Abstract
Periostin is a modular glycoprotein frequently observed to be a major constituent of the extracellular milieu of mass-forming intrahepatic cholangiocarcinoma and other desmoplastic malignant tumors. In intrahepatic cholangiocarcinoma, as well as in desmoplastic pancreatic ductal adenocarcinoma, periostin is overexpressed and hypersecreted in large part, if not exclusively, by cancer-associated fibroblasts within the tumor stroma. Through its interaction with specific components of the extracellular tumor matrix, particularly collagen type I and tenascin-C, and with cell surface receptors, notably integrins leading to activation of the Akt and FAK signaling pathways, this TGF-β family-inducible matricellular protein appears to be functioning as a key extracellular matrix molecule regulating such critically important and diverse malignant tumor behaviors as tumor fibrogenesis and desmoplasia, invasive malignant cell growth, chemoresistance, and metastatic colonization. This review will discuss current evidence and basic molecular mechanisms implicating periostin as a mediator of intrahepatic cholangiocarcinoma invasive growth. In addition, its significance as a potential prognostic biomarker for intrahepatic cholangiocarcinoma patients, as well as future possibilities and challenges as a molecular target for cholangiocarcinoma therapy and/or prevention, will be critically evaluated.
Keywords: periostin, cancer-associated fibroblasts, tumor desmoplasia, cholangiocarcinoma cell migration and invasion, metastasis, Akt, integrins
Intrahepatic cholangiocarcinoma (ICC) is a highly lethal primary epithelial cancer of the hepatobiliary tract that exhibits characteristics of cholangiocyte differentiation. It represents the second most common primary hepatic cancer after hepatocellular carcinoma, accounting for about 10% of all primary liver malignant neoplasms and 3% of gastrointestinal cancers. Although considered to be an orphan cancer, ICC is increasingly being recognized worldwide as a cancer of rising clinical importance and certainly one which continues to present significant biological and therapeutic challenges. This increased interest is largely due to the increasing incidence of ICC in many Western countries, its often insidious development and late onset of symptoms, high recurrence rates after surgical resection, limited treatment options for the vast majority of patients who present with advanced metastatic disease, and continuingly high mortality rates [1–3].
Unlike conventional hepatocellular carcinoma, ICC is frequently characterized by a prominent desmoplastic stroma, which has long been recognized as a hallmark histological feature. Although the biological significance and clinical implications of the desmoplastic reaction in ICC are only just beginning to be addressed at the cellular and molecular levels, it is becoming increasingly evident that this complex and evolving tumor stromal reaction may be playing a crucial role in promoting ICC progression [4,5]. The desmoplastic reaction in ICC is notably marked by the accumulation of cancer-associated fibroblasts (CAFs) positive for α-smooth muscle actin (α-SMA), which are associated with concomitant increases within the tumor microenvironment of secreted structural and non-structural matrix proteins, proinvasive growth factors and cytokines, matrix modifying enzymes, and angiogenesis regulatory factors interacting in various ways to modulate malignant cell proliferation, invasion and metastasis, apoptosis resistance, and/or epithelial-mesenchymal transition (EMT) [4, 5].
Included among the non-structural protein constituents of the tumor microenvironment having potential clinicopathological relevance as potentially important modulators of ICC progression and metastasis is the matricellular protein periostin (Postn). This review will critically discuss the current and mounting evidence implicating Postn in ICC invasive growth and metastasis, as well as its potential as a prognostic biomarker in patients with resected ICC. In addition, data derived from recent biological and molecular studies of ICC and other gastrointestinal tract cancers will be described in an effort to provide mechanistic insight as to how Postn may be functioning to modulate malignant ICC cancer cell invasiveness and metastatic potential. Last, the possibility of Postn to serve as a novel molecular target for metastatic ICC therapy will be addressed.
Modular Structural-Functional Domains
Postn, a disulfide-linked extracellular matrix (ECM) glycoprotein with a molecular weight of approximately 90 kDA, was first identified in 1993 by Takeshita et al. [6] in a screen of the cDNA library from the MC3T3-E1 mouse osteoblastic cell line. Originally termed osteoblast specific factor-2 (OSF-2), this secreted ECM-associated factor was renamed Postn by Horiuchi et al. [7], based as reported on its high level of localized expression in the periosteum and periodontal ligament of adult mice. The designation of Postn over OSF-2 is now the preferred term for this connective tissue protein, since it prevents it from being confused with Osf2 (Osf2/Cbfa1; Runx2), a member of the Runt-related family of transcription factors that has been identified as having a key functional role in skeletogenesis [8] and more recently linked to metastatic tumor and cancer cell interactions with bone [9].
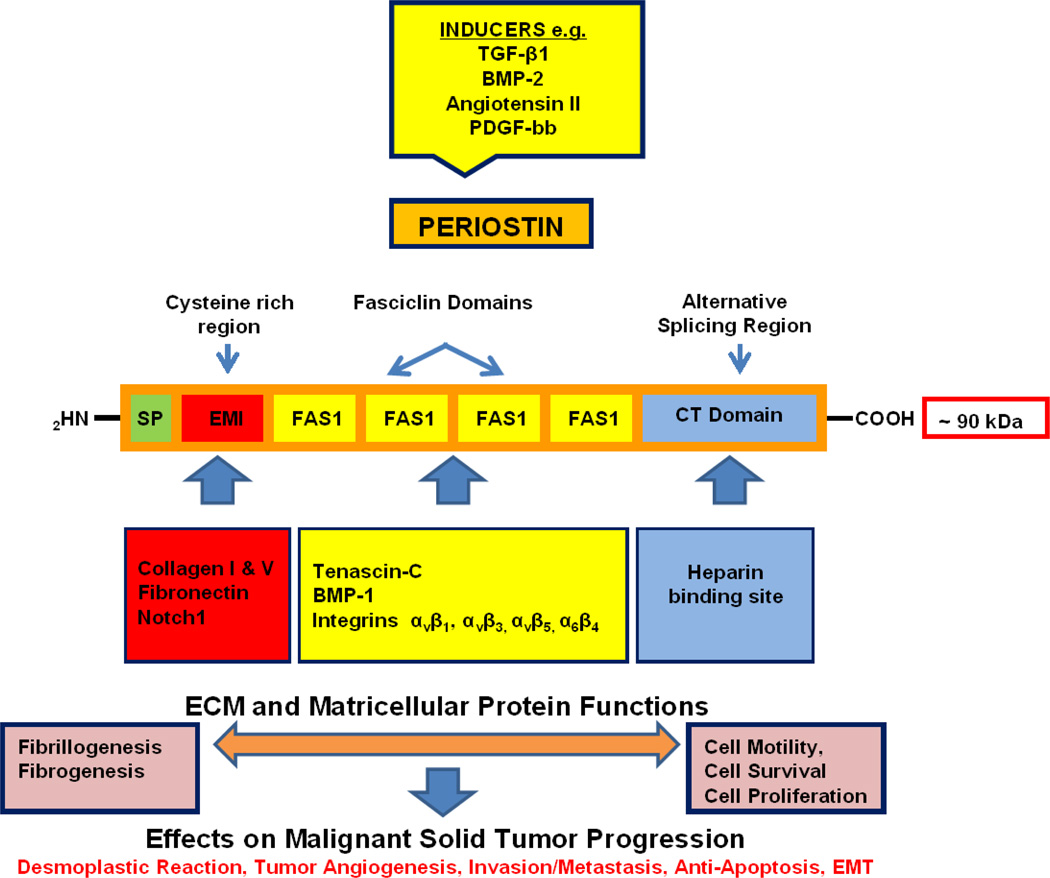
As structurally depicted in Figure 1, Postn is a modular glycoprotein [6, 7, 10, 11] comprised of a typical NH2 - terminal secretory signal sequence, but lacking in a transmembrane domain, that is adjacent to a cysteine-rich EMI domain (in reference to its presence in proteins of the EMILIN family). The EMI domain is followed by tandem repeats of four FAS1 domains homologous to the insect adhesion protein fasciclin 1, functionally linked in Drosophila to axonal growth guidance, migration, and differentiation [10]. Postn is thus classified as being a member of the fasciclin family of proteins, that also includes βig-h3 (TGF-β-induced clone 3), the stabilins, and periostin-like factor [10, 12]. Among the members of this family, Postn and βig-h3 are the most similar [13], each having four uninterrupted repeats of FAS1. Contained within these FAS1-like domains are recognition sites for the vitamin-K-dependent enzyme γ-glutamylcarboxylase, catalyzing within the molecule post-translational modification of glutamic acid to γ-carboxyglutamic acid [14].
Figure 1.
Schematic representation of the modular structure of periostin showing its domain-specific binding sites for extracellular matrix proteins promoting fibrillogenesis and fibrogenesis, as well as cell surface signaling molecules, notably integrins, whose activation by their interaction with this matricellular glycoprotein regulate diverse cellular functions, including cell motility, survival and proliferation. Overexpression of periostin induced by fibrogenic factors that include transforming growth factor-β family members and angiotensin II in cancers such as intrahepatic cholangiocarcinoma and pancreatic ductal adenocarcinoma may be playing a key role in facilitating desmoplasia and creating a tumor-supportive microenvironment that promotes malignant cell invasion, metastatic colonization, and chemotherapeutic resistance. See text for specific details.
In contrast to the NH2 portion of the molecule, which is evolutionarily conserved, the hydrophilic COOH-terminal domain of Postn is structurally variable and has been reported to show increased evolutionary plasticity due to alternative splicing, giving rise to multiple Postn isoforms [10, 15]. Currently, five and possibly up to eight isoforms of Postn have been recognized as a result of alternative splicing in the COOH-terminal domain [15–17]. As proposed by Hoersch and Andrade-Navarro [15], the sequence variation imparted by alternative splicing within the COOH-terminal domain of Postn could be a key determinant of its biological function, and possibly a likely explanation for how Postn has been observed to function either as a promoter [10] or suppressor [18, 19] of cancer cell invasiveness in different carcinoma types.
Figure 1 further illustrates recognition sites on the Postn molecule that permit domain-specific interactions with other ECM proteins, including collagen types I and V, fibronectin, tenascin-C (Tnc), and heparin, as well as with cell surface receptors, notably integrins and Notch1. These modular interactions, in turn, function to regulate or induce cell-specific responses affecting cyto-differentiation, proliferation, survival, and/or motility [10, 11]. In addition, Postn plays a crucial role in collagen fibrillogenesis [13, 20] and in fibrogenesis associated with development and tissue and organ remodeling [13, 20, 21], connective tissue wound repair [20, 22, 23], cardiovascular disease [12, 21, 24] and fibrogenic disorders, including idiopathic pulmonary fibrosis [25], renal fibrosis [26], scleroderma [27], epiretinal fibrous membrane formation in proliferative vitreoretinopathy [28], allergic inflammation [29] and desmoplastic neoplasia [30].
Kudo and his colleagues have shown that Postn interacting through its FAS1 domain with bone morphogenic protein (BMP)-1 enhances the proteolytic activation of lysyl oxidase to promote collagen cross-linking [31]. Postn acting via its EMI domain binding collagen type I and fibronectin and its adjacent FAS1 domain binding Tnc was further shown to function as a molecular bridge to support the incorporation of Tnc into the ECM, thereby acting to form a specific hexabrachion structure that may increase and stabilize bifurcations of collagen type I and fibronectin fibrils integrated into an extracellular meshwork architecture [32]. It should be further noted that heparin sulfate glycosaminoglycans, which bind to the COOH domain of Postn have also been demonstrated to be essential for the deposition of Tnc into the ECM [33].
Postn is a more recently added member of the matricellular protein family, a class of non-structural ECM proteins that also includes Tnc, βig-h3, osteopontin, secreted protein acidic and rich in cysteine (SPARC), thrombospondin-1, as well as others [21]. In terms of its matricellular protein activities, Postn is known to modulate cell-matrix interactions and cell regulatory functions by either binding to cell surface receptors or other ECM proteins to activate specific cell signaling pathways capable of regulating diverse biological processes, such as tissue morphogenesis and remodeling, fibrogenesis, cell motility, angiogenesis, tumor invasiveness and metastasis [10, 11, 16, 20, 21, 34]. Specifically, Postn has been demonstrated to play a crucial role as a pro-fibrogenic molecule during tissue morphogenesis [21], to facilitate myofibroblast differentiation and contraction associated with the profibrotic phase of cutaneous wound repair [22], and to perpetuate fibrogenesis in desmoplastic pancreatic cancer by acting to sustain fibrogenic stellate (myofibroblast) cell activity in the tumor microenvironment [30]. Postn binding to integrins has further been shown to initiate cross-talk between integrins and receptor tyrosine kinases like epidermal growth factor at the plasma membrane to co-activate the serine threonine Akt/PKB (Akt) and focal adhesion kinase (FAK) cell signaling pathways modulating cell motility, proliferation, and/or survival [10,16, 34–37]. Dysregulation of these down-stream signaling events associated with over-expression of Postn in the microenvironment of malignant neoplastic cells has important implications for malignant tumor progression and its role in regulating metastatic potential. In this context, it is particularly notable that Postn and Tnc have been proposed to collaboratively function as key metastatic niche molecules promoting metastatic colonization by enhancing Wnt and Notch signaling in stem-like metastasizing initiating cancer cells [38, 39]. Moreover, Postn has been found to be an inducer of EMT change in various epithelial cancer cell lines [16] and more recently reported to promote a stem cell-like phenotype, as well as a mesenchymal phenotype in human mammary epithelial cells and breast cancer cells [40]. EMT and cancer stem-like cells both factor into malignant cell heterogeneity within tumors and their progression to metastatic cancer.
In ICC, like in pancreatic ductal adenocarcinoma (PDAC), Postn has been largely, if not exclusively localized to the desmoplastic connective tissue stroma [30, 41–45]. Moreover, various methods including real-time-PCR, immunohistochemistry, and Western blotting have demonstrated myofibroblastic CAFs positive for α-SMA to be a major cellular source of Postn secreted into the desmoplastic stroma of human and rat cholangiocarcinoma in liver [43–46].
Although there have been as yet no definitive studies to identify specific regulators of Postn expression in ICC myofibroblastic cells, TGF-β1 and BMP-2, both of which are known inducers of Postn, were shown to be strong stimulants of α-SMA in human pancreatic myofibroblastic (stellate) cells, whereas platelet derived growth factor-BB and fibroblast growth factor-B were found in this same study to be potent secretagogues for Postn [30]. In vitro, hypoxia was also determined to significantly increase α-SMA expression in pancreatic stellate cells together with inducing an increased synthesis and secretion of Postn by these cells [47]. The hypoxia-responsive factor angiotensin II, which has been shown to induce myofibroblastic cell activation in the LI-90 human hepatic stellate cell line [48], has also been reported to increase Postn expression via Ras/p38 MAPK/CREB and Erk1/2/TGF-β1 pathways in cardiac fibroblasts [49]. Here it is of further relevance that angiotensin II has been reported to facilitate the profibrotic activity of TGF-β1 during hepatic fibrogenesis [50], and was shown in vitro to enhance EMT through cross-talk between activated LI-90 myofibroblastic cells and stromal cell-derived factor-1/CXCR4 axis in cultured human ICC cells [51]. Other demonstrated regulators of Postn in fibroblastic cells include interleukin-4 and −13, which are signature cytokines of the type 2 immune response [29].
Aberrant interactive signaling mediated in part by TGF-β family members, angiotensin II, platelet derived growth factors, and chemokines like stromal cell-derived factor-1 are regarded as being important regulators of fibrogenesis, potentially acting to promote the desmoplasia/hypoxia milieu and tumor progression in ICC. In this context, it would now be useful to experimentally assess if such factors as these may be interacting in a cooperative manner to regulate Postn synthesis and secretion by myofibroblastic CAFs in ICC, contributing in turn to a potentially more aggressive malignant phenotype.
Prognostic Significance of Postn in ICC
In recent years, there has been an increasing number of single center studies reporting Postn overexpression in various human gastrointestinal (GI) carcinoma types, including ICC, to be an independent prognostic factor for predicting decreased overall survival rates following tumor resection (Table 1). Postn serum levels were also reported to be significantly elevated in preoperative patients with cholangiocarcinoma compared with those patients with normal liver, liver cirrhosis, hepatocellular carcinoma, and other hepatic malignancies [57]. Similarly, elevated serum Postn levels were detected in patients with pancreatic ductal adenocarcinoma (PDAC) compared to sera from patients with various inflammatory diseases and healthy serum samples [58]. High preoperative levels of serum Postn were also found to be associated with poor prognosis in patients with hepatocellular carcinoma after hepatectomy [59] and elevated levels of preoperative serum Postn levels in colorectal carcinoma were reported to correlate with distant metastases, advanced stage disease, and poor prognosis [60].
Table 1.
Potential Prognostic Value of Postn Overexpression in Human ICC and Other GI Cancers After Surgical Resection
| Study Site |
Tum or Type a |
No Cases Analyzed |
% Tumors with Strong Postn Expression |
Compartment d |
Endpoint e |
Statistics |
Result | Reference |
|---|---|---|---|---|---|---|---|---|
| Switzerland | BDC | 116b | 67.2 33.6 |
S C |
OS | Kaplan-Meier, Multivariate Cox analysis | High C, but not S, Postn expression independent prognostic factor for reduced OS (P=0.033) | [52] |
| Thailand | ICC | 52 | 58 0 |
S C |
OS | Kaplan-Meier, Multivariate Cox analysis | High S Postn expression independent prognostic factor for shorter OS times (P=0.026) | [43] |
| Switzerland | HCC | 91 | 11 20.9 |
S C |
OS | Kaplan-Meier | No significant correlation between Postn expression and OS | [52] |
| P.R. China | HCC | 71 | 73.2c 19.7 |
C PC |
OS DFS |
Kaplan-Meier, Multivariate Cox analysis | High Postn positive expression independent prognostic factor for OS and DFS rates (P=0.001) | [53] |
| Germany | PDAC | 41 | 100 0 |
S C |
OS | Kaplan-Meier | Tumors with increased Postn mRNA levels had a tendency towards shorter median survival times (P=0.14) | [30] |
| P.R. China | PDAC | 94 | 80 30 |
S C |
OS | Kaplan-Meier | Elevated expression of Postn in S as well as in C associated with poorer OS rates (P=0.035 and 0.022, respectively) | [54] |
| P.R. China | ESCC | 68 | 73.5 | C | OS DSF |
Kaplan-Meier, Multivariate Cox analysis | Postn expression independent predictor of poor prognosis for both OS and DFS (P=0.027 and 0.026, respectively) | [55] |
| P.R. China | CRC | 720 | 30.28 | C | OS | Kaplan-Meier, Multivariate Cox analysis | Postn expression independent prognostic factor for post- operative liver metastasis (P=0.001) and poor prognosis for OS (P=0.001) | [56] |
BDC, bile duct carcinomas; ICC, intrahepatic cholangiocarcinoma; HCC, hepatocellular carcinoma; PDAC, pancreatic ductal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; CRC, colorectal adenocarcinoma
Survival data available for 111 patients
Postn expression scored only as positive or negative
S, stromal cell; C, cancer cell; PC, paracarcinomatous tissue
OS, overall survival; DFS, disease free survival
There have been conflicting reports regarding whether elevated expression of Postn localized to tumor stroma versus cancer cell is better suited as a possible biomarker of tumor progression and poorer patient survival outcomes after potentially curative resection for ICC and other biliary tract carcinomas. As summarized in Table 1, the results of Utispan et al. [43] demonstrating resected ICC patients with high levels of Postn (n=29) in stromal fibroblasts to have significantly shorter survival times than those with low Postn levels (n=22) are in sharp contrast to the findings of Riener et al. [52] showing no association between high stromal Postn expression and overall survival for a cohort of patients with various biliary tract carcinomas that included 19 cases of ICC, 59 cases of extrahepatic cholangiocarcinoma, and 38 cases of gallbladder carcinomas. On the other hand, Reiner et al. reported that biliary tract carcinoma patients with strong Postn expression in the tumor epithelia had reduced overall survival, whereas Utispan et al. did not detect evidence of Postn expression in the cancer cells nor immune cells in the 51 cases of ICC analyzed in their survival study.
These seemingly contradictory results could possibly relate in part to the fact that in the Utispan et al. study, all of the cholangiocarcinoma tissues analyzed for Postn expression were from hepatectomized ICC patients, whereas in the Reiner et al. study only 16% of the 116 tumors analyzed were diagnosed as ICC, with the greater majority of tumors in this cohort being comprised of extrahepatic and gallbladder carcinomas.
Since both studies used similar immunohistochemical methods to detect Postn immunoreactivity in the analyzed biliary tumor tissues, it presumes the need for more comprehensive and randomized studies focused on analyzing Postn expression in large multi- rather than single center samplings of ICC rather than in mixed cohorts of biliary tract carcinomas to resolve the prognostic value of stromal versus cancer cell Postn expression for ICC compared with other biliary tract carcinomas. Such an approach would take into account biological differences between intrahepatic and extrahepatic cholangiocarcinoma, as well as gallbladder cancer. A combination of methodologies that include determinations of both Postn mRNA and protein expression levels as part of the study design would also provide a more robust molecular evaluation of the potential prognostic value of stromal versus cancer cell Postn expression in patients with resected ICC. Risk of bias and meta-analyses applied to well designed, randomized multi-center studies, together with adherence to reporting recommendations for tumor marker prognostic studies guidelines would further serve to provide a more definitive assessment and validation of Postn as a prognostic biomarker potentially useful for monitoring ICC recurrence in patients with resected primary tumors [61].
As already noted in the previous section of this review, in the case of both ICC and PDAC, there are very convincing mRNA and protein data to support tumor stromal CAFs as being the primary if not exclusive source of Postn overexpressed in these respective desmoplastic GI cancers [41, 43, 45]. In agreement with the findings of Utispan et al. [43], and using mRNA and protein methodologies, we independently determined CAFs to be the principal if not sole source of overexpressed Postn secreted into to the tumor stromal microenvironment of rat cholangiocarcinomas formed in the livers of syngeneic rats after bile duct inoculation of highly malignant neu-transformed (BDEneu) or of lower grade malignant spontaneously-transformed (BDEsp) rat cholangiocytes [42, 44, 46]. Also, consistent with the findings of Utispan et al. for human ICC, we simultaneously observed in our rat orthotopic model of cholangiocarcinoma that Postn mRNA and protein levels were more highly expressed in high grade malignant BDEneu liver cholangiocarcinoma and associated metastatic tumor tissues than in the less aggressive BDEsp liver cholangiocarcinoma [42, 44, Figure 2]. Thus, our experimental animal findings using both mRNA and protein determinations support the findings of Utispan et al. for human ICC, correlating tumor stromal fibroblastic cell Postn overexpression with increased malignant aggressiveness.
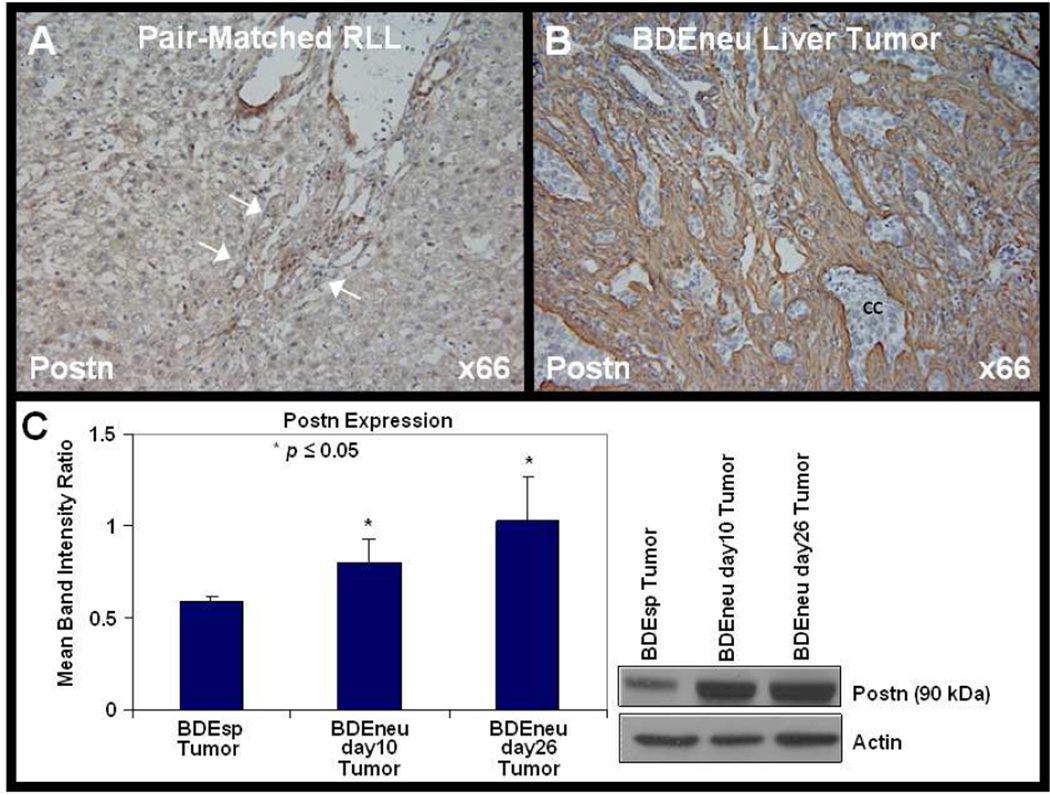
Figure 2.
Periostin (Postn) is exclusively and strongly overexpressed in the desmoplastic stroma in an orthotopic, syngeneic rat model of cholangiocarcinoma. As depicted in the representative photomicrograph in [B], the tumor stroma, but not nests of cancer cells (cc), within a cholangicarcinoma formed in rat liver following bile duct inoculation of highly malignant neu-transformed rat cholangiocytes to be strongly immunoreactive for Postn compared with that in [A] of pair-matched right liver lobe tissue (RLL) without tumor [A]. Note that Postn immunostaining is completely negative in the RLL non-tumorous liver parenchymal tissue and in hyperplastic bile ductules (arrows) related to tumor-associated bile duct obstruction at the liver hilus (80). Quantitative Western blot analysis [C] demonstrating protein levels of Postn to be significantly higher in rat BDEneu liver tumor than in less aggressive, lower grade desmoplastic BDEsp tumor formed in rat liver after bile duct inoculation of spontaneously-transformed rat cholangiocytes. Postn protein expression was not detected by Western blotting in normal rat liver, icteric liver after bile duct ligation, or in pair-matched non-tumorous RLL from a BDEneu tumor-bearing rat, but was detected in peritoneal metastases from BDEneu tumor (42). Significantly higher levels of Postn mRNA were also demonstrated in BDEneu liver tumor tissue over those expressed in BDEsp liver tumor tissue, but not detected in either cultured tumorigenic BDEneu or BDEsp cholangiocytes from which the tumors were respectively derived (44). The immunochemical data were generated using Postn antibody (ab14041) from Abcam Inc., Cambridge, MA.
The prospect of developing elevated serum levels of Postn into a specific serodiagnostic biomarker assay for ICC, as has been suggested [57], seems unlikely since as described above, high levels of circulating Postn have also been detected in patients with various carcinoma types, including hepatocellular carcinoma and other GI cancers (e.g. pancreatic, colorectal) that characteristically metastasize to liver. However, the possibility that serum Postn levels might be used to monitor ICC recurrence after surgical resection or as an indicator of therapeutic response is potentially feasible. More extensive and better controlled random testing will now be needed to validate the clinical usefulness of measuring circulating levels of Postn to help identify ICC recurrence, as well as assess adjuvant treatment outcomes following curative resection.
That Postn has been demonstrated to co-localize with α-SMA in CAFs in the ICC stroma (43, 45, 46) together with the results of recent independent single studies demonstrating high levels of α-SMA expression in the ICCs to also significantly correlate with poor survival outcomes in ICC patients following surgical resection [5, 61–63] provides an additional variable that needs to be factored into study designs aimed at evaluating clinical value of Postn as a prognostic biomarker for ICC. Specifically, do elevated levels of expression of Postn in ICC represent a functional manifestation of accumulating numbers of α-SMA-positive CAFs in the evolution and remodeling of the tumor stroma or may they also reflect an up-regulation of Postn expression in CAFs that is induced by secreted factors such as TGF-β members or angiotensin II into tumor stromal microenvironment. Further mechanistic studies are clearly needed to address this issue.
Postn and ICC Invasiveness
Consistent with its potential as a prognostic factor for predicting overall patient survival outcomes for patients with various malignant neoplasms, clinicopathological studies have revealed Postn overexpression to correlate with tumor invasiveness and metastatic growth in a variety of human carcinomas, including breast, prostate, and ovarian carcinomas [11, 64, 65], nasopharyngeal carcinoma [66] esophageal carcinoma [67], PDAC [54], hepatocellular carcinoma [53], and colon carcinoma [35]. In the cases of human ICC analyzed by Utispan et al. [43], other than overall survival, lymph node metastasis and other clinical data showed no association with Postn expression. However, in the same study, these investigators using the Matrigel invasion assay demonstrated exogeneous recombinant Postn to significantly increase in vitro cell invasiveness of the human cholangiocarcinoma cell lines KKU-M213 and KKU-M156 over control cells without Postn treatment.
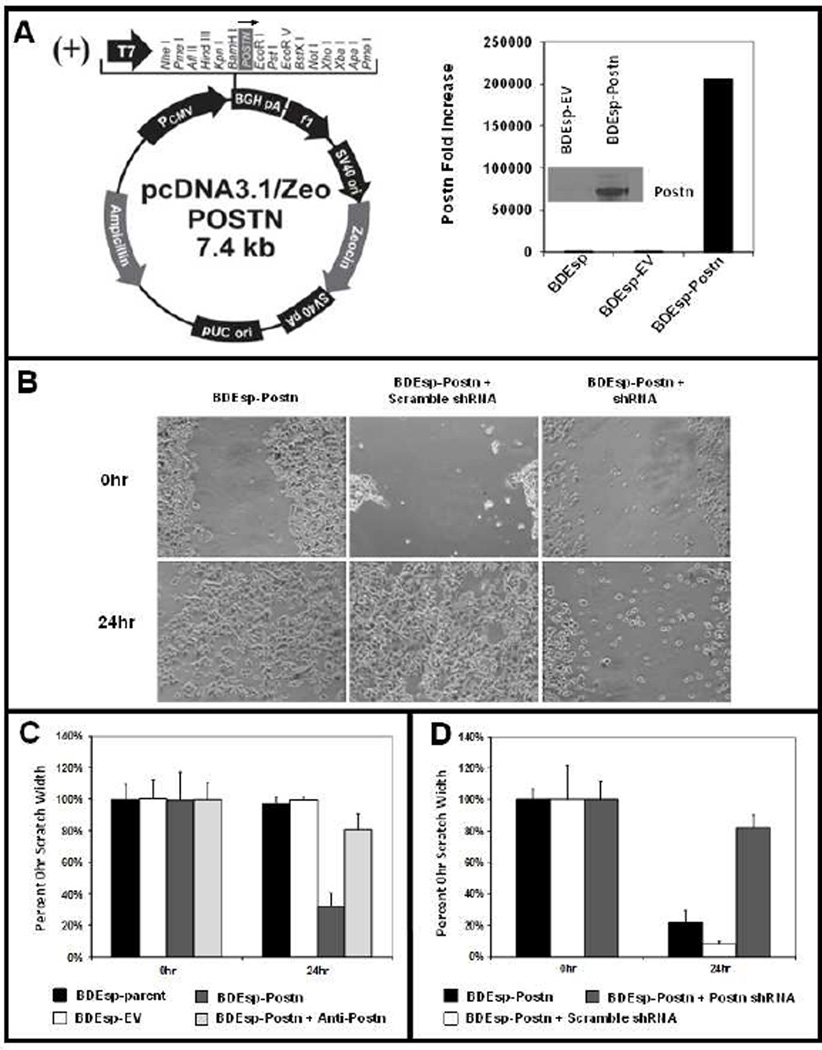
As shown in Figure 3A, we have now established from spontaneously transformed tumorigenic rat BDEsp cholangiocytes a novel cell strain (BDEsp-Postn cells) genetically engineered to constitutively overexpress rat periostin cDNA. As a further proof of principal, the data presented in Figure 3B–D demonstrate that BDEsp-Postn cells exhibit a marked increase in cell migration compared with parent BDEsp cells, as well as empty vector control BDEsp cells, when assayed in vitro in the scratch wound assay. As further shown in Figure 3C & D, this enhanced cell migration, which is a feature of cancer cell invasiveness, could be largely blocked by anti-periostin antibody treatment or by shRNA silencing of Postn expression.
Figure 3.
[A] Plasmid-based stable transfection of spontaneously-transformed, tumorigenic rat BDEsp cholangiocytes to constitutively overexpress normal rat periostin cDNA (2400bp). Real-time RT-PCR and Western blotting were used to validate resulting BDEsp-Postn clonal cell line (selected for zeocin resistance) for overexpression of normal rat Postn mRNA (graph) and secreted protein (insert) in culture medium. [B] Photomicrographs showing wound closure responses over a 24 hour period exhibited by BDEsp-Postn cells, BDEsp-Postn cells transfected with scrambled shRNA, and BDEsp-Postn cells transfected with rat Postn shRNA, respectively, in the scratch motility assay. [C] Scratch motility assay quantitative data demonstrating dramatically increased scratch wound closure, a reflection of cell migration, by cultured BDEsp-Postn cells compared with parent BDEsp and BDEsp- empty vector (EV) control cells. Treatment with anti-Postn antibody (ab14041 at 5.0 µg /ml) blocks the enhanced wound closure response of the BDEsp-Postn cells. [D] Similarly, as exemplified in [B], BDEsp-Postn cells and BDEsp-Postn cells transfected with scrambled shRNA, each exhibited prominent scratch wound closure responses at 24hr after the initial scrape, but this cell migration response is almost completely blocked in BDEsp-Postn cells transfected with rat periostin shRNA.
The mechanism by which Postn promotes cell migration and invasiveness in cholangiocarcinoma cells is only just beginning to be addressed, but as been determined for other carcinoma cell types, integrin- Akt activation is now believed to play a key role. More recent data from Utispan et al. [68] have provided evidence implicating the integrin α5β1 /PI3K/Akt signaling pathway in Postn–stimulated invasion of human KKU-M213 cholangiocarcinoma cells in vitro. It was also demonstrated in this study that KKU-M213 cells expressed a high level of integrin α6β4, which like integrin α5β1 functioned as a favorable receptor for Postn. It was further shown by Baril et al. [58] that the integrin α6β4 complex acts as the cell receptor for Postn in human pancreatic adenocarcinoma cells, resulting in activation of the PI3K/Akt and FAK pathways, which correlated with Postn-stimulated cellular motility, increased invasion through Matrigel matrix, and enhanced resistance to hypoxia-induced cell death of the cultured cancer cells. Although these findings are limited in scope, they suggest that Postn may be affecting ICC progression through its interaction with either integrin α5β1 or α6β4. Here, it is noteworthy that integrin β4, which binds to integrin α6 and which is prominently expressed in human and rat cholangiocarcinoma cells [46, 68], has been found to amplify Met and ErbB2 receptor tyrosine kinase signaling in tumorigenesis [69–71]. Since overexpression of activated ErbB2 and/or Met have been linked to ICC progression, it seems reasonable to now want to investigate if Postn through its interaction with α6β4 may be acting as an enhancer of Met and/or ErbB2 tyrosine kinase receptor signaling in relation to cholangiocarcinogenesis and invasive malignant growth.
Another novel mechanism by which Postn may be cooperating to mediate tumor invasion is suggested by the recent findings of Wong et al. [72], who demonstrated in an organotypic 3-dimensional culture model of esophageal squamous cell carcinoma (ESCC) invasion that Postn cooperates with mutant p53 to mediate invasion through the induction of the signal transducer and activator of transcription 1 signaling network. Furthermore, the effects of the interaction between Postn and Tnc on ICC progression need to be elucidated at the mechanistic level, since as noted above, both of these stromal matricellular proteins appear to be important collaborators in promoting invasive tumor growth and metastasis [39].
Postn as a Potential Target for Inhibiting ICC Invasive Growth and Metastasis
While additional studies are clearly needed to elucidate Postn’s function as a stromal mediator of cholangiocarcinoma cell migration and invasion, there is a growing, albeit limited, but compelling body of preclinical evidence to suggest that Postn could potentially serve as a target for inhibiting invasive cancer growth and metastasis of stromal enriched cancers, such as ICC. Kyutoku et al. [73] have reported that a neutralizing antibody (PN1-Ab) against full length Postn suppressed both primary and metastatic growth of Postn-expressing 4T1 mouse breast cancer cells and significantly increased the survival rate of the treated tumor bearing animals in a murine model. Similarly, Zhu et al. [74] have demonstrated a neutralizing monoclonal antibody (MZ-1) to Postn significantly inhibited the growth and reduced the metastatic potential of the Postn-expressing A2780 ovarian cancer cell line implanted either subcutaneously or intraperitoneally into SCID mice.
A potentially promising therapeutic approach to targeting Postn mediated cancer growth and metastasis is suggested by the recent preclinical findings of Lee et al. [75], who utilized a novel benzyl-d(U)TP-modified DNA aptamer (PNDA-3) capable of binding to human Postn with high affinity to inhibit biological functions related to cancer growth and metastasis. Using the Matrigel invasion assay, these investigators demonstrated PNDA-3 to significantly inhibit in vitro cell migration and invasion of three different human breast cancer cell lines. They further showed that PNDA-3 binding to the third or fourth FAS-1 domain in Postn blocked its binding to integrins, mostly to αvβ5 integrin in 4T1 and MDA-MB-231 human breast cancer cells, leading to a dose-dependent inhibition of FAK and Src phosphorylation that correlated with PNDA-3 concentration in the aptamer-treated cells. In a xenograft model of 4T1cell implantation into the mammary fat pads of female BALB/c mice, intratumorally injected PNDA-3 at 500µg/kg, three times a week for 16 days was observed to significantly decrease primary tumor volume, as well as to significantly reduce the number of distant nodular and focal metastases formed in lung. Moreover, the PDNA-3-treated primary 4T1 tumors were found to exhibit a significant decrease in Ki-67-positive cells, an indicator of proliferative activity, together with a significant reduction in microvascular density when compared with vehicle- or control aptamer-treated groups. Interestingly, single intraveneous injection of 500µg/kg Cy3-labeled PDNA-3 to the tumor bearing mice was determined to diffuse throughout the primary tumor stroma within 6 hours after the initial injection, with fluorescent signal still being detected in the tumor until 72 hours. This latter result suggests that systemic delivery of the Postn-binding DNA aptamer can concentrate in tumor overexpressing Postn, although it remains to be determines if intravenously delivered PDNA-3 is capable of producing a therapeutic response comparable to or greater than that achieved with intratumoral injection.
The use of novel relatively non-invasive imaging strategies combined with gene expression profiling to identify cancer phenotypes of differing malignant potential represents a promising approach to screen for molecular targets like Postn correlating with cellular invasion. The potential clinical value of using such imaging strategies to reveal Postn as a molecular determinant of tumor progression and invasion is suggested by results of two published studies, one preclinical [76] and the other clinical [77]. In the preclinical study Anil Rustgi and his collaborators described the development of an optical imaging method that uses near-infrared fluorescent imaging with upper-GI endoscopy to detect fluorescent signals from Cy 5.5-labeled Postn antibody localized to neoplastic lesions developed in a genetic mouse model of ESCC. In this model, the highest fluorescent signal was observed in mice with severe dysplasia compared with a lower level of signal intensity in those with mild dysplasia and no signal in control mice, thereby correlating with disease progression. Of further importance, this group of investigators had also demonstrated Postn mRNA and protein overexpression to be a key component of a novel tumor-invasive signature that annotates invasive human primary ESCC as being distinctive from adjacent normal human esophageal mucosa [78]. Interestingly, the results of this analysis further suggested that the induction of Postn could also arise in the stroma during ESCC progression.
The clinical study reported by Zinn et al. [77] combined gene expression profiling together with MRI-FLAIR (fluid attenuated inversion recovery) to screen for cancer subtypes and genomic correlates of cellular invasion in Glioblastoma Multiforme (GMB). Using this radiogenomic mapping approach, high Postn and low microRNA (miR) −219 were determined to be significantly associated with the mesenchymal GMB subset and malignant cell invasion, both of which are major features of GMB aggressiveness and therapy failure. It was further pointed out by these investigators that miR-219 is known to have a potential binding site in the 3’UTR of the Postn gene, suggesting that it may play a role as a down-regulator of Postn expression.
Although the potential therapeutic effects of targeting Postn in ICC still need to be determined, the preclinical data described above for other tumor types suggest that molecular suppression of Postn up-regulation and/or interactive signaling may have future promise as a testable adjuvant strategy for combating invasive ICC growth and metastasis. Moreover, utilizing current diagnostic and pre-therapeutic imaging methods to stage ICC, such as endoscopic retrograde cholangiopancreatography assisted biopsy or MRI/MRCP imaging, in conjunction with measurements of elevated serum Postn levels and Postn immunohistochemistry of biopsied ICC tissue might prove to be a useful approach to early identification of subsets of primary invasive or recurrent ICCs as potential candidates for intervention with Postn-specific targeted agents, that may include RNA interference molecules [79, Figure 3], inhibitory Postn binding aptamers, or therapeutic antibodies.
Future Challenges and Possibilities
While it seems increasingly apparent that Postn overexpression in the microenvironment of ICC and other types of solid tumors is playing an important contributing role in promoting cancer cell invasion and metastasis, leading to poor patient survival outcomes, critical challenges remain as to how to effectively exploit this overexpressed ECM protein as a potential prognostic biomarker and/or molecular target for ICC therapy and/or prevention. Future multicenter, randomly controlled human studies aimed at achieving non-biased validation of serum and tumor tissue Postn as a biomarker of primary ICC progression or recurrence after curative resection would do much to clarify its clinical usefulness as a potential predictor of ICC patient survival rates following primary tumor resection, as well as act as a possible molecular monitor of treatments aimed at targeting pro-invasive elements of CAF-enriched tumor stroma as a strategy for impairing ICC progression and metastasis.
It is also challenging that at present there have been no experimental animal studies specifically aimed at evaluating the therapeutic effects of in vivo silencing Postn expression or suppressing its cell signaling activity on ICC invasive growth and metastasis. In this regard, a need now exists to conduct rationally designed, mechanism-based preclinical studies in which animal models of ICC that closely mimic the human invasive/metastatic disease are used as preclinical platforms to identify and test novel molecular strategies for anti-metastatic ICC therapy based on selective knockdown of stromal cell Postn expression or inhibition of its pro-invasive signaling activity. The establishment of in vivo rodent cholangiocarcinoma models, such as the orthotopic rat BDE cholangiocarcinoma model [80] , available siRNAs for rat and mouse Postn together with advances in targeted siRNA delivery systems [81, 82], the development of a reproducible hepatic artery catheterization method for preclinical investigations of liver-directed therapies in rodent models of liver cancer [83], and commercial ELISA assay kits to measure serum Postn levels in rat and mouse models, all support the feasibility of now being able to preclinically investigate the effects of therapeutic targeting of Postn on cholangiocarcinoma invasive growth and metastasis.
Current evidence suggest that molecular components of the tumor microenvironment can act in a “Jekyll and Hyde” manner to either promote or retard carcinoma progression and metastasis [84, 85], as well as to increase or decrease the sensitivity of desmoplastic tumors to chemotherapeutic drugs. In terms of developing a therapeutic intervention strategy, this dichotomy is best exemplified at present by recent efforts to deplete tumor stromal based on targeting the Hedgehog (Hh) signaling pathway to suppress the accumulation of CAFs in the tumor microenvironment. Olive et al. [86] using a genetically engineered mouse model of PDAC were the first to demonstrate that pharmacological inhibition of Hh signaling inhibited the formation of CAF-enriched desmoplastic stroma, resulting in a transient increase in intratumoral microvascularity and concomitant increased in intratumoral concentration of the chemotherapeutic agent gemcitabine, leading to a transient stabilization of the disease. Pharmacological inhibition of Hh signaling in the syngeneic orthotopic rat BDEneu cholangiocarcinoma model has also been recently shown to be tumor and metastatic suppressive [87], although it is not yet known as to what extent this therapeutic effect was stromal dependent. Targeting CAFs for cell death by the BH3 mimetic navitoclax leading to CAF depletion in cholangiocarcinoma was further demonstrated to be therapeutic in the rat orthotopic BDEneu model (88), supporting CAF depletion as a possible general strategy potentially applicable to the treatment of solid tumors. In sharp contrast, Rhim and Olive and their colleagues [89] recently showed in mouse PDAC that deletion of CAFs from tumor microenvironment, either by genetic or pharmacological targeting of the Hh pathway, actually enhanced malignant aggressiveness by increasing vascularity and cancer cell proliferation, together with promoting a more undifferentiated histopathology. This study also demonstrated that the modest survival benefit afforded by improved gemcitabine availability to the tumor following short-term Hh signaling inhibition [86] was overcome by the negative effects of long-term inhibition of Hh signaling. This latter result is also consistent with the poor results obtained with Hh (Smoothened) inhibitors in PDAC clinical trials.
In lieu of the possibility that targeting some elements of the tumor stroma may accelerate rather than restrain the invasive growth and metastasis of desmoplastic carcinomas, such as ICC or PDAC, future approaches should focus on identifying and testing new tumor stromal therapeutic strategies that are specifically designed to abrogate interactive molecular pathways that are known to promote malignant cell invasion and metastasis. In this regard, selective targeting of interactive metastatic niche proteins represented by Postn and Tnc, both of which are strongly overexpressed in ICC stroma, would appear to be a rational choice for future translational studies aimed at developing novel stromal-based molecular treatment strategies for ICC and other solid desmoplastic cancers.
Acknowledgments
Financial Support: This work was supported by NIH grant R01 CA083650.
Abbreviations
- ICC
intrahepatic cholangiocarcinoma
- CAFs
cancer-associated fibroblasts
- α-SMA
α-smooth muscle actin
- EMT
epithelial-mesenchymal transition
- Postn
periostin
- OSF-2
osteoblast specific factor-2
- BMP-1
bone morphogenic protein-1
- Tnc
tenascin-C
- Akt
serine/threonine Akt/PKB
- FAK
focal adhesion kinase
- PI3K
phosphoinositide 3-kinase
- TGF
transforming growth factor, βig-h3, TGF-β induced clone 3
- SPARC
secreted protein acidic and rich in cysteine
- GI
gastrointestinal
- PDAC
pancreatic ductal adenocarcinoma
- ESCC
esophageal squamous cell carcinoma
- GMB
Glioblastoma Multiforme
- miR
micro-RNA, Hh, Hedgehog
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None to report
References
- 1.Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013;32:4861–4870. doi: 10.1038/onc.2012.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dodson RM, Weiss MJ, Cosgrove D, Herman JM, Kamel I, Anders R, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg. 2013;217:736–750. doi: 10.1016/j.jamcollsurg.2013.05.021. e4. [DOI] [PubMed] [Google Scholar]
- 3.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2012;9:44–54. doi: 10.1038/nrgastro.2011.222. [DOI] [PubMed] [Google Scholar]
- 5.Sirica AE, Gores GJ. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology. 2014;59:2397–2402. doi: 10.1002/hep.26762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294:271–278. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 8.Thirunavukkarasu K, Mahajan M, McLarren KW, Stifani S, Karsenty G. Two domains unique to osteoblast-specific transcription factor Osf2/Cbfa1 contribute to its transactivation function and its inability to heterodimerize with Cbfβ. Mol Cell Biol. 1998;18:4197–4208. doi: 10.1128/mcb.18.7.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pratap J, Lian JB, Javed A, Barnes GL, van Wijnen AJ, Stein JL, et al. Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Rev. 2006;25:589–600. doi: 10.1007/s10555-006-9032-0. [DOI] [PubMed] [Google Scholar]
- 10.Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 2009;66:2219–2230. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo Y, Siriwardena BSMS, Hatano H, Ogawa I, Takata T. Periostin: novel diagnostic and therapeutic target for cancer. Histol Histopathol. 2007;22:1167–1174. doi: 10.14670/HH-22.1167. [DOI] [PubMed] [Google Scholar]
- 12.Litvin J, Zhu S, Norris R, Markwald R. Periostin family of proteins: therapeutic targets for heart disease. Anat Rec. 2005;287A:1205–1212. doi: 10.1002/ar.a.20237. [DOI] [PubMed] [Google Scholar]
- 13.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci. 2011;68:3201–3207. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coutu DL, Wu JH, Monette A, Rivard G-É, Blostein MD, Galipeau J. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J Biol Chem. 2008;283:17991–18001. doi: 10.1074/jbc.M708029200. [DOI] [PubMed] [Google Scholar]
- 15.Hoersch S, Andrade-Navarro MA. Periostin shows increased evolutionary plasticity in its alternatively spliced region. BMC Evol Biol. 2010;10:30. doi: 10.1186/1471-2148-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011;459:465–475. doi: 10.1007/s00428-011-1151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morra L, Rechsteiner M, Casagrande S, von Teichman A, Schraml P, Moch H, et al. Characterization of periostin isoform pattern in non-small cell lung cancer. Lung Cancer. 2012;76:183–190. doi: 10.1016/j.lungcan.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Kim CJ, Isono T, Tambe Y, Chano T, Okabe H, Okada Y, et al. Role of alternative splicing of periostin in human bladder carcinogenesis. Int J Oncol. 2008;32:161–169. [PubMed] [Google Scholar]
- 19.Isono T, Kim CJ, Ando Y, Sakurai H, Okada Y, Inoue H. Suppression of cell invasiveness by periostin via TAB1/TAK1. Int J Oncol. 2009;35:425–432. [PubMed] [Google Scholar]
- 20.Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal. 2008;2:9–17. doi: 10.1007/s12079-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR. The many facets of the matricellular protein periostin during cardiac development, remodeling, and pathophysiology. J Cell Commun Signal. 2009;3:275–286. doi: 10.1007/s12079-009-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott CG, Wang J, Guo X, Xu S-W, Eastwood M, Guan J, et al. Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J Cell Sci. 2012;125:121–132. doi: 10.1242/jcs.087841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ontsuka K, Kotobuki Y, Shiraishi H, Serada S, Ohta S, Tanemura A, et al. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol. 2012;21:331–336. doi: 10.1111/j.1600-0625.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 24.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1046–L1056. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mael-Ainin M, Abed A, Conway SJ, Dussaule J-C, Chatziantoniou C. Inhibition of periostin expression protects against the development of renal inflammation and fibrosis. J Am Soc Nephrol. 2014;25:1724–1736. doi: 10.1681/ASN.2013060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S, et al. Periostin facilitates skin sclerosis via PI3K/Ak mechanism in a mouse model of scleroderma. PLOS One. 2012;7:e41994. doi: 10.1371/journal.pone.0041994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa K, Yoshida S, Nakao S, Nakama T, Kita T, Asato R, et al. Periostin promotes the generation of fibrous membranes in proliferative vitreoretinopathy. FASEB J. 2014;28:131–142. doi: 10.1096/fj.13-229740. [DOI] [PubMed] [Google Scholar]
- 29.Izuhara K, Arima K, Ohta S, Suzuki S, Inamitsu M, Yamamoto K. Periostin in allergic inflammation. Allergol Int. 2014;63:143–151. doi: 10.2332/allergolint.13-RAI-0663. [DOI] [PubMed] [Google Scholar]
- 30.Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I, et al. Periostin creates a tumor-supportive micoenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–1464. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 31.Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem. 2010;285:13294–13303. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, et al. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem. 2010;285:2028–2039. doi: 10.1074/jbc.M109.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung CY, Erickson HP. Glycosaminoglycans modulate fibronectin matrix assembly and are essential for matrix incorporation of tenascin-C. J Cell Sci. 1997;110:1413–1419. doi: 10.1242/jcs.110.12.1413. [DOI] [PubMed] [Google Scholar]
- 34.Wong GS, Rustgi AK. Matricellular proteins: priming the tumour microenvironment for cancer development and metastasis. Br J Cancer. 2013;108:755–761. doi: 10.1038/bjc.2012.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–339. doi: 10.1016/s1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 36.Ouyang G, Liu M, Ruan K, Song G, Mao Y, Bao S. Upregulated expression of periostin by hypoxia in non-small-cell lung cancer cells promotes cell survival via the Akt/PKB pathway. Cancer Lett. 2009;281:213–219. doi: 10.1016/j.canlet.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, et al. Periostin mediates vascular smooth muscle cell migration through the integrins av(33 and av(35 and focal adhesion kinase (FAK) pathway. Atherosclerosis. 2010;208:358–365. doi: 10.1016/j.atherosclerosis.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr H-A, Delaloye J-F, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 39.Oskarsson T, Massagué J. Extracellular matrix players in metastatic niches. EMBO J. 2012;31:254–256. doi: 10.1038/emboj.2011.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Liu J, Wang Z, Huang Y, Liu W, Zhu X, et al. Periostin contributes to the acquisition of multipotent stem cell-like properties in human mammary epithelial cells and breast cancer cells. PLOS One. 2013;8:e72962. doi: 10.1371/journal.pone.0072962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukushima N, Kikuchi Y, Nishiyama T, Kudo A, Fukayama M. Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Mod Pathol. 2008;21:1044–1053. doi: 10.1038/modpathol.2008.77. [DOI] [PubMed] [Google Scholar]
- 42.Sirica AE, Dumur CI, Campbell DJW, Almenara JA, Ogunwobi OO, DeWitt JL. Intrahepatic cholangiocarcinoma progression: prognostic factors and basic mechanisms. Clin Gastroenterol Hepatol. 2009;7:S68–S78. doi: 10.1016/j.cgh.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Utispan K, Thuwajit P, Abiko Y, Charngkaew K, Paupairoj A, Chau-in S, et al. Gene expression profiling of cholangiocarcinoma-derived fibroblast reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Mol Cancer. 2010;9:13. doi: 10.1186/1476-4598-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dumur CI, Campbell DJW, DeWitt JL, Oyesanya RA, Sirica AE. Differential gene expression profiling of cultured neu-transformed versus spontaneously-transformed rat cholangiocytes and of corresponding cholangiocarcinomas. Exp Mol Pathol. 2010;89:227–235. doi: 10.1016/j.yexmp.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darby IA, Vuillier-Devillers K, Pinault É, Sarrazy V, Lepreux S, Balabaud C, et al. Proteomic analysis of differentially expressed proteins in peripheral cholangiocarcinoma. Cancer Microenviron. 2010;4:73–91. doi: 10.1007/s12307-010-0047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell DJW, Dumur CI, Lamour NF, DeWitt JL, Sirica AE. Novel organotypic culture model of cholangiocarcinoma progression. Hepatol Res. 2012;42:1119–1130. doi: 10.1111/j.1872-034X.2012.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erkan M, Reiser-Erkan C, Michalski CW, Deucker S, Sauliunaite D, Streit S, et al. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia. 2009;11:497–508. doi: 10.1593/neo.81618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okamoto K, Tajima H, Ohta T, Nakanuma S, Hayashi H, Nakagawara H, et al. Angiotensin II induces tumor progression and fibrosis in intrahepatic cholangiocarcinoma through an interaction with hepatic stellate cells. Int J Oncol. 2010;37:1251–1259. doi: 10.3892/ijo_00000776. [DOI] [PubMed] [Google Scholar]
- 49.Li L, Fan D, Wang C, Wang J-Y, Cui X-B, Wu D, et al. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-β1 pathways in cardiac fibroblasts. Cardiovasc Res. 2011;91:80–89. doi: 10.1093/cvr/cvr067. [DOI] [PubMed] [Google Scholar]
- 50.Li Y-S, Ni S-Y, Meng Y, Shi X-L, Zhao X-W, Luo H-H, et al. Angiotensin II facilitates fibrogenic effect of TGF-β1 through enhancing the down-regulation of BAMBI caused by LPS: a new pro-fibrotic mechanism of angiotensin II. PLOS One. 2013;8:e76289. doi: 10.1371/journal.pone.0076289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okamoto K, Tajima H, Nakanuma S, Sakai S, Makino I, Kinoshita J, et al. Angiotensin II enhances epithelial-to-mesenchymal transition through the interaction between activated hepatic stellate cells and the stromal cell-derived factor-1/CXCR4 axis in intrahepatic cholangiocarcinoma. Int J Oncol. 2012;41:573–582. doi: 10.3892/ijo.2012.1499. [DOI] [PubMed] [Google Scholar]
- 52.Riener M-O, Fritzsche FR, Soll C, Pestalozzi BC, Probst-Hensch N, Clavien P-A, et al. Expression of the extracellular matrix protein periostin in liver tumours and bile duct carcinomas. Histopathology. 2010;56:600–606. doi: 10.1111/j.1365-2559.2010.03527.x. [DOI] [PubMed] [Google Scholar]
- 53.Lv Y, Wang W, Jia W-D, Sun Q-K, Li J-S, Ma J-L, et al. High-level expression of periostin is closely related to metastatic potential and poor prognosis of hepatocellular carcinoma. Med Oncol. 2013;30:385. doi: 10.1007/s12032-012-0385-7. [DOI] [PubMed] [Google Scholar]
- 54.Ben Q-W, Jin X-L, Liu J, Cai X, Yuan F, Yuan Y-Z. Periostin, a matrix specific protein, is associated with proliferation and invasion of pancreatic cancer. Oncol Rep. 2011;25:709–716. doi: 10.3892/or.2011.1140. [DOI] [PubMed] [Google Scholar]
- 55.Wang W, Sun Q-K, He Y-F, Ma D-C, Xie M-R, Ji C-S, et al. Overexpression of periostin is significantly correlated to the tumor angiogenesis and poor prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:593–601. [PMC free article] [PubMed] [Google Scholar]
- 56.Wu G, Wang X, Xhang X. Clinical implications of periostin in the liver metastasis of colorectal cancer. Cancer Biother Radiopharm. 2013;28:298–302. doi: 10.1089/cbr.2012.1374. [DOI] [PubMed] [Google Scholar]
- 57.Fujimoto K, Kawaguchi T, Nakashima O, Ono J, Ohta S, Kawaguchi A, et al. Periostin, a matrix protein, has potential as a novel serodiagnostic marker for cholangiocarcinoma. Oncol Rep. 2011;25:1211–1216. doi: 10.3892/or.2011.1194. [DOI] [PubMed] [Google Scholar]
- 58.Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, Harada T, et al. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the β4 integrin and the PI3K pathway. Oncogene. 2007;26:2082–2094. doi: 10.1038/sj.onc.1210009. [DOI] [PubMed] [Google Scholar]
- 59.Lv Y, Wang W, Jia WD, Sun QK, Huang M, Zhou HC, et al. High preoparative levels of serum periostin are associated with poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Eur J Surg Oncol. 2013;39:1129–1135. doi: 10.1016/j.ejso.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 60.Ben Q-W, Zhao Z, Ge S-F, Zhou J, Yuan F, Yuan Y-Z. Circulating levels of periostin may help identify patients with more aggressive colorectal cancer. Int J Oncol. 2009;34:821–828. doi: 10.3892/ijo_00000208. [DOI] [PubMed] [Google Scholar]
- 61.Ruys AT, Koerkamp BG, Wiggers JK, Klümpen H-J, ten Kate FJ, van Gulik TM. Prognostic biomarkers in patients with resected cholangiocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21:487–500. doi: 10.1245/s10434-013-3286-x. [DOI] [PubMed] [Google Scholar]
- 62.Chuaysri C, Thuwajit P, Paupairoj A, Chau-In S, Suthiphongchai T, Thuwajit C. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep. 2009;21:957–969. doi: 10.3892/or_00000309. [DOI] [PubMed] [Google Scholar]
- 63.Okabe H, Beppu T, Hayashi H, Horino K, Masuda T, Komori H, et al. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:2555–2564. doi: 10.1245/s10434-009-0568-4. [DOI] [PubMed] [Google Scholar]
- 64.Nuzzo PV, Rubagotti A, Zinoli L, Ricci F, Salvi S, Boccardo S, et al. Prognostic value of stromal and epithelial periostin expression in human prostate cancer: correlation with clinical pathological features and the risk of biochemical relapse or death. BMC Cancer. 2012;12:625. doi: 10.1186/1471-2407-12-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu M, Fejzo MS, Anderson L, Dering J, Ginther C, Ramos L, et al. Periostin promotes ovarian cancer angiogenesis and metastasis. Gynecol Oncol. 2010;119:337–344. doi: 10.1016/j.ygyno.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 66.Li M, Li C, Li D, Xie Y, Shi J, Li G, et al. Periostin, a stroma-associated protein, correlates with tumor invasiveness and progression in nasopharyngeal carcinoma. Clin Exp Metastasis. 2012;29:865–877. doi: 10.1007/s10585-012-9465-5. [DOI] [PubMed] [Google Scholar]
- 67.Luo J-H, Zhou J, Gao Y. Correlation between periostin and SNCG and esophageal cancer invasion, infiltration and apoptosis. Asian Pac J Trop Med. 2013;6:516–519. doi: 10.1016/S1995-7645(13)60088-7. [DOI] [PubMed] [Google Scholar]
- 68.Utispan K, Sonongbua J, Thuwajit P, Chau-In S, Pairojkul C, Wongkham S, et al. Periostin activates integrin α5β1 through a PI3K/AKT-dependent pathway in invasion of cholangiocarcinoma. Int J Oncol. 2012;41:1110–1118. doi: 10.3892/ijo.2012.1530. [DOI] [PubMed] [Google Scholar]
- 69.Bertotti A, Comoglio PM, Trusolino L. β4 integrin is a transforming molecule that unleashes Met tyrosine kinase tumorigenesis. Cancer Res. 2005;65:10674–10679. doi: 10.1158/0008-5472.CAN-05-2827. [DOI] [PubMed] [Google Scholar]
- 70.Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, et al. β4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 71.Yoshioka T, Otero J, Chen Y, Kim Y-M, Koutcher JA, Satagopan J, et al. β4 integrin signaling induces expansion of prostate tumor progenitors. J Clin Invest. 2013;123:682–699. doi: 10.1172/JCI60720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong GS, Lee J-S, Park Y-Y, Klein-Szanto AJ, Waldron TJ, Cukierman E, et al. Periostin cooperates with mutant p53 to mediate invasion through the induction of STAT1 signaling in the esophageal tumor microenvironment. Oncogenesis. 2013;2:e59. doi: 10.1038/oncsis.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kyutoku M, Taniyama Y, Katsuragi N, Shimizu H, Kunugiza Y, Iekushi K, et al. Role of periostin in cancer progression and metastasis: inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int J Mol Med. 2011;28:181–186. doi: 10.3892/ijmm.2011.712. [DOI] [PubMed] [Google Scholar]
- 74.Zhu M, Saxton RE, Ramos L, Chang DD, Karlan BY, Gasson JC, et al. Neutralizing monoclonal antibody to periostin inhibits ovarian tumor growth and metastasis. Mol Cancer Ther. 2011;10:1500–1508. doi: 10.1158/1535-7163.MCT-11-0046. [DOI] [PubMed] [Google Scholar]
- 75.Lee YJ, Kim IS, Park S-A, Kim Y, Lee JE, Noh D-Y, et al. Periostin-binding DNA aptamer inhibits breast cancer growth and metastasis. Mol Ther. 2013;21:1004–1013. doi: 10.1038/mt.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong GS, Habibollahi P, Heidari P, Lee J-S, Klein-Szanto AJ, Waldron TJ, et al. Optical imaging of periostin enables early endoscopic detection and characterization of esophageal cancer in mice. Gastroenterology. 2013;144:294–297. doi: 10.1053/j.gastro.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zinn PO, Majadan B, Sathyan P, Singh SK, Majumder S, Jolesz FA, et al. Radiogenomic mapping of edema/cellular invasion MRI-phenotypes in glioblastoma multiforme. Plos One. 2011;6:e25451. doi: 10.1371/journal.pone.0025451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Michaylira CZ, Wong GS, Miller CG, Gutierrez CM, Nakagawa H, Hammond R, et al. Periostin, a cell adhesion molecule, facilitates invasion in the tumor microenvironment and annotates a novel tumor-invasive signature in esophageal cancer. Cancer Res. 2010;70:5281–5292. doi: 10.1158/0008-5472.CAN-10-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun C, Zhao X, Xu K, Gong J, Liu W, Ding W, et al. Periostin: a promising target of therapeutic intervention for prostate cancer. J Transl Med. 2011;9:99. doi: 10.1186/1479-5876-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sirica AE, Zhang Z, Lai G-H, Asano T, Shen X-N, Ward DJ, et al. A novel “patientlike” model of cholangiocarcinoma progression based on bile duct inoculation of tumorigenic rat cholangiocyte cell lines. Hepatology. 2008;47:1178–1190. doi: 10.1002/hep.22088. [DOI] [PubMed] [Google Scholar]
- 81.Fonseca NA, Gregório AC, Valério-Fernandes A, Simões S, Moreira JN. Bridging cancer biology and the patient’s needs with nanotechnology-based approaches. Cancer Treat Rev. 2014 doi: 10.1016/j.ctrv.2014.02.002. Epub 2014 Feb 22. [DOI] [PubMed] [Google Scholar]
- 82.Williford JM, Wu J, Ren Y, Archang MM, Leong KW, Mao HQ. Recent advances in nanoparticle-mediated siRNA delivery. Annu Rev Biomed Eng. 2014 doi: 10.1146/annurev-bioeng-071813-105119. Epub 2014 Jun 2. [DOI] [PubMed] [Google Scholar]
- 83.Sheu AY, Zhang Z, Omary RA, Larson AC. Invasive catheterization of the hepatic artery for preclinical investigation of liver-directed therapies in rodent models of liver cancer. Am J Transl Res. 2013;5:269–278. [PMC free article] [PubMed] [Google Scholar]
- 84.Bierie B, Moses HL. Tumor microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 85.Allen M, Louse Jones J. Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J Pathol. 2011;223:162–176. doi: 10.1002/path.2803. [DOI] [PubMed] [Google Scholar]
- 86.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;12:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Razumilava N, Gradilone SA, Smoot RL, Mertens JC, Bronk SF, Sirica AE, et al. Non-canonical Hedgehog signaling contributes to chemotaxis in cholangiocarcinoma. J Hepatol. 2014;60:599–605. doi: 10.1016/j.jhep.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2012;73:897–907. doi: 10.1158/0008-5472.CAN-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support pancreatic ductal carcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]