exchange from DMPA-treated (+) and cycling (-) macaques. Residual TDF was measured from rings after removal at weeks 4.5, 8.5 and 12.5. Release rate was calculated by dividing the amount of TDF released by 28 days. Filled circles depict the breakthrough animal T6. Horizontal bars are the average for the six animals. C) Kaplan-Meier plot for the current TDF/DMPA study (black lines) and the previous study of cycling macaques (red lines). P-values for survival and median time to infection were calculated using a log-rank test. Each treated group was compared to its own control animals. There was no statistical difference in median time to infection between the two control groups. D) Median viral load of all infected animals. Peak viral load was aligned for comparison of controls from both studies and the single breakthrough animal, T6. Blunted viremia was observed in the breakthrough animal that retained its TDF ring after infection.
Susceptibility of DMPA-treated TDF and placebo IVR macaques to repeated challenges
All placebo IVR macaques became infected with a median of 2 exposures compared to median of 4 exposures for cycling animals (Figure 2C). In contrast, only 1 of 6 DMPA TDF IVR treated macaques became infected (T6 at week 8) (83% protection, log rank test, p=0.0012). Animals were monitored for SHIV by PCR through week 21 and no additional animals became infected. There was a trend towards higher plasma viral loads in the placebo IVR DMPA-treated macaques compared to historic cycling control animals (Figure 2D and Figure S1)) that was not statistically significant (log rank test, p=0.301). Interestingly, peak viral load in the one breakthrough animal, T6, was ~100-fold lower than the median viral load of DMPA, placebo IVR treated macaques (2.6×105 copies/ml vs. 2.4×107 copies/ml). Furthermore, T6 had undetectable plasma virus beginning 5 weeks after peak viremia whereas the placebo + DMPA animals continued to have detectable plasma virus 12 weeks after peak viremia (median, 3.4×105 copies/ml). TDF-associated resistance (K65R mutation) was not detected in the TDF IVR infected animal at any time where plasma virus was detected11.
Discussion
The current study tested the efficacy of a TDF IVR in a model that combined multiple high-dose injections of DMPA with weekly exposures to virus. We hypothesized that DMPA treatment would increase the susceptibility to SHIV by inducing an exaggerated and sustained luteal phase. Prior studies indicate that cycling animals are more susceptible in the luteal phase of the menstrual cycle and that the window of susceptibility in cycling macaques encompasses approximately one half of the menstrual cycle13. In this study, DMPA-treated macaques in the placebo arm became infected after a median of 2 challenges compared to 4 challenges in historical control cycling animals. This trend is consistent with previous studies reporting an increased risk of acquisition of SIV with DMPA. Notably, the dose and intervals of DMPA administration were not designed to simulate the hormone levels or vaginal histology of women using DMPA for contraception, but rather to provide a “worst-case” scenario by maintaining a reduced vaginal epithelial layer throughout the exposure period5. The small group sizes in this study did not offer sufficient power to statistically evaluate the role of DMPA in differences observed in time to infection, peak viral load, or viral clearance from the plasma.
Although no IVR has yet proven effective against HIV acquisition in women, several have provided varying degrees of protection in nonhuman primates with PK and efficacy studies informing IVR design2,9, 14. For example, results from PK studies prompted the advancement of the current TDF reservoir ring rather than a matrix IVR design11.. The reservoir TDF ring provided complete protection in cycling macaques against 16 weekly virus exposures9 and protected 5 of 6 DMPA-treated macaques from 12 weekly challenges in the current study. The addition of DMPA to the repeat challenge model may provide a highly stringent model of the efficacy of drug delivery systems if infections consistently occur earlier in the control animals as observed here.
The TDF IVR produced high TFV levels in macaque vaginal fluids. We focused on measuring TFV rather than TDF levels because luminal TDF is hydrolytically converted into TFV and thus less variable than TDF levels9. Importantly, the TFV levels exceeded those in the CAPRISA 004 study (1,000 ng/ml) that correlated with protection in women receiving 1% TFV gel15. Similar TFV levels were observed proximal and distal to the ring indicating well-dispersed tissue coverage with sustained delivery. Notably, high levels of TFV persisted one week after ring removal (week 15), suggesting that women may be protected for several days after ring removal. The TFV levels in vaginal secretions of DMPA-treated animals were significantly higher at several time points in this study compared to those observed in the cycling animals, which may reflect either greater drug release and/or thinning of the epithelial barrier allowing drug to more easily penetrate the tissues and more readily return to the vaginal vault. However, because we did not obtain biopsies and thus did not measure intracellular TFV-diphosphate levels, we cannot exclude the possibility that differences in drug levels reflect differences in drug metabolism.
The one TDF animal that became infected at the eighth exposure cannot be explained with the data available. The TFV levels in vaginal secretions in this animal were within the range of those in protected animals. However, this macaque did have an unusual pharmacological response to the DMPA injections; plasma MPA levels were within range of other DMPA-treated animals and consistent with levels that have been previously shown to suppress progesterone production5, yet T6 continued to produce progesterone (Figure 1). This aberrant response might be expected to decrease the risk of SHIV if the vaginal epithelium fluctuated in thickness with the changes in progesterone levels. Unfortunately, we were unable to sample the vaginal epithelium during the exposure period. It is also possible that lower than expected intracellular TFV-diphosphate levels or increased numbers of activated immune cells contributed to the lack of protection in this one animal.
More intensive PK studies in DMPA-treated animals are planned to assess the impact of sustained DMPA treatment on vaginal epithelium, mucosal immune cell populations and levels of TFV-diphosphate in lymphocytes from cervicovaginal tissue and lymph nodes. These studies should include a range of DMPA dosing and intervals as the current regimen was not designed to simulate the hormone levels or vaginal histology of women using DMPA for hormonal contraception, but rather to provide a “worst-case” scenario by maintaining a reduced vaginal epithelial layer throughout the exposure period 5.
In summary, we describe a stringent model combining high dose DMPA with repeated viral exposures to evaluate the potential efficacy of a TDF IVR. The predictive value of this and other models requires testing of other PrEP options and validation with clinical studies. The findings further support the clinical advancement of this polyurethane reservoir TDF IVR, which is currently being evaluated in a Phase 1 clinical study (ClinicalTrials.gov Identifier: NCT02006264) and suggest that protective levels of TDF may be observed in cycling women as well as women receiving DMPA hormonal contraception.
Supplementary Material
Acknowledgements
We acknowledge Gilead Sciences for providing the tenofovir disoproxil fumarate. We would like to thank CDC contributions from R. Michael Hendry and Janet M. McNicholl for their support and helpful discussions; David Garber, James Mitchell, Frank Deyounks, Shanon Ellis, and Leecresia Jenkins for all animal procedures; Chou-Pong Pau for expertise and help with the analytical chemistry; and Jeffrey A. Johnson and Jonathan Lipscomb for the resistance testing. We would also like to acknowledge the contributions of Rachna Rastogi for the ring design and helpful discussions. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
This study was supported by U19AI03461. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Centers for Disease Control or NIH.
Footnotes
The authors do not have a commercial or other association that might pose a conflict of interest.
Presented in part: Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, 10 September–13 September, 2013
References
- 1.Gengiah TN, Moosa A, Naidoo A, Mansoor LE. Adherence challenges with drugs for pre-exposure prophylaxis to prevent HIV infection. Int J Clin Pharm. 2014 Feb;36(1):70–85. doi: 10.1007/s11096-013-9861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiser PF, Johnson TJ, Clark JT. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev. 2012 Jan-Mar;14(1):62–77. [PubMed] [Google Scholar]
- 3.Heffron R, Mugo N, Ngure K, et al. Hormonal contraceptive use and risk of HIV-1 disease progression. Aids. 2013 Jan 14;27(2):261–267. doi: 10.1097/QAD.0b013e32835ad473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polis CB, Westreich D, Balkus JE, Heffron R. Assessing the effect of hormonal contraception on HIV acquisition in observational data: challenges and recommended analytic approaches. AIDS. 2013 Oct;27(Suppl 1):S35–43. doi: 10.1097/QAD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radzio J, Hanley K, Mitchell J, et al. Physiologic doses of depot-medroxyprogesterone acetate do not increase acute plasma simian HIV viremia or mucosal virus shedding in pigtail macaques. Aids. 2014 Apr 22; doi: 10.1097/QAD.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 6.Friend DR, Doncel GF. Combining prevention of HIV-1, other sexually transmitted infections and unintended pregnancies: Development of dual-protection technologies. Antiviral Res. 2010 Dec;88(Suppl 1):S47–54. doi: 10.1016/j.antiviral.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Romano J, Manning J, Hemmerling A, McGrory E, Holt BY. Prioritizing multipurpose prevention technology development and investments using a target product profile. Antiviral Res. 2013 Dec;100(Suppl):S32–38. doi: 10.1016/j.antiviral.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Lerma JG, Heneine W. Animal models of antiretroviral prophylaxis for HIV prevention. Curr Opin HIV AIDS. 2012 Nov;7(6):505–513. doi: 10.1097/COH.0b013e328358e484. [DOI] [PubMed] [Google Scholar]
- 9.Smith JM, Rastogi R, Teller RS, et al. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):16145–16150. doi: 10.1073/pnas.1311355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veazey RS. Animal models for microbicide safety and efficacy testing. Curr Opin HIV AIDS. 2013 Jul;8(4):295–303. doi: 10.1097/COH.0b013e328361d096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson JA, Rompay KK, Delwart E, Heneine W. A rapid and sensitive real-time PCR assay for the K65R drug resistance mutation in SIV reverse transcriptase. AIDS Res Hum Retroviruses. 2006 Sep;22(9):912–916. doi: 10.1089/aid.2006.22.912. [DOI] [PubMed] [Google Scholar]
- 12.Mesquita PM, Rastogi R, Segarra TJ, et al. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother. 2012 Jul;67(7):1730–1738. doi: 10.1093/jac/dks097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vishwanathan SA, Guenthner PC, Lin CY, et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr. 2011 Aug 1;57(4):261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 14.Moss JA, Malone AM, Smith TJ, et al. Safety and pharmacokinetics of intravaginal rings delivering tenofovir in pig-tailed macaques. Antimicrob Agents Chemother. 2012 Nov;56(11):5952–5960. doi: 10.1128/AAC.01198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karim SS, Kashuba AD, Werner L, Karim QA. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011 Jul 16;378(9787):279–281. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


